Quantitative Magnetic Resonance Imaging and Patient-Reported Outcomes in Patients Undergoing Hip Labral Repair or Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
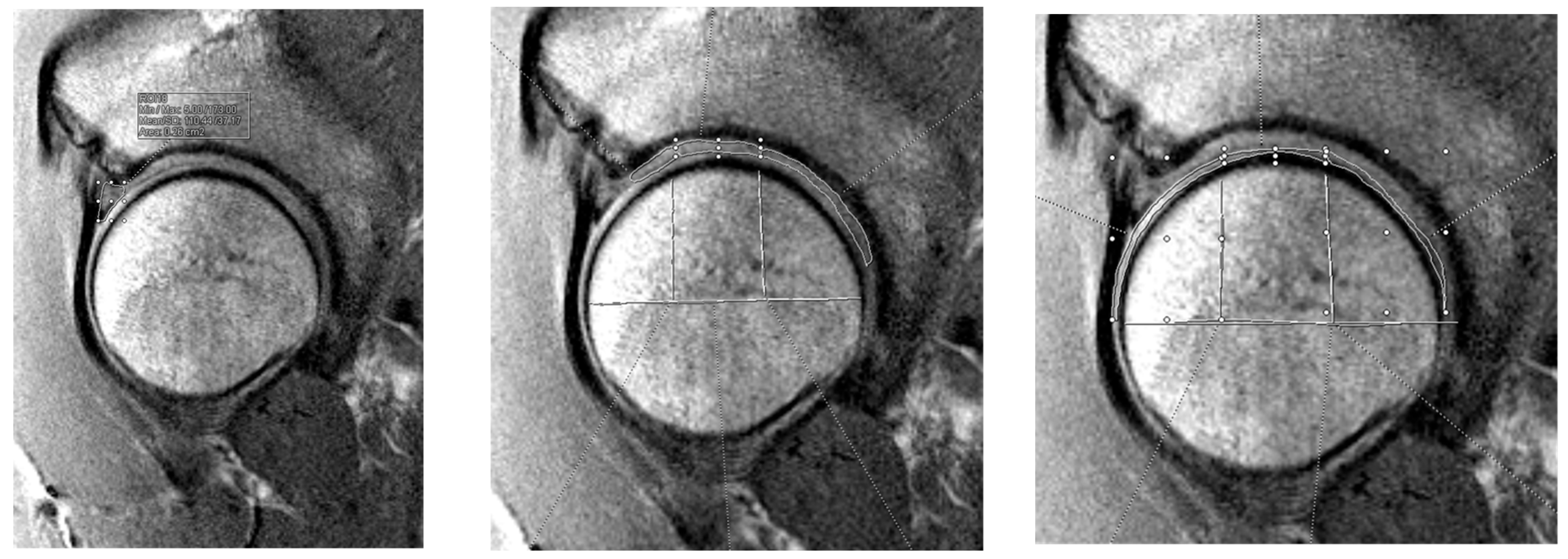
2.2. Preoperative Imaging Technique
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. T2 Mapping Results—All Patients
3.2. T2 Mapping Results—Labral Repair Group
3.3. T2 Mapping Results—Labral Reconstruction Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| iHOT-12 | International hip outcome tool—12 question version |
| dGEMRIC | Delayed gadolinium-enhanced MRI of Cartilage |
| PRO | Patient reported outcome |
| MESE | Multi-echo spin-echo |
| TR | Repetition time |
| TE | Echo time |
| VS | Voxel size |
| FOV | Field of view |
| AT | Acquisition time |
| MLM | Mixed linear models |
| CI | Confidence interval |
References
- Groh, M.M.; Herrera, J. A comprehensive review of hip labral tears. Curr. Rev. Musculoskelet. Med. 2009, 2, 105–117. [Google Scholar] [CrossRef]
- Duthon, V.B.; Charbonnier, C.; Kolo, F.C.; Magnenat-Thalmann, N.; Becker, C.D.; Bouvet, C.; Coppens, E.; Hoffmeyer, P.; Menetrey, J. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy 2013, 29, 411–419. [Google Scholar] [CrossRef]
- Philippon, M.J.; Nepple, J.J.; Campbell, K.J.; Dornan, G.J.; Jansson, K.S.; LaPrade, R.F.; Wijdicks, C.A. The hip fluid seal—Part I: The effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 722–729. [Google Scholar] [CrossRef]
- Scanaliato, J.P.; Green, C.K.; Salfiti, C.E.; Wolff, A.B. Hip Labral Reconstruction: Techniques and Outcomes. Curr. Rev. Musculoskelet. Med. 2021, 14, 340–350. [Google Scholar] [CrossRef]
- Bsat, S.; Frei, H.; Beaulé, P.E. The acetabular labrum: A review of its function. Bone Jt. J. 2016, 98-B, 730–735. [Google Scholar] [CrossRef]
- Dwyer, M.K.; Jones, H.L.; Hogan, M.G.; Field, R.E.; McCarthy, J.C.; Noble, P.C. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am. J. Sports Med. 2014, 42, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Domb, B.G.; Hartigan, D.E.; Perets, I. Decision Making for Labral Treatment in the Hip: Repair Versus Débridement Versus Reconstruction. J. Am. Acad. Orthop. Surg. 2017, 25, e53–e62. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.; Guermazi, A. Articular cartilage in the knee: Current MR imaging techniques and applications in clinical practice and research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 relaxation time mapping: Overview and applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, R.K.; Lucas, E.P.; Wilson, K.J.; Saroki, A.J.; Ho, C.P. Clinically Relevant Subregions of Articular Cartilage of the Hip for Analysis and Reporting Quantitative Magnetic Resonance Imaging: A Technical Note. Cartilage 2014, 5, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.P.; Surowiec, R.K.; Ferro, F.P.; Lucas, E.P.; Saroki, A.J.; Dornan, G.J.; Fitzcharles, E.K.; Anz, A.W.; Smith, W.S.; Wilson, K.J.; et al. Subregional Anatomical Distribution of T2 Values of Articular Cartilage in Asymptomatic Hips. Cartilage 2014, 5, 154–164. [Google Scholar] [CrossRef]
- Ho, C.P.; Surowiec, R.K.; Frisbie, D.D.; Ferro, F.P.; Wilson, K.J.; Saroki, A.J.; Fitzcharles, E.K.; Dornan, G.J.; Philippon, M.J. Prospective In Vivo Comparison of Damaged and Healthy-Appearing Articular Cartilage Specimens in Patients with Femoroacetabular Impingement: Comparison of T2 Mapping, Histologic Endpoints, and Arthroscopic Grading. Arthroscopy 2016, 32, 1601–1611. [Google Scholar] [CrossRef]
- Lee, J.H.; Houck, D.A.; Gruizinga, B.A.; Garabekyan, T.; Jesse, M.K.; Kraeutler, M.J.; Mei-Dan, O. Correlation of Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) Value with Hip Arthroscopy Intraoperative Findings and Midterm Periacetabular Osteotomy Outcomes. Orthop. J. Sports Med. 2022, 10, 23259671221117606. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Vemula, S.P.; Lindner, D.; Lodhia, P.; Suarez-Ahedo, C.; Domb, B.G. Preoperative Delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage (dGEMRIC) for Patients Undergoing Hip Arthroscopy: Indices Are Predictive of Magnitude of Improvement in Two-Year Patient-Reported Outcomes. J. Bone Joint Surg. Am. 2015, 97, 1305–1315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, D.S.; Durkan, M.G.; Foss, E.W.; Szumowski, J.; Crawford, D.C. Temporal in vivo assessment of fresh osteochondral allograft transplants to the distal aspect of the femur by dGEMRIC (delayed gadolinium-enhanced MRI of cartilage) and zonal T2 mapping MRI. J. Bone Joint Surg. Am. 2014, 96, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Tadenuma, T.; Uchio, Y.; Kumahashi, N.; Fukuba, E.; Kitagaki, H.; Iwasa, J.; Ochi, M. Delayed gadolinium-enhanced MRI of cartilage and T2 mapping for evaluation of reparative cartilage-like tissue after autologous chondrocyte implantation associated with Atelocollagen-based scaffold in the knee. Skeletal Radiol. 2016, 45, 1357–1363. [Google Scholar] [CrossRef]
- Hesper, T.; Neugroda, C.; Schleich, C.; Antoch, G.; Hosalkar, H.; Krauspe, R.; Zilkens, C.; Bittersohl, B. T2*-Mapping of Acetabular Cartilage in Patients with Femoroacetabular Impingement at 3 Tesla: Comparative Analysis with Arthroscopic Findings. Cartilage 2018, 9, 118–126. [Google Scholar] [CrossRef]
- Morgan, P.; Nissi, M.J.; Hughes, J.; Mortazavi, S.; Ellermann, J. T2* Mapping Provides Information That Is Statistically Comparable to an Arthroscopic Evaluation of Acetabular Cartilage. Cartilage 2018, 9, 237–240. [Google Scholar] [CrossRef]
- Griffin, D.R.; Parsons, N.; Mohtadi, N.G.; Safran, M.R. A short version of the International Hip Outcome Tool (iHOT-12) for use in routine clinical practice. Arthroscopy 2012, 28, 611–616, quiz 616–618. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, N.G.; Griffin, D.R.; Pedersen, M.E.; Chan, D.; Safran, M.R.; Parsons, N.; Sekiya, J.K.; Kelly, B.T.; Werle, J.R.; Leunig, M.; et al. The Development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: The International Hip Outcome Tool (iHOT-33). Arthroscopy 2012, 28, 595–610.e1. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.C.; Wait, T.J.; Wipf, C.J.; Keeter, C.L.; Peszek, A.; Mayer, S.W.; Ho, C.P.; Orahovats, A.; Genuario, J.W. Preoperative quantitative imaging use in predicting intraoperative decision for hip labral repair versus reconstruction. J. Hip Preserv. Surg. 2024, 11, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Cui, J.; Huang, S.; Liu, W.; Qi, J.; He, K.; Li, T. An interpretable radiomics-based machine learning model for predicting reverse left ventricular remodeling in STEMI patients using late gadolinium enhancement of myocardial scar. Eur. Radiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Song, F.; Zhang, W.; Gong, T.; Zhao, S.; Lv, F. Prediction of benign and malignant pulmonary nodules using preoperative CT features: Using PNI-GARS as a predictor. Front. Immunol. 2024, 15, 1446511. [Google Scholar] [CrossRef] [PubMed]
- Ilizaliturri, V.M.; Byrd, J.T.; Sampson, T.G.; Guanche, C.A.; Philippon, M.J.; Kelly, B.T.; Dienst, M.; Mardones, R.; Shonnard, P.; Larson, C.M. A geographic zone method to describe intra-articular pathology in hip arthroscopy: Cadaveric study and preliminary report. Arthroscopy 2008, 24, 534–539. [Google Scholar] [CrossRef]

| Characteristic | Overall (N = 29) | Repair (N = 22) | Recon (N = 7) | p-Value |
|---|---|---|---|---|
| Age (yrs) | 0.183 | |||
| Mean ± SD | 32.5 ± 10.8 | 30.8 ± 11.2 | 37.6 ± 8.0 | |
| Range | 14–50 | 14–50 | 23–45 | |
| Sex, n (%) | 0.755 | |||
| Male | 15 (51.7) | 11 (50) | 4 (57.1) | |
| Female | 14 (48.3) | 11 (50) | 3 (42.9) | |
| BMI (kg/m2) | 0.196 | |||
| Mean ± SD | 24.3 ± 2.6 | 23.9 ± 2.5 | 25.5 ± 2.6 | |
| Range | 19.2–29.3 | 19.2–27.5 | 21.5–29.3 | |
| Laterality, n (%) | 0.108 | |||
| Left | 16 (55.2) | 14 (63.6) | 2 (28.6) | |
| Right | 13 (44.8) | 8 (36.4) | 5 (71.4) |
| Baseline | 3 Months | 6 Months | 12 Months | 24 Months | ||
|---|---|---|---|---|---|---|
| Labrum | β | 0.045 | 0.007 | 0.050 | −0.101 | −0.189 |
| 95% CI | (0.009, 0.081) | (−0.03, 0.045) | (0.014, 0.086) | (−0.143, −0.058) | (−0.236, −0.142) | |
| p-value | 0.006 | >0.90 | 0.002 | <0.001 | <0.001 | |
| Acetabulum | ||||||
| β | 0.036 | 0.064 | −0.014 | −0.086 | −0.158 | |
| 95% CI | (0.011, 0.061) | (0.038, 0.099) | (−0.039, 0.011) | (−0.112, −0.059) | (−0.189, −0.127) | |
| p-value | 0.001 | <0.001 | 0.591 | <0.001 | <0.001 | |
| Femur | ||||||
| β | 0.024 | 0.037 | −0.009 | −0.063 | −0.104 | |
| 95% CI | (0.004, 0.044) | (0.016, 0.058) | (−0.03, 0.011) | (−0.085, −0.041) | (−0.129, −0.079) | |
| p-value | 0.011 | <0.001 | 0.763 | <0.001 | <0.001 |
| Baseline | 3 Months | 6 Months | 12 Months | 24 Months | ||
|---|---|---|---|---|---|---|
| Labrum | β | −0.010 | 0.030 | 0.087 | −0.132 | −0.160 |
| 95% CI | (−0.051, 0.031) | (−0.014, 0.073) | (0.046, 0.129) | (−0.183, −0.081) | (−0.216, −0.104) | |
| p-value | 0.9 | 0.336 | <0.001 | <0.001 | <0.001 | |
| Acetabulum | ||||||
| β | −0.012 | 0.115 | 0.002 | −0.139 | −0.136 | |
| 95% CI | (−0.042, 0.017) | (0.084, 0.146) | (−0.028, 0.031) | (−0.171, −0.107) | (−0.173, −0.099) | |
| p-value | 0.807 | <0.001 | >0.90 | <0.001 | <0.001 | |
| Femur | ||||||
| β | −0.011 | 0.062 | 0.009 | −0.095 | −0.084 | |
| 95% CI | (−0.034, 0.012) | (0.038, 0.086) | (−0.014, 0.032) | (−0.121, −0.070) | (−0.113, −0.055) | |
| p-value | 0.676 | <0.001 | 0.837 | <0.001 | <0.001 |
| Baseline | 3 Months | 6 Months | 12 Months | 24 Months | ||
|---|---|---|---|---|---|---|
| Labrum | β | 0.139 | −0.033 | 0.098 | −0.083 | −0.51 |
| 95% CI | (0.056, 0.221) | (−0.121, 0.055) | (0.015, 0.181) | (−0.168, 0.001) | (−0.61, −0.41) | |
| p-value | <0.001 | 0.867 | 0.012 | 0.056 | <0.001 | |
| Acetabulum | ||||||
| β | 0.049 | −0.026 | 0.105 | 0 | −0.449 | |
| 95% CI | (−0.005, 0.103) | (−0.081, 0.028) | (0.051, 0.159) | (−0.056, 0.057) | (−0.513, −0.385) | |
| p-value | 0.099 | 0.691 | <0.001 | >0.90 | <0.001 | |
| Femur | ||||||
| β | 0.04 | −0.014 | 0.076 | −0.003 | −0.388 | |
| 95% CI | (−0.011, 0.091) | (−0.065, 0.037) | (0.026, 0.127) | (−0.056, 0.05) | (−0.447, −0.329) | |
| p-value | 0.208 | >0.90 | 0.001 | >0.90 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamar, K.S.J.; Peszek, A.; Alder, C.C.; Wait, T.J.; Wipf, C.J.; Keeter, C.L.; Mayer, S.W.; Ho, C.P.; Genuario, J.W. Quantitative Magnetic Resonance Imaging and Patient-Reported Outcomes in Patients Undergoing Hip Labral Repair or Reconstruction. J. Imaging 2025, 11, 261. https://doi.org/10.3390/jimaging11080261
Jamar KSJ, Peszek A, Alder CC, Wait TJ, Wipf CJ, Keeter CL, Mayer SW, Ho CP, Genuario JW. Quantitative Magnetic Resonance Imaging and Patient-Reported Outcomes in Patients Undergoing Hip Labral Repair or Reconstruction. Journal of Imaging. 2025; 11(8):261. https://doi.org/10.3390/jimaging11080261
Chicago/Turabian StyleJamar, Kyle S. J., Adam Peszek, Catherine C. Alder, Trevor J. Wait, Caleb J. Wipf, Carson L. Keeter, Stephanie W. Mayer, Charles P. Ho, and James W. Genuario. 2025. "Quantitative Magnetic Resonance Imaging and Patient-Reported Outcomes in Patients Undergoing Hip Labral Repair or Reconstruction" Journal of Imaging 11, no. 8: 261. https://doi.org/10.3390/jimaging11080261
APA StyleJamar, K. S. J., Peszek, A., Alder, C. C., Wait, T. J., Wipf, C. J., Keeter, C. L., Mayer, S. W., Ho, C. P., & Genuario, J. W. (2025). Quantitative Magnetic Resonance Imaging and Patient-Reported Outcomes in Patients Undergoing Hip Labral Repair or Reconstruction. Journal of Imaging, 11(8), 261. https://doi.org/10.3390/jimaging11080261







