Computer Assisted Examination of Infrared and Near Infrared Spectra to Assess Structural and Molecular Changes in Biological Samples Exposed to Pollutants: A Case of Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation for FTIR and FTNIR Spectroscopic Analysis
2.2. Acquisition of FTIR and FTNIR Spectra of Vicia Faba Samples
2.3. Basic Theory of the Proposed Digital Examination of FTIR and FTNIR Spectra
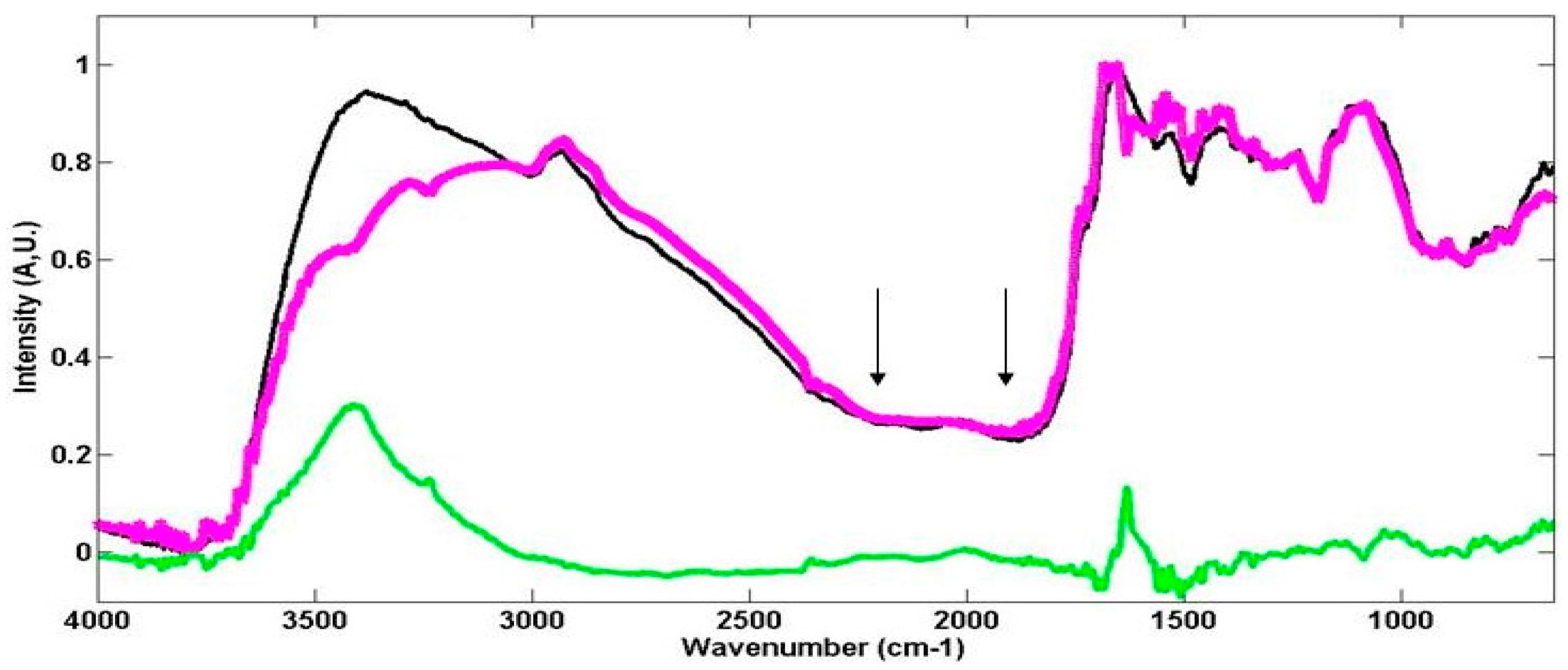
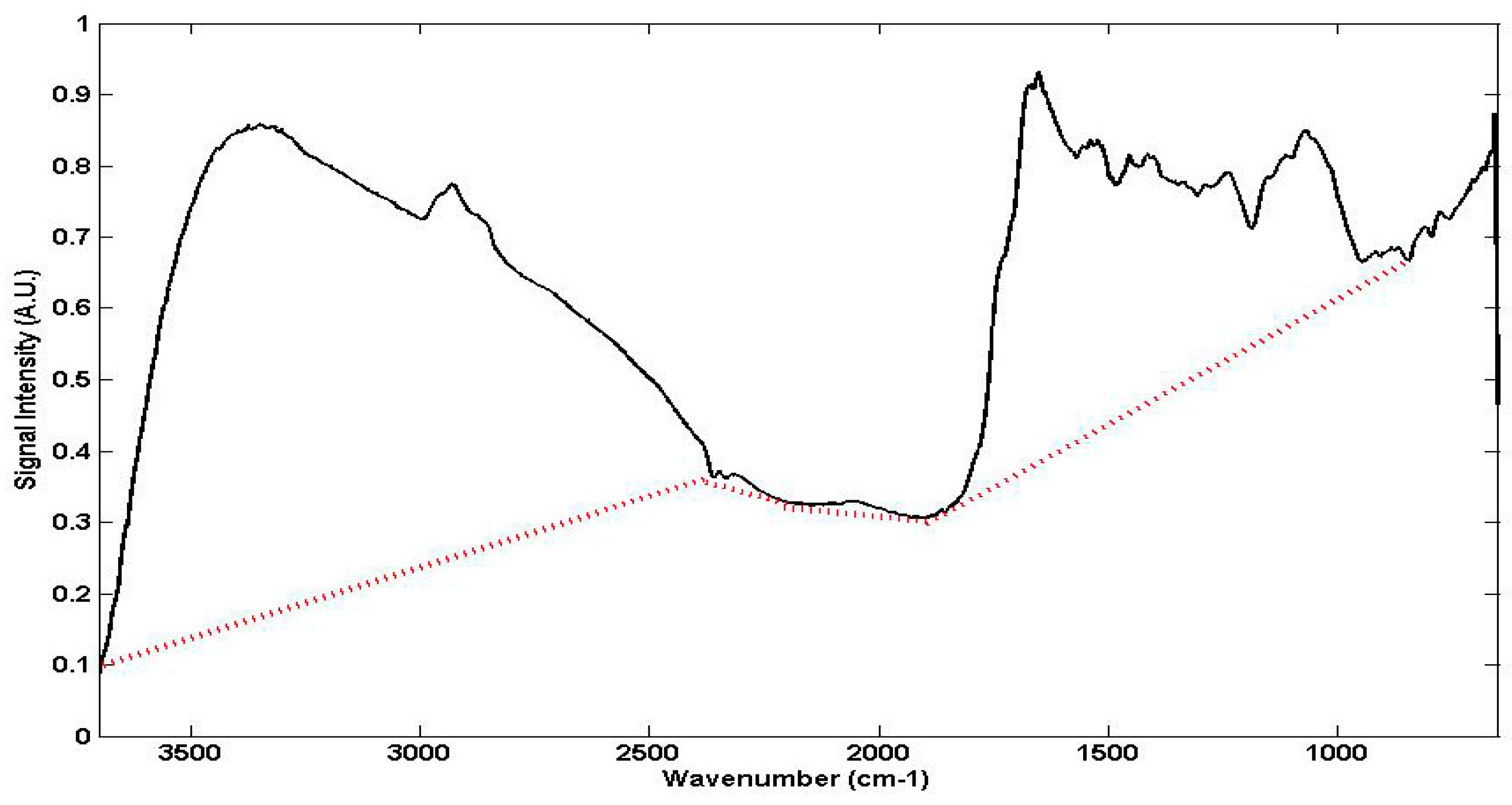
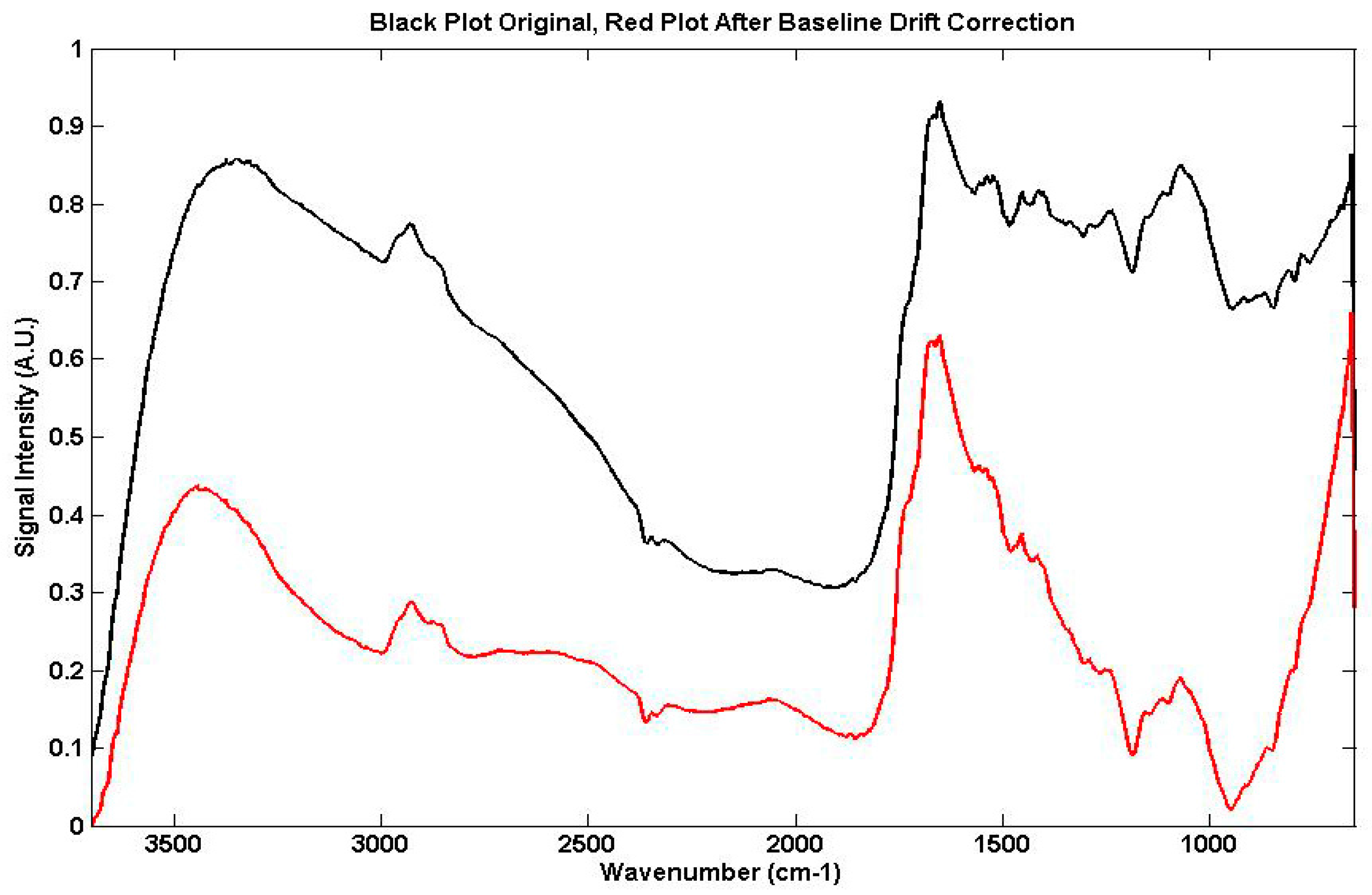
2.3.1. The Examination of Digitised FTIR Spectra
2.3.2. The Examination of Digitised FTNIR Spectra
2.4. Chemical Reagents
3. Results
3.1. Conventional Interpretation of FTIR and FTNIR Spectra for Living Organisms
3.1.1. FTIR Spectroscopy
3.1.2. FTNIR Spectra
4. Discussion
4.1. As Effects on Vicia Faba Roots by the Proposed Computer Assisted Method
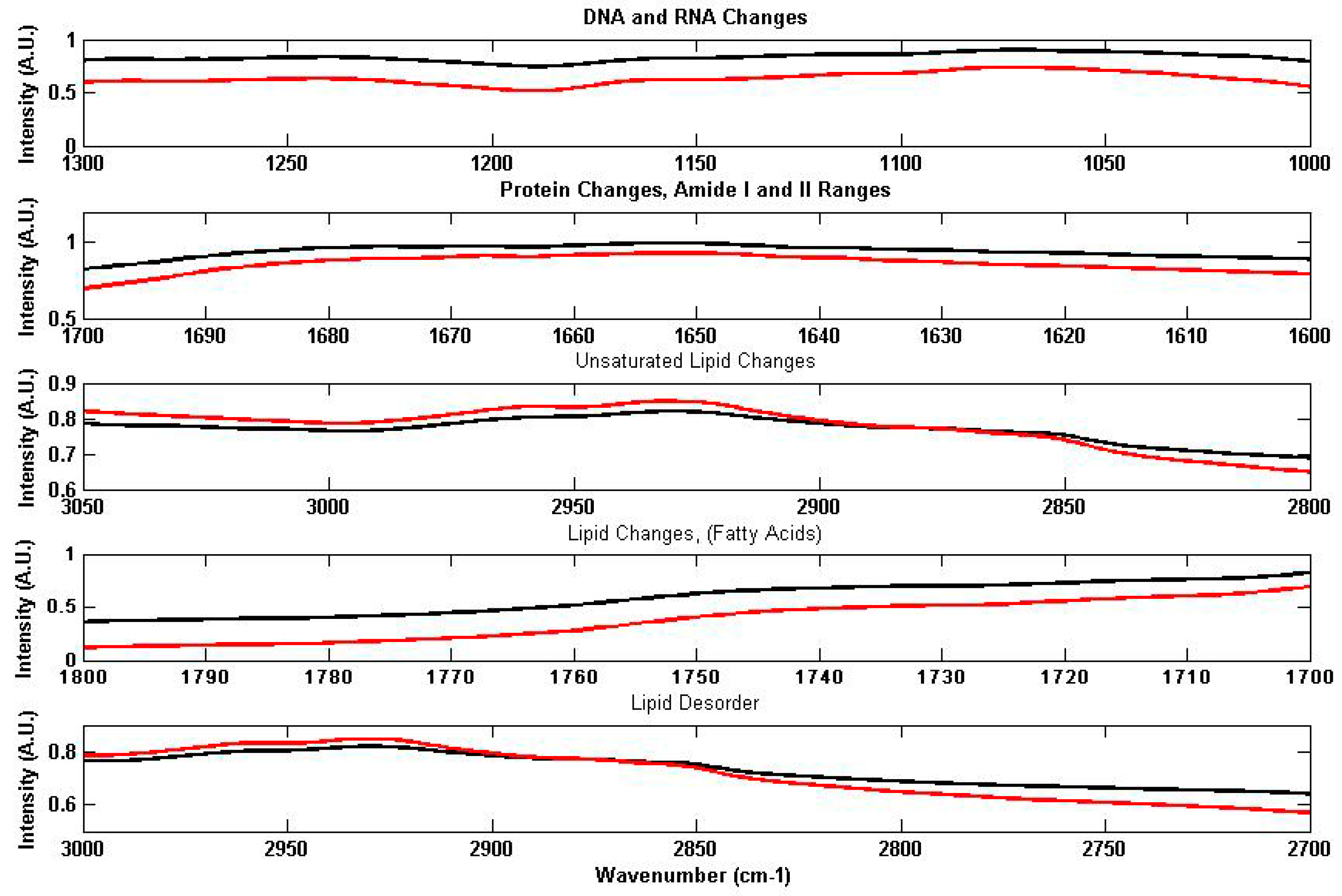
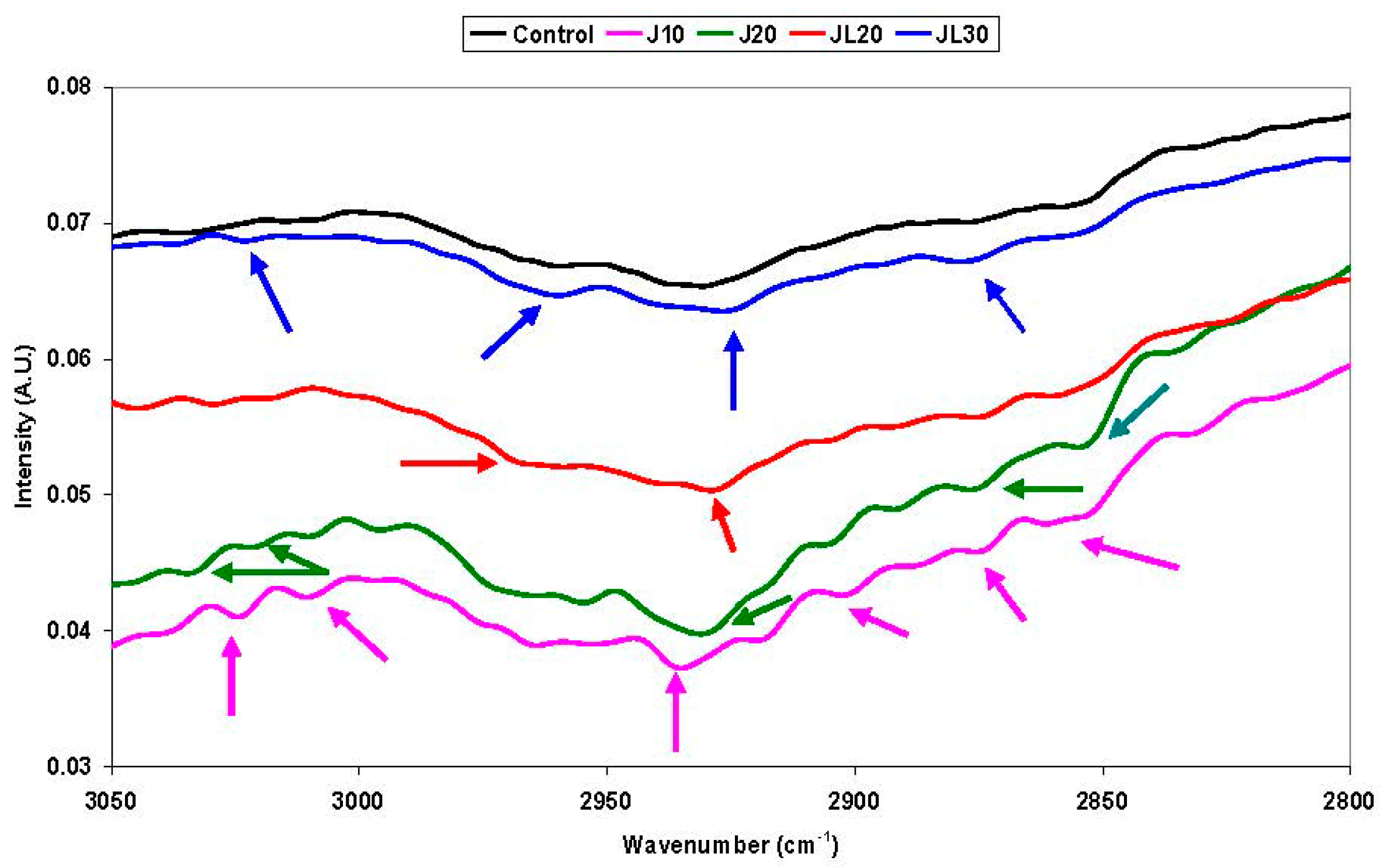
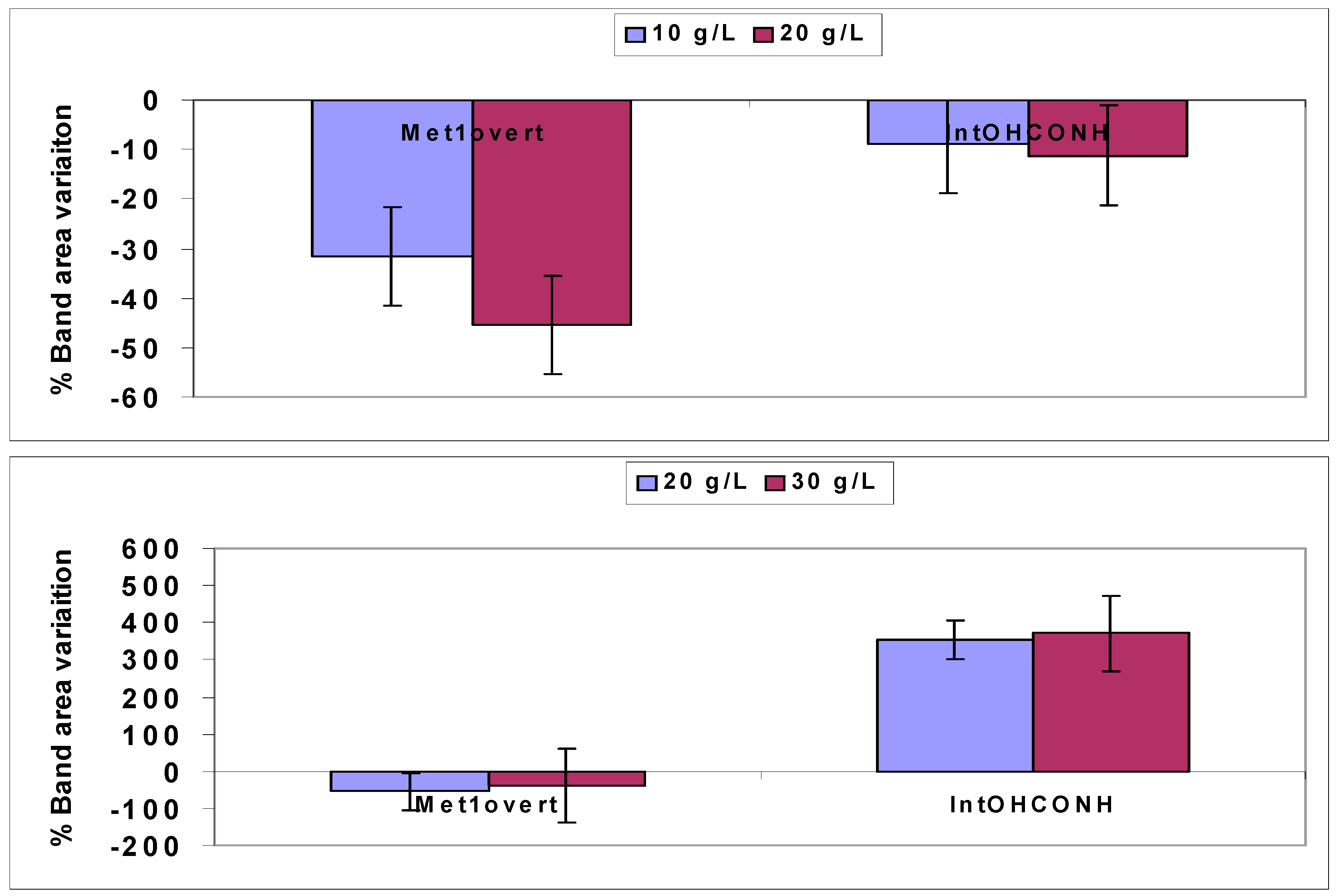
4.1.1. Quantitative Changes Detected by FTIR Spectroscopy in Vicia Faba Roots
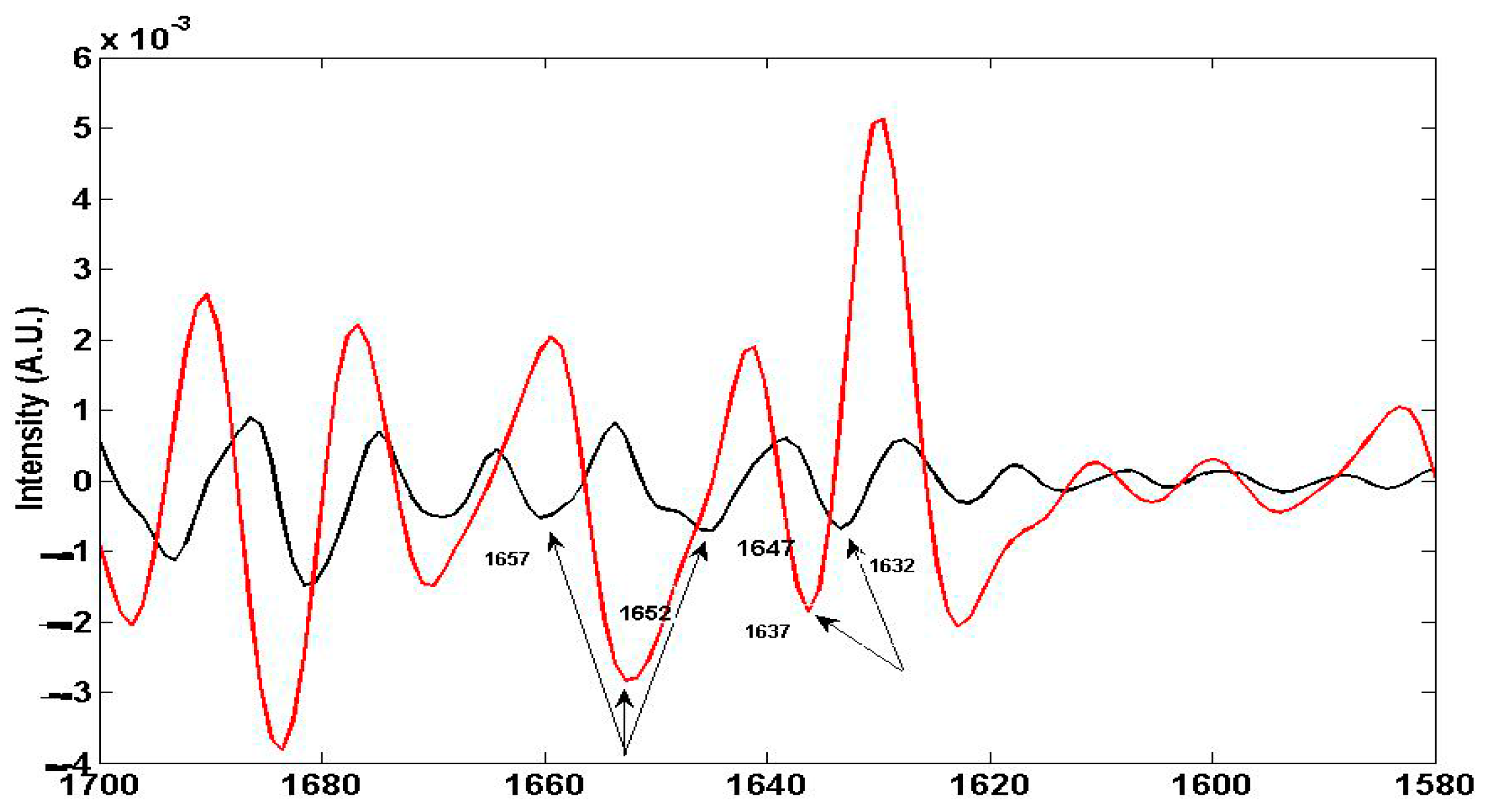
4.1.2. Non Specific Structural Changes Detected by FTIR Spectroscopy in Vicia faba Roots
4.2.1. FTNIR Results
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Stuart, B. Modern Infrared Spectroscopy; John Wiley & Sons: Chichester, UK, 1996. [Google Scholar]
- Yu, C.; Irudayaraj, J. Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers 2005, 77, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Togan, I.; Severcan, F. 17-βEstradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FTIR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. 2006, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bezirci, G.; Akkas, S.B.; Karsten Rinke, K.; Feriha Yildirim, F.; Kalaylioglu, Z.; Severcan, F.; Beklioglu, M. Impacts of salinity and fish-exuded kairomone on the survival and macromolecular profile of Daphnia pulex. Ecotoxicology 2012, 21, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Palanniappan, P.L.; Vijayasurandam, V. FTIR study of Arsenic induced biochemical changes on the liver tissues of fresh water fingerlings Labeo rohita. Rom. J. Biophys. 2008, 18, 135–144. [Google Scholar]
- Boccia, P.; Meconi, C.; Mecozzi, M.; Sturchio, E. Molecular modifications induced by inorganic Arsenic in Vicia faba investigated by FTIR, FTNIR spectroscopy and genotoxicity testing. J. Toxicol. Environ. Health Part A 2013, 76, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y. Near-Infrared spectroscopy—Its versatility in analytical chemistry. Anal. Sci. 2012, 28, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, M.; Pietroletti, M.; Tornambè, A. Molecular and structural characteristics in toxic algae cultures of Ostreopsis ovata and Ostreopsis spp. evidenced by FTIR and FTNIR spectroscopy. Spectrochim. Acta Part A 2011, 78, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Sturchio, E.; Napolitano, P.; Beni, C.; Mecozzi, M. Evaluation of arsenic effects in Vicia faba by FTIR and FTNIR spectroscopy. Glob. NEST J. 2012, 14, 86–92. [Google Scholar]
- Wagner, H.; Dunker, S.; Liu, Z.; Wilhelm, C. Subcommunity FTIR-spectroscopy to determine physiological cell states. Curr. Opin. Biotechnol. 2013, 24, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J. Algorithms for Chemists; John Wiley & Sons: Chichester, UK, 1989. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by a simplified least squares method. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Mecozzi, M.; Sturchio, E. Effects of essential oil treatments on the secondary protein structure of Vicia faba: A mid-infrared spectroscopic study supported by two-dimensional correlation analysis. Spectrochim. Acta Part A 2015, 137, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kondepati, V.R.; Keese, M.; Mueller, R.; Backhaus, J. Near-infrared spectroscopic detection of human colon diverticulis: A pilot study. Vib. Spectrosc. 2007, 44, 56–61. [Google Scholar] [CrossRef]
- Christy, A.A.; Egeberg, P.K. Quantitative determination of saturated and unsaturated fatty acids in edible oils by infrared spectroscopy and chemometrics. Chemom. Int. Lab. Syst. 2006, 82, 130–136. [Google Scholar] [CrossRef]
- Bertoluzza, A.; Bottura, G.; Filippetti, P.; Tosi, M.R.; Vasina, M.; Pratella, G.C.; Folchi, A.; Gallerani, G. Vibrational spectroscopy for the evaluation of molecular perturbation indiced in fruit lipids by cold storage. J. Mol. Struct. 1994, 324, 177–188. [Google Scholar] [CrossRef]
- Lee, D.C.; Chapman, D. Infrared spectroscopic studies of biomembranes and model membranes. Biosci. Rep. 1986, 6, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Triba, M.N.; Deveaux, P.F.; Warscheawski, D.E. Effects of lipid chain length and unsaturation on bicelles stability. A phosphorous NMR study. Biophys. J. 2006, 91, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Borchman, D.; Bicknell-Brown, E. Lipid-protein ratios by infrared spectroscopy. Anal. Biochem. 1984, 136, 382–389. [Google Scholar] [CrossRef]
- Stehfest, K.; Toepel, J.; Wihlhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Phys. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Ball, L.A.; Beardall, J.; Giordano, M.; Maberly, S.C. Article lacking carbon-concentrating mechanisms. Can. J. Bot. 2005, 83, 879–890. [Google Scholar] [CrossRef]
- Soto, P.; Gaete, H.; Hidalgo, M.E. Assessment of catalase activity, lipid peroxidation, chlorophyll-a, and growth rate in the freshwater green algae Pseudokirchneriella subcapata exposed to copper and zinc. Lat. Am. J. Aquat. Res. 2011, 39, 280–285. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R. Configurations of polypeptide chains with favoured orientations around single bonds. Proc. Natl. Acad. Sci. USA 1951, 37, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Grimaldi, P.; Udroiu, I.; Bedini, A.; Giliberti, C.; Giuliani, L.; Palomba, R.; Congiu Castellano, A. Effects induced in cells by ultrasound revealed by ATR-FTIR spectroscopy. Vib. Spectrosc. 2010, 52, 79–84. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, Y.; Hielscher, R.; Voicescu, M.; Gross, J.; Hellwig, P. On the specificity of the amide VI band for the secondary structure of proteins. Vib. Spectrosc. 2011, 55, 258–266. [Google Scholar] [CrossRef]
- Palanniappan, P.L.; Vijayasurandam, V. Fourier transform infrared study of protein secondary structural changes in the muscle of Labeo rohita due to arsenic intoxication. Food Chem. Toxicol. 2008, 46, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Palanniappan, P.L.; Vijayasurandam, V. The effect of Arsenic exposure on the biochemical and mineral contents of Labeo rohita bones: An FTIR study. Infrared Phys. Technol. 2009, 52, 32–36. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, T.K. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Yedjou, C.; Thuisseu, L.; Tchounwou, C.; Gomes, M.; Howard, C.; Tchounwou, P. Ascorbic Acid Potentiation of Arsenic Trioxide Anticancer Activity Against Acute Promyelocytic Leukemia. Arch. Drug Inf. 2009, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Swindell, E.P.; Hankins, P.L.; Chen, H.; Miodragović, D.U.; O’Hallora, T. Anticancer Activity of Small-Molecule and Nanoparticulate Arsenic(III) Complexes. Inorg. Chem. 2013, 52, 12292–12304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Ann. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, A.B.; Sandhi, A.; Bhattacharya, P. Arsenic uptake by plants and possible phytoremediation applications: A brief overview. Environ. Chem. Lett. 2012, 10, 217–224. [Google Scholar] [CrossRef]
- Aja, M.; Jaya, M.; Vijayakumaran Nair, K.; Joe, I.H. FT-IR spectroscopy as a sentinel technology in earthworm toxicology. Spectrochim. Acta Part A 2014, 120, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Szalontai, B.; Nishyama, Y.; Gombos, Z.; Murata, N. Membrane dynamics as seen by Fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803. The effects of lipid unsaturation and the protein-to-lipid ratio. Biochim. Biophys. Acta 2000, 1509, 409–419. [Google Scholar] [CrossRef]
- Kóta, Z.; Debreczeny, M.; Szalontai, B. Separable contributions of ordered and disordered lipid fatty acyl chain segments to –CH2 bands in model and biological membranes: A Fourier transform infrared spectroscopic study. Biospectroscopy 1999, 5, 168–178. [Google Scholar] [CrossRef]

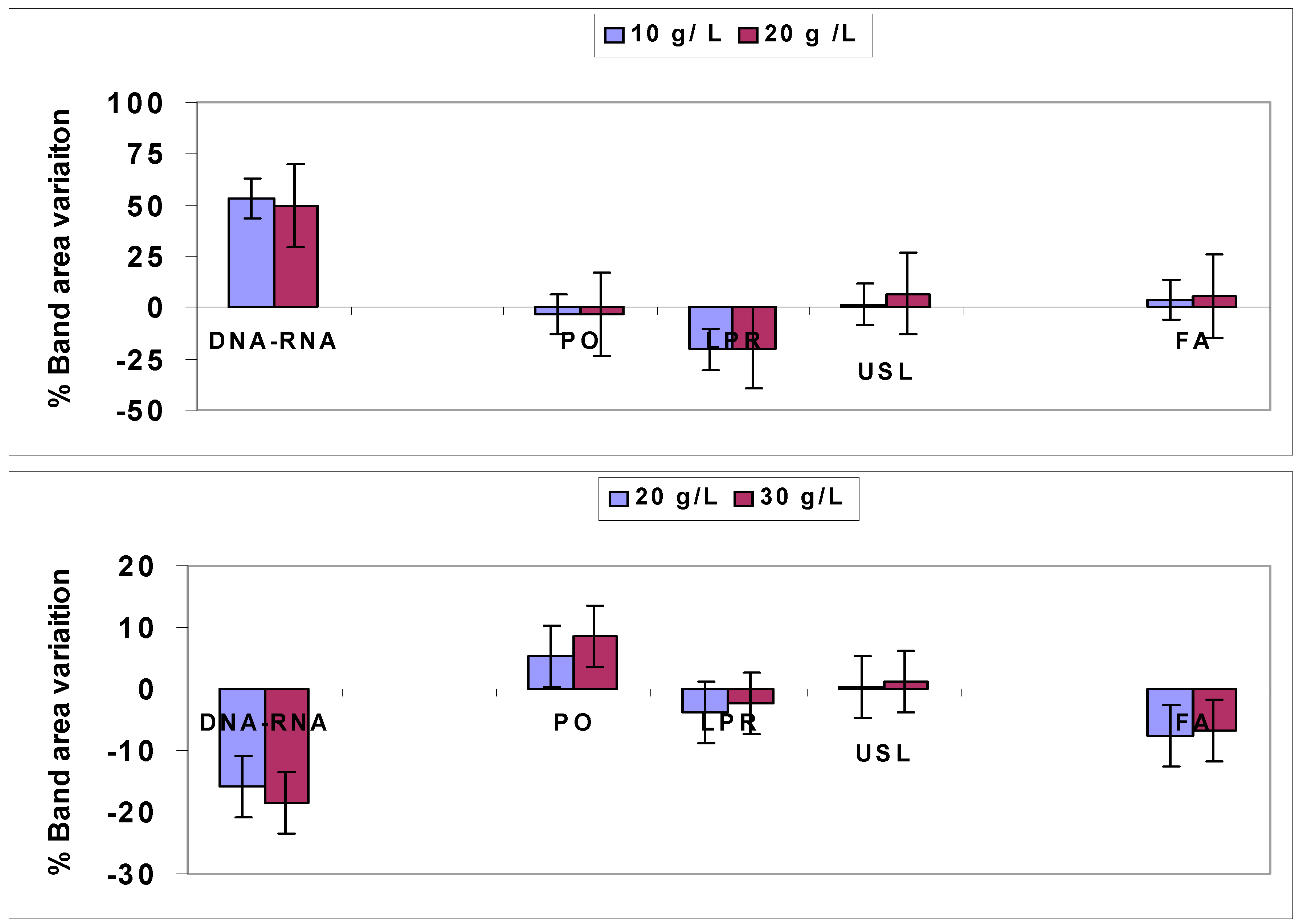
| Wavenumber cm−1 | Functional Group |
|---|---|
| 3350–3450 | OH of carbohydrates, proteins, and polyphenols |
| 3200–3250 | NH2 aminoacidic group |
| 3010–3020 | CH alkene group |
| 3020–3060 | CH of aromatic ring |
| 2850–2950 | CH and CH2 aliphatic stretching group |
| 2100–2500 | C=C conjugated and C≡C |
| 1730–1740 | C=O ester fatty acid group |
| 1700–1715 | C=O fatty acid group |
| 1620–1670 | C=O Amide I band |
| 1670 * | beta turns Amide I band |
| 1650 * | alpha helix Amide I band |
| 1635 * | beta sheet Amide I band |
| 1625–1630 * | random coil Amide I band (i.e., denaturation of proteins) |
| 1540–1550 | C–N Amide II band |
| 1510 * | Lignin skeletal band (aromatic) |
| 1400–1460 | stretching –C=O inorganic carbonate |
| 1350–1440 | CH and CH2 aliphatic bending group |
| 1240–1340 | C–N Amide III band |
| 1120–1160 | C–O–C polysaccharide and DNA and RNA backbones |
| 1085–1080 | P=O phospholipids in DNA and RNA |
| 1080–1060 | C–O carbohydrates in DNA and RNA backbones |
| 900–800 | C=C, C=N, C–H in ring structure, DNA and RNA backbones |
| Wavelength (cm−1) | Functional Group |
|---|---|
| 8400–8800 | –CH and –CH2 second overtone aliphatic chains |
| 6600–6900 | –OH first overtone proteins and lipids |
| 6500–6600 | –NH2 amino acid first overtone |
| 5100–5200 | Combination Amide I and –OH proteins |
| 4800–5000 | Combination –OH and Amide II proteins |
| 4200–4800 | Combination –NH2, –CH, C–C, and –OH |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mecozzi, M.; Sturchio, E. Computer Assisted Examination of Infrared and Near Infrared Spectra to Assess Structural and Molecular Changes in Biological Samples Exposed to Pollutants: A Case of Study. J. Imaging 2017, 3, 11. https://doi.org/10.3390/jimaging3010011
Mecozzi M, Sturchio E. Computer Assisted Examination of Infrared and Near Infrared Spectra to Assess Structural and Molecular Changes in Biological Samples Exposed to Pollutants: A Case of Study. Journal of Imaging. 2017; 3(1):11. https://doi.org/10.3390/jimaging3010011
Chicago/Turabian StyleMecozzi, Mauro, and Elena Sturchio. 2017. "Computer Assisted Examination of Infrared and Near Infrared Spectra to Assess Structural and Molecular Changes in Biological Samples Exposed to Pollutants: A Case of Study" Journal of Imaging 3, no. 1: 11. https://doi.org/10.3390/jimaging3010011
APA StyleMecozzi, M., & Sturchio, E. (2017). Computer Assisted Examination of Infrared and Near Infrared Spectra to Assess Structural and Molecular Changes in Biological Samples Exposed to Pollutants: A Case of Study. Journal of Imaging, 3(1), 11. https://doi.org/10.3390/jimaging3010011





