Quantifiable Measures of Abdominal Wall Motion for Quality Assessment of Cine-MRI Slices in Detection of Abdominal Adhesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Technique of Cine-MRI
2.3. Subjective Quality Grading
2.4. Development of Image Processing Algorithm for Biomarkers
2.5. Correlation between Biomarker and Quality Grading
2.6. Impact of Patient-Related Factors on Movement on Cine-MRI
3. Results
3.1. Subjective Quality Grading
3.2. Biomarker and Manual Measurements
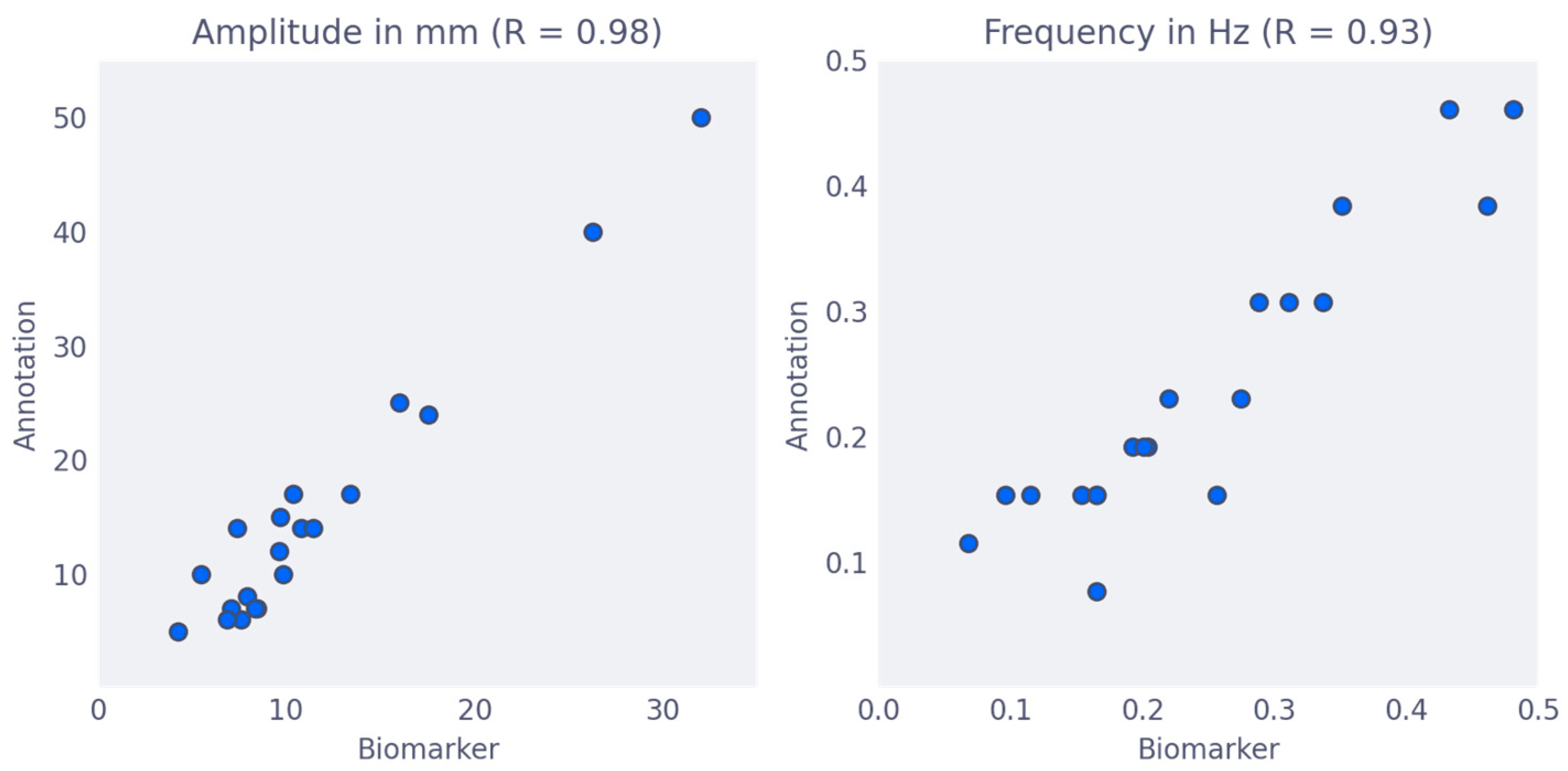
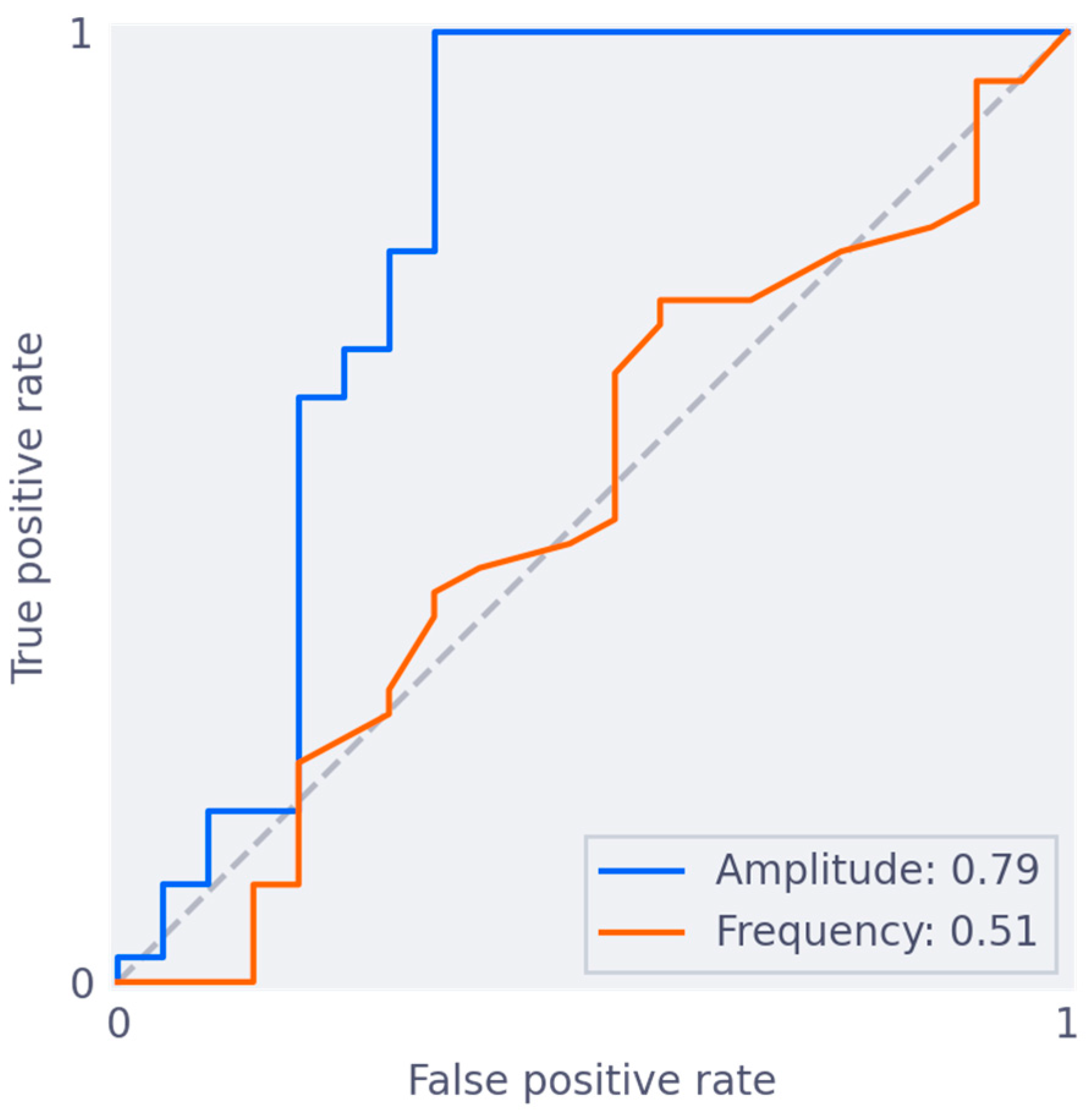
3.3. Association between Biomarker and Subjective Quality
3.4. Patient-Related Factors and Movement on Cine-MRI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van den Beukel, B.A.W.; Stommel, M.W.J.; van Leuven, S.; Strik, C.; MA, I.J.; Joosten, F.; van Goor, H.; Ten Broek, R.P.G. A Shared Decision Approach to Chronic Abdominal Pain Based on Cine-MRI: A Prospective Cohort Study. Am. J. Gastroenterol. 2018, 113, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lienemann, A.; Sprenger, D.; Steitz, H.O.; Korell, M.; Reiser, M. Detection and mapping of intraabdominal adhesions by using functional cine MR imaging: Preliminary results. Radiology 2000, 217, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Stommel, M.W.J.; Ten Broek, R.P.G.; Strik, C.; Slooter, G.D.; Verhoef, C.; Grünhagen, D.J.; van Duijvendijk, P.; Bemelmans, M.H.A.; den Dulk, M.; Sietses, C.; et al. Multicenter Observational Study of Adhesion Formation After Open-and Laparoscopic Surgery for Colorectal Cancer. Ann. Surg. 2018, 267, 743–748. [Google Scholar] [CrossRef] [PubMed]
- ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. Bmj 2013, 347, f5588. [Google Scholar] [CrossRef]
- Cheong, Y.; William Stones, R. Chronic pelvic pain: Aetiology and therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 695–711. [Google Scholar] [CrossRef]
- Gerner-Rasmussen, J.; Donatsky, A.M.; Bjerrum, F. The role of non-invasive imaging techniques in detecting intra-abdominal adhesions: A systematic review. Langenbeck’s Arch. Surg. 2019, 404, 653–661. [Google Scholar] [CrossRef]
- van den Beukel, B.A.; de Ree, R.; van Leuven, S.; Bakkum, E.A.; Strik, C.; van Goor, H.; Ten Broek, R.P.G. Surgical treatment of adhesion-related chronic abdominal and pelvic pain after gynaecological and general surgery: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 276–288. [Google Scholar] [CrossRef]
- Lang, R.A.; Buhmann, S.; Hopman, A.; Steitz, H.O.; Lienemann, A.; Reiser, M.F.; Jauch, K.W.; Hüttl, T.P. Cine-MRI detection of intraabdominal adhesions: Correlation with intraoperative findings in 89 consecutive cases. Surg. Endosc. 2008, 22, 2455–2461. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S. Prediction of intra-abdominal adhesions using the visceral slide test: A prospective observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 22–25. [Google Scholar] [CrossRef]
- Zinther, N.B.; Zeuten, A.; Marinovskij, E.; Haislund, M.; Friis-Andersen, H. Detection of abdominal wall adhesions using visceral slide. Surg. Endosc. 2010, 24, 3161–3166. [Google Scholar] [CrossRef]
- Randall, D.; Fenner, J.; Gillott, R.; Ten Broek, R.; Strik, C.; Spencer, P.; Bardhan, K.D. A Novel Diagnostic Aid for Detection of Intra-Abdominal Adhesions to the Anterior Abdominal Wall Using Dynamic Magnetic Resonance Imaging. Gastroenterol. Res. Pract. 2016, 2016, 2523768. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.; Joosten, F.; Ten Broek, R.P.; Gillott, R.; Bardhan, K.D.; Strik, C.; Prins, W.; van Goor, H.; Fenner, J.W. A novel diagnostic aid for intra-abdominal adhesion detection in cine-MRI: Pilot study and initial diagnostic impressions. Br. J. Radiol. 2017, 90, 20170158. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.G.; Lima, C.S.; de Sá, R.B.; Reinaux, C.M.; Braz Júnior, D.S.; Teixeira, A.L.; de Andrade, A.D.; Marinho, P.E. Influence of posture on the ventilatory pattern and the thoraco-abdominal kinematics of patients with chronic obstructive pulmonary disease (COPD). Physiother. Theory Pract. 2014, 30, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Blaise, H.; Remen, T.; Ambarki, K.; Weiland, E.; Kuehn, B.; Orry, X.; Laurent, V. Comparison of respiratory-triggered 3D MR cholangiopancreatography and breath-hold compressed-sensing 3D MR cholangiopancreatography at 1.5 T and 3 T and impact of individual factors on image quality. Eur. J. Radiol. 2021, 142, 109873. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, Y.; Xie, F.; Shi, W.; Yang, Y.; Yang, A.; Wu, D. Educating Outpatients for Bowel Preparation Before Colonoscopy Using Conventional Methods vs. Virtual Reality Videos Plus Conventional Methods: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2135576. [Google Scholar] [CrossRef] [PubMed]
- Tarroni, G.; Oktay, O.; Bai, W.; Schuh, A.; Suzuki, H.; Passerat-Palmbach, J.; de Marvao, A.; O’Regan, D.P.; Cook, S.; Glocker, B.; et al. Learning-Based Quality Control for Cardiac MR Images. IEEE Trans. Med. Imaging 2019, 38, 1127–1138. [Google Scholar] [CrossRef]
- Buhmann-Kirchhoff, S.; Lang, R.; Kirchhoff, C.; Steitz, H.O.; Jauch, K.W.; Reiser, M.; Lienemann, A. Functional cine MR imaging for the detection and mapping of intraabdominal adhesions: Method and surgical correlation. Eur. Radiol. 2008, 18, 1215–1223. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Andrews, D.W.K.; Buchinsky, M. A three-step method for choosing the number of bootstrap repetitions. Econometrica 2000, 68, 23–51. [Google Scholar] [CrossRef]
- van den Beukel, B.A.W.; Toneman, M.K.; van Veelen, F.; van Oud-Alblas, M.B.; van Dongen, K.; Stommel, M.W.J.; van Goor, H.; Ten Broek, R.P.G. Elective adhesiolysis for chronic abdominal pain reduces long-term risk of adhesive small bowel obstruction. World J. Emerg. Surg. 2023, 18, 8. [Google Scholar] [CrossRef]
- Stommel, M.W.; Strik, C.; ten Broek, R.P.; de Wilt, J.H.; van Goor, H. Impact of Adhesiolysis on Outcome of Colorectal Surgery. Dig. Surg. 2016, 33, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yasemin, A.; Mehmet, B.; Omer, A. Assessment of the diagnostic efficacy of abdominal ultrasonography and cine magnetic resonance imaging in detecting abdominal adhesions: A double-blind research study. Eur. J. Radiol. 2020, 126, 108922. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McDonnell, R.; Jacques, A.; Fender, L.; Lo, G. MRI sliding sign: Using MRI to assess rectouterine mobility in pelvic endometriosis. J. Med. Imaging Radiat. Oncol. 2022, 66, 54–59. [Google Scholar] [CrossRef]
- Zheng, D.; He, X.; Jing, J. Overview of Artificial Intelligence in Breast Cancer Medical Imaging. J. Clin. Med. 2023, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Miranda, J.; El Homsi, M.; Peoples, J.J.; Long, N.M.; Simpson, A.L.; Do, R.K.G. A primer on texture analysis in abdominal radiology. Abdom. Radiol. 2022, 47, 2972–2985. [Google Scholar] [CrossRef]
- Del Negro, C.A.; Funk, G.D.; Feldman, J.L. Breathing matters. Nat. Rev. Neurosci. 2018, 19, 351–367. [Google Scholar] [CrossRef]
- Ball, H.J.; Santanam, L.; Senan, S.; Tanyi, J.A.; van Herk, M.; Keall, P.J. Results from the AAPM Task Group 324 respiratory motion management in radiation oncology survey. J. Appl. Clin. Med. Phys. 2022, 23, e13810. [Google Scholar] [CrossRef]
- Yuksen, C.; Sawatmongkornkul, S.; Tuangsirisup, J.; Sawanyawisuth, K.; Sittichanbuncha, Y. The CPR outcomes of online medical video instruction versus on-scene medical instruction using simulated cardiac arrest stations. BMC Emerg. Med. 2016, 16, 25. [Google Scholar] [CrossRef]
- de Wilde, B.; Joosten, F.; Venderink, W.; Davidse, M.E.J.; Geurts, J.; Kruijt, H.; Vermeulen, A.; Martens, B.; Schyns, M.V.P.; Huige, J.; et al. Inter- and Intra-Observer Variability and the Effect of Experience in Cine-MRI for Adhesion Detection. J. Imaging 2023, 9, 55. [Google Scholar] [CrossRef]

| Factors | N (%)/(±SD)/(Range) |
|---|---|
| Male | 138 (24.7%) |
| Female | 422 (75.3%) |
| Age (in years) | 50.7 (±14.0) |
| BMI (kg/m2) | 26.0 (±5.2) |
| Stoma | 49 (8.8%) |
| Ventral abdominal wall hernia | 21 (3.2%) |
| Adhesions | 378 (68.9%) |
| Gastric bypass | 24 (4.3%) |
| Artifacts | 6 (1.1%) |
| Length (in cm) | 169.9 (±9.1) |
| Weight (in kg) | 75.3 (±17.6) |
| Video instruction | 134 (23.9%) |
| Number of slices per patient | 6 (4–18) |
| Number of sufficient slices per patient | 5 (0–16) |
| Percentage of sufficient slices per patient | 60.1 (±38.7) |
| Total number of unreadable slices | 124 (3.5%) |
| Total number of slices | 3535 (100%) |
| Number of scans | 560 |
| N (%) or Median (range) or mean (±SD) | |
| Factor | Univariate Regression Coefficient | 95% CI | p-Value | Multivariate Regression Coefficient | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Sex (male) | 0.020 | −0.047–0.088 | 0.550 | 0.110 | 0.015–0.204 | 0.023 |
| Age (for each year) | 0.002 | 0.000–0.004 | 0.052 | 0.003 | 0.001–0.006 | 0.005 |
| Stoma (yes) | −0.096 | −0.198–0.006 | 0.064 | −0.178 | −0.292–−0.063 | 0.002 |
| Abdominal wall hernia (yes) | −0.056 | −0.208–0.096 | 0.469 | - | - | - |
| Gastric Bypass (yes) | −0.010 | −0.153–0.133 | 0.892 | - | - | - |
| Adhesions (yes) | −0.005 | −0.068–0.058 | 0.867 | - | - | - |
| Artifacts (yes) | −0.194 | −0.475–0.087 | 0.175 | - | - | - |
| BMI (for each kg/m2) | 0.001 | −0.006–0.007 | 0.848 | - | - | - |
| Length (for each cm) | 0.005 | 0.001–0.009 | 0.007 | 0.009 | 0.004–0.013 | 0.001 |
| Weight (for each Kg) | 0.001 | −0.001–0.003 | 0.163 | - | - | - |
| Video instruction (yes) | −0.011 | −0.079–0.057 | 0.752 | - | - | - |
| Number of slices per patient | −0.004 | −0.014–0.021 | 0.675 | - | - | - |
| Factor | Univariate Regression Coefficient | 95% CI | p-Value | Multivariate Regression Coefficient | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Sex (male) | −0.091 | −0.549–0.367 | 0.697 | 0.627 | 0.062–1.191 | 0.030 |
| Age (for each year) | 0.012 | −0.002–0.026 | 0.098 | 0.014 | 0.000−0.028 | 0.044 |
| Stoma (yes) | −0.890 | −1.584–−0.195 | 0.012 | −1.203 | −1.887–−0.519 | 0.001 |
| Abdominal wall hernia (yes) | −0.568 | −1.605–0.470 | 0.283 | - | - | - |
| Gastric Bypass (yes) | −0.117 | −1.091–0.857 | 0.814 | - | - | - |
| Adhesions (yes) | 0.128 | −0.230–0.558 | 0.558 | - | - | - |
| Artifacts (yes) | −1.587 | −3.499–0.325 | 0.104 | - | - | - |
| BMI (for each kg/m2) | 0.006 | −0.038–0.050 | 0.786 | - | - | - |
| Length (for each cm) | 0.037 | 0.012–0.061 | 0.004 | 0.044 | 0.017–0.070 | 0.001 |
| Weight (for each kg) | 0.010 | −0.003–0.023 | 0.122 | - | - | - |
| Video instruction (yes) | 0.804 | 0.346–1.261 | 0.001 | - | - | - |
| Number of slices per patient | 0.750 | 0.649–0.852 | 0.001 | 0.729 | 0.612–0.846 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Beukel, B.A.W.; de Wilde, B.; Joosten, F.; van Goor, H.; Venderink, W.; Huisman, H.J.; ten Broek, R.P.G. Quantifiable Measures of Abdominal Wall Motion for Quality Assessment of Cine-MRI Slices in Detection of Abdominal Adhesions. J. Imaging 2023, 9, 92. https://doi.org/10.3390/jimaging9050092
van den Beukel BAW, de Wilde B, Joosten F, van Goor H, Venderink W, Huisman HJ, ten Broek RPG. Quantifiable Measures of Abdominal Wall Motion for Quality Assessment of Cine-MRI Slices in Detection of Abdominal Adhesions. Journal of Imaging. 2023; 9(5):92. https://doi.org/10.3390/jimaging9050092
Chicago/Turabian Stylevan den Beukel, Bastiaan A. W., Bram de Wilde, Frank Joosten, Harry van Goor, Wulphert Venderink, Henkjan J. Huisman, and Richard P. G. ten Broek. 2023. "Quantifiable Measures of Abdominal Wall Motion for Quality Assessment of Cine-MRI Slices in Detection of Abdominal Adhesions" Journal of Imaging 9, no. 5: 92. https://doi.org/10.3390/jimaging9050092
APA Stylevan den Beukel, B. A. W., de Wilde, B., Joosten, F., van Goor, H., Venderink, W., Huisman, H. J., & ten Broek, R. P. G. (2023). Quantifiable Measures of Abdominal Wall Motion for Quality Assessment of Cine-MRI Slices in Detection of Abdominal Adhesions. Journal of Imaging, 9(5), 92. https://doi.org/10.3390/jimaging9050092






