Optical Coherence Tomography (OCT) Findings in Post-COVID-19 Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- Post-COVID-19 group (n = 25): HCWs with a prior PCR-confirmed SARS-CoV-2 infection.
- Non-COVID-19 group (n = 30): HCWs without prior COVID-19 infection.
2.2. Ophthalmological Examination and OCT Analysis
- Macular thickness measurements were performed using the “Macular Cube 512 × 128 A-scan” protocol covering a 6 mm × 6 mm scanning area, divided into 9 zones according to the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. All values, including CMT and parafoveolar and perifoveolar thickness, were automatically calculated by the Zeiss Cirrus HD-OCT system using built-in analysis software (11.5.2.54532).
- Ganglion Cell Complex Analysis: Based on a macular protocol centered on the fovea with automated (GCL+IPL) measurements divided into 6 quadrants.
- Optic Nerve Head and RNFL Thickness: Optic Disc Cube 200 × 200 protocol measuring RNFL thickness for a 2.4 mm diameter circle around the optic disc.
2.3. Dataset Description
2.4. Statistical Analysis
2.5. Software and Visualization Tools
3. Results
3.1. Demographic and Clinical Characteristics
3.2. OCT Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, A.; Trieu, M.-C.; Eriksson, E.M.; Zhou, F.; McVernon, J.; Brokstad, K.A.; Cox, R.J. SARS-CoV-2 Infection Rates and Associated Risk Factors in Healthcare Workers: Systematic Review and Meta-Analysis. Sci. Rep. 2025, 15, 4705. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Karnik, S.; Saef, J.; Bergmann, C.; Barnard, J.; Lederman, M.M.; Tilton, J.; Cheng, F.; Harding, C.V.; Young, J.B.; et al. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 2020, 58, 102907. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Johnston, C.I.; Forbes, J.M.; Burns, W.C.; Thomas, M.C.; Lew, R.A.; Yarski, M.; Smith, A.I.; Cooper, M.E. Identification of angiotensin converting enzyme 2 in the rodent retina. Curr. Eye Res. 2004, 29, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Luhtala, S.; Vaajanen, A.; Oksala, O.; Valjakka, J.; Vapaatalo, H. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine ocular tissues. J. Ocul. Pharmacol. Ther. 2009, 25, 23–28. [Google Scholar] [CrossRef]
- Senanayake, P.D.; Drazba, J.; Shadrach, K.; Milsted, A.; Rungger-Brandle, E.; Nishiyama, K.; Miura, S.-I.; Karnik, S.; Sears, J.E.; Hollyfield, J.G. Angiotensin II and its receptor subtypes in the human retina. Investig. Opthalmol. Vis. Sci. 2007, 48, 3301–3311. [Google Scholar] [CrossRef]
- Schnichels, S.; Rohrbach, J.M.; Bayyoud, T.; Thaler, S.; Ziemssen, F.; Hurst, J. Kann SARS-CoV-2 das Auge infizieren?—Ein Überblicküber den Rezeptorstatus in okularemGewebe. Ophthalmologe 2020, 117, 618–621. [Google Scholar] [CrossRef]
- de Figueiredo, C.S.; Raony, Í.; Giestal-de-Araujo, E. SARS-CoV-2 Targeting the Retina: Host–virus Interaction and Possible Mechanisms of Viral Tropism. Ocul. Immunol. Inflamm. 2020, 28, 1301–1304. [Google Scholar] [CrossRef]
- Casagrande, M.; Fitzek, A.; Püschel, K.; Aleshcheva, G.; Schultheiss, H.-P.; Berneking, L.; Spitzer, M.S.; Schultheiss, M. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul. Immunol. Inflamm. 2020, 28, 721–725. [Google Scholar] [CrossRef]
- Albertos-Arranz, H.; Martínez-Gil, N.; Sánchez-Sáez, X.; Noailles, A.; MonferrerAdsuara, C.; RemolíSargues, L.; Pérez-Santonja, J.J.; Lax, P.; Calvo Andrés, R.; Cuenca, N. Microglia Activation and Neuronal Alterations in Retinas from COVID-19 Patients: Correlation with Clinical Parameters. Eye Vis. 2023, 10, 12. [Google Scholar] [CrossRef]
- Karampelas, M.; Dalamaga, M.; Karampela, I. Does COVID-19 Involve the Retina? Ophthalmol. Ther. 2020, 9, 693–695. [Google Scholar] [CrossRef]
- Virgo, J.; Mohamed, M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye 2020, 34, 2352–2353. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Diamond, M.; Anwar, S.; Glaser, A.; Tyagi, P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 2020, 21, e00867. [Google Scholar] [CrossRef] [PubMed]
- Insausti-García, A.; Reche-Sainz, J.A.; Ruiz-Arranz, C.; López Vázquez, Á.; Ferro-Osuna, M. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur. J. Ophthalmol. 2020, 32, NP168–NP172. [Google Scholar] [CrossRef]
- Sheth, J.U.; Narayanan, R.; Goyal, J.; Goyal, V. Retinal vein occlusion in COVID-19: A novel entity. Indian J. Ophthalmol. 2020, 68, 2291–2293. [Google Scholar] [CrossRef]
- Raony, Í.; de Figueiredo, C.S. Retinal outcomes of COVID-19: Possible role of CD147 and cytokine storm in infected patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 165, 108280. [Google Scholar] [CrossRef]
- Savastano, A.; Crincoli, E.; Savastano, M.C.; Younis, S.; Gambini, G.; De Vico, U.; Cozzupoli, G.M.; Culiersi, C.; Rizzo, S. Gemelli against COVID-19 post-acute care study group peripapillary retinal vascular involvement in early post-COVID-19 patients. J. Clin. Med. 2020, 9, 2895. [Google Scholar] [CrossRef]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R., Jr. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef]
- Pereira, L.A.; Soares, L.C.M.; Nascimento, P.A.; Cirillo, L.R.N.; Sakuma, H.T.; da Veiga, G.L.; Fonseca, F.L.A.; Lima, V.L.; Abucham-Neto, J.Z. Retinal findings in hospitalised patients with severe COVID-19. Br. J. Ophthalmol. 2022, 106, 102–105. [Google Scholar] [CrossRef]
- Abrishami, M.; Emamverdian, Z.; Shoeibi, N.; Omidtabrizi, A.; Daneshvar, R.; Rezvani, T.S.; Saeedian, N.; Eslami, S.; Mazloumi, M.; Sadda, S.; et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: A case-control study. Can. J. Ophthalmol. 2021, 56, 24–30. [Google Scholar] [CrossRef]
- Yildiz, A.M.; Gunduz, G.U.; Yalcinbayir, O.; Ozturk, N.A.A.; Avci, R.; Coskun, F. SD-OCT assessment of macular and optic nerve alterations in patients recovered from COVID-19. Can. J. Ophthalmol. 2022, 57, 75–81. [Google Scholar] [CrossRef]
- Kaim, M.; Kır, M.B.; Uzun, F.; Findik, H. Evaluation of Retinal and Optic Nerve Parameters in Recovered COVID-19 Patients: Potential Neurodegenerative Impact on the Ganglion Cell Layer. Diagnostics 2025, 15, 1195. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.E.; Alhalel, J.; Busza, A.; Suen, E.; Gill, N.; Decker, N.; Suchy, S.; Orban, Z.; Jimenez, M.; Perez Giraldo, G.; et al. Non-Hospitalized Long COVID Patients Exhibit Reduced Retinal Capillary Perfusion: A Prospective Cohort Study. J. Imaging 2025, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Kalaw, F.G.P.; Warter, A.; Cavichini, M.; Knight, D.; Li, A.; Deussen, D.; Galang, C.; Heinke, A.; Mendoza, V.; Borooah, S.; et al. Retinal tissue and microvasculature loss in COVID-19 infection. Sci. Rep. 2023, 13, 5100. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance; WHO: Geneva, Switzerland, 2020; Available online: https://iris.who.int/handle/10665/332196 (accessed on 3 October 2020).
- Saban, O.; Levy, J.; Chowers, I. Risk of SARS-CoV-2 transmission to medical staff and patients from an exposure to a COVID-19-positive ophthalmologist. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2271–2274. [Google Scholar] [CrossRef]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Zhan, M.; Qin, Y.; Xue, X.; Zhu, S. Death from Covid-19 of 23 health care workers in China. N. Engl. J. Med. 2020, 382, 2267–2268. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Pérez-Bartolomé, F.; Sánchez-Quirós, J. Ocular manifestations of SARS-CoV-2: Literaturereview. Arch. Soc. Esp. Oftalmol. 2020, 96, 32–40. [Google Scholar] [CrossRef]
- Sanjay, S.; Agrawal, S.; Jayadev, C.; Kawali, A.; Gowda, P.B.; Shetty, R.; Mahendradas, P. Posterior segment manifestations and imaging features post-COVID-19. Med. Hypothesis Discov. Innov. Ophthalmol. 2021, 10, 95–106. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef]
- Chen, L.; Deng, C.; Chen, X.; Zhang, X.; Chen, B.; Yu, H.; Qin, Y.; Xiao, K.; Zhang, H.; Sun, X. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: A cross-sectional study. Acta Ophthalmol. 2020, 98, e951–e959. [Google Scholar] [CrossRef]
- Wei, J.; Chen, X.; Wang, L.; Wang, Z.; Li, J.; Wang, Z. A Cross-Sectional Study of Fundus Lesion Characteristics in Patients with Acute Visual Impairment Caused by COVID-19 Infection. Sci. Rep. 2024, 14, 28134. [Google Scholar] [CrossRef]
- Landecho, M.F.; Yuste, J.R.; Gándara, E.; Sunsundegui, P.; Quiroga, J.; Alcaide, A.B.; García-Layana, A. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J. Intern. Med. 2021, 289, 116–120. [Google Scholar] [CrossRef]
- Sumer, F.; Subasi, S. Effects of COVID-19 on Retinal and Choroidal Thickness by Optical Coherence Tomography. J. Glaucoma 2023, 32, 569–574. [Google Scholar] [CrossRef]
- Noor, M.; McGrath, O.; Drira, I.; Aslam, T. Retinal Microvasculature Image Analysis Using Optical Coherence Tomography Angiography in Patients with Post-COVID-19 Syndrome. J. Imaging 2023, 9, 234. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Donate-Lopez, J.; Vidal-Villegas, B.; García-Feijóo, J. Optic nerve analysis in COVID-19 patients. J. Med. Virol. 2021, 93, 190–191. [Google Scholar] [CrossRef]
- Ugurlu, A.; Agcayazi, S.B.; Icel, E.; Budakoglu, O.; Unver, E.; Barkay, O.; Karakeçili, F.; Bayrakceken, K. Assessment of the optic nerve, macular, and retinal vascular effects of COVID-19. Can. J. Ophthalmol. 2023, 58, 570–576. [Google Scholar] [CrossRef]
- Kılıçarslan, O.; Çebi, A.Y.; Uçar, D. Retinal nerve fiber layer thickness and peripapillary vasculature of post-COVID-19 patients with and without olfactory/gustatory dysfunction symptoms. Taiwan J. Ophthalmol. 2023, 14, 102–107. [Google Scholar] [CrossRef]
- Seker, E.D.; Timur, I.E.E. COVID-19: More than a respiratory virus, an optical coherence tomography study. Int. Ophthalmol. 2021, 41, 3815–3824. [Google Scholar] [CrossRef]
- Mauschitz, M.M.; Bonnemaijer, P.W.M.; Diers, K.; Rauscher, F.G.; Elze, T.; Engel, C.; Loeffler, M.; Colijn, J.M.; Ikram, M.A.; Vingerling, J.R.; et al. Systemic and Ocular Determinants of Peripapillary Retinal Nerve Fiber Layer Thickness Measurements in the European Eye Epidemiology (E3) Population. Ophthalmology 2018, 125, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Kazanci, L.; Balikoglu Yilmaz, M.; Akcay, F.A.; Bayata, S. Analysis of central macular thickness and choroidal thickness changes in patients with cardiovascular risk factors. Eye 2020, 34, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Talebnejad, M.R.; Badie, M.R.; Shahriari, H.; Nowroozzadeh, M.H. Analysis of Retinal Sublayers in Patients with Systemic COVID-19 Illness with Varying Degrees of Severity. Sci. Rep. 2025, 15, 2877. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef]
- Teberik, K. The Effect of Smoking on Macular, Choroidal, and Retina Nerve Fiber Layer Thickness. Turk. J. Ophthalmol. 2019, 49, 20–24. [Google Scholar] [CrossRef]
- Paniri, A.; Hosseini, M.M.; Rasoulinejad, A.; Akhavan-Niaki, H. Molecular effects and retinopathy induced by hydroxychloroquine during SARS-CoV-2 therapy. Eur. J. Pharmacol. 2020, 886, 173454. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine retinopathy. Eye 2017, 31, 828–845. [Google Scholar] [CrossRef]
- Mesquida, M.; Molins, B.; Llorenç, V.; Hernández, M.V.; Espinosa, G.; de la Maza, M.S.; Adán, A. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina 2018, 38, 1361–1370. [Google Scholar] [CrossRef]
- Savastano, M.C.; Gambini, G.; Cozzupoli, G.M.; Crincoli, E.; Savastano, A.; De Vico, U.; Culiersi, C.; Falsini, B.; Martelli, F.; Minnella, A.M.; et al. Retinal capillary involvement in early post-COVID-19 patients: A healthy controlled study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2157–2165. [Google Scholar] [CrossRef]
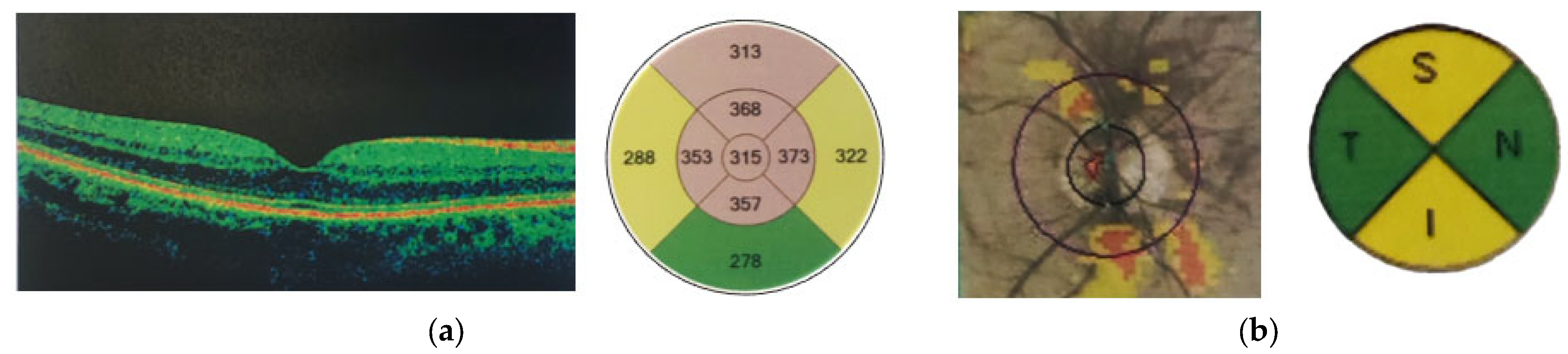
| Characteristics | Post-COVID-19 | Non-COVID-19 |
|---|---|---|
| Sex | ||
| Female | 28% | 88% |
| Male | 72% | 12% |
| Age (years) | 37.68 ± 10.12 | 38.92 ± 11.06 |
| Smoking | ||
| Yes | 24% | 36% |
| No | 76% | 64% |
| Comorbidity | ||
| Cardiovascular diseases | 8% | 12% |
| Endocrine diseases | 16% | 32% |
| Respiratory system diseases | 8% | 4% |
| Other | 0% | 4% |
| Clinical presentation | ||
| Fever | 76% | |
| Malaise | 32% | |
| Anosmia | 24% | |
| Ageusia | 20% | |
| Severity of COVID-19 | ||
| Asymptomatic | 16% | |
| Mild | 56% | |
| Moderate | 24% | |
| Severe | 4% | |
| Critical | 0% | |
| Treatment recieved | ||
| No medication | 36% | |
| Symptomatic | 52% | |
| Hydroxychloroquine | 20% | |
| Azytromycine | 48% | |
| Remdesevir | 0% | |
| Favipiravir | 0% | |
| Methylprednisolone | 8% | |
| Tocilizumab | 12% |
| OCT Findings | Post-COVID-19 | Non-COVID-19 | p-Value |
|---|---|---|---|
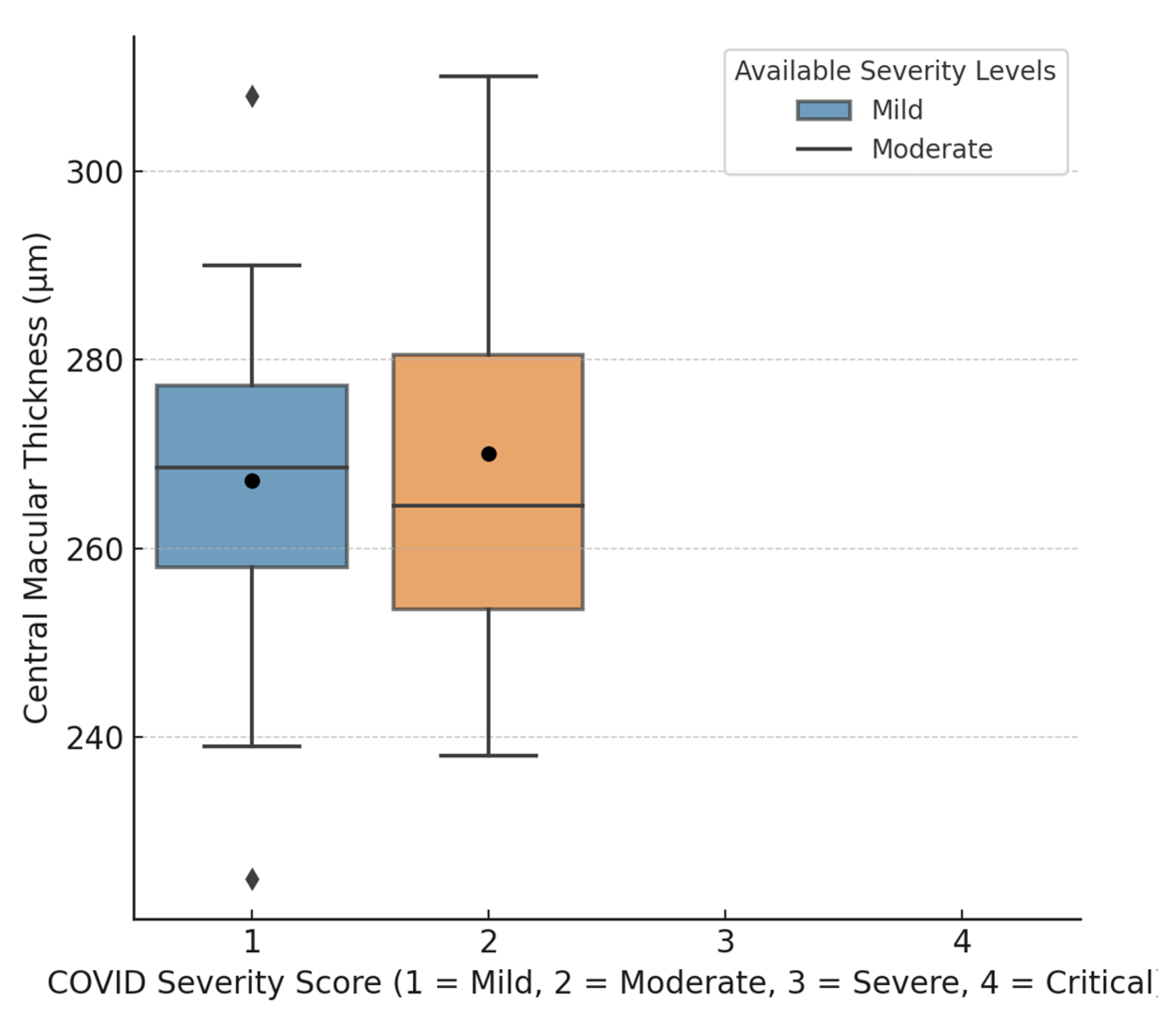
| Central macular thickness (CMT) | 272.94 ± 20.79 µm | 259.99 ± 24.83 µm | <0.05 * |
| RNFL thickness | |||
| Superior quadrant | 115.02 ± 28.72 µm | 120.95 ± 15.17 µm | >0.05 |
| Temporal quadrant | 62.08 ± 20.17 µm | 67.03 ± 21.16 µm | >0.05 |
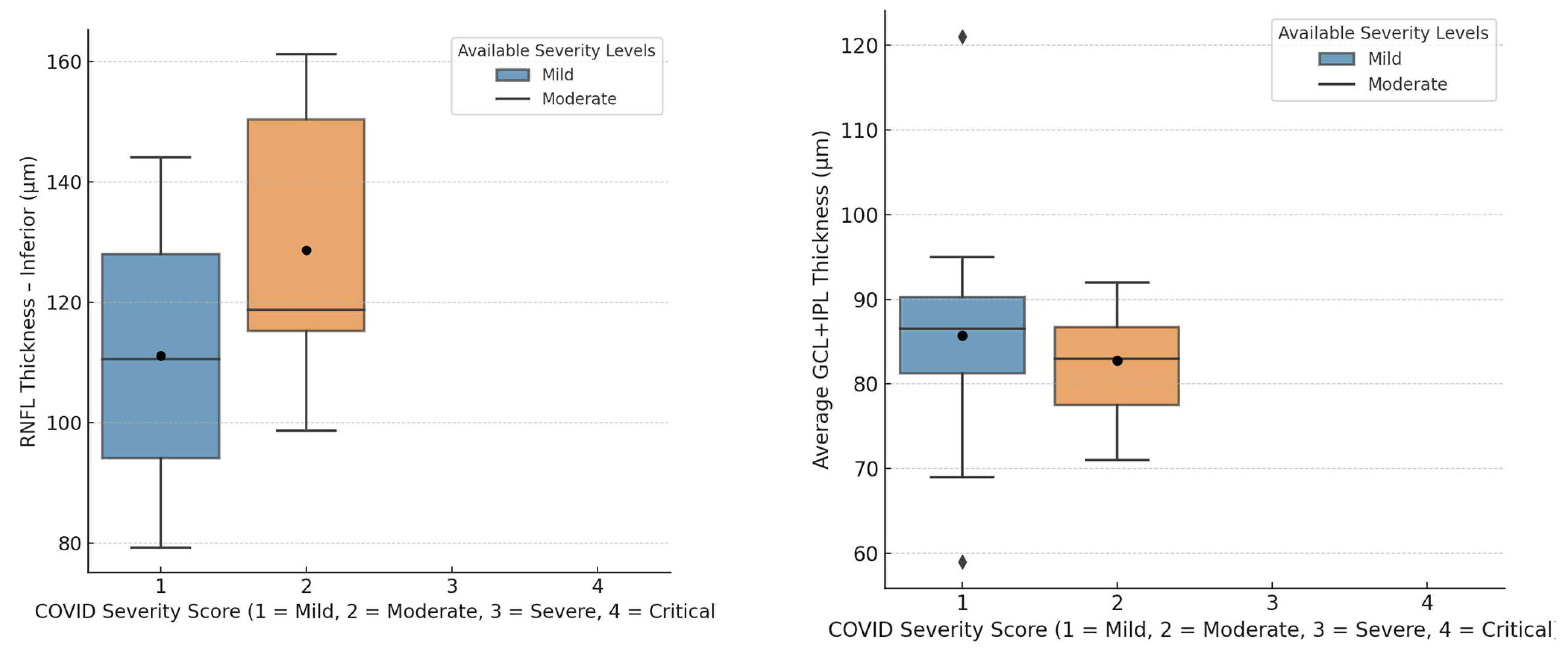
| Inferior quadrant | 120.94 ± 35.43 µm | 130.00 ± 36.31 µm | >0.05 |
| Nasal quadrant | 72.90 ± 12.40 µm | 71.98 ± 12.88 µm | >0.05 |
| GCL+IPL thickness | |||
| Average | 85.00 ± 10.99 µm | 84.81 ± 10.12 µm | >0.05 |
| Minimum | 80.96 ± 10.45 µm | 81.38 ± 12.81 µm | >0.05 |
| Cube volume | 13.96 ± 3.75 mm3 | 10.43 ± 3.64 mm3 | >0.05 |
| Cube average thickness | 287.04 ± 17.96 µm | 288.98 ± 15.34 µm | >0.05 |
| <38 Years Old | ≥38 Years Old | p-Value | |
|---|---|---|---|
| Central macular thickness (CMT) | 269.44 ± 22.41 µm | 265.95 ± 14.46 µm | >0.05 |
| RNFL thickness | |||
| Superior quadrant | 118.82 ± 20.71 µm | 109.00 ± 35.34 µm | >0.05 |
| Temporal quadrant | 59.46 ± 12.82 µm | 63.10 ± 2.85 µm | >0.05 |
| Inferior quadrant | 119.34 ± 37.61µm | 126.67 ± 15.50 µm | >0.05 |
| Nasal quadrant | 74.34 ± 10.12 µm | 73.72 ± 13.52 µm | >0.05 |
| Average GCL+IPL thickness | 83.24 ± 10.04 µm | 87.48 ± 12.24 µm | >0.05 |
| Post-COVID-19 with Comorbidities | Non-COVID-19 with Comorbidities | p-Value | |
|---|---|---|---|
| Central macular thickness (CMT) | 282.12 ± 17.77 µm | 264.09 ± 23.75 µm | <0.01 * |
| Parafoveolar superior sector | 336.58 ± 21.35 µm | 339.45 ± 23.48 µm | >0.05 |
| Parafoveolar temporal sector | 330.06 ± 6.93 µm | 318.31 ± 29.02 µm | <0.05 * |
| Parafoveolar inferior sector | 332.05 ± 21.12 µm | 337.42 ± 24.61 µm | >0.05 |
| Parafoveolar nasal sector | 336.82 ± 24.55 µm | 338.13 ± 26.24 µm | >0.05 |
| RNFL thickness | |||
| Superior quadrant | 108.75 ± 25.75 µm | 124.38 ± 19.46 µm | <0.05 * |
| Temporal quadrant | 63.25 ± 14.16 µm | 63.83 ± 11.14 µm | >0.05 |
| Inferior quadrant | 103.38 ± 8.40 µm | 135.56 ± 9.75 µm | <0.001 * |
| Nasal quadrant | 76.62 ± 14.85 µm | 73.53 ± 5.22 µm | >0.05 |
| GCL+IPL thickness | |||
| Average | 83.44 ± 13.57 µm | 86.12 ± 10.59 µm | >0.05 |
| Minimum | 80.62 ± 10.12 µm | 82.88 ± 9.49 µm | >0.05 |
| Hydroxychloroquine/Tocilizumab | No Treatment/Standard Treatment | p-Value | |
|---|---|---|---|
| Central macular thickness (CMT) | 271.06 ± 20.00 µm | 267.85 ± 15.29 µm | >0.05 |
| Parafoveolar superior sector | 335.88 ± 11.35 µm | 332.26 ± 17.28 µm | >0.05 |
| Parafoveolar temporal sector | 324.25 ± 10.26 µm | 320.44 ± 12.17 µm | >0.05 |
| Parafoveolar inferior sector | 333.94 ± 18.20 µm | 329.06 ± 17.63 µm | >0.05 |
| Parafoveolar nasal sector | 338.19 ± 10.82 µm | 333.03 ± 24.08 µm | >0.05 |
| RNFL thickness | |||
| Superior quadrant | 123.25 ± 14.43 µm | 122.76 ± 41.68 µm | >0.05 |
| Temporal quadrant | 64.50 ± 3.85 µm | 64.65 ± 16.23 µm | >0.05 |
| Inferior quadrant | 96.81 ± 7.65 µm | 127.62 ± 35.56 µm | <0.05 * |
| Nasal quadrant | 75.25 ± 12.07 µm | 71.71 ± 7.87 µm | >0.05 |
| GCL+IPL thickness | |||
| Average | 81.81 ± 7.45 µm | 83.50 ± 10.20 µm | >0.05 |
| Minimum | 79.44 ± 7.27 µm | 81.94 ± 10.19 µm | >0.05 |
| OCT Parameters | Post-COVID-19 | Non-COVID-19 | p-Value | |||
|---|---|---|---|---|---|---|
| Smoker | Non-Smoker | Smoker | Non-Smoker | COVID | Non-COVID | |
| Central macular thickness | 263.80 ± 18.53 µm | 268.09 ± 14.79 µm | 237.00 ± 18.03 µm | 269.43 ± 21.62 µm | >0.05 | >0.05 |
| Parafov. superior sector | 327.40 ± 17.42 µm | 334.90 ± 18.43 µm | 309.02 ± 15.00 µm | 332.50 ± 16.61 µm | <0.05 * | >0.05 |
| Parafov. temporal sector | 318.07 ± 14.05 µm | 324.22 ± 12.62 µm | 289.07 ± 15.01 µm | 321.64 ± 15.75 µm | <0.05 * | >0.05 |
| Parafov. inferior sector | 323.55 ± 15.83 µm | 330.01 ± 18.18 µm | 303.01 ± 17.01 µm | 330.14 ± 17.06 µm | >0.05 | >0.05 |
| Parafov. nasal sector | 327.10 ± 20.89 µm | 332.90 ± 23.20 µm | 308.03 ± 19.08 µm | 332.57 ± 18.22 µm | >0.05 | >0.05 |
| RNFL thickness | ||||||
| Superior quadrant | 113.88 ± 29.35 µm | 115.69 ± 21.53 µm | 117.09 ± 19.98 µm | 114.50 ± 11.45 µm | >0.05 | >0.05 |
| Temporal quadrant | 61.70 ± 11.44 µm | 58.48 ± 12.46 µm | 63.40 ± 9.89 µm | 70.00 ± 8.15 µm | >0.05 | >0.05 |
| Inferior quadrant | 108.90 ± 39.95 µm | 104.28 ± 42.84 µm | 138.11 ± 20.08 µm | 129.86 ± 15.98 µm | >0.05 | >0.05 |
| Nasal quadrant | 77.53 ± 12.41 µm | 76.36 ± 10.66 µm | 93.31 ± 13.92 µm | 69.71 ± 6.59 µm | >0.05 | <0.05 * |
| GCL + IPL thickness | ||||||
| Average | 84.97 ± 15.27 µm | 82.65 ± 10.90 µm | 78.21 ± 10.13 µm | 84.50 ± 9.17 µm | >0.05 | >0.05 |
| Minimum | 77.62 ± 11.20 µm | 80.27 ± 13.30 µm | 75.08 ± 11.02 µm | 77.07 ± 19.34 µm | >0.05 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgić, S.S.; Resan, M.; Mavija, M.; Smoljanović Skočić, S.; Grgić, S.; Tadić, D.; Pajic, B. Optical Coherence Tomography (OCT) Findings in Post-COVID-19 Healthcare Workers. J. Imaging 2025, 11, 195. https://doi.org/10.3390/jimaging11060195
Burgić SS, Resan M, Mavija M, Smoljanović Skočić S, Grgić S, Tadić D, Pajic B. Optical Coherence Tomography (OCT) Findings in Post-COVID-19 Healthcare Workers. Journal of Imaging. 2025; 11(6):195. https://doi.org/10.3390/jimaging11060195
Chicago/Turabian StyleBurgić, Sanela Sanja, Mirko Resan, Milka Mavija, Saša Smoljanović Skočić, Sanja Grgić, Daliborka Tadić, and Bojan Pajic. 2025. "Optical Coherence Tomography (OCT) Findings in Post-COVID-19 Healthcare Workers" Journal of Imaging 11, no. 6: 195. https://doi.org/10.3390/jimaging11060195
APA StyleBurgić, S. S., Resan, M., Mavija, M., Smoljanović Skočić, S., Grgić, S., Tadić, D., & Pajic, B. (2025). Optical Coherence Tomography (OCT) Findings in Post-COVID-19 Healthcare Workers. Journal of Imaging, 11(6), 195. https://doi.org/10.3390/jimaging11060195








