Comparison of Piezoelectric and Optical Projection Imaging for Three-Dimensional In Vivo Photoacoustic Tomography
Abstract
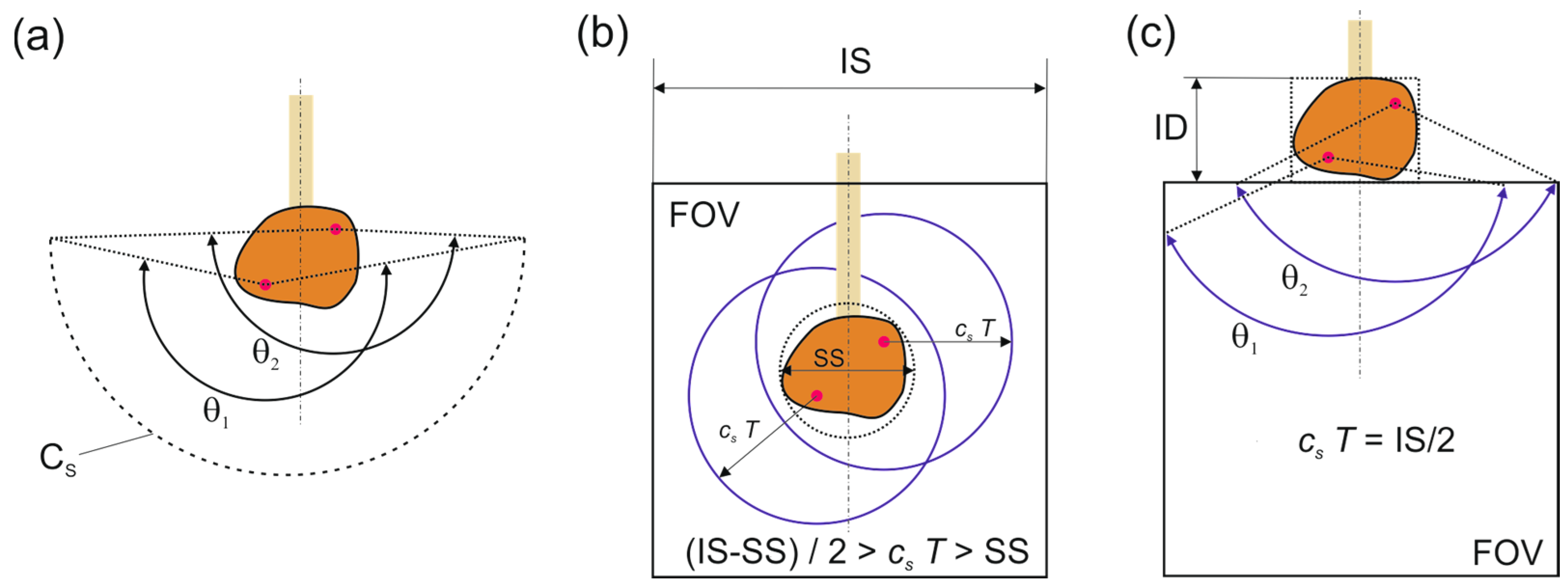
1. Introduction
2. Materials and Methods
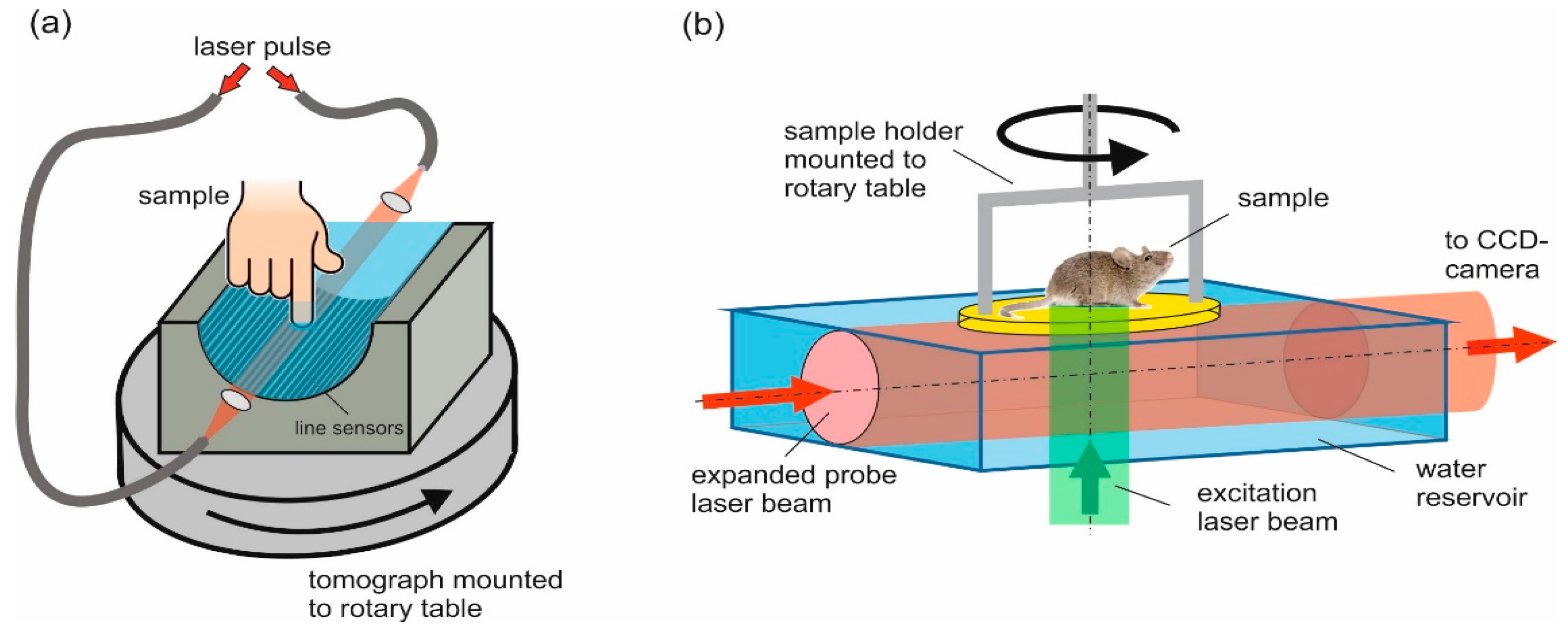
2.1. Imaging Setups
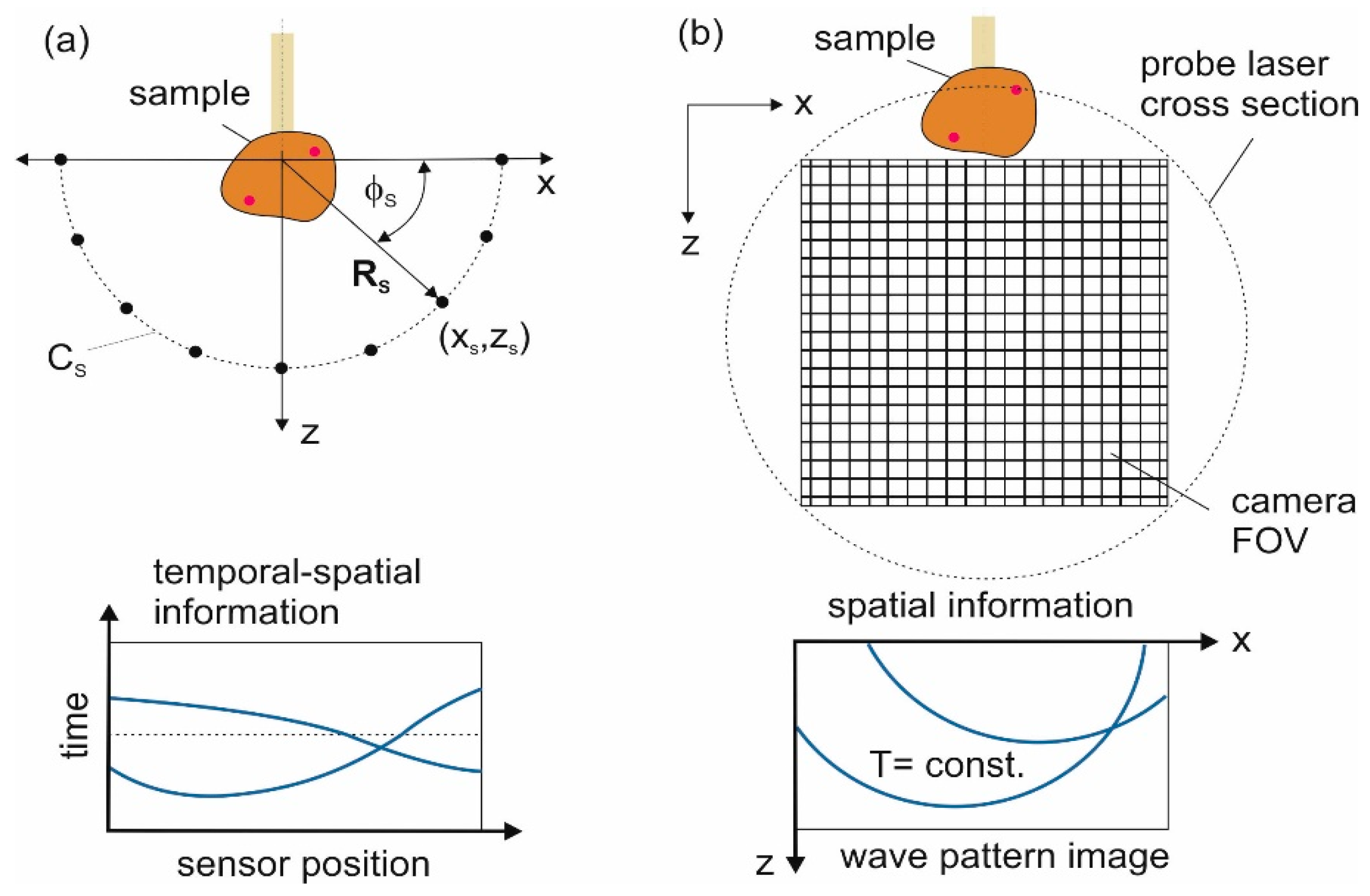
2.2. Image Reconstruction
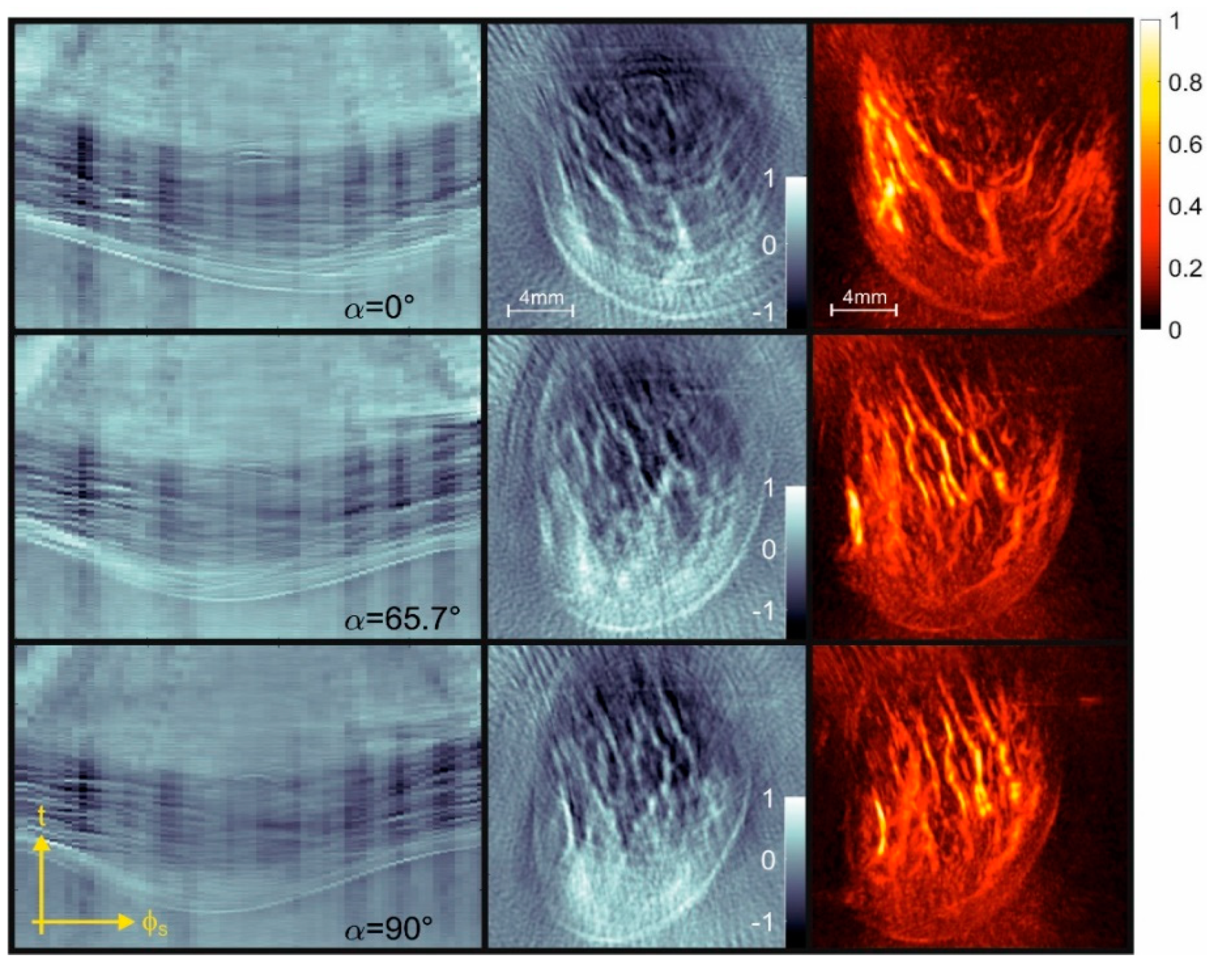
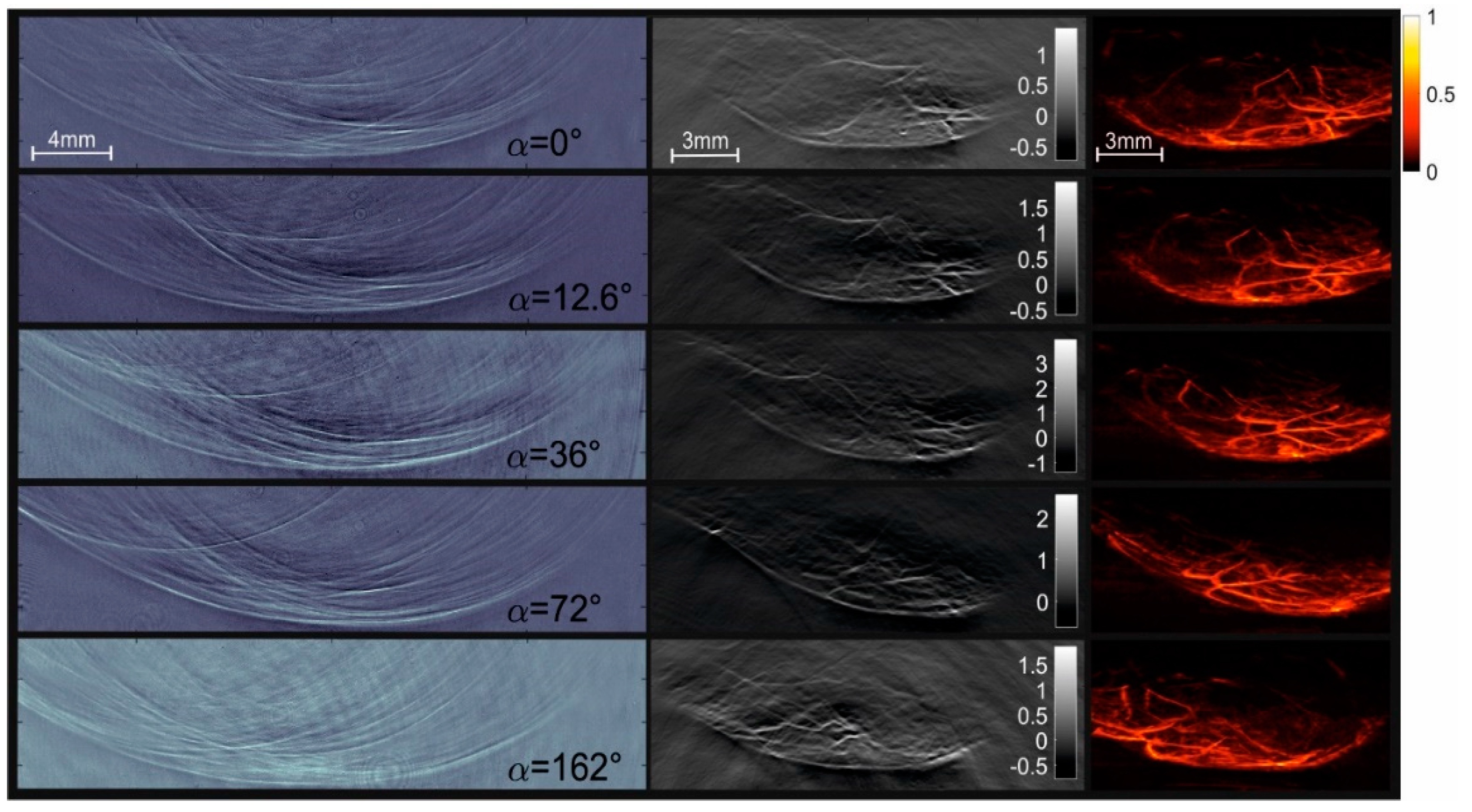
3. Results
4. Discussion
4.1. Image Resolution
4.2. Sensitivity
4.3. Artifacts
4.4. Operation
4.5. Manufacturing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, E.Z.; Laufer, J.G.; Pedley, R.B.; Beard, P.C. In vivo high-resolution 3D photoacoustic imaging of superficial vascular anatomy. Phys. Med. Biol. 2009, 54, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Paltauf, G.; Nuster, R.; Haltmeier, M.; Burgholzer, P. Photoacoustic tomography using a Mach-Zehnder interferometer as acoustic line detector. Appl. Opt. 2007, 46, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Deán-Ben, X.L.; Sela, G.; Lauri, A.; Kneipp, M.; Ntziachristos, V.; Westmeyer, G.G.; Shoham, S.; Razansky, D. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci. Appl. 2016, 5, e16201. [Google Scholar] [CrossRef] [PubMed]
- Buehler, A.; Herzog, E.; Razansky, D.; Ntziachristos, V. Video rate optoacoustic tomography of mouse kidney perfusion. Opt. Lett. 2010, 35, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Brecht, H.-P.; Su, R.; Fronheiser, M.; Ermilov, S.A.; Conjusteau, A.; Oraevsky, A.A. Whole-body three-dimensional optoacoustic tomography system for small animals. J. Biomed. Opt. 2009, 14, 64007. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, J.J.; Jaeger, M.; Lemor, R.; Weber, P.; Frenz, M. Combined Ultrasound and Optoacoustic System for Real-Time High-Contrast Vascular Imaging in Vivo. IEEE Trans. Med. Imaging 2005, 24, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Paltauf, G.; Nuster, R.; Haltmeier, M.; Burgholzer, P. Experimental evaluation of reconstruction algorithms for limited view photoacoustic tomography with line detectors. Inverse Probl. 2007, 23, S81–S94. [Google Scholar] [CrossRef]
- Berer, T.; Veres, I.A.; Grün, H.; Bauer-Marschallinger, J.; Felbermayer, K.; Burgholzer, P. Characterization of broadband fiber optic line detectors for photoacoustic tomography. J. Biophotonics 2012, 5, 518–528. [Google Scholar] [CrossRef]
- Paltauf, G.; Hartmair, P.; Kovachev, G.; Nuster, R. Piezoelectric line detector array for photoacoustic tomography. Photoacoustics 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Bauer-Marschallinger, J.; Felbermayer, K.; Berer, T. All-optical photoacoustic projection imaging. Biomed. Opt. Express 2017, 8, 3938–3951. [Google Scholar] [CrossRef] [PubMed]
- Nuster, R.; Zangerl, G.; Haltmeier, M.; Paltauf, G. Full field detection in photoacoustic tomography. Opt. Express 2010, 18, 6288–6299. [Google Scholar] [CrossRef] [PubMed]
- Nuster, R.; Slezak, P.; Paltauf, G. High resolution three-dimensional photoacoutic tomography with CCD-camera based ultrasound detection. Biomed. Opt. Express 2014, 5, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, S.; Chao, C.Y.; Guo, L.J.; O’donnell, M. Ultrasound Detection Using Polymer Microring Optical Resonator. Appl. Phys. Lett. 2004, 85, 5418–5420. [Google Scholar] [CrossRef]
- Maswadi, S.M.; Ibey, B.L.; Roth, C.C.; Tsyboulski, D.A.; Beier, H.T.; Glickman, R.D.; Oraevsky, A.A. All-optical optoacoustic microscopy based on probe beam deflection technique. Photoacoustics 2016, 4, 91–101. [Google Scholar] [CrossRef]
- Kostli, K.P.; Frenz, M.; Weber, H.P.; Paltauf, G.; Schmidt-Kloiber, H. Optoacoustic Tomography: Time-Gated Measurement of Pressure Distributions and Image Reconstruction. Appl. Opt. 2001, 40, 3800–3809. [Google Scholar] [CrossRef]
- Dong, B.; Sun, C.; Zhang, H.F. Optical Detection of Ultrasound in Photoacoustic Imaging. IEEE Trans. Bio-Med. Eng. 2017, 64, 4–15. [Google Scholar] [CrossRef]
- Wissmeyer, G.; Pleitez, M.A.; Rosenthal, A.; Ntziachristos, V. Looking at sound: Optoacoustics with all-optical ultrasound detection. Light Sci. Appl. 2018, 7, 219. [Google Scholar] [CrossRef]
- Beard, P.C.; Hurrell, A.M.; Mills, T.N. Characterization of a polymer film optical fiber hydrophone for use in the range 1 to 20 MHz: A comparison with PVDF needle and membrane hydrophones. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2000, 47, 256–264. [Google Scholar] [CrossRef]
- Nuster, R.; Gratt, S.; Passler, K.; Grün, H.; Berer, T.; Burgholzer, P.; Paltauf, G. Comparison of optical and piezoelectric integrating line detectors. Proc. SPIE 2009, 7177, 71770T. [Google Scholar]
- Xu, M.H.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography (Vol 71, Art No 016706, 2005). Phys. Rev. E 2007, 75, 59903. [Google Scholar] [CrossRef]
- Burgholzer, P.; Matt, G.; Haltmeier, M.; Paltauf, G. Exact and approximative imaging methods for photoacoustic tomography using an arbitrary detection surface. Phys. Rev. E 2007, 75, 46706. [Google Scholar] [CrossRef]
- Kostli, K.P.; Frenz, M.; Bebie, H.; Weber, H.P. Temporal Backward Projection of Optoacoustic Pressure Transients Using Fourier Transform Methods. Phys. Med. Biol. 2001, 46, 1863–1872. [Google Scholar] [CrossRef]
- Paltauf, G.; Nuster, R.; Burgholzer, P. Weight factors for limited angle photoacoustic tomography. Phys. Med. Biol. 2009, 54, 3303–3314. [Google Scholar] [CrossRef]
- Roitner, H.; Haltmeier, M.; Nuster, R.; O’Leary, D.P.; Berer, T.; Paltauf, G.; Grun, H.; Burgholzer, P. Deblurring algorithms accounting for the finite detector size in photoacoustic tomography. J. Biomed. Opt. 2014, 19, 56011. [Google Scholar] [CrossRef]
- Xu, M.H.; Wang, L.V. Analytic explanation of spatial resolution related to bandwidth and detector aperture size in thermoacoustic or photoacoustic reconstruction. Phys. Rev. E 2003, 67, 56605. [Google Scholar] [CrossRef]
- Haltmeier, M.; Zangerl, G. Spatial resolution in photoacoustic tomography: Effects of detector size and detector bandwidth. Inverse Probl. 2010, 26, 125002. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.V.; Ambartsoumian, G.; Kuchment, P. Reconstructions in limited-view thermoacoustic tomography. Med. Phys. 2004, 31, 724–733. [Google Scholar] [CrossRef]
| Piezoelectric Array | Camera Setup | ||
|---|---|---|---|
| Detection Bandwidth | DC—12 MHz (non-uniform) | 1.1–23 MHz (flat) | |
| Sensitivity (NEPLP)/Directivity | 0.4 kPa·mm/incident angle weighted response | 5.1 kPa·mm/omnidirectional response | |
| Artifact Level | High (streak artifacts, sparse sampling) | Low (dense spatial sampling) | |
| Image Resolution | 220 µm | 80 µm | |
| Imaging Frame Rate | Projection | capability for real-time, single shot imaging | |
| 3D Imaging | 80 s 1 | 160 s 1 | |
| about 2 s 2 | |||
| User-friendliness | high | moderate | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuster, R.; Paltauf, G. Comparison of Piezoelectric and Optical Projection Imaging for Three-Dimensional In Vivo Photoacoustic Tomography. J. Imaging 2019, 5, 15. https://doi.org/10.3390/jimaging5010015
Nuster R, Paltauf G. Comparison of Piezoelectric and Optical Projection Imaging for Three-Dimensional In Vivo Photoacoustic Tomography. Journal of Imaging. 2019; 5(1):15. https://doi.org/10.3390/jimaging5010015
Chicago/Turabian StyleNuster, Robert, and Günther Paltauf. 2019. "Comparison of Piezoelectric and Optical Projection Imaging for Three-Dimensional In Vivo Photoacoustic Tomography" Journal of Imaging 5, no. 1: 15. https://doi.org/10.3390/jimaging5010015
APA StyleNuster, R., & Paltauf, G. (2019). Comparison of Piezoelectric and Optical Projection Imaging for Three-Dimensional In Vivo Photoacoustic Tomography. Journal of Imaging, 5(1), 15. https://doi.org/10.3390/jimaging5010015





