Detection by Infrared Thermography of the Effect of Local Cryotherapy Exposure on Thermal Spreadin Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology
- -
- passive: the subject undergoes the environment (22°C, H = 60%) cooling only (control group);
- -
- an application of a cold pack for 5 min behind the popliteal fossa (group ice 5 min);
- -
- an application of a cold pack for 10 min behind the popliteal fossa (group ice 10 min);
2.2. Material
- -
- emissivity of the body: 0.976 [26];
- -
- environment temperature: 22 °C;
- -
- object distance: 1.4 m.
3. Results and Discussion
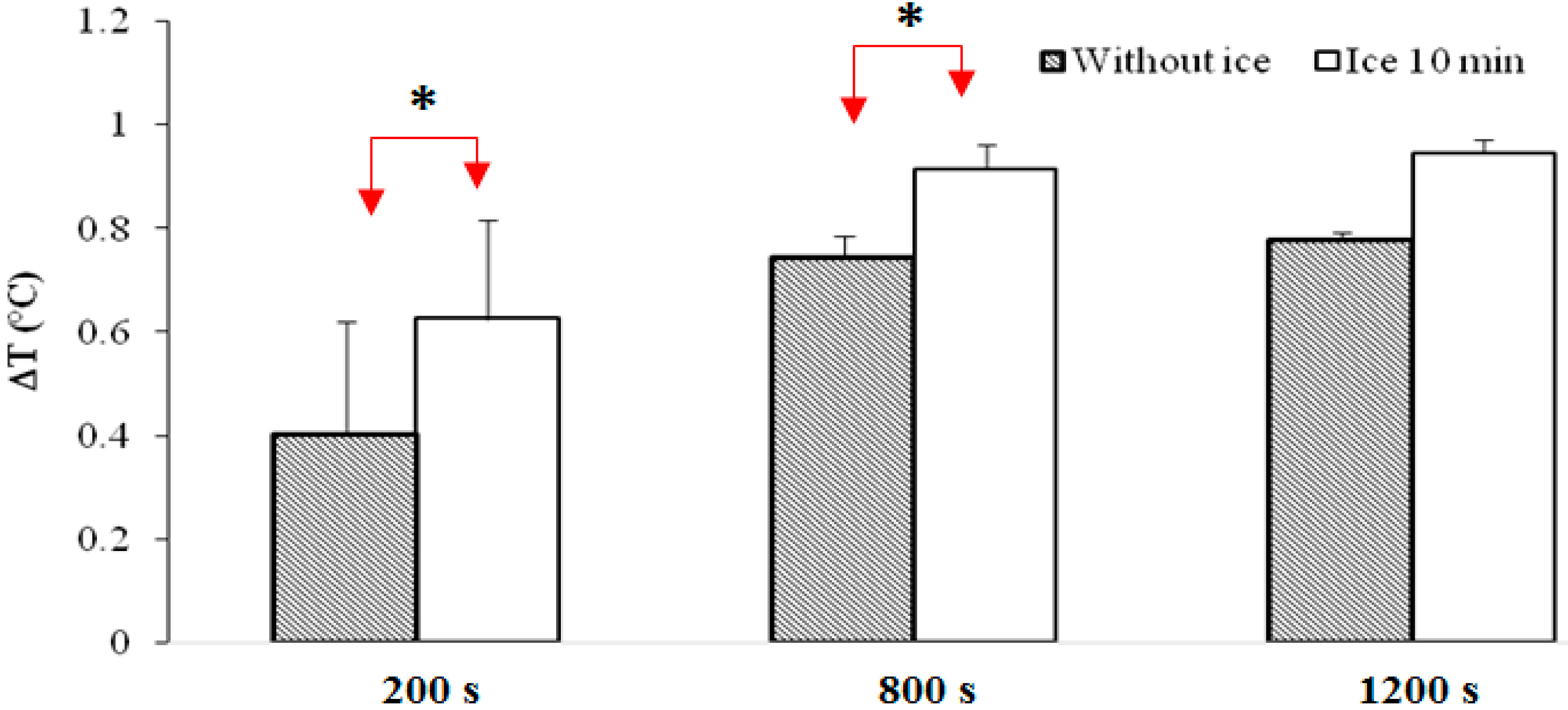
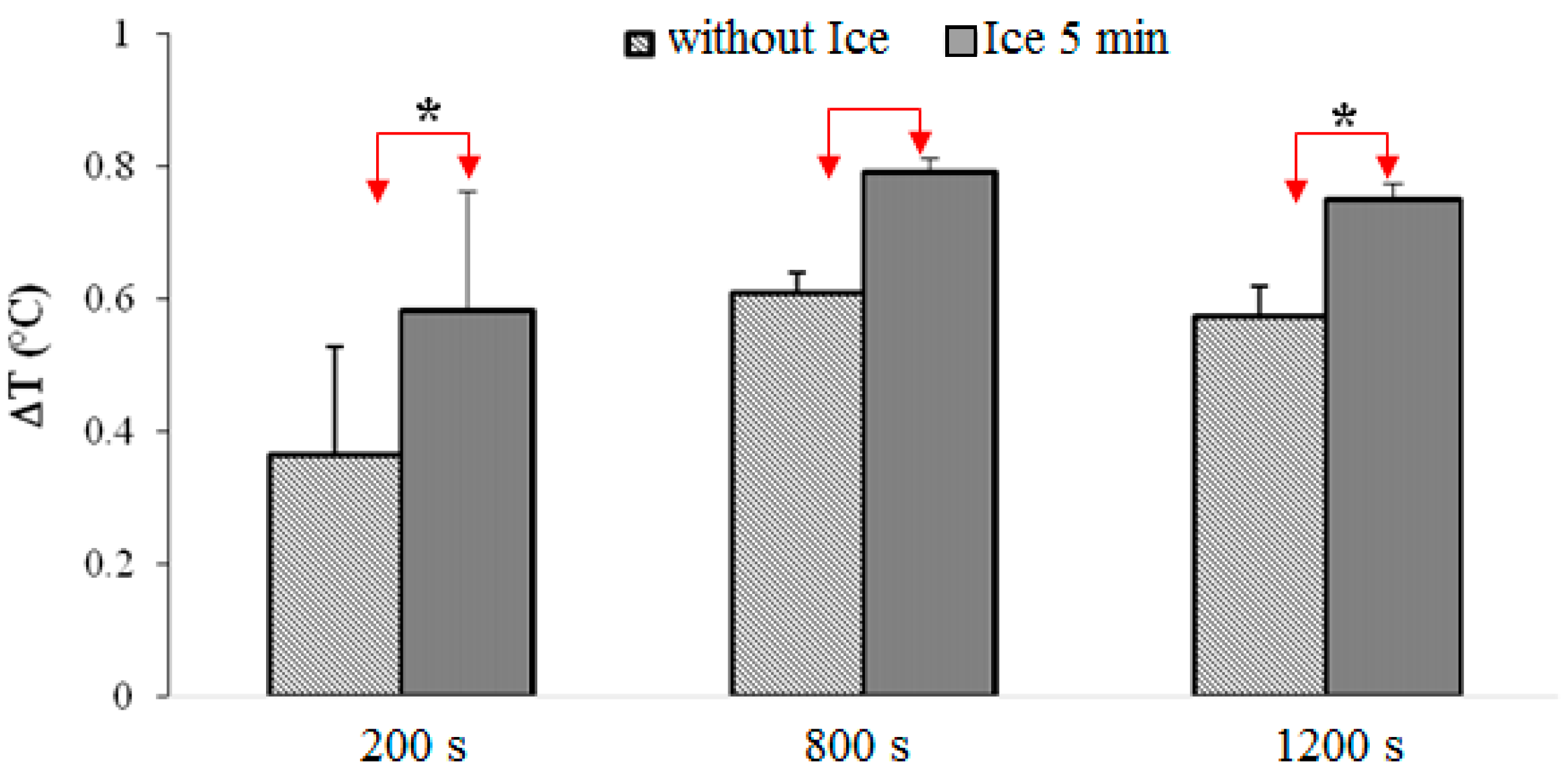
3.1. Results
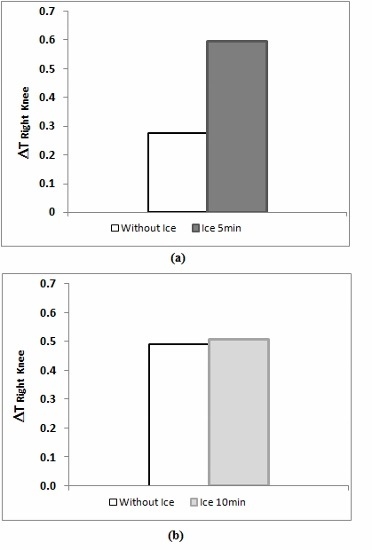
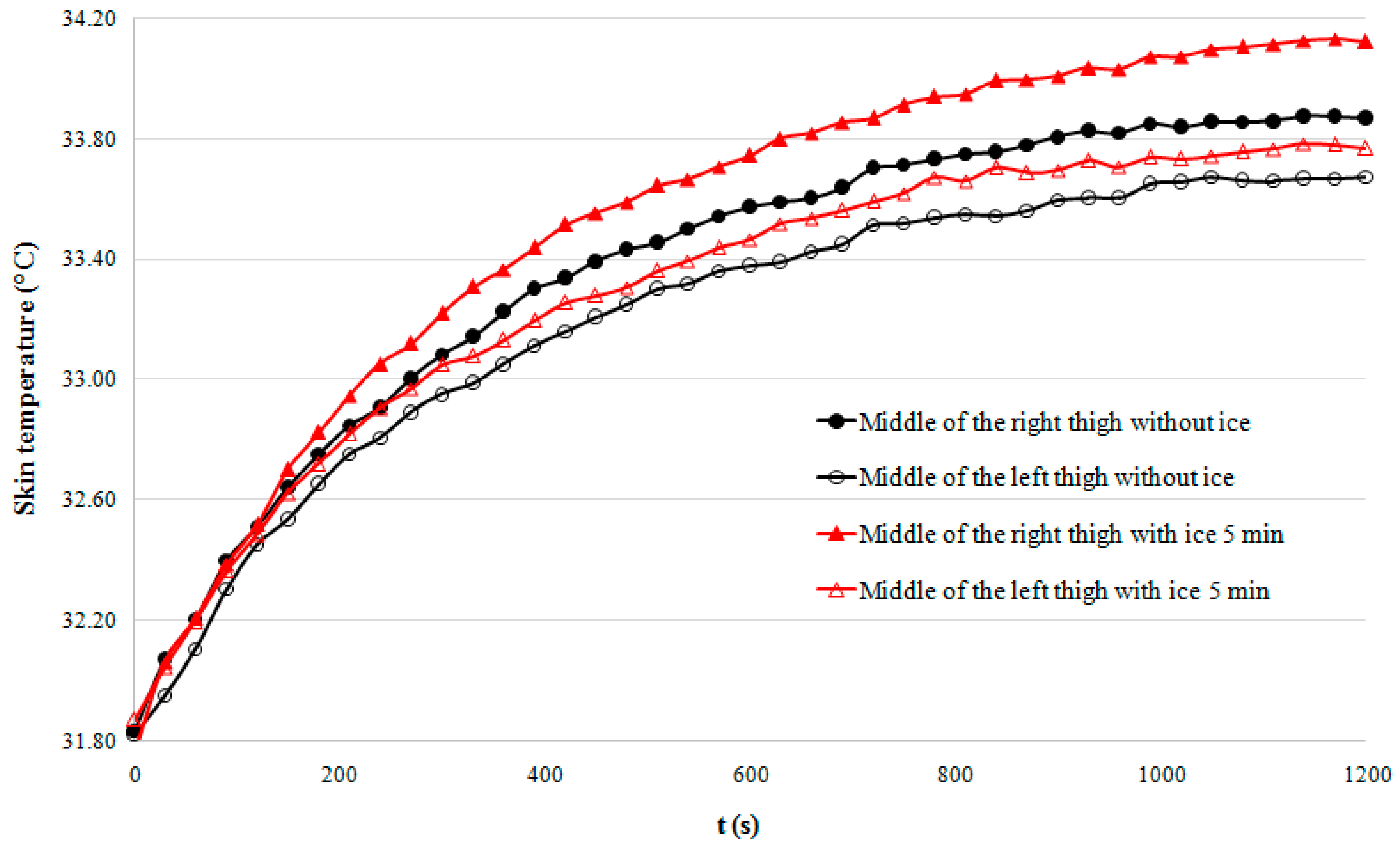
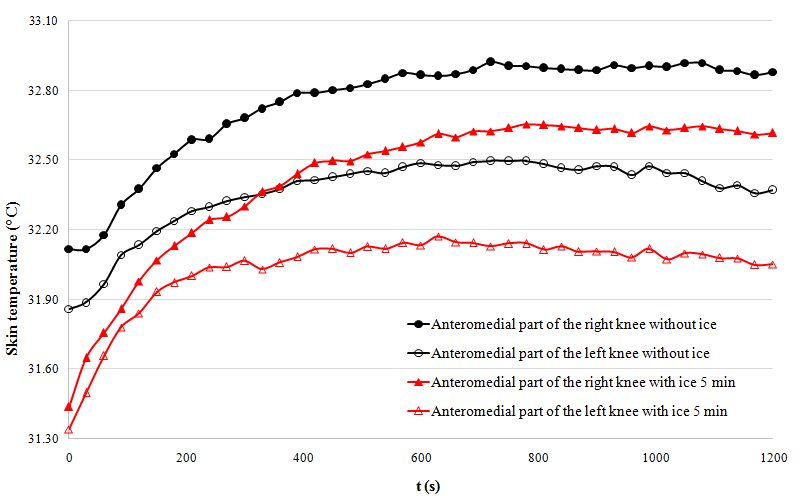
- -
- The 5-min cooling group shows significantly higher temperatures than the group that did not undergo cryotherapy (p < 0.001).
- -
- Putting an ice pack for 5 min during the post-exercise rest seems to provide significantly higher temperatures compared to the other experiments.
3.2. Discussion
- -
- that cryotherapy application increases skin temperature in specific areas;
- -
- that the phenomenon does not only have a local impact.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galliera, E.; Dogliotti, G.; Melegati, G.; MCorsi, M.; Cabitza, P.; Banfi, G. Bone remodelling biomarkers after whole body cryotherapy (WBC) in elite rugby players. Injury 2013, 44, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Melegati, G.; Barassi, A.; Dogliotti, G.; Melzid’Eril, G.; Dugue, B.; MCorsi, M. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J. Therm. Biol. 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Pinho Júnior, E.A.; Brito, C.J.; Costa Santos, W.O.; Nardelli Valido, C.; Lacerda Mendes, E.; Franchini, E. Influence of cryotherapy on muscle damage markers in jiujitsu fighters after competition: Acrossover study. Rev. Andal. Med. Deporte 2014, 7, 7–12. [Google Scholar] [CrossRef]
- Aza, M.; Miller, S.; Douglas-Moore, J.; Miller, M. Cryotherapy and its applications in the management of urologic malignancies: A review of its use in prostate and renal cancers. Urol. Oncol. Semin. Orig. Investig. 2014, 32, e19–e27. [Google Scholar]
- Chuan-Hang, Y.; Hung-Pin, L.; Shih-Jung, C.; Andy, S.; Hsin-Ming, C. Cryotherapy for oral precancers and cancers. J. Formos. Med. Assoc. 2014, 113, 272–277. [Google Scholar]
- Bleakley, C.M.; Bieuzen, F.; Davison, G.W.; Costello, J.T. Whole-body cryotherapy: Empirical evidence and theoretical perspectives. J. Sports Med. 2014, 5, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Lombardi, G.; Colombini, A.; Melegati, G. Whole-Body Cryotherapy in Athletes. Sports Med. 2010, 40, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Mars, M.; Hadebe, B.; Tufts, M. The effect of icepack cooling on skin and muscle temperature at rest and after exercise. S. Afr. J. Sports Med. 2006, 18. [Google Scholar] [CrossRef]
- Jeri, E.; Zemke, J.C.; Andersen, W.; Guion, K.; McMillan, J.; Joyner, A.B. Intramuscular Temperature Responses in the Human Leg to Two Forms of cryotherapy: Ice Massage and Ice Bag. J. Orthop. Sports Phys. Ther. 1998, 27, 301–307. [Google Scholar]
- Ho, S.S.; Illgen, R.L.; Meyer, R.W.; Torok, P.J.; Cooper, M.D.; Reider, B. Comparison of Various Icing Times in Decreasing Bone Metabolism and Blood Flowin the Knee. Am. J. Sports Med. 1995, 23, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, O.; Lilja, B.; Ahlgren, L.; Hemdal, B.; Westlin, N. The effect of local cold application on intramuscular blood flow at rest and after running. Med. Sci. Sports Exerc. 1985, 17, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.J.; Ammer, K. The technique of infrared imaging in medicine. Thermol. Int. 2000, 10, 7–14. [Google Scholar]
- Hoover, K.C.; Burlingame, S.E.; Lautz, C.H. Opportunities and challenges in concrete with thermal imaging. Concr. Int. 2004, 26, 23–27. [Google Scholar]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef] [PubMed]
- Bouzida, N.; Bendada, A.; Maldague, X. Visualization of body thermoregulation by infrared imaging. J. Therm. Biol. 2009, 34, 120–126. [Google Scholar] [CrossRef]
- Barcelos, E.Z.; Caminhas, W.M.; Ribeiro, E.; Pimenta, E.M.; Palhares, R.M. A Combined Method for Segmentation and Registration for an Advanced and Progressive Evaluation of Thermal Images. Sensors 2014, 14, 21950–21967. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Yu, K.L.; Chuang, H.Y.; Huang, M.H.; Chen, T.W.; Chen, C.H. The application of Infrared Thermography in the assessment of patients with Coccygodynia before and after manual therapy combined with Diathermy. J. Manip. Physiol. Ther. 2009, 32, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Ammer, K. The Glamorgan Protocol for recording and evaluation of thermal images of the human body. Thermol. Int. 2008, 18, 125–144. [Google Scholar]
- Jones, B.F.; Plassmann, P. Digital infrared thermal imaging of human skin. IEEE Eng. Med. Biol. 2002, 21, 41–48. [Google Scholar] [CrossRef]
- Ludwig, N.; Formenti, D.; Gargano, M.; Alberti, G. Skin temperature evaluation by infrared thermography: Comparison of image analysis methods. Infrared Phys. Technol. 2014, 62, 1–6. [Google Scholar] [CrossRef]
- Costello, J.T.; McInerney, C.D.; Bleakley, C.M.; Selfe, J.; Donnelly, A.E. The Use of Thermal Imaging in Assessing Skin Temperature Following Cryotherapy: A Review. J. Therm. Biol. 2012, 37, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Monod, H.; Flandrois, R.; Vandewalle, H. Bases physiologiques des activités physiques et sportives. In Physiologie du Sport, 6th ed.; Elsevier Masson: Paris, France, 2007. [Google Scholar]
- Schwartz, R.G. Guidelines for Neuromusculoskeletal Thermography. Thermol. Int. 2006, 16, 5–9. [Google Scholar]
- Harriss, D.J.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research. Int. J. Sports Med. 2009, 30, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Infratec Infrared. Available online: http://www.infratec-infrared.com/fileadmin/downloads/pdf/VC_hr/VC_hr_headen.pdf (assessed on 1 September 2015).
- Villaseñor-Mora, C.; Sánchez-Marin, F.J.; Calixto-Carrera, S. An indirect skin emissivity measurement in the infrared thermal range through reflection of a CO2 laser beam. Rev. Mex. Fis. 2009, 55, 387–392. [Google Scholar]
- Foda, E.; Almesri, I.; Awbi, H.B.; Sirén, K. Models of human thermoregulation and the prediction of local and overall thermal sensations. Build. Environ. 2011, 46, 2023–2032. [Google Scholar] [CrossRef]
- Laporte, S.; Cucherat, M.; Mismetti, P. Méthodologie des essais cliniques: Une perspective critique. Méd. Thér. 2006, 12, 19–28. [Google Scholar]
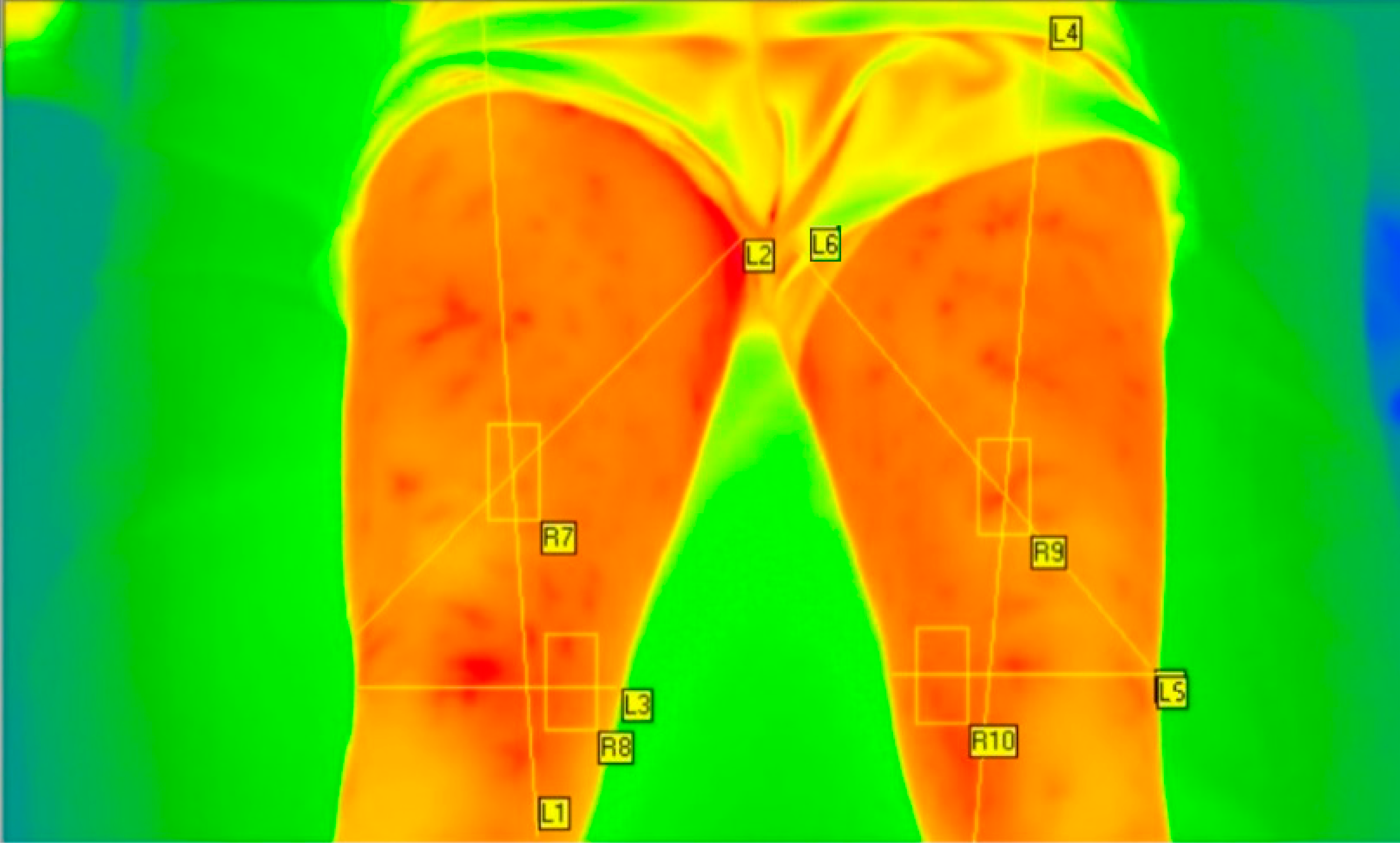

| Age (years) | Height (m) | Weight (kg) | BMI (kg∙m−2) | % of Body Fat | |
|---|---|---|---|---|---|
| Averages | 23.20 | 1.755 | 68.30 | 22.13 | 18.65 |
| SD | 1.69 | 0.077 | 7.10 | 1.33 | 4.65 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellard, M.; Arfaoui, A. Detection by Infrared Thermography of the Effect of Local Cryotherapy Exposure on Thermal Spreadin Skin. J. Imaging 2016, 2, 20. https://doi.org/10.3390/jimaging2020020
Vellard M, Arfaoui A. Detection by Infrared Thermography of the Effect of Local Cryotherapy Exposure on Thermal Spreadin Skin. Journal of Imaging. 2016; 2(2):20. https://doi.org/10.3390/jimaging2020020
Chicago/Turabian StyleVellard, Matthieu, and Ahlem Arfaoui. 2016. "Detection by Infrared Thermography of the Effect of Local Cryotherapy Exposure on Thermal Spreadin Skin" Journal of Imaging 2, no. 2: 20. https://doi.org/10.3390/jimaging2020020
APA StyleVellard, M., & Arfaoui, A. (2016). Detection by Infrared Thermography of the Effect of Local Cryotherapy Exposure on Thermal Spreadin Skin. Journal of Imaging, 2(2), 20. https://doi.org/10.3390/jimaging2020020