Abstract
The remediation of contaminated groundwater is a critical component of environmental management. In situ bioremediation (ISB) is a technique used to treat contaminated groundwater by promoting the activity of microorganisms, which transform harmful substances into less toxic or non-toxic forms. The present study investigates social issues regarding the sustainability approach applied to the remediation of contaminated sites by analyzing occupational health and safety (OH&S) impacts on workers involved in the remediation process. Occupational chemical risk is analyzed by comparing two techniques for the remediation of groundwater contaminated by chlorinated aliphatic hydrocarbons (CAHs): the pump-and-treat system and biological barriers. A contaminated Italian site, located near an industrial waste disposal site, was analyzed, applying a quantitative approach for chemical risk assessment. This approach is based on the use of a validated algorithm (SNPA software) and environmental measurement campaigns of chemical agents. This study (as far as the authors know) is the first research carried out in this field and shows that the adoption of bio-barriers for remediation is intrinsically safer in terms of OH&S impacts on workers.
1. Introduction
Sustainability encompasses the concept of the three Ps: profit, planet and people. However, the majority of organizations and researchers is mainly concerned with the first two topics: economic- (i.e., life cycle cost assessment, cost–benefit analysis, etc.) and energy-related matters (such as energy consumption, emissions reductions, etc.) [1,2]. In this context, researchers have recently focused their efforts on the third concept, as it is connected with social development. In particular, the main goal of social development is based on ensuring the health and safety (H&S) of human beings, because a human’s quality of life strongly depends on reducing accidents related to H&S. Considering this aspect, Nawaz et al. [1] proved that there was a connection between people’s safety and sustainable development, as shown via examining different case studies.
The issue of social development can be a very important matter in reference to the remediation of contaminated sites. Chlorinated aliphatic hydrocarbons (CAHs) contaminate soils and groundwater via leaching from industrial sites and landfills and improper disposal. It is estimated that there are 650,000 contaminated sites in Europe, which require remediation. There are 2600 of these sites in Italy [3]. In particular, chlorinated organic compounds are present in 10% of contaminated water and 8% of contaminated soil [4]. The specific European directives for water indicate that perchloroethylene (PCE) and trichlorethylene (TCE) are the main contaminants [5].
Several techniques can be used to remediate sites contaminated by CAHs. Chlorinated solvents are compounds that are resistant to traditional treatments, but they can be effectively transformed by microorganisms. Chloroethenes (CE) can be biodegraded via reductive dechlorination under anaerobic conditions as well as via oxidation under aerobic conditions [6]. The strategy of stimulating natural biological activity is based on the injection of essential nutrients and electron donors in order to maximize the metabolism of the microbiome [7]. Chloroethenes’ tendency to undergo reductive dechlorination decreases with a decreasing number of chlorine substituents. On the other hand, in the presence of fewer chlorine substituents, chloroethenes more readily undergo oxidative degradation. In fact, less-chlorinated aliphatic hydrocarbons (CAHs), produced by the degradation of highly CAHs, are usually recalcitrant under anaerobic conditions and more easily biodegraded under aerobic conditions. This is the case of vinyl chloride (VC), which is more toxic than the more-chlorinated compounds and classified by the International Agency for Research on Cancer (IARC) as a cancerogenic compound [8]. Sequential anaerobic/aerobic biodegradation can compensate for the disadvantages of reductive dechlorination and leads to the complete mineralization of the chlorinated pollutants.
The pump-and-treat (P&T) system is a method for cleaning up contaminated groundwater containing chemicals such as industrial solvents, metals and fuel oil. Groundwater is pumped from wells or trenches to the treatment plant, which removes the contaminants. This pumping prevents contaminants from reaching drinking-water wells, wetlands, streams and other natural resources.
P&T plants usually consist of one or more wells designed to extract contaminated groundwater. Groundwater is pumped from the “extraction wells” into a treatment system, which may involve several different cleaning methods [9]. The pumped water is treated through a sequential combination of filtering and precipitation, air stripping, catalytic oxidation and activated carbon adsorption. Therefore, P&T systems involve the use of different chemicals, which could pose OH&S risks. An example of a P&T system is depicted in Figure 1.
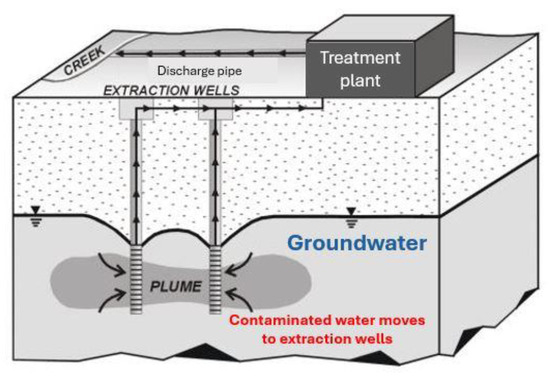
Figure 1.
A pump-and-treat system with two extraction wells (reproduced from [9]).
P&T plants have been in operation at several sites for many years. It has to be highlighted that data collected from these sites reveal that although P&T systems can be promising during the initial stages of implementation, their performance suddenly decreases in later stages. As a result, a significant quantity of contamination can remain unaffected by the treatment [10].
In situ bioremediation (ISB) offers several advantages from a sustainability perspective:
- Energy efficiency;
- Reduced environmental impact, particularly with regard to the surrounding ecosystem;
- Cost-effectiveness due to the absence of extensive infrastructure and energy consumption associated with P&T systems;
- Long-term effectiveness.
In this framework, INAIL (National Institute for Insurance against Accidents at Work) funded a research project through the call INAIL-BRIC 2019 (project ID: 52). The studied system was aimed at remediating a solute plume, which contained CAHs in an Italian alluvial aquifer.
The sustainability integration into site remediation studies requires a comprehensive approach, which considers not only environmental aspects but also the H&S of workers involved in the remediation process.
The goal of this study is to assess the occupational chemical risk, comparing two techniques for the remediation of groundwater contaminated by CAHs: the P&T system and biological barriers.
2. The Case Study
The site (170,000 m2) is located in Italy, near an industrial waste disposal site (160,000 m2 and the total waste mass is about 1,700,000 tons).
The zone lies three meters below the sea level. The site is bounded to the south by a lagoon and to the north by the agricultural land reclamation canal, which is an artificial drainage system built to allow agricultural activities [11]. In Figure 2, a site map is reported. Since 1995, the aquifer has been secured by a hydraulic barrier operating with a P&T system.
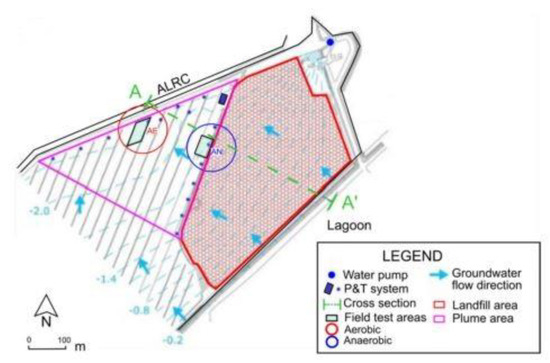
Figure 2.
The site map.
The groundwater below the landfill is affected by several contaminants (chlorinated compounds, petroleum hydrocarbons and BTEX, arsenic and heavy metals). Background information, related to the plume source and the aquifer configuration, is reported in Casiraghi et al. [11]. The P&T system was used for the hydraulic control of the contaminated plume in the aquifer. In order to assess in situ aerobic and anaerobic bioremediation processes, two pilot bio-barriers were installed and monitored.
2.1. The Groundwater Treatment Plant (P&T Process)
In the examined site, the hydraulic confinement system of the groundwater contamination plume consists of a hydraulic barrier, which is composed of 13 wells and 2 piezometers. The treatment plant is located in a shed, and it is composed of the following units:
- Oxidation and metal filtration section: Sodium hyplochlorite (NaClO) and hydroxide (NaOH) are injected in the spilled groundwater. The first compound favors the arsenic precipitation, whereas NaOH generates suitable conditions for precipitation.
- Desorption (stripping) section: The previously treated groundwater undergoes acidification based on the hydrochloric acid dosage, and it is successively fed to the desorption section. In particular, two strippers are used, and the air flow allows to remove the volatile substances. From the second stripper bottom, the water is moved to the sand filter, and it is successively moved to charcoal canisters.
- Section of final water treatment by adsorption: Contaminants and micro-pollutants, contained in the water, are trapped by an adsorption system, which uses charcoal canisters.
- Unit of gaseous effluents treatment: It is composed of the oxidation section and scrubber. In the first component, hydrocarbon and chlorine-based compounds are oxidized to hydrochloric acid, carbon dioxide and water vapor. The scrubber removes the chemicals produced via oxidation from the gaseous stream.
- Air treatment section (charcoal canisters): In the case of an oxidation section failure, the air, exiting from the desorption unit, is automatically moved to two couples of charcoal canisters. The air flow is released in the atmosphere by a stack.
- Section of the treated water reinjection: The treated water is stored in a tank, and a part of its quantity is used to create a physical barrier near the channel.
2.2. The Bioremediation System
The bioremediation system consists of two bio-barriers arranged in sequence and perpendicular to the direction of the groundwater flow:
- A barrier under anaerobic conditions designed to promote the degradation of more-chlorinated compounds [12];
- A barrier under aerobic conditions aimed at promoting the degradation of less-chlorinated compounds (mainly VC) produced by the anaerobic bio-barrier.
The first bio-barrier is anaerobic and placed immediately downstream from the landfill, which runs along the north-eastern side (its length is about 390 m). The bio-barrier consists of 39 wells, which allow for the addition of a reducing substrate, consisting of soluble condensed molasse, to stimulate anaerobic microbial activity (Figure 3).
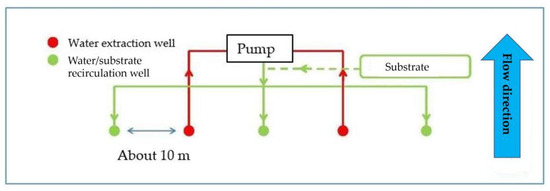
Figure 3.
Anaerobic bio-barrier layout.
The recirculation water flow for each injection well is 0.05–0.07 m3/h. The volume of the substrate injected into the aquifer varies between 1.8 and 2.8 L/day. In the full-scale application, the substrate quantity to be injected is about 14,000 kg/year.
The feeding of the reducing substrate through recirculation wells allows for homogeneous substrate diffusion via a groundwater recirculation system. Furthermore, the substrate can be continuously dosed. The substrate dosage takes place using three independent dosing units, each of which controls approximately 39 wells (19 for water extraction and 20 for water/substrate injection).
The second barrier is aerobic and aimed at promoting the biodegradation of VC and BTEX [13]. It is located downstream from the first and parallel to the drainage channel with a length of 400 m. In Figure 4, the aerobic barrier layout is reported.
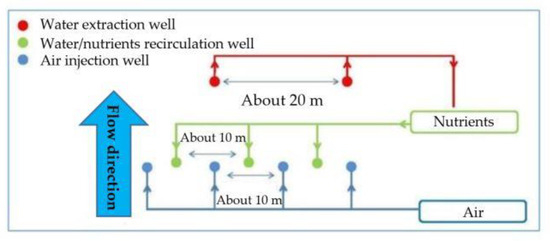
Figure 4.
Aerobic bio-barrier layout.
The bio-barrier consists of 39 wells (19 for water extraction and 20 for its reinjection after dosing with nitrogen- and phosphorus-based compounds. The average recirculation rate is about 0.93 m3/h per well. Downstream from the aerobic bio-barrier and parallel to it, there is a P&T system, which treats the water, coming from the bio-barriers, to eliminate residual contaminants from the sequential treatment before returning the water to the drainage channel.
To stimulate the process of aerobic biodegradation, it is necessary to supply the aquifer with oxygen and nutrients (urea and ammonium phosphate), which release nitrogen and phosphorus. The nutrients fed into the aquifer occur through recirculation wells. The water recirculation and nutrient addition occur via electric pumps. During full-scale application, the nutrient quantity, which is fed, is approximately 3000 kg/year (phosphorus/nitrogen ratio = approximately 1:3). Oxygen is supplied to the aquifer by blowing atmospheric air into injection wells. The amount of air blown in each well is about 0.6–1.5 m3/h.
3. Materials and Methods
3.1. Methodological Approaches of Chemical Risk Assessment
The site taken into account by the project offered the chance of studying the use of bio-barriers compared to the adoption of P&T plant, as the two processes have been active since 2005 (P&T) and 2016 (bio-barriers), allowing them to treat contaminated groundwater.
Analysis of the professional chemical risk was based on European occupational health and safety directives but also taking into account European product regulations (Regulation EC No 1272/2008) on classification, labeling and packaging of chemicals [14] and Technical Standard (EN 689) on assessment of exposure to chemical agents in the workplace [15].
In accordance with Directive 98/24/EC (art. 4), the occupational chemical risk has to consider several factors, including the following: hazardous substance properties; information on safety and health that shall be provided by the supplier (e.g., the relevant Safety Data Sheet); the level, type and duration of exposure; the circumstances of work involving such agents (their quantity); occupational exposure limit values or biological limit values established by law; the effect of prevention measures taken or to be taken; and, where available, the conclusions derived from the health surveillance.
According to the same directive, the assessment of professional risk from dangerous substances presupposes the acquisition of information on the following:
- Production cycle and process layout;
- Chemical agents, i.e., substances and mixtures, which are present in the production cycle: raw materials, pollutants in the groundwater, products and intermediates of chemical reactions between raw materials and pollutants, by-products, waste;
- Job description of staff potentially exposed to risk. Each task should be defined in terms of type and sequence of operations carried out and worker exposure times. This also allows us to define the Similar Exposure Group (SEG) referred to in the EN 689 standard [15].
A SEG is a group of workers, who have common risks and similar exposure profiles [15]. SEGs are created on knowledge of employee activities, hypothesizing that similar tasks with similar regularity will result in similar exposures (qualitative approach), or based on exposure measurements (quantitative).
In the framework of the occupational chemical risk assessment, for each SEG and for each individual substance, health risks will be distinguished from the safety ones.
There are different methodological approaches for the assessment: qualitative, semi-quantitative and quantitative.
The quantitative approach is based on the use of validated algorithms or environmental measurement campaigns of chemical agents to verify the compliance with the occupational exposure limit values (OELVs).
3.2. Validated Algorithms
The chemical risk analysis can be performed applying special tools, which are able to help the employer to carry out the assessment and set preventive measures.
In this study, risk assessment was performed applying a guideline provided by Italian National System for Environmental Protection (SNPA) for the protection of operators from risks deriving from chemical agents [16]. The guideline meets the requirements of Community legislation and the updates introduced by EC Regulations 1907/2006 (REACH Regulation) [17] and 1272/2008 (CLP Regulation). They provide a methodology for chemical risk assessment, distinguishing between risks for safety, related to reactivity and chemical–physical properties of substances, and risks for health, which could be caused by the toxicological properties of chemical agents. This guideline was developed for risk assessment in research and didactic laboratories of SNPA [18], but it has been applied to P&T systems because of the exiguous quantity of chemicals handled during the week.
In the following paragraph, the SNPA tool is described.
3.2.1. Risk for Health
The risk assessment for health includes three steps: hazard assessment, exposure assessment and risk characterization [18].
The first point requires the collection and evaluation of any available information on substances and their properties in order to identify danger, potential effects on workers’ health and occupational limit value.
The assessment of dangerous substances and mixtures requires estimating and/or measuring the concentration to which workers are or may be exposed.
The risk characterization (exposure level), lastly, depends on exposure limit values and protective measures taken to decrease them. This characterization can be carried out using the calculation model shown below. The calculation model compares the risk factors with all preventive and protective measures used to reduce the risk. The methodology used for the assessment of risks for health is focused on identification of the workers’ exposure level to hazardous chemical agents. It adopts the assessment criteria defined by the REACH-compatible ECETOC TRA (Targeted Risk Assessment) tool [18].
The first step of the assessment procedure is the collection of information [18]:
- Chemical agents contained in the workplace;
- Analysis of tasks, activities and workplaces;
- Prevention and protection measures already adopted in the working environment.
The procedure has to be applied to each worker or SEG of workers. An algorithm has been employed for the calculation of risk index or exposure level L, based on all parameters reported in Directive 98/24/EC (art. 4):
where:
- L: worker exposure level to the n dangerous chemical agents;
- Hi: sum of the factors of danger characterizing risky properties H of the i-th dangerous chemical agent (H phrases under CLP Regulation);
- Ti: sum of exposure factors T characterizing the route of exposure (dermal and/or by inhalation);
- Si: physical state factor corresponding to the physical substance state;
- Ei: exposure length of time factor corresponding to the duration of exposure in a reference week;
- Qi: amount factor corresponding to the chemical agent amount used in a reference week;
- Ui: condition of use factor taking into account the possibility of release into the air of the substance;
- Di: stored quantity factor corresponding to the chemical agent amount in the workplace (excluding the amount contained in safety cabinets and in specific storage areas) in a reference week;
- Ai: work factor depending on the work activity (usual work, maintenance, cleaning, waste management);
- Ki: worker prevention and protection factor corresponding to all the measures (fume hood and extraction systems, collective and personal protection devices, written procedures, specific training of workers, etc.) taken to decrease the risks;
- VLi: threshold limit value (ppm), if any, set by European Directives for the i-th substance [18].
For each i-th hazardous substance handled by an operator, the IT tool calculates a Li value; all Li values are summed for calculating the overall L exposure for the worker according to Equation (1). The relationship is based on the conservative assumption that all chemical agents used in a week are handled in a single day. If, according to the parameters employed in the calculation of risk index, the overall exposure level (L) for an operator or a SEG is lower than 1, it can be deduced that preventive and protective measures are able to contain the risks for health or that the risks are decreased to an acceptable level. In this case, there is no obligation to carry out a deeper risk assessment in order to apply specific protective and preventive measures, arrangements to deal with accidents and emergencies, health surveillance and exposure records, as required by legislation. Higher L and Li values clearly signify higher-risk situations, as shown in Table 1 and Table 2 [18].

Table 1.
L values.

Table 2.
Li values.
3.2.2. Risks for Safety
The assessment of risk for safety was based on qualitative remarks on chemical–physical properties of the compounds used by the workers and on workplace characteristics. The assessment has to be carried out whenever dangerous chemical agents characterized by hazard statements (phrases H2XX) and by supplemental hazard information on fire, explosion and corrosion danger (phrases EUHXXX) are handled in a workspace [18]. In particular, the risk assessment includes the following:
- Fire risk;
- Explosion risk (formation of potentially explosive atmospheres);
- Risk from incompatibility among chemicals.
The explosion risk can derive from accidental releases of flammable gases/vapors, which could generate potentially explosive atmospheres. According to the ATEX (ATmosphere EXplosive—ATEX) Directive 99/92/EC, the explosion risk has to be assessed, and the workplace zones, where potentially explosive mixtures could occur, have to be classified and identified by a warning sign [19], reported in Annex III of the mentioned directive.
Risk from incompatibility among chemicals depends on their reactivity. It is well known that the outcomes of an unintentional mixing of different compounds could result in a fast chemical reaction or an explosion, the production of unwanted by-products, flammable or toxic gases and the generation of hazardous substances in case of contact with the skin.
In order to assess these dangerous scenarios, the software uses two-dimension matrix, analyzing the interactions of chemicals with other compounds in the workplace, other than air and water.
3.3. Environmental Measurement Campaigns of Chemical Agents
In addition to the output of a validated algorithm, the present study takes into consideration the results of environmental measurement campaigns of chemical agents carried out in the case study site. The European Standard EN 689 specifies a strategy aimed at performing representative measurements of exposure by the inhalation of chemical agents in order to verify the compliance with OELVs.
The standard application is assigned to the figure of the appraiser, who is sufficiently trained and experienced in occupational hygiene principles, working and measurement techniques, to conduct the part of the assessment they are performing according to the state of the art.
In accordance with the standard, the occupational exposure assessment involves a basic characterization, which consists of three steps:
- Identification of chemical agents and other information (raw materials, primary products, intermediates, final products, reaction and process products or by-products, identifications of chemicals by EC or CAS numbers, hazardous properties, classification and labelling, appropriate OELVs, whether dermal and oral exposure of the chemical agents is relevant, used amount, vapor pressure, temperature, dustiness, etc.);
- Review of workplace factors (work organization, processes and techniques, workplace layout and configuration, ventilation system and other engineering control measures, emission sources, work load, worker behaviour, etc.);
- Estimation of exposure (potential information sources include: previous measurements results in the same workplace, measurements results from similar installations or processes, calculations based on relevant quantitative information, etc.).
All the information collected during the basic characterization shall be used to:
- Decide whether measurements are necessary or not;
- Generate the different SEGs.
If measurements are necessary, the appraiser has to develop a sampling strategy consisting of:
- Constitution of SEGs on the basis of information on the profile of exposure and duration of the tasks performed in the work shifts throughout the year.
- Choice of sampling and measuring procedure in order to obtain valid and representative measurements of the operators’ exposure in comparison with the OELVs. The EN 482 standard [20] dictates the performance requirements of procedures for the chemical agent measurements. Among the other performance characteristics of a measurement procedure, special attention should be paid to sampling duration to represent the exposure for the task/activity (Annex D of EN 689).
Once the measurements have been carried out, it is necessary to analyze the validity of each measurement and to validate the constitution of each SEG.
The comparison of results with OELVs may be performed applying a preliminary test (Section 5.5.2 of the standard), which requires from three to five valid exposure measurements on workers, belonging to a SEG or, alternatively, a statistical test (Section 5.5.3) to check whether the SEG exposure complies with the OELV. The test should measure, with at least 70% confidence, whether less than 5% of exposures in the SEG exceed the OELV.
It is worth noting that, according to Italian legislation, it is not mandatory to carry out the measurement of hazardous chemical agents for the purpose of occupational risk assessment. Instead, the measurement is always mandatory in case of exposure to carcinogenic and/or mutagenic chemical agents.
4. Results
4.1. P&T Plant
4.1.1. Chemical Agents
Table 3 shows the list of hazardous substances and mixtures used in the operation of the P&T plant. The substances’ hazard characteristics are reported in Appendix A (Table A1).

Table 3.
P&T plant: hazardous substances.
The following contaminants (Table 4) in groundwater should be added to the chemical agents listed above:
- Chlorinated solvents (PCE, TCE, cis 1,2 DCE and VC);
- Aromatic and aliphatic hydrocarbons (total petroleum hydrocarbons).
The substances’ characteristics are reported in Appendix A (Table A2).

Table 4.
Contaminants.
Table 4.
Contaminants.
| Chemical Agent/Acronym | CAS No. |
|---|---|
| Tetrachloroethylene (Perchloroethylene)/PCE | 127-18-4 |
| Trichloroethylene/TCE | 79-01-6 |
| cis-1,2-Dichloroethylene (cis-1,2-Dichloroethene)/cis 1,2 DCE | 156-59-2 |
| Vinyl chloride (Chloroethene)/VC | 75-01-4 |
4.1.2. Management and Operating of the Groundwater Treatment Plant
The operating parameters of the groundwater treatment plant are continuously monitored: highly skilled workers ensure that the plant is operating correctly daily. Indeed, they verify the absence of leakages from pipelines and pumps and the non-activation of alarms, and, furthermore, they report the main parameters of machine operations. The main activities of management and operating of the groundwater treatment plant are as follows [21]:
- The visual inspection of the correct plant operating;
- The check of leakages from the pipeline;
- The alarms’ absence check in the instruments panel;
- The check of water, hydrochloric acid and sodium hydroxide levels in the tanks;
- Water (once every fifteen days) and gas sampling (this last sampling is required when the air flow is filtered by the charcoal canisters);
- The weekly measurement of the main physical and chemical parameters of the treated groundwater;
- The charcoal canister substitution (air and final water treatment sections);
- The management of the reagents (hydrochloric acid, sodium hydroxide, etc.) supply chain;
- Operational maintenance of the plant components (pumps, valves, flanges, pipeline, sand filter, scrubber and stripping columns, etc.).
The activities, referred to as points 2, 5, 7, 8 and 9, can generate a potential risk of exposure to dangerous substances.
4.1.3. Tasks and Exposure Profiles
Table 5 shows an overview of the activities carried out by the personnel assigned to run the P&T plant and the characterization of the chemical exposure profiles.

Table 5.
P&T plant: Activities carried out by the workers.
These are plant maintenance operations, to which two skilled workers are dedicated for 8 h/day and 4 days/week.
4.1.4. Risk Assessment
The SNPA tool was applied, making the following assumptions:
- A single SEG corresponding to the operator of the P&T maintenance working group has been identified in the workplace;
- The assessment of risk for health investigates the following dangerous chemical agents: HCl 33%, NaOH 30%, NaClO 15%, C2Cl4, C2H2Cl2;
- Operating temperature (maximum value in summer) equal to 35 °C has been assumed;
- The exposure duration to HCl, NaOH and NaClO can be inferred from Table 5, while for groundwater pollutants, an exposure of 24 h/week was assumed (4 days/week for 6 h/day) corresponding to 1440 min/week;
- In order to calculate the highest quantity of each handled chemical agent to which the worker can be exposed on weekly basis, the annual feedstock amounts provided for HCl, NaOH and NaClO were assumed; with reference to chlorinated solvents, the values of the maximum concentrations (µg/L) measured in October 2021 were considered valid;
- In reference to the use condition, it has been assumed that the system is closed (chemicals are used and/or stored in watertight reactors or containers and transferred from one container to another through watertight piping).
It has been assumed that the following risk prevention and protection measures are applied to the plant:
- Natural ventilation, guaranteed by the opening of the shed doors, is efficient to dilute the airborne pollutants, and there is a forced ventilation system;
- Special written procedures regulate the execution of operations with the highest exposure risk;
- PPE (Personal Protective Equipment) for protection of the body, eyes, hands and airways is provided and correctly used;
- Workers have been provided with training on the specific risk associated with the dangerous substances they are handling;
- Substances, which are incompatible with each other, are adequately managed.
With reference to each substance and the assumptions listed above, the software returned the Li values shown in Table 6.

Table 6.
Results: Li values.
In the case study, the chemicals used to treat the groundwater mainly cause potential risks to health. On the other hand, the hydrogen and propane use can generate a risk (fire or explosion) to safety. In the area of the groundwater treatment plant, there is an above-ground tank (its volume is about 12,500 L) of propane storage and a hydrogen bottle (its volume is 8 Nm3 and the gas pressure is about 200 bar). The propane storage tank is about 30 m from the shed, which includes the above-mentioned plant. The bottle is located in a building near the shed. Because of the presence of the mentioned hazardous compounds, according to Atex Directive 99/92/EC, the employer is obliged to classify the areas where potentially explosive atmospheres could occur. This classification procedure is based on technical Standard IEC EN 60079-10-1 and detects the hazardous zones (zone 0, zone 1 or zone 2), which could be generated by the potential sources (flanges, valves, compressors, etc.) of hydrogen and propane release. Furthermore, the hazardous area extent (expressed in meters) has to be estimated.
In the examined site, there are no incompatibilities among the chemicals.
Finally, this study, using the software focused on the groundwater treatment process, assigned a non-low value to a risk to safety.
The following analyte (grouped by families) concentrations were measured in the workplace air:
- Aliphatic, saturated and unsaturated, chlorinated and brominated organic compounds;
- Light hydrocarbons (C5 ÷ C8 and C9 ÷ C12 fractions);
- Heavy hydrocarbons (C13 ÷ C18 fractions);
- Mineral acids (hydrochloric acid).
The measurements were carried out (April 2023) with reference to the representative GOE of the personnel, running the P&T plant on three different days. In reference to quantitative analysis, three analytical methods were adopted:
- NIOSH 7907: 2014 (volatile acids via ion chromatography: hydrogen chloride, hydrogen bromide, nitric acid) for hydrochloric acid;
- NIOSH 1550:1997 (Naphthas) for heavy hydrocarbons (C13 ÷ C18 fractions);
- ISO 16200-1:2001 (workplace air quality—sampling and analysis of volatile organic compounds via solvent desorption/gas chromatography—Part 1: pumped sampling method) for all the other analytes.
Table 7 shows the measurement results.

Table 7.
Measurement results.
Applying the preliminary test of EN 689 (Section 5.5.2 of the standard) to the measured concentration values, all analyte compliance with the OELVs was verified.
4.2. Bio-Barriers
4.2.1. Chemical Agents
An organic anaerobic barrier substrate, which consists of soluble condensed molasses, is used as a raw material for the process. The substance, known by several trade names, is classified as UVCB (unknown or variable composition, complex reaction products or biological materials). Based on the classifications provided in the registration dossiers to ECHA (there are currently 27 active registrations under REACH), the substance is recognized to be responsible for severe eye irritation (H319).
Urea phosphate and monoammonium phosphate (MAP) are also used (Table 8) during the aerobic barrier operation.

Table 8.
Aerobic barrier: chemical agents.
The substances’ hazardous characteristics are reported in Appendix A (Table A3).
4.2.2. Management and Operation of Bio-Barriers
Bio-barrier inspection and maintenance are carried out by trained technicians (four days/week).
The main operating parameters, which have to be checked, are as follows:
- Recirculated flow rates;
- Nutrient quantities;
- Air flow rates;
- Well-head pressures.
Furthermore, injection well backwashing is carried out, especially with regard to the anaerobic barrier, in order to limit any biomass accumulation inside the well and drainage as much as possible, which would cause losses in the wells’ operation efficiency. Finally, measurements of the main chemical–physical parameters are carried out.
4.2.3. Tasks and Exposure Profiles
Table 9 reports the bio-barrier management activities, which are associated with a potential chemical risk with the exclusion of the operations of supplying the products to the dosing units. In addition to the chemical agents reported in Table 3, the dangerous substances to which workers are exposed, are chlorinated solvents contained in the groundwater.

Table 9.
Bio-barrier management activities.
The main routes of exposure for workers are as follows:
- Inhalation, although for short exposure times;
- Dermal and ingestion (rarely): the risk may arise during sample collection due to accidental contact.
With regard to accidental events, it must be highlighted that product splashes/jets could occur during the sampling and well purging phases.
This study also entailed a detailed analysis of the activities carried out by personnel involved in the operation and periodic and extraordinary maintenance of bio-barriers, highlighting the tasks that could result in increased exposure to hazardous substances.
4.2.4. Risk Assessment
The products fed into the production cycle (urea phosphate, monoammonium phosphate and soluble condensed molasses) are added in solid and liquid forms, which severely limit the potential for aerodispersion at bio-barrier management stations.
The low volatility of the raw materials and the only knowledge of the levels of tetrachloroethylene and dichloroethylene concentration made the SNPA software (https://www.snpambiente.it/snpa/manuale-per-la-valutazione-del-rischio-da-esposizione-ad-agenti-chimici-pericolosi-e-ad-agenti-cancerogeni-e-mutageni/ accessed on 10 March 2024) application impractical and, in any case, not very meaningful. Similarly to the P&T system, measurements of airborne pollutant concentrations were carried out with reference to the representative GOE of the bio-barrier personnel. Three days in April 2023 were chosen to carry out the measurements. With reference to quantitative measurements, the same analytical methods applied to the P&T plant were used. Table 10 shows the measurement results.

Table 10.
Measurement results.
The preliminary test of EN 689 (Section 5.5.2) was applied to the measured concentration values, and analyte compliance with the OELVs was verified.
4.3. Comparison between P&T System and Bio-Barriers
With regard to the P&T plant, the software application returned the values shown in Table 6. With reference to the criteria given in Table 1 and Table 2, it follows that there is a slight risk to the health for chlorinated solvents, medium risk for hydrochloric acid and sodium hydroxide and, finally, a high risk for sodium hypochlorite.
Furthermore, as the overall exposure level L per individual worker (obtained by sum of the Li associated with each chemical agent) is <1, the conclusions reached by the software certify that the risk is slight for health, although it is still necessary to take specific prevention and protection measures for substances characterized by medium or high risk levels.
With reference to the P&T plant, a comparison of the two risk assessment approaches shows the following:
- The two methods cannot be directly compared, because the environmental monitoring did not examine NaClO and NaOH.
- In reference to HCl, the software indicates a medium risk (Li = 0.064), whereas the environmental monitoring highlights a higher risk level associated with the SEG operator of the P&T maintenance.
A comparison between the P&T and bio-barrier processes shows the following:
- Both processes can expose operators to halogenated hydrocarbons included in groundwater: the concentrations of these pollutants in a liquid matrix and the duration and frequency of “risk operations” carried out by personnel engaged in the two processes do not pose an inhalation exposure risk, as attested by the results of the environmental investigations carried out according to the technical standards (EN 689 and EN 482).
- The SNPA software application for the P&T process shows a slight risk for the operators’ health. However, the intrinsic danger of hydrochloric acid, sodium hydroxide and sodium hypochlorite requires the application of careful work procedures. There is also a significant safety risk associated with the use of hydrogen and propane.
Bio-barrier management involves exposure to soluble condensed molasses, urea phosphate and mono-ammonium phosphate, but the intrinsic danger (corrosiveness and possible eye damage) is easily manageable. Indeed, these substances are solid and liquid, and, therefore, the operators could be exposed essentially via the dermal route. However, the PPE (protective glasses, Tyvek overalls and gloves for chemical protection) used by staff during these activities guarantees, if appropriately worn and correctly maintained, a sufficient level of protection.
Therefore, with regard to the impacts on worker health, regardless of the risk assessment, the hazard source examination already makes the bio-barrier adoption intrinsically safer.
Similar considerations are also applied to impacts on safety. Indeed, while the open area, where the wells and piezometers are located, is considered to be at low fire risk, the P&T system area can be classified as medium risk.
Furthermore, the classification of areas of the groundwater treatment plant, where potentially explosive atmospheres (explosion risk) could occur, detected Atex zones, generated by the potential emission sources of propane and hydrogen.
5. Discussion
P&T plants are characterized by long remediation times as well as high operational and management costs [9,22].
Bioremediation techniques are cheaper if compared to physicochemical treatments [7], and they have low impacts on the environment as well as on worker health. Indeed, most of the bioremediation treatments are carried out in situ, avoiding direct contact between the operators and the contaminated matrix [22]. The ISB can contribute to the sustainability of the groundwater remediation efforts and can promote natural processes, decrease the energy consumption and minimize the environmental impact.
In the case study, the P&T technology was useful for the hydraulic confinement of the site, but it was not efficient for its cleaning. Therefore, the effectiveness of the permeable reactive bio-barriers in the biological remediation of aquifers contaminated by chlorinated solvents and the sustainability of the biological process in terms of environmental safety were preliminary evaluated. The feasibility of ISB technology based on a microbial organohalide respiration process was tested at the laboratory and pilot scales [11,12,13].
In Casiraghi et al., the results, derived from a combination of multi-scale analyses, were reported, and they were propaedeutic for the creation of Italy’s largest in situ sequential bioremediation system. Monitored experiments, which included microcosms and in situ pilot-scale bio-barriers, suggested that natural biodegradation and a single anaerobic bio-barrier were not sufficient to achieve CAH degradation [11]. Sequential anaerobic–aerobic bioremediation barriers and bio-stimulation improved the effectiveness of the bioremediation products, as key contaminant concentrations were lower than the Maximum Contaminant Level (MCL) set by Italian law. This included the less-chlorinated products (e.g., VC), which derived from the degradation of highly chlorinated compounds (e.g., PCE).
The hazard characteristics and physical state of all substances, examined in the work environments, influence the choice of strategies, criteria and methods of risk assessment, making P × D matrix analysis generally preferable to the application of specific software or measurements as part of environmental monitoring.
The software used to assess the professional chemical risk, while ensuring adherence to regulations, represents extremely conservative approaches, and, like all methodologies for calculating risk levels, the provided results are not absolute but essentially comparative. Therefore, with reference to the risk assessment, they can be used as an initial approach aimed at identifying chemical agents, for which action is required as a priority, thereby generating deep investigations.
When a substance’s OELV is available, environmental monitoring is always preferable (possibly of personal type), and it has to be repeated according to criteria and periodicity indicated by the EN 689 standard.
6. Conclusions
A fundamental component of the social sustainability of a remediation process is the assessment of the OH&S of the adopted technologies in order to protect workers and local communities. In the opinion of the authors, the coming research on this issue should be aimed at considering, in an increasingly systematic way, the remediation technique’s sustainability from the point of view of the social development of human beings. In particular, the coming studies’ goal has to consider the best choice of remediation technique, taking into account the potential outcomes on workers’ health and safety.
Author Contributions
Conceptualization, B.P., E.I. and R.L.; methodology, B.P. and E.I.; software, E.I.; validation, B.P., E.I. and R.L.; formal analysis, E.I.; investigation, E.I. and R.L.; resources, B.P. and E.I.; data curation, B.P., E.I. and R.L.; writing—original draft preparation, B.P., E.I. and R.L.; writing—review and editing, B.P., E.I. and R.L.; visualization, B.P., E.I. and R.L.; supervision, B.P., E.I. and R.L.; project administration, B.P.; funding acquisition, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by INAIL (Istituto Nazionale per l’Assicurazione contro gli Infortuni sul Lavoro—National Institute for Insurance against Accidents at Work) through the call INAIL-BRIC 2019 (project ID: 52).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Contaminants’ Hazard Characteristics According to CLP Regulation

Table A1.
P&T plant: raw material.
Table A1.
P&T plant: raw material.
| Chemical Agent | Formula | CAS No. | Hazard Classification |
|---|---|---|---|
| Hydrochloric acid solution 33% | HCl | 7647-01-0 | Corrosive to metals, Category 1; H290 Skin corrosion, Category 1B; H314 Serious eye damage, Category 1; H318 Specific Target Organ Toxicity (single exposure), Category 3; H335 |
| Sodium hypochlorite, aqueous solution containing active chlorine 14–15% | NaClO | 7681-52-9 | Corrosive to metals, Category 1; H290 Skin corrosion, Category 1B; H314 Serious eye damage, Category 1; H318 Specific Target Organ Toxicity (single exposure), Category 3; H335 Hazardous to the aquatic environment, Acute Category 1; H400 Hazardous to the aquatic environment, Chronic Category 1; H410 |
| Sodium hydroxide 30% | NaOH | 1310-73-2 | Corrosive to metals, Category 1; H290 Skin corrosion, Category 1A; H314 |
| Hydrogen | H2 | 1333-74-0 | Flammable gases, Category 1; H220 Gases under pressure, compressed gas; H280 |
| Propane | C3H8 | 74-98-6 | Flammable gases, Category 1; H220 Gases under pressure, liquefied gas; H280 |

Table A2.
Groundwater: contaminants.
Table A2.
Groundwater: contaminants.
| Chemical Agent/ Acronym | Formula | CAS No. | Hazard Classification |
|---|---|---|---|
| Tetrachloroethylene (Perchloroethylene)/PCE | C2Cl4 | 127-18-4 | Skin irritation, Category 2; H315 Skin sensitisation, Category 1; H317 Eye irritation, Category 2; H319 Specific Target Organ Toxicity (single exposure), Category 3; H336 Carcinogenicity, Category 2; H351 Hazardous to the aquatic environment, Chronic Category 2; H411 |
| Trichloroethylene/TCE | C2HCl3 | 79-01-6 | Skin irritation, Category 2; H315 Skin sensitisation, Category 1; H317 Eye irritation, Category 2; H319 Specific Target Organ Toxicity (single exposure), Category 3; H336 Germ cell mutagenicity, Category 2; H341 Carcinogenicity, Category 1B; H350 Hazardous to the aquatic environment, Chronic Category 3; H412 |
| cis-1,2-Dichloroethylene (cis-1,2-Dichloroethene)/cis 1,2 DCE | C2H2Cl2 | 156-59-2 | Flammable liquids, Category 2; H225 Acute toxicity, Category 4, oral; H302 Acute toxicity, Category 4, inhalation; H332 Skin irritation, Category 2; H315 Hazardous to the aquatic environment, Chronic Category 3; H412 |
| Vinyl chloride (Chloroethene)/VC | C2H3Cl | 75-01-4 | Flammable gases, Category 1; H220 Gases under pressure, liquefied gas; H280 Carcinogenicity, Category 1A; H350 |

Table A3.
Aerobic barrier: raw material.
Table A3.
Aerobic barrier: raw material.
| Chemical Agent/ Acronym | Formula | CAS No. | Hazard Classification |
|---|---|---|---|
| Urea phosphate | CH7N2O5P/ CH4N2O · H3PO4 | 4861-19-2 | Skin corrosion, Category 1B; H314 |
| Ammonium dihydrogen phosphate (monoammonium phosphate)/MAP | H6NO4P/ (NH4)H2PO4 | 7722-76-1 | Not a dangerous substance according to GHS. Registration entry of the manufacturer on the ECHA website |
References
- Nawaz, W.; Linke, P.; Koç, M. Safety and sustainability nexus: A review and appraisal. J. Clean. Prod. 2019, 216, 74–87. [Google Scholar] [CrossRef]
- Mohandes, S.R.; Zhang, X. Developing a Holistic Occupational Health and Safety risk assessment model: An application to a case of sustainable construction project. J. Clean. Prod. 2021, 291, 125934. [Google Scholar] [CrossRef]
- European Commission. JRC Technical Reports. Status of Local Soil Contamination in Europe; Pérez, P., Eugenio, N.R., Eds.; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-80072-6. [Google Scholar]
- European Commission. JRC Technical Reports. Progress in the Management of Contaminated Sites in Europe; Van Liedekerke, M., Prokop, G., Rabl-Berger, S., Kbblewhite, M., Louwagie, G., Eds.; Publications Office of the European Union: Luxembourg, 2014; ISBN 978-92-79-34846-4. [Google Scholar]
- European Commission. Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Off. J. Eur. Union 2006, L 372, 19–31. [Google Scholar]
- Aulenta, F.; Majone, M.; Tandoi, V. Enhanced anaerobic bioremediation of chlorinated solvents: Environmental factors influencing microbial activity and their relevance under field conditions. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1463–1474. [Google Scholar] [CrossRef]
- Majone, M.; Verdini, R.; Aulenta, F.; Rossetti, S.; Tandoi, V.; Kalogerakis, N.; Agathos, S.; Puig, S.; Zanaroli, G.; Fava, F. In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnol. 2015, 32, 133–146. [Google Scholar] [CrossRef] [PubMed]
- IARC. Vinyl Chloride; International Agency for Research on Cancer: Ottawa, ON, Canada, 1987; ARC Monographs on the Identification of Carcinogenic Hazards to Humans; 2020; Volume 1–127, Available online: https://monographs.iarc.fr/list-of-classifications (accessed on 5 September 2022).
- EPA 542-F-12-017; A Citizen’s Guide to Pump and Treat. Environmental Protection Agency: Washington, DC, USA, 2012. Available online: https://19january2021snapshot.epa.gov/sites/static/files/2015-04/documents/a_citizens_guide_to_pump_and_treat.pdf (accessed on 18 July 2024).
- Speight, J.G. Remediation technologies. In Natural Water Remediation; Elsevier: Amsterdam, The Netherland, 2020. [Google Scholar]
- Casiraghi, G.; Pedretti, D.; Beretta, G.P.; Bertolini, M.; Bozzetto, G.; Cavalca, L.; Ferrari, L.; Masetti, M.; Terrenghi, J. Piloting Activities for the Design of a Large-scale Biobarrier involving In Situ Sequential Anaerobic–aerobic Bioremediation of Organochlorides and Hydrocarbons. Water Air Soil Pollut. 2022, 233, 425. [Google Scholar] [CrossRef]
- Bertolini, M.; Zecchin, S.; Beretta, G.P.; De Nisi, P.; Ferrari, L.; Cavalca, L. Effectiveness of Permeable Reactive Bio-Barriers for Bioremediation of an Organohalide-Polluted Aquifer by Natural-Occurring Microbial Community. Water 2021, 13, 2442. [Google Scholar] [CrossRef]
- Bertolini, M.; Zecchin, S.; Cavalca, L. Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination. Water 2023, 15, 1406. [Google Scholar] [CrossRef]
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (Text with EEA relevance). Off. J. Eur. Union 2008, L 353, 1–1355.
- BS EN 689:2018; Workplace Exposure. Measurement of Exposure by Inhalation to Chemical Agents. Strategy for Testing Compliance with Occupational Exposure Limit Values. European Standard: Plzen, Czech Republic, 2018.
- SNPA Sistema Nazionale per la Protezione dell’Ambiente. Manuale per la Valutazione del Rischio da Esposizione ad Agenti Chimici Pericolosi e ad Agenti Cancerogeni e Mutageni. Linee Guida SNPA|05 2017. Terza Revisione. Available online: https://www.isprambiente.gov.it/files2017/pubblicazioni/manuali-linee-guida/MLG_164_17_Man_rischio_chimico.pdf (accessed on 10 March 2024).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union 2006, L 396. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1907 (accessed on 3 February 2024).
- Incocciati, E.; Lauri, R.; Pietrangeli, B. Innovative mixed microbial culture processes for PHA production at pilot scale: Professional chemical risk assessment. Chem. Eng. Trans. 2020, 79, 49–54. [Google Scholar]
- Directive 99/92/EC of the European Parliament and of the Council of 16 December 1999 on minimum requirements for improving the safety and health protection of workers potentially at risk from explosive atmospheres. Off. J. Eur. Union 2000, L 23, 57–64.
- BS EN 482:2021; Workplace Exposure—Procedures for the Determination of the Concentration of Chemical Agents—Basic Performance Requirements. European Standard: Plzen, Czech Republic, 2021.
- Lauri, R. Biomethane compression unit: A methodological approach aimed at decreasing the Atex zones hazardousness. Chem. Eng. Trans. 2023, 104, 121–126. [Google Scholar]
- Casasso, A.; Tosco, T.; Bianco, C.; Bucci, A.; Sethi, R. How can we make pump and treat systems more energetically sustainable? Water 2020, 1, 67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).