Ultrasound Imaging in Dentistry: A Literature Overview
Abstract
1. Introduction
- Sound waves move in straight lines.
- Reflections are generated from structures along the central axis of the beam.
- Amplitude of reflection corresponds to the reflector scattering strength.
- Sound moves at exactly 1540 m/s.
- Sound moves directly to the reflector and back.
- Reverberation: these artifacts manifest as multiple lines at the same distance to a ray line, generated by multiple sound echoes from the same interfaces.
- Ring down: these images are generated when small bodies produce the same resonance of ultrasound for the emitted sound. This sound is produced after the first reflection, and when it is received by the probe, the device interprets this signal as coming from deeper interfaces in the medium.
- Mirror images: sound can bounce off a strong, smooth reflector such as the diaphragm. The surface acts as mirror and reflects the pulse to another tissue interface, and from this image, it seems that the second interface is beyond the first.
- Reflections: a mechanism that can be superimposed on the mirror image, different in a peculiar way in how it appears, and is generated by different reflections, producing the effect that there are deeper structures than the one studied.
- Enhancement: this artifact is seen as an abnormally high brightness. This occurs when sound travels through a medium with an attenuation rate lower than surrounding tissue.
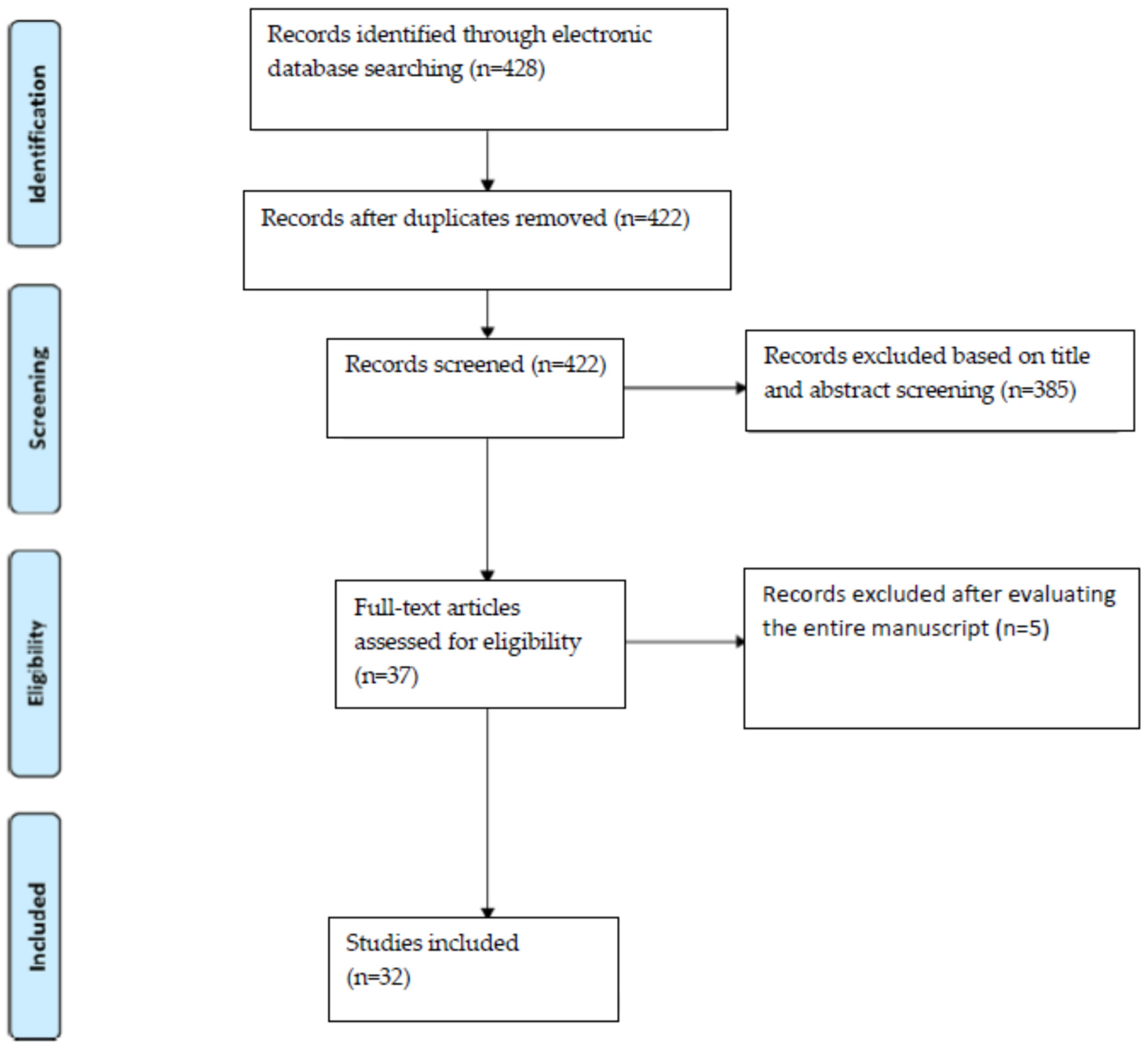
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aanenson, J.W.; Till, J.E.; Grogan, H.A. Understanding and communicating radiation dose and risk from cone beam computed tomography in dentistry. J. Prosthet. Dent. 2018, 120, 353–360. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Scarfe, W.C.; Vaughn, V.M.; Jacobs, R. Cone beam computed tomography in implant dentistry: A systematic review focusing on guidelines, indications, and radiation dose risks. Int. J. Oral Maxillofac. Implant. 2014, 29, 55–77. [Google Scholar] [CrossRef]
- Alhammadi, M.; Al-Mashraqi, A.; Alnami, R.; Ashqar, N.; Alamir, O.; Halboub, E.; Reda, R.; Testarelli, L.; Patil, S. Accuracy and Reproducibility of Facial Measurements of Digital Photographs and Wrapped Cone Beam Computed Tomography (CBCT) Photographs. Diagnostics 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, G.; Baccaglione, G.; Clauser, T.; Scaini, R.; Grassi, R.; Testarelli, L.; Reda, R.; Testori, T.; Del Fabbro, M. Total Face Approach (TFA) 3D Cephalometry and Superimposition in Orthognathic Surgery: Evaluation of the Vertical Dimensions in a Consecutive Series. Methods Protoc. 2021, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Alkahtani, A.; Bhandi, S.; Mashyakhy, M.; Alvarez, M.; Alroomy, R.; Hendi, A.; Varadarajan, S.; Reda, R.; Raj, A.; et al. Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. Diagnostics 2021, 11, 1208. [Google Scholar] [CrossRef]
- Di Nardo, D.; Gambarini, G.; Capuani, S.; Testarelli, L. Nuclear Magnetic Resonance Imaging in Endodontics: A Review. J. Endod. 2018, 44, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.; Zanza, A.; Mazzoni, A.; Cicconetti, A.; Testarelli, L.; Di Nardo, D. An Update of the Possible Applications of Magnetic Resonance Imaging (MRI) in Dentistry: A Literature Review. J. Imaging 2021, 7, 75. [Google Scholar] [CrossRef]
- Valenti-Obino, F.; DI Nardo, D.; Quero, L.; Miccoli, G.; Gambarini, G.; Testarelli, L.; Galli, M. Symmetry of root and root canal morphology of mandibular incisors: A cone-beam computed tomography study in vivo. J. Clin. Exp. Dent. 2019, 11, e527–e533. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; Plotino, G.; Grande, N.M.; Testarelli, L.; Prencipe, M.; Messineo, D.; Fratini, L.; D’Ambrosio, F. Differential diagnosis of endodontic-related inferior alveolar nerve paraesthesia with cone beam computed tomography: A case report. Int. Endod. J. 2010, 44, 176–181. [Google Scholar] [CrossRef]
- Gambarini, G.; Ropini, P.; Piasecki, L.; Costantini, R.; Carneiro, E.; Testarelli, L.; Dummer, P.M.H. A preliminary assessment of a new dedicated endodontic software for use with CBCT images to evaluate the canal complexity of mandibular molars. Int. Endod. J. 2017, 51, 259–268. [Google Scholar] [CrossRef]
- Aldrich, J.E. Basic physics of ultrasound imaging. Crit. Care Med. 2007, 35, S131–S137. [Google Scholar] [CrossRef] [PubMed]
- Haubrich, W.S. Doppler of the Doppler ultrasound effect. Gastroenterology 2003, 125, 1590. [Google Scholar]
- Burns, P.N. Principles of Doppler and color flow. Radiol. Med. 1993, 85, 3–16. [Google Scholar] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.V.; Baldini, C. Major Salivary Gland Ultrasonography in the Diagnosis of Sjögren’s Syndrome: A Place in the Diagnostic Criteria? Rheum. Dis. Clin. 2016, 42, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Afzelius, P.; Nielsen, M.-Y.; Ewertsen, C.; Bloch, K.P. Imaging of the major salivary glands. Clin. Physiol. Funct. Imaging 2014, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heřman, J.; Sedláčková, Z.; Fürst, T.; Vachutka, J.; Salzman, R.; Vomáčka, J.; Heřman, M. The Role of Ultrasound and Shear-Wave Elastography in Evaluation of Cervical Lymph Nodes. BioMed Res. Int. 2019, 2019, 4318251. [Google Scholar] [CrossRef]
- Akturk, E.S.; Eren, H.; Gorurgoz, C.; Orhan, K.; Karasu, H.A.; Akat, B.; Memikoglu, T.U.T. Electromyographic, Ultrasonographic, and Ultrasound Elastographic Evaluation of the Masseter Muscle in Class III Patients Before and After Orthognathic Surgery. J. Craniofacial Surg. 2020, 31, 2049–2053. [Google Scholar] [CrossRef]
- Su, N.; Van Wijk, A.J.; Visscher, C.M.; Lobbezoo, F.; Van Der Heijden, G.J.M.G. Diagnostic value of ultrasonography for the detection of disc displacements in the temporomandibular joint: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2599–2614. [Google Scholar] [CrossRef]
- Tzoumpas, M.; Mohr, B.; Kurtulus-Waschulewski, I.; Wahl, G. The Use of High-Frequency Ultrasound in the Measurement of Thickness of the Maxillary Attached Gingiva. Int. J. Prosthodont. 2015, 28, 374–382. [Google Scholar] [CrossRef][Green Version]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Oranges, T.; Dini, V.; Nisi, M.; Graziani, F.; Gabriele, M.; Caramella, D. Discovering a new anatomy: Exploration of oral mucosa with ultra-high frequency ultrasound. Dentomaxillofacial Radiol. 2020, 49, 20190318. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Musu, D.; Goddi, A.; Dettori, C.; Campisi, G.; Shemesh, H. Ultrasound Examination to Visualize and Trace Sinus Tracts of Endodontic Origin. J. Endod. 2019, 45, 1184–1191. [Google Scholar] [CrossRef]
- Chan, H.-L.; Kripfgans, O.D. Ultrasonography for diagnosis of peri-implant diseases and conditions: A detailed scanning protocol and case demonstration. Dentomaxillofacial Radiol. 2020, 49, 20190445. [Google Scholar] [CrossRef]
- Bohner, L.; Habor, D.; Tortamano, P.; Radermacher, K.; Wolfart, S.; Marotti, J. Assessment of Buccal Bone Surrounding Dental Implants Using a High-Frequency Ultrasound Scanner. Ultrasound Med. Biol. 2019, 45, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Bohner, L.; Habor, D.; Gremse, F.; Tortamano, P.; Wolfart, S.; Marotti, J. Accuracy of High-Frequency Ultrasound Scanner in Detecting Peri-implant Bone Defects. Ultrasound Med. Biol. 2019, 45, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Sinjab, K.; Li, J.; Chen, Z.; Wang, H.; Kripfgans, O.D. Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions. J. Clin. Periodontol. 2018, 45, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.O.; Choi, M.; Singh, R.S.; White, S.N. Ultrasound imaging of dental implants. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 456–459. [Google Scholar]
- Vayron, R.; Soffer, E.; Anagnostou, F.; Haïat, G. Ultrasonic evaluation of dental implant osseointegration. J. Biomech. 2014, 47, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.-H.; Bosc, R.; Naili, S.; Haïat, G. Assessment of the biomechanical stability of a dental implant with quantitative ultrasound: A three-dimensional finite element study. J. Acoust. Soc. Am. 2016, 139, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Albini Lomami, H.; Meningaud, J.-P.; Bosc, R.; Haiat, G. Comparison of Resonance Frequency Analysis and of Quantitative Ultrasound to Assess Dental Implant Osseointegration. Sensors 2018, 18, 1397. [Google Scholar] [CrossRef]
- Vayron, R.; Nguyen, V.-H.; Lecuelle, B.; Haiat, G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 470–478. [Google Scholar] [CrossRef]
- Bayrakdar, I.; Caglayan, F. The Intraoral Ultrasonography in Dentistry. Niger. J. Clin. Pr. 2016, 21, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Natanasabapathy, V.; Arul, B.; Mishra, A.; Varghese, A.; Padmanaban, S.; Elango, S.; Arockiam, S. Ultrasound imaging for the differential diagnosis of periapical lesions of endodontic origin in comparison with histopathology—A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Musu, D.; Rossi-Fedele, G.; Campisi, G.; Cotti, E. Ultrasonography in the diagnosis of bone lesions of the jaws: A systematic review. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e19–e29. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Z.B.; Demir, H.; Berker Yıldız, D.; Yaşar, F. Diagnostic accuracy of panoramic radiography and ultrasonography in detecting periapical lesions using periapical radiography as a gold standard. Dentomaxillofacial Radiol. 2020, 49, 20190290. [Google Scholar] [CrossRef]
- Sönmez, G.; Kamburoğlu, K.; Yılmaz, F.; Koc, C.; Barış, E.; Tüzüner, A. Versatility of high resolution ultrasonography in the assessment of granulomas and radicular cysts: A comparative in vivo study. Dentomaxillofacial Radiol. 2019, 48, 20190082. [Google Scholar] [CrossRef] [PubMed]
- Davide, M.; Hagay, S.; Michela, B.; Claudia, D.; Elisabetta, C. The effectiveness of ultrasound examination to assess the healing process of bone lesions of the jaws: A systematic review. Clin. Oral Investig. 2020, 24, 3739–3747. [Google Scholar] [CrossRef]
- Gad, K.; Ellabban, M.; Sciubba, J. Utility of Transfacial Dental Ultrasonography in Evaluation of Cystic Jaw Lesions. J. Ultrasound Med. 2018, 37, 635–644. [Google Scholar] [CrossRef]
- Prince, C.N.; Annapurna, C.S.; Sivaraj, S.; Ali, I.M. Ultrasound imaging in the diagnosis of periapical lesions. J. Pharm. Bioallied Sci. 2012, 4, S369–S372. [Google Scholar] [CrossRef]
- Curvers, F.; Meschi, N.; Vanhoenacker, A.; Strijbos, O.; Van Mierlo, M.; Lambrechts, P. Ultrasound Assessment of Bone Healing after Root-end Surgery: Echoes Back to Patient’s Safety. J. Endod. 2018, 44, 32–37. [Google Scholar] [CrossRef]
- Demirturk Kocasarac, H.; Angelopoulos, C. Ultrasound in Dentistry: Toward a Future of Radiation-Free Imaging. Dent. Clin. N. Am. 2018, 62, 481–489. [Google Scholar] [CrossRef]
- Marotti, J.; Heger, S.; Tinschert, J.; Tortamano, P.; Chuembou, F.; Radermacher, K.; Wolfart, S. Recent advances of ultrasound imaging in dentistry--a review of the literature. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.-C.T.; Le, L.H.; Kaipatur, N.R.; Zheng, R.; Lou, E.H.; Major, P.W. High-Resolution Ultrasonic Imaging of Dento-Periodontal Tissues Using a Multi-Element Phased Array System. Ann. Biomed. Eng. 2016, 44, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, V.; Chan, H.-L.; MacEachern, M.; Kripfgans, O.D. Updates on ultrasound research in implant dentistry: A systematic review of potential clinical indications. Dentomaxillofacial Radiol. 2018, 47, 20180076. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, J.; Xing, D.; Lu, H.; Yue, R.; Li, S.; Wu, D. Ultrasonic Measurement of Lingual Artery and Its Application for Midline Glossectomy. Ann. Otol. Rhinol. Laryngol. 2020, 129, 856–862. [Google Scholar] [CrossRef]
- Di Bari, R.; Coronelli, R.; Cicconetti, A. Intraosseous vascularization of anterior mandible: A radiographic analysis. J. Craniofacial Surg. 2014, 25, 872–879. [Google Scholar] [CrossRef]
- Gonçalves, T.M.S.V.; Campos, C.H.; Gonçalves, G.M.; De Moraes, M.; Garcia, R.C.M.R. Mastication improvement after partial implant-supported prosthesis use. J. Dent. Res. 2013, 92, 189S–194S. [Google Scholar] [CrossRef]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Nisi, M.; Oranges, T.; Dini, V.; Ferro, F.; Baldini, C.; Romanelli, M.; Caramella, D.; et al. Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. Can. Assoc. Radiol. J. 2021, 72, 418–431. [Google Scholar] [CrossRef]
- Yi, J.; Nguyen, K.-C.T.; Wang, W.; Yang, W.; Pan, M.; Lou, E.; Major, P.W.; Le, L.H.; Zeng, H. Polyacrylamide/Alginate double-network tough hydrogels for intraoral ultrasound imaging. J. Colloid Interface Sci. 2020, 578, 598–607. [Google Scholar] [CrossRef]
- Kim, J.; Shin, T.; Kong, H.; Hwang, J.; Hyun, H. High-Frequency Ultrasound Imaging for Examination of Early Dental Caries. J. Dent. Res. 2019, 98, 363–367. [Google Scholar] [CrossRef]
- Gambarini, G.; Miccoli, G.; Seracchiani, M.; Khrenova, T.; Donfrancesco, O.; D’Angelo, M.; Galli, M.; Di Nardo, D.; Testarelli, L. Role of the Flat-Designed Surface in Improving the Cyclic Fatigue Resistance of Endodontic NiTi Rotary Instruments. Materials 2019, 12, 2523. [Google Scholar] [CrossRef]
- Bhandi, S.; Seracchiani, M.; Donfrancesco, O.; Reda, R.; Mazzoni, A.; Nottola, S.; Familiari, G.; Testarelli, L.; Gambarini, G. Nickel-Titanium Rotary Instruments: An In Vitro Comparison (Torsional Resistance of Two Heat-treated Reciprocating Files). J. Contemp. Dent. Pract. 2021, 22, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; Miccoli, G.; Seracchiani, M.; Morese, A.; Piasecki, L.; Gaimari, G.; DI Nardo, D.; Testarelli, L. Fatigue Resistance of New and Used Nickel-Titanium Rotary Instruments: A Comparative Study. La Clin. Ter. 2018, 169, e96–e101. [Google Scholar]
- Di Nardo, D.; Galli, M.; Morese, A.; Seracchiani, M.; Ferri, V.; Miccoli, G.; Gambarini, G.; Testarelli, L. A comparative study of mechanical resistance of two reciprocating files. J. Clin. Exp. Dent. 2019, 11, e231–e235. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; Piasecki, L.; Miccoli, G.; Dds, G.G.; Dds, R.D.G.; Di Nardo, D.; Azim, A.; Testarelli, L. Classification and cyclic fatigue evaluation of new kinematics for endodontic instruments. Aust. Endod. J. 2018, 45, 154–162. [Google Scholar] [CrossRef]
- Kondrashova, T.; De Wan, D.; Briones, M.U.; Kondrashov, P. Integration of ultrasound imaging into pre-clinical dental education. Eur. J. Dent. Educ. 2017, 21, 228–234. [Google Scholar] [CrossRef] [PubMed]
| Title | Authors | Year |
|---|---|---|
| Major Salivary Gland Ultrasonography in the Diagnosis of Sjögren’s Syndrome: A Place in the Diagnostic Criteria? | Jonsson MV, Baldini C. | 2016 |
| Diagnostic imaging in salivary gland disease. | Afzelius P, Nielsen MY, Ewertsen C, Bloch KP. | 2016 |
| Electromyographic, Ultrasonographic, and Ultrasound Elastographic Evaluation of the Masseter Muscle in Class III Patients Before and After Orthognathic Surgery. | Sunal Akturk E, Eren H, Gorurgoz C, Orhan K, Karasu HA, Akat B, Toygar Memikoglu TU. | 2020 |
| Ultrasonography for diagnosis of peri-implant diseases and conditions: a detailed scanning protocol and case demonstration. | Chan HL, Kripfgans OD. | 2020 |
| Diagnostic value of ultrasonography for the detection of disc displacements in the temporomandibular joint: a systematic review and meta-analysis. | Su N, van Wijk AJ, Visscher CM, Lobbezoo F, van der Heijden GJMG. | 2018 |
| Ultrasound Assessment of Bone Healing after Root-end Surgery: Echoes Back to Patient’s Safety. | Curvers F, Meschi N, Vanhoenacker A, Strijbos O, Van Mierlo M, Lambrechts P. | 2018 |
| The Intraoral Ultrasonography in Dentistry. | Caglayan F, Bayrakdar IS. | 2018 |
| Recent advances of ultrasound imaging in dentistry—A review of the literature. | Marotti J, Heger S, Tinschert J, Tortamano P, Chuembou F, Radermacher K, Wolfart S. | 2013 |
| Ultrasound in Dentistry: Toward a Future of Radiation-Free Imaging. | Demirturk Kocasarac H, Angelopoulos C. | 2018 |
| Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. | Patil S, Alkahtani A, Bhandi S, Mashyakhy M, Alvarez M, Alroomy R, Hendi A, Varadarajan S, Reda R, Raj AT, Testarelli L. | 2021 |
| Assessment of Buccal Bone Surrounding Dental Implants Using a High-Frequency Ultrasound Scanner. | Bohner L, Habor D, Tortamano P, Radermacher K, Wolfart S, Marotti J. | 2019 |
| Polyacrylamide/Alginate double-network tough hydrogels for intraoral ultrasound imaging. | Yi J, Nguyen KT, Wang W, Yang W, Pan M, Lou E, Major PW, Le LH, Zeng H. | 2020 |
| Ultrasonography in the diagnosis of bone lesions of the jaws: a systematic review. | Musu D, Rossi-Fedele G, Campisi G, Cotti E. | 2016 |
| Diagnostic accuracy of panoramic radiography and ultrasonography in detecting periapical lesions using periapical radiography as a gold standard. | Arslan ZB, Demir H, Berker Yıldız D, Yaşar F. | 2020 |
| Ultrasonic Measurement of Lingual Artery and Its Application for Midline Glossectomy. | Liu C, Qin J, Xing D, Lu H, Yue R, Li S, Wu D. | 2020 |
| Ultrasound Examination to Visualize and Trace Sinus Tracts of Endodontic Origin. | Cotti E, Musu D, Goddi A, Dettori C, Campisi G, Shemesh H. | 2019 |
| Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. | Izzetti R, Vitali S, Aringhieri G, Nisi M, Oranges T, Dini V, Ferro F, Baldini C, Romanelli M, Caramella D, Gabriele M. | 2021 |
| Discovering a new anatomy: exploration of oral mucosa with ultra-high frequency ultrasound. | Izzetti R, Vitali S, Aringhieri G, Oranges T, Dini V, Nisi M, Graziani F, Gabriele M, Caramella D. | 2020 |
| Accuracy of High-Frequency Ultrasound Scanner in Detecting Peri-implant Bone Defects. | Bohner L, Habor D, Gremse F, Tortamano P, Wolfart S, Marotti J. | 2019 |
| The Role of Ultrasound and Shear-Wave Elastography in Evaluation of Cervical Lymph Nodes. | Heřman J, Sedláčková Z, Fürst T, Vachutka J, Salzman R, Vomáčka J, Heřman M. | 2019 |
| Versatility of high resolution ultrasonography in the assessment of granulomas and radicular cysts: a comparative in vivo study. | Sönmez G, Kamburoğlu K, Yılmaz F, Koç C, Barış E, Tüzüner A. | 2019 |
| Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions. | Chan HL, Sinjab K, Li J, Chen Z, Wang HL, Kripfgans OD. | 2018 |
| The effectiveness of ultrasound examination to assess the healing process of bone lesions of the jaws: a systematic review | Davide M, Hagay S, Michela B, Claudia D, Elisabetta C. | 2020 |
| Integration of ultrasound imaging into pre-clinical dental education. | Kondrashova T, De Wan D, Briones MU, Kondrashov P. | 2017 |
| High-Frequency Ultrasound Imaging for Examination of Early Dental Caries. | Kim J, Shin TJ, Kong HJ, Hwang JY, Hyun HK. | 2019 |
| Mastication improvement after partial implant-supported prosthesis use. | Gonçalves TM, Campos CH, Gonçalves GM, de Moraes M, Rodrigues Garcia RC. | 2013 |
| Updates on ultrasound research in implant dentistry: a systematic review of potential clinical indications. | Bhaskar V, Chan HL, MacEachern M, Kripfgans OD. | 2018 |
| Utility of Transfacial Dental Ultrasonography in Evaluation of Cystic Jaw Lesions. | Gad K, Ellabban M, Sciubba J. | 2018 |
| Ultrasound imaging of dental implants. | Culjat MO, Choi M, Singh RS, White SN. | 2012 |
| High-Resolution Ultrasonic Imaging of Dento-Periodontal Tissues Using a Multi-Element Phased Array System. | Nguyen KT, Le LH, Kaipatur NR, Zheng R, Lou EH, Major PW. | 2016 |
| Ultrasound real-time imaging in the differential diagnosis of periapical lesions. | Prince CN, Annapurna CS, Sivaraj S, Ali IM. | 2012 |
| The Use of High Frequency Ultrasound in the Measurement of Thickness of the Maxillary Attached Gingiva. | Tzoumpas M, Mohr B, Kurtulus-Waschulewski I, Wahl G. | 2015 |
| Title | Year | Types of Transducers | Range of Frequencies | Advantages/Disadvantages of the Different Ultrasound Systems |
|---|---|---|---|---|
| Major Salivary Gland Ultrasonography in the Diagnosis of Sjögren’s Syndrome: A Place in the Diagnostic Criteria? | 2016 | Being user-friendly, rapidly performed, repeatable, noninvasive, and nonradiating, SG-US has emerged as a promising diagnostic and prognostic tool. | ||
| Diagnostic imaging in salivary gland disease | 2016 | 7–15 MHz | It can be used for image guided biopsies, and can be performed in the emergency setting. Ultrasound has limitations in evaluating structures behind bone and the deep parts of the parotid gland. | |
| Electromyographic, Ultrasonographic, and Ultrasound Elastographic Evaluation of the Masseter Muscle in Class III Patients Before and After Orthognathic Surgery | 2020 | Convex transducers | 3–5 MHz | Muscle length, thickness, cross-sectional area, and volume measurements can be obtained with ultrasound imaging. |
| Ultrasonography for diagnosis of peri-implant diseases and conditions: a detailed scanning pro-tocol and case demonstration | 2020 | Toothbrush-sized (~30 mm × 18 mm × 12 mm) probe | 25 MHz | It displays images of peri-implant tissues of various health conditions in live humans. |
| Diagnostic value of ultrasonography for the detection of disc displacements in the temporomandibular joint: a systematic review and meta-analysis | 2018 | Ultrasound can be considered as a relevant imaging tool to supplement clinical examination in patients with suspected disc displacement in selected cases. Combined static and dynamic examinations using high-resolution ultrasound should be preferred. | ||
| Ultrasound Assessment of Bone Healing after Root-end Surgery: Echoes Back to Patient’s Safety | 2018 | Linear ultrasonic probe operating | 12 MHz | It can detect initial bone healing processes. |
| The Intraoral Ultrasonography in Dentistry | 2016 | 2.5–10 MHz, up to 40 MHz | Intraoral ultrasound examination is limited to the anterior aspects of the jaws, as the presently available probes are not ideal for use in the posterior jaws in areas of thick cortical plates. | |
| Recent advances of ultrasound imaging in dentistry—a review of the literature | 2013 | 2–20 MHz | Ultrasonography may provide a significant benefit to patients by allowing early detection of tooth lesions and defects, measurement of mucosa and gingival thickness, dental implant locations, and dental scanning. | |
| Ultrasound in Dentistry: Toward a Future of Radi-ation-Free Imaging | 2018 | 3–12 MHz | It provides real-time and simultaneous imaging of both hard and soft tissues. | |
| Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review | 2021 | 6–12 MHz | Within the limitations of the studies included, this review indicates that it provides better diagnostic accuracy for differentiating endodontic lesions compared to radiographic imaging. | |
| Assessment of Buccal Bone Surrounding Dental Implants Using a High-Frequency Ultrasound Scanner | 2019 | Transducer spherically focused with an aperture of 6 mm and focus of 13.2 mm | 28 MHz | High-frequency ultrasound was able to measure buccal bone dimensions surrounding dental implants with a trueness similar to that of cone-beam computed tomography. |
| Polyacrylamide/alginate double-network tough hydrogels for intraoral ultrasound imaging | 2020 | 6.35 mm diameter unfocused transducers | 20 MHz | PAM/alginate tough hydrogels were explored as potential couplants for intraoral ultrasound imaging by a comprehensive comparison of their physical, mechanical, frictional, and ultrasound properties, as well as biocompatibility with the commercial couplant. |
| Ultrasonography in the diagnosis of bone lesions of the jaws: a systematic review | 2016 | The results demonstrated the value of ultrasonography for the evaluation of the nature of intra-osseous lesions in the jaws. | ||
| Diagnostic accuracy of panoramic radiography and ultrasonography in detecting periapical lesions using periapical radiography as a gold standard | 2020 | Linear ultrasonic probe | 7–10 MHz | These results showed that although the ultrasound has a higher value than the panoramic, the two techniques have similar diagnostic accuracy values, and there is no significant difference between the two techniques in the detection of periapical lesions. |
| Ultrasonic Measurement of Lingual Artery and Its Application for Midline Glossectomy | 2020 | In conclusion, preoperative US can show the course of the lingual artery clearly for preoperative planning. | ||
| Ultrasound Examination to Visualize and Trace Sinus Tracts of Endodontic Origin | 2019 | Linear and multifrequency probes | 7–12 MHz | Ultrasound real-time examination can be successfully used to detect the STs of endodontic origin and to trace their route of drainage from the periapical lesion to the opening within the oral mucosa or the skin. |
| Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience | 2021 | 30–70 MHz | The literature on UHFUS is still evolving, but ultrahigh frequencies seem to be the answer to several clinical problems related to the high-resolution investigation of both normal anatomy and disease processes. | |
| Discovering a new anatomy: exploration of oral mucosa with ultra-high frequency ultrasound | 2020 | 70 MHz | It is considered to be a diagnostic support in the management of oral soft tissue lesions, in terms of diagnosis, surgical procedure, postoperative discomfort reduction, and prevention/early detection of malignant transformation. | |
| Accuracy of High-Frequency Ultrasound Scanner in Detecting Peri-implant Bone Defects | 2019 | Custom spherically focused transducer with an aperture of 4 mm | 42 MHz | High-frequency ultrasound in association with the a priori information technique was accurate in measuring the width of peri-implant defects. |
| The Role of Ultrasound and Shear-Wave Elastography in Evaluation of Cervical Lymph Nodes | 2019 | Linear probe | 4–15 MHz | Good results in discriminating benign from malignant cervical lymph nodes. |
| Versatility of high resolution ultrasonography in the assessment of granulomas and radicular cysts: a comparative in vivo study | 2019 | (1) Linear (2) Hockey probes | (1) 9 MHz (2) 15 MHz | It provides useful information for the diagnosis and assessment of granulomas and radicular cysts. |
| Ultrasonography for noninvasive and real-time evaluation of peri-implant tissue dimensions | 2018 | 25 MHz | It could become a valuable method to evaluate peri-implant tissue biotype and peri-implant diseases. | |
| The effectiveness of ultrasound examination to assess the healing process of bone lesions of the jaws: a systematic review | 2020 | Mainly linear | 5–12 MHz | The USE implemented with CPD is an advanced imaging technique feasible for monitoring the early and long-term response of intra-osseous jaw lesions in both surgical and nonsurgical treatments. |
| Integration of ultrasound imaging into pre-clinical dentaleducation | 2017 | Results of the current study suggested that ultrasound could be integrated into dental education. | ||
| High-Frequency Ultrasound Imaging for Examination of Early Dental Caries | 2018 | Press-focused HFUS transducer | 40 MHz | The invasion depths of WSLs obtained with HFUS images had good agreement with those of WSLs obtained with the micro-CT images within the limits of the study. |
| Mastication Improvement After Partial Implant-Supported Prosthesis Use | 2013 | Linear probe | 7–18 MHz | The IRDPs and IFDPs significantly increased MBF and FCI, with the magnitude of the masticatory improvements closely related to prosthesis type. |
| Updates on Ultrasound Research in Implant Dentistry: A Systematic Review ofPotential Clinical Indications | 2018 | Limitations of ultrasound include the need of a medium for sound conduction, inability to penetrate into bone, and narrow field of view. Acoustic gel is needed. | ||
| Utility of Transfacial Dental Ultrasonography in Evaluation of Cystic Jaw Lesions | 2018 | Linear transducer | 7–12 MHz | On transfacial dental US supplemented by a Doppler study with either a power or color display, vascular flow could be enhanced, and can be determinant in differential diagnosis. |
| Ultrasound imaging of dental implants | 2012 | 16 Mhz | This experiment demonstrated that ultrasonography could be used to measure tissue depth over acoustically diffuse cancellous bone before implant placement, and to locate and measure soft tissue thickness over submerged implants. | |
| High-Resolution Ultrasonic Imaging of Dento-Periodontal Tissues Using a Multi-Element Phased Array System | 2016 | Broadband array transducer | 8–40 MHz | High-quality ultrasound images of the tooth and the surrounding periodontium. |
| Ultrasound imaging in the diagnosis of periapical lesions | 2012 | Linear transducer | 7–11 MHz | With its potential usefulness to differentiate the periapical lesions, ultrasonography can be considered as a better imaging modality with improved efficacy when compared to conventional radiography. |
| The Use of High Frequency Ultrasound in the Measurement of Thickness of the Maxillary Attached Gingiva | 2015 | Linear probe | 20 MHz | It has better characteristics, with the same results compared to a trans-mucosal probing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, R.; Zanza, A.; Cicconetti, A.; Bhandi, S.; Miccoli, G.; Gambarini, G.; Di Nardo, D. Ultrasound Imaging in Dentistry: A Literature Overview. J. Imaging 2021, 7, 238. https://doi.org/10.3390/jimaging7110238
Reda R, Zanza A, Cicconetti A, Bhandi S, Miccoli G, Gambarini G, Di Nardo D. Ultrasound Imaging in Dentistry: A Literature Overview. Journal of Imaging. 2021; 7(11):238. https://doi.org/10.3390/jimaging7110238
Chicago/Turabian StyleReda, Rodolfo, Alessio Zanza, Andrea Cicconetti, Shilpa Bhandi, Gabriele Miccoli, Gianluca Gambarini, and Dario Di Nardo. 2021. "Ultrasound Imaging in Dentistry: A Literature Overview" Journal of Imaging 7, no. 11: 238. https://doi.org/10.3390/jimaging7110238
APA StyleReda, R., Zanza, A., Cicconetti, A., Bhandi, S., Miccoli, G., Gambarini, G., & Di Nardo, D. (2021). Ultrasound Imaging in Dentistry: A Literature Overview. Journal of Imaging, 7(11), 238. https://doi.org/10.3390/jimaging7110238










