Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation
Abstract
1. Introduction
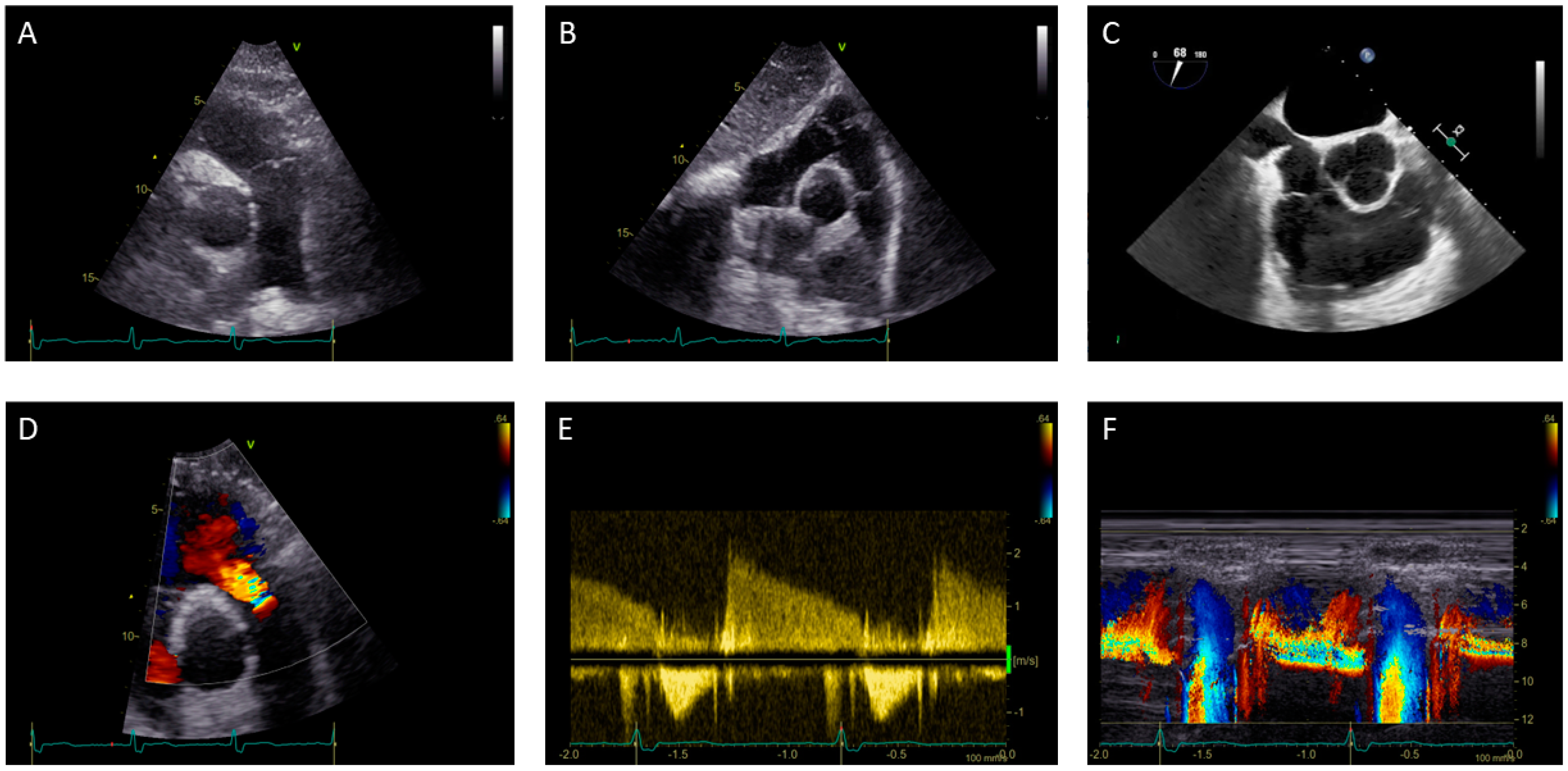
1.1. Pulmonary Valve Stenosis: Role of Echocardiography
1.2. Pulmonary Valve Regurgitation: Role of Echocardiography
1.3. Right Ventricle Assessment in Pulmonary Stenosis and Regurgitation: Role of Echocardiography
2. Role of Cardiac Magnetic Resonance in Pulmonary Stenosis and Regurgitation
2.1. Pulmonary Stenosis: Role of CMR
2.2. Pulmonary Regurgitation: Role of CMR
2.3. Right Ventricle Assessment in Pulmonary Stenosis and Regurgitation: Role of Cardiac Magnetic Resonance
3. Role of CT on Pulmonary Stenosis and Regurgitationt Assessment
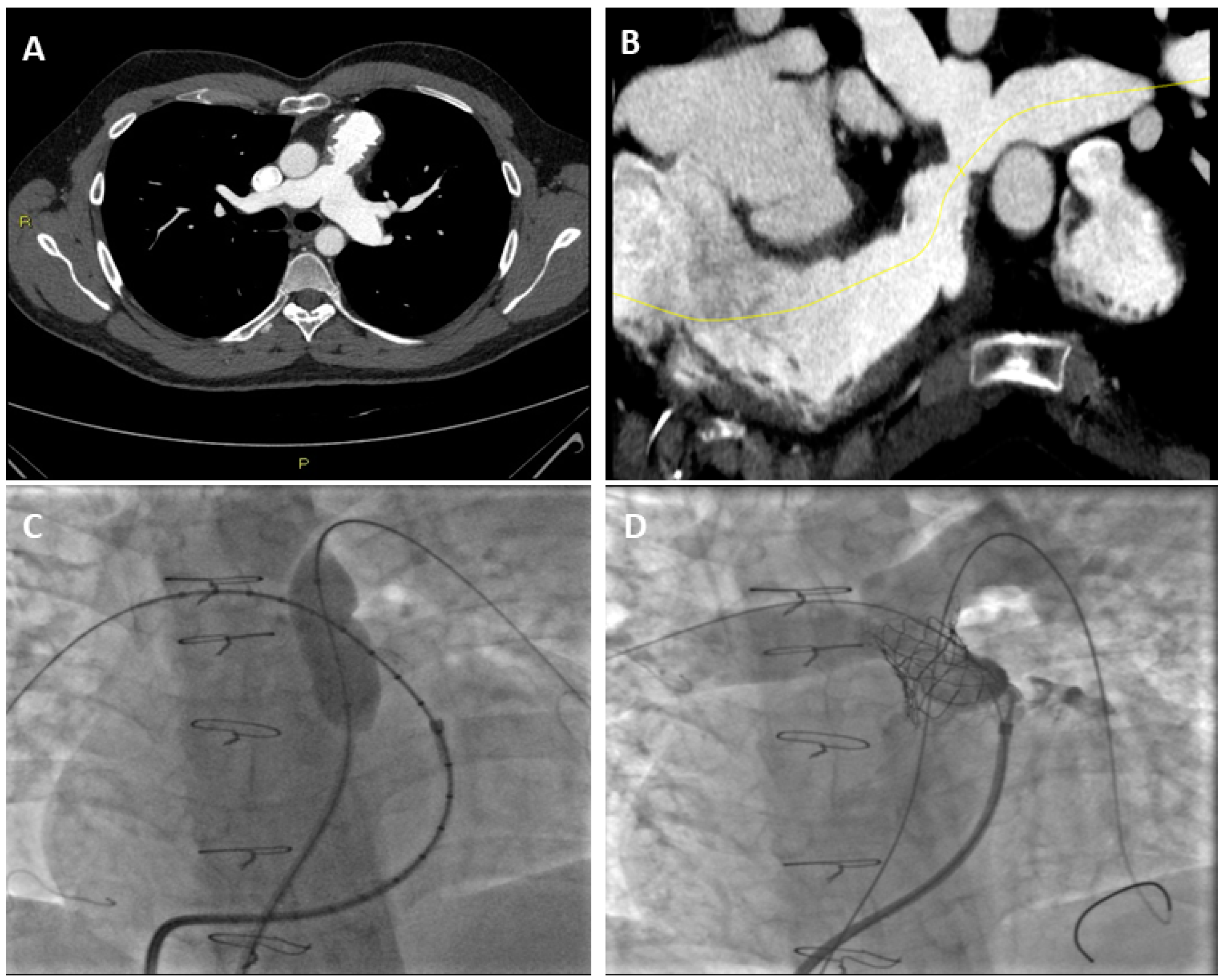
3.1. Pulmonary Stenosis and CT
3.2. Pulmonary Regurgitation and CT
3.3. Right Ventricle Assessment with Cardiac CT
4. Prenatal Diagnosis of Pulmonary Stenosis and Regurgitation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Scientific Document Committee of the European Association of Cardiovascular Imaging Recommendations for the Echocardiographic Assessment of Native Valvular Regurgitation: An Executive Summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P.; et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 3–46. [Google Scholar] [CrossRef]
- Schicchi, N.; Secinaro, A.; Muscogiuri, G.; Ciliberti, P.; Leonardi, B.; Santangelo, T.; Napolitano, C.; Agliata, G.; Basile, M.C.; Guidi, F.; et al. Multicenter Review: Role of Cardiovascular Magnetic Resonance in Diagnostic Evaluation, Pre-Procedural Planning and Follow-up for Patients with Congenital Heart Disease. Radiol. Med. 2016, 121, 342–351. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23, quiz 101–102. [Google Scholar] [CrossRef]
- Ruckdeschel, E.; Kim, Y.Y. Pulmonary Valve Stenosis in the Adult Patient: Pathophysiology, Diagnosis and Management. Heart 2019, 105, 414–422. [Google Scholar] [CrossRef]
- Baumgartner, H.; de Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; la Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-Modality Imaging Assessment of Native Valvular Regurgitation: An EACVI and ESC Council of Valvular Heart Disease Position Paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Saremi, F.; Gera, A.; Ho, S.Y.; Hijazi, Z.M.; Sánchez-Quintana, D. CT and MR Imaging of the Pulmonary Valve. Radiographics 2014, 34, 51–71. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef]
- Law, M.A.; Chatterjee, A. Transcatheter Pulmonic Valve Implantation: Techniques, Current Roles, and Future Implications. World J. Cardiol. 2021, 13, 117–129. [Google Scholar] [CrossRef]
- Feltes, T.F.; Bacha, E.; Beekman, R.H.; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; de Moor, M.; Morrow, W.R.; et al. Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease: A Scientific Statement from the American Heart Association. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef] [PubMed]
- Hascoët, S.; Acar, P.; Boudjemline, Y. Transcatheter Pulmonary Valvulation: Current Indications and Available Devices. Arch. Cardiovasc. Dis. 2014, 107, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Mick, S.L. The Tricuspid Valve: No Longer Forgotten but Still Not Well Understood. J. Am. Coll. Cardiol. 2021, 77, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Weyman, A.E.; Hurwitz, R.A.; Girod, D.A.; Dillon, J.C.; Feigenbaum, H.; Green, D. Cross-Sectional Echocardiographic Visualization of the Stenotic Pulmonary Valve. Circulation 1977, 56, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Weyman, A.E.; Dillon, J.C.; Feigenbaum, H.; Chang, S. Echocardiographic Differentiation of Infundibular from Valvular Pulmonary Stenosis. Am. J. Cardiol. 1975, 36, 21–26. [Google Scholar] [CrossRef]
- Hahn, R.T.; Abraham, T.; Adams, M.S.; Bruce, C.J.; Glas, K.E.; Lang, R.M.; Reeves, S.T.; Shanewise, J.S.; Siu, S.C.; Stewart, W.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic Examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J. Am. Soc. Echocardiogr. 2013, 26, 921–964. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, M.D.; Askovich, B.; Sower, C.T.; Williams, R.V.; Minich, L.L.; Tani, L.Y. Pulmonary Regurgitation: Determining Severity by Echocardiography and Magnetic Resonance Imaging. Congenit. Heart Dis. 2008, 3, 168–175. [Google Scholar] [CrossRef]
- Renella, P.; Aboulhosn, J.; Lohan, D.G.; Jonnala, P.; Finn, J.P.; Satou, G.M.; Williams, R.J.; Child, J.S. Two-Dimensional and Doppler Echocardiography Reliably Predict Severe Pulmonary Regurgitation as Quantified by Cardiac Magnetic Resonance. J. Am. Soc. Echocardiogr. 2010, 23, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.H.; Chen, J.J.; Ko, Y.L.; Cheng, J.J.; Kuan, P.; Lien, W.P. Reappraisal of Quantitative Evaluation of Pulmonary Regurgitation and Estimation of Pulmonary Artery Pressure by Continuous Wave Doppler Echocardiography. Cardiology 1995, 86, 249–256. [Google Scholar] [CrossRef]
- Silversides, C.K.; Veldtman, G.R.; Crossin, J.; Merchant, N.; Webb, G.D.; McCrindle, B.W.; Siu, S.C.; Therrien, J. Pressure Half-Time Predicts Hemodynamically Significant Pulmonary Regurgitation in Adult Patients with Repaired Tetralogy of Fallot. J. Am. Soc. Echocardiogr. 2003, 16, 1057–1062. [Google Scholar] [CrossRef]
- Li, W.; Davlouros, P.A.; Kilner, P.J.; Pennell, D.J.; Gibson, D.; Henein, M.Y.; Gatzoulis, M.A. Doppler-Echocardiographic Assessment of Pulmonary Regurgitation in Adults with Repaired Tetralogy of Fallot: Comparison with Cardiovascular Magnetic Resonance Imaging. Am. Heart J. 2004, 147, 165–172. [Google Scholar] [CrossRef]
- Goldberg, S.J.; Allen, H.D. Quantitative Assessment by Doppler Echocardiography of Pulmonary or Aortic Regurgitation. Am. J. Cardiol. 1985, 56, 131–135. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Surkova, E.; Cosyns, B.; Gerber, B.; Gimelli, A.; la Gerche, A.; Ajmone Marsan, N. The Dysfunctional Right Ventricle: The Importance of Multi-Modality Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 885–897. [Google Scholar] [CrossRef]
- Mathew, R.C.; Löffler, A.I.; Salerno, M. Role of Cardiac Magnetic Resonance Imaging in Valvular Heart Disease: Diagnosis, Assessment, and Management. Curr. Cardiol. Rep. 2018, 20, 119. [Google Scholar] [CrossRef]
- Chelu, R.G.; Wanambiro, K.W.; Hsiao, A.; Swart, L.E.; Voogd, T.; van den Hoven, A.T.; van Kranenburg, M.; Coenen, A.; Boccalini, S.; Wielopolski, P.A.; et al. Cloud-Processed 4D CMR Flow Imaging for Pulmonary Flow Quantification. Eur. J. Radiol. 2016, 85, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D Flow Cardiovascular Magnetic Resonance Consensus Statement. J. Cardiovasc. Magn. Reason. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Singh, A.; McCann, G.P. Cardiovascular Magnetic Resonance in the Evaluation of Heart Valve Disease. BMC Med. Imaging 2017, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, J.A.A.E.; Witsenburg, M.; van der Linde, D.; Roos-Hesselink, J.W. Pulmonary Stenosis: Update on Diagnosis and Therapeutic Options. Heart 2013, 99, 339–347. [Google Scholar] [CrossRef]
- Oosterhof, T.; Mulder, B.J.M.; Vliegen, H.W.; de Roos, A. Cardiovascular Magnetic Resonance in the Follow-up of Patients with Corrected Tetralogy of Fallot: A Review. Am. Heart J. 2006, 151, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.M.; Cook, S.; Festa, P.; Ko, H.H.; Krishnamurthy, R.; Taylor, A.M.; Warnes, C.A.; Kreutzer, J.; Geva, T. Multimodality Imaging Guidelines for Patients with Repaired Tetralogy of Fallot: A Report from the American Society of Echocardiography: Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J. Am. Soc. Echocardiogr. 2014, 27, 111–141. [Google Scholar] [CrossRef]
- Guglielmo, M.; Rovera, C.; Rabbat, M.G.; Pontone, G. The Role of Cardiac Magnetic Resonance in Aortic Stenosis and Regurgitation. J. Cardiovasc. Dev. Dis. 2022, 9, 108. [Google Scholar] [CrossRef]
- Geva, T. Repaired Tetralogy of Fallot: The Roles of Cardiovascular Magnetic Resonance in Evaluating Pathophysiology and for Pulmonary Valve Replacement Decision Support. J. Cardiovasc. Magn. Reson. 2011, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. Circ Cardiovasc Imaging 2022, 15, e014415. [Google Scholar] [CrossRef]
- Imazio, M.; Andriani, M.; Nigro, M.; Bodoni, L.L. Manuale Pratico di Risonanza Magnetica Cardiaca, 1st ed.; Il Pensiero Scientifico: Rome, Italy, 2018. [Google Scholar]
- Lee, C.; Kim, Y.M.; Lee, C.H.; Kwak, J.G.; Park, C.S.; Song, J.Y.; Shim, W.S.; Choi, E.Y.; Lee, S.Y.; Baek, J.S. Outcomes of Pulmonary Valve Replacement in 170 Patients with Chronic Pulmonary Regurgitation after Relief of Right Ventricular Outflow Tract Obstruction: Implications for Optimal Timing of Pulmonary Valve Replacement. J. Am. Coll. Cardiol. 2012, 60, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Purmah, Y.; Lei, L.Y.; Dykstra, S.; Mikami, Y.; Cornhill, A.; Satriano, A.; Flewitt, J.; Rivest, S.; Sandonato, R.; Seib, M.; et al. Right Ventricular Ejection Fraction for the Prediction of Major Adverse Cardiovascular and Heart Failure-Related Events. Circ. Cardiovasc. Imaging 2021, 14, e011337. [Google Scholar] [CrossRef]
- Orwat, S.; Diller, G.-P.; Kempny, A.; Radke, R.; Peters, B.; Kühne, T.; Boethig, D.; Gutberlet, M.; Dubowy, K.-O.; Beerbaum, P.; et al. Myocardial Deformation Parameters Predict Outcome in Patients with Repaired Tetralogy of Fallot. Heart 2016, 102, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, Y.; Fujioka, T.; Ide, H.; Yazaki, K.; Honjo, O.; Sun, M.; Friedberg, M.K. Impaired Right and Left Ventricular Function and Relaxation Induced by Pulmonary Regurgitation Are Not Reversed by Tardive Antifibrosis Treatment. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H38–H51. [Google Scholar] [CrossRef] [PubMed]
- Babu-Narayan, S.V.; Goktekin, O.; Moon, J.C.; Broberg, C.S.; Pantely, G.A.; Pennell, D.J.; Gatzoulis, M.A.; Kilner, P.J. Late Gadolinium Enhancement Cardiovascular Magnetic Resonance of the Systemic Right Ventricle in Adults with Previous Atrial Redirection Surgery for Transposition of the Great Arteries. Circulation 2005, 111, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Ylitalo, P.; Pitkänen, O.M.; Lauerma, K.; Holmström, M.; Rahkonen, O.; Heikinheimo, M.; Sairanen, H.; Jokinen, E. Late Gadolinium Enhancement (LGE) Progresses with Right Ventricle Volume in Children after Repair of Tetralogy of Fallot. Int. J. Cardiology. Heart Vessel. 2014, 3, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kozak, M.F.; Redington, A.; Yoo, S.-J.; Seed, M.; Greiser, A.; Grosse-Wortmann, L. Diffuse Myocardial Fibrosis Following Tetralogy of Fallot Repair: A T1 Mapping Cardiac Magnetic Resonance Study. Pediatr. Radiol. 2014, 44, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Alì, M.; Monti, C.B.; Greiser, A.; Pluchinotta, F.R.; Carminati, M.; Sardanelli, F. Right and Left Ventricle Native T1 Mapping in Systolic Phase in Patients with Congenital Heart Disease. Acta Radiol. 2021, 62, 334–340. [Google Scholar] [CrossRef]
- Pignatelli, R.H.; Noel, C.; Reddy, S.C.B. Imaging of the Pulmonary Valve in the Adults. Curr. Opin. Cardiol. 2017, 32, 529–540. [Google Scholar] [CrossRef]
- Kerl, J.M.; Ravenel, J.G.; Nguyen, S.A.; Suranyi, P.; Thilo, C.; Costello, P.; Bautz, W.; Schoepf, U.J. Right Heart: Split-Bolus Injection of Diluted Contrast Medium for Visualization at Coronary CT Angiography. Radiology 2008, 247, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Nazarian, J.; Vogelius, E.; Gilkeson, R.C. CT and MRI of Pulmonary Valvular Abnormalities. Clin. Radiol. 2014, 69, 630–638. [Google Scholar] [CrossRef]
- Tops, L.F.; Wood, D.A.; Delgado, V.; Schuijf, J.D.; Mayo, J.R.; Pasupati, S.; Lamers, F.P.L.; van der Wall, E.E.; Schalij, M.J.; Webb, J.G.; et al. Noninvasive Evaluation of the Aortic Root with Multislice Computed Tomography Implications for Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Imaging 2008, 1, 321–330. [Google Scholar] [CrossRef]
- Jonas, S.N.; Kligerman, S.J.; Burke, A.P.; Frazier, A.A.; White, C.S. Pulmonary Valve Anatomy and Abnormalities: A Pictorial Essay of Radiography, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI). J. Thorac. Imaging 2016, 31, W4–W12. [Google Scholar] [CrossRef]
- Kochav, J.; Simprini, L.; Weinsaft, J.W. Imaging of the Right Heart—CT and CMR. Echocardiography 2015, 32 (Suppl. 1), S53–S68. [Google Scholar] [CrossRef]
- Han, B.K.; Lesser, A.M.; Vezmar, M.; Rosenthal, K.; Rutten-Ramos, S.; Lindberg, J.; Caye, D.; Lesser, J.R. Cardiovascular Imaging Trends in Congenital Heart Disease: A Single Center Experience. J. Cardiovasc. Comput. Tomogr. 2013, 7, 361–366. [Google Scholar] [CrossRef]
- Sánchez Ramírez, C.J.; Pérez de Isla, L. Tetralogy of Fallot: Cardiac Imaging Evaluation. Ann. Transl. Med. 2020, 8, 966. [Google Scholar] [CrossRef]
- Entrikin, D.W.; Gupta, P.; Kon, N.D.; Carr, J.J. Imaging of Infective Endocarditis with Cardiac CT Angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 399–405. [Google Scholar] [CrossRef]
- Feuchtner, G.M.; Stolzmann, P.; Dichtl, W.; Schertler, T.; Bonatti, J.; Scheffel, H.; Mueller, S.; Plass, A.; Mueller, L.; Bartel, T.; et al. Multislice Computed Tomography in Infective Endocarditis: Comparison with Transesophageal Echocardiography and Intraoperative Findings. J. Am. Coll. Cardiol. 2009, 53, 436–444. [Google Scholar] [CrossRef]
- Gebhard, C.; Maredziak, M.; Messerli, M.; Buechel, R.R.; Lin, F.; Gransar, H.; Achenbach, S.; Al-Mallah, M.H.; Andreini, D.; Bax, J.J.; et al. Increased Long-Term Mortality in Women with High Left Ventricular Ejection Fraction: Data from the CONFIRM (COronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) Long-Term Registry. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 363–374. [Google Scholar] [CrossRef]
- Kovalchin, J.P.; Silverman, N.H. The impact of fetal echocardiography. Pediatr. Cardiol. 2004, 25, 299–306. [Google Scholar] [CrossRef]
- Vesel, S.; Rollings, S.; Jones, A.; Callaghan, N.; Simpson, J.; Sharland, G.K. Prenatally diagnosed pulmonary atresia with ventricular septal defect: Echocardiography, genetics, associated anomalies and outcome. Heart 2006, 92, 1501–1505. [Google Scholar] [CrossRef]
- Knapp, J.; Tavares de Sousa, M.; Schonnagel, B.P. Fetal Cardiovascular MRI—A Systemic Review of the Literature: Challenges, New Technical Developments, and Perspectives. Rofo 2022, 194, 841–851. [Google Scholar] [CrossRef]
- Roy, C.W.; van Amerom, J.F.P.; Marini, D.; Seed, M.; Macgowan, C.K. Fetal Cardiac MRI: A Review of Technical Advancements. Top Magn. Reason. Imaging 2019, 28, 235–244. [Google Scholar] [CrossRef]
- Ryd, D.; Fricke, K.; Bhat, M.; Arheden, H.; Liuba, P.; Hedstrom, E. Utility of Fetal Cardiovascular Magnetic Resonance for Prenatal Diagnosis of Complex Congenital Heart Defects. JAMA Netw. Open 2021, 4, e213538. [Google Scholar] [CrossRef]
- Dong, S.Z.; Zhu, M.; Ji, H.; Ren, J.Y.; Liu, K. Fetal cardiac MRI: A single center experience over 14-years on the potential utility as an adjunct to fetal technically inadequate echocardiography. Sci. Rep. 2020, 10, 12373. [Google Scholar] [CrossRef]

| PS Severity Classes | Mild | Moderate | Severe |
|---|---|---|---|
| Peak velocity (m/s) | <3 | 3–4 | >4 |
| Peak gradient (mmHg) | <36 | 36–64 | >64 |
| PR Severity Classes | Mild | Moderate | Severe |
|---|---|---|---|
| Echo Qualitative parameters | |||
| Pulmonic valve morphology | Normal | Normal/Abnormal | Abnormal |
| Color flow PR jet width | Small, usually <10 mm in length with a narrow origin | Intermediate | Large, with a wide origin; may be brief in duration |
| Reversal flow in pulmonary artery branches | Absent | Absent | Present |
| CW signal of PR jet | Faint/Slow deceleration | Dense/variable | Dense/steep deceleration, early termination of diastolic flow |
| Pulmonic vs. aortic flow by PW | Normal or slightly increased | Intermediate | Greatly increased |
| Echo Semi-quantitative parameters | |||
| VC width (mm) | Not defined | Not defined | Not defined |
| Deceleration time of the PR | Not defined | Not defined | <260 ms |
| Pressure half-time | Not defined | Not defined | <100 ms |
| Jet width/annulus ratio | Not defined | Not defined | >65% |
| PR index | Not defined | Not defined | <0.77 |
| Echo Quantitative parameters | |||
| EROA (mm2) | Not defined | Not defined | Not defined |
| R Vol (mL) | Not defined | Not defined | Not defined |
| RF (%) | <20 | 20–40 | >40 |
| CMR parameters | |||
| RF (%) | <20 | 20–40 | >40 |
| Echo/CMR Structural parameters | |||
| Structural parameters, RV size | Usually normal | Normal or dilated | Usually dilated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, P.; Perone, F.; Siani, A.; Groenhoff, L.; Muscogiuri, G.; Sironi, S.; Marra, P.; Carriero, S.; Pavon, A.G.; Guglielmo, M. Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation. J. Imaging 2022, 8, 278. https://doi.org/10.3390/jimaging8100278
Costantini P, Perone F, Siani A, Groenhoff L, Muscogiuri G, Sironi S, Marra P, Carriero S, Pavon AG, Guglielmo M. Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation. Journal of Imaging. 2022; 8(10):278. https://doi.org/10.3390/jimaging8100278
Chicago/Turabian StyleCostantini, Pietro, Francesco Perone, Agnese Siani, Léon Groenhoff, Giuseppe Muscogiuri, Sandro Sironi, Paolo Marra, Serena Carriero, Anna Giulia Pavon, and Marco Guglielmo. 2022. "Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation" Journal of Imaging 8, no. 10: 278. https://doi.org/10.3390/jimaging8100278
APA StyleCostantini, P., Perone, F., Siani, A., Groenhoff, L., Muscogiuri, G., Sironi, S., Marra, P., Carriero, S., Pavon, A. G., & Guglielmo, M. (2022). Multimodality Imaging of the Neglected Valve: Role of Echocardiography, Cardiac Magnetic Resonance and Cardiac Computed Tomography in Pulmonary Stenosis and Regurgitation. Journal of Imaging, 8(10), 278. https://doi.org/10.3390/jimaging8100278







