Towards the Performance Characterization of a Robotic Multimodal Diagnostic Imaging System
Abstract
1. Introduction
2. Materials and Methods
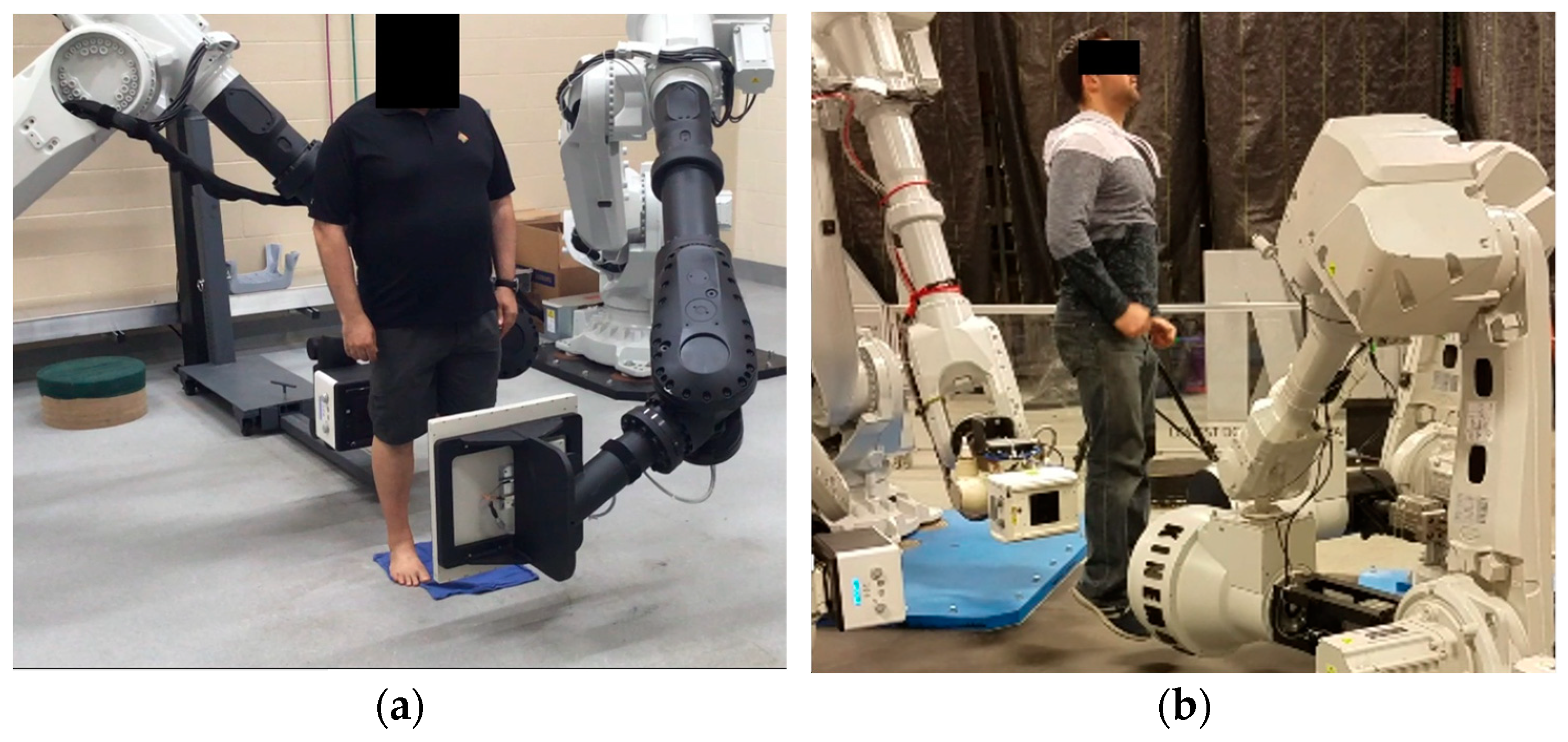
2.1. System Overview and Data Acquisition
2.2. Emitters and Detectors
2.3. Calibration
- (a)
- Distortion correction, while field imaging baseline and system geometric alignment with local and global coordinate systems;
- (b)
- Calibration for cupping effects, scatter correction (SC), streaking, and beam-hardening correction (BHC);
- (c)
- Radiation dose measurements with a specialized custom frame that included a pediatric whole-body phantom;
- (d)
- Dose–area product (DAP) measurements for the fluoroscopy mode;
- (e)
- Comparative burst progressive pulse fluoro (BPPF) mode radiographic dose experiment;
- (f)
- Radiologic dose experiments and their relationship to X-ray power and energy generation;
- (g)
- Over-and-under-exposure imaging for diagnostic quality.
- (a)
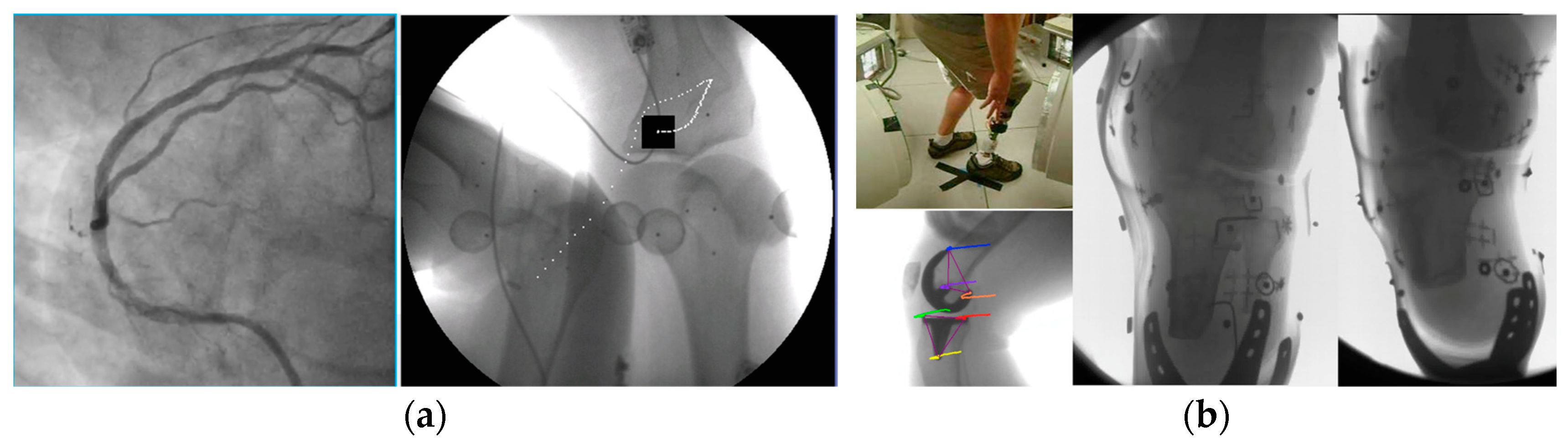
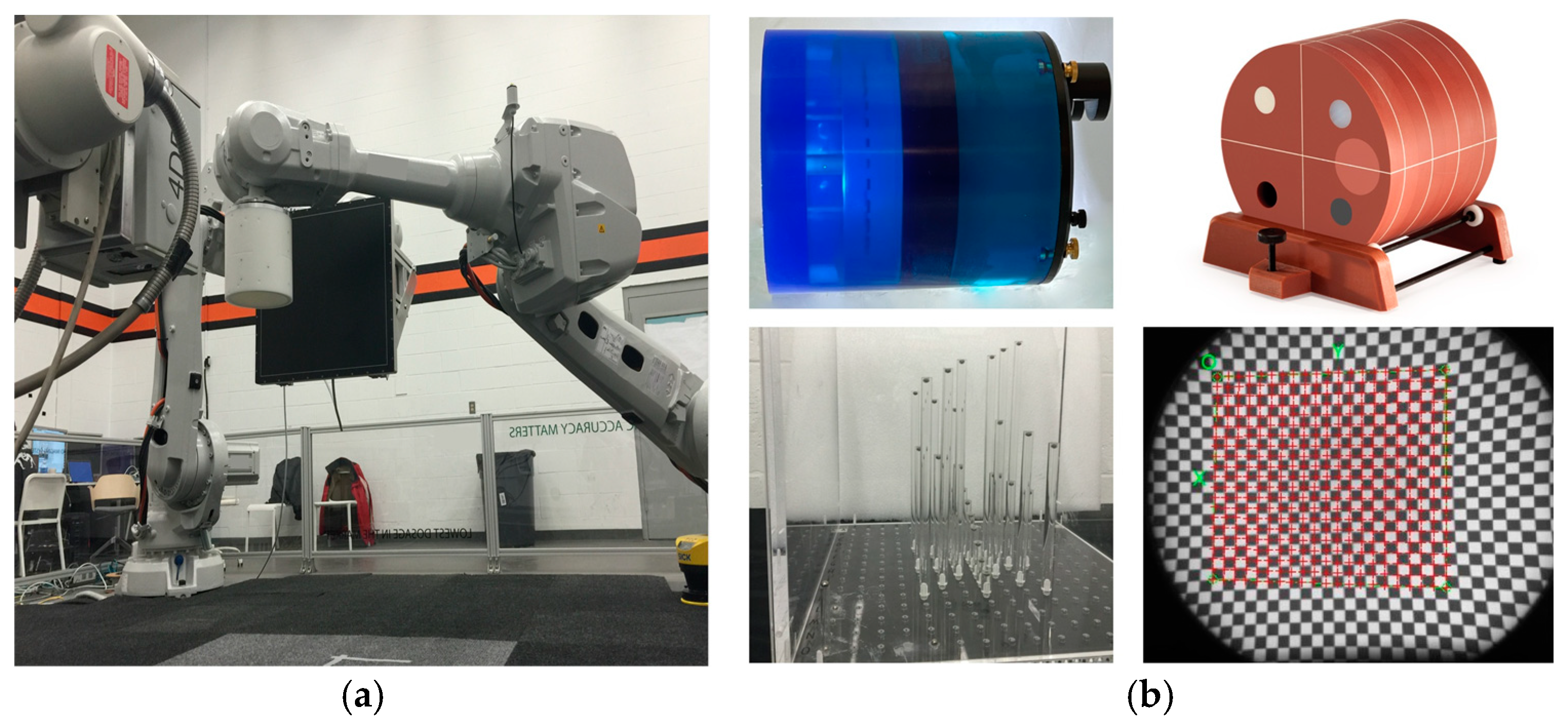
- We initially performed distortion correction, while field imaging baseline tests and system geometric alignment with local and global coordinate systems. Two custom-made 1145 tantalum marker 3D grids (Figure 7) were used to correct (a) the distortion introduced by the image intensifiers and associated optics and (b) align the geometry of the tissue kinematics tracking software with that of the robotic hardware as prescribed by the manufacturer [52]. Additionally, a white-field image (acquired from the X-ray system with nothing in the FOV) was used to perform a log-based correction for image intensity nonuniformity.
- (b)
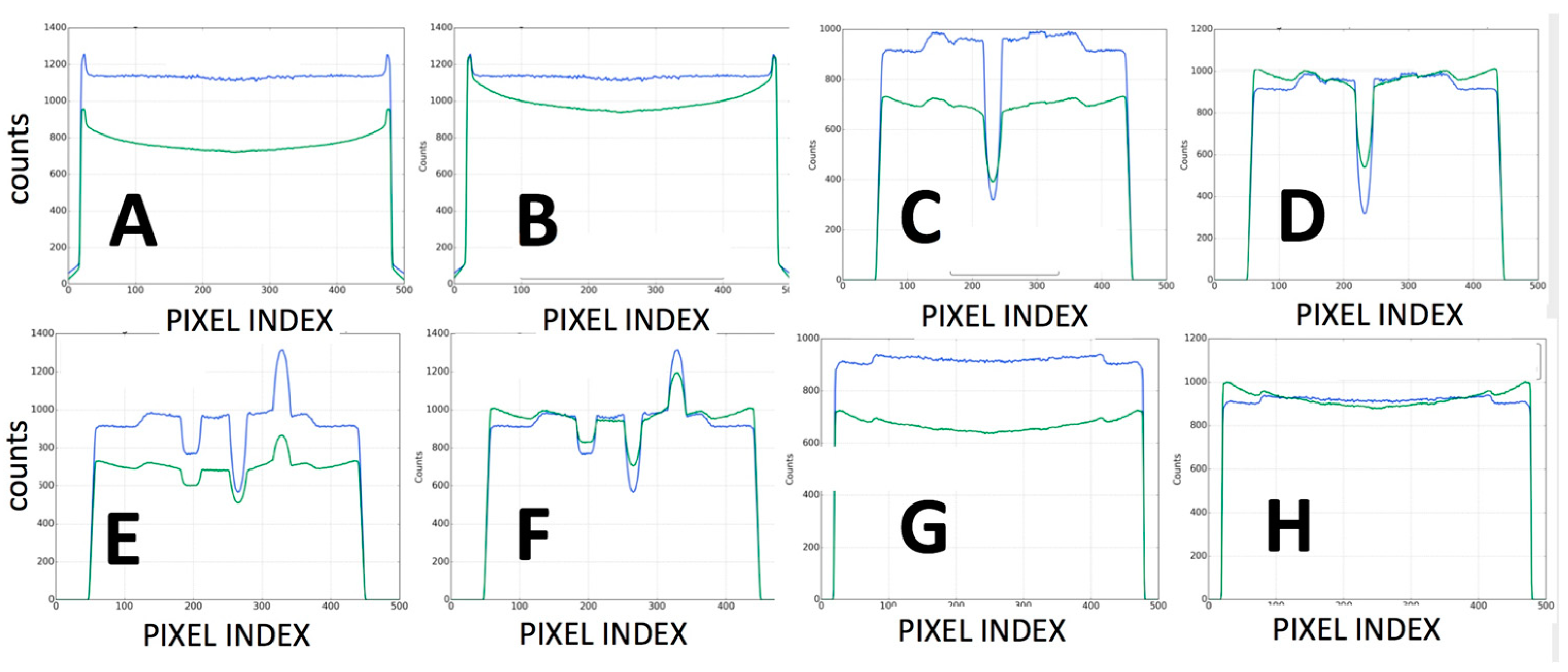
- The second type of calibration used an ACR 464 CT (Sun Nuclear) and a Catphan 500 CT (Phantomlab) phantom (Figure 7). This calibration included the correction of the issue of scatter-to-primary ratios whereas optimal large cone angles and large detector apertures were chosen in those tests. These tests reported (a) cupping in the Hounsfield Unit (HU) profiles of uniform objects and (b) streaking between high- and low-density objects. For mitigation and correction, the device used software-based stepwise mechanical corrections, an anti-scatter grid on the detector, a bowtie filter at the source, and a protocol-based extension of the patient-to-detector distance based on previous testing with a specialized custom frame (Figure 7b) [52]. The calibration included projection normalization with water scans, empirical scatter kernel corrections on projection data, and scatter correction using a deconvolution method (results were reported on uniformity of ±7 HU across a 20 cm water equivalent phantom) (Figure 8). A third resolution evaluation with resolution test pattern tools (2 to 20 lp/mm) was conducted for all three optional detectors (Figure 8D–F).
- (c)
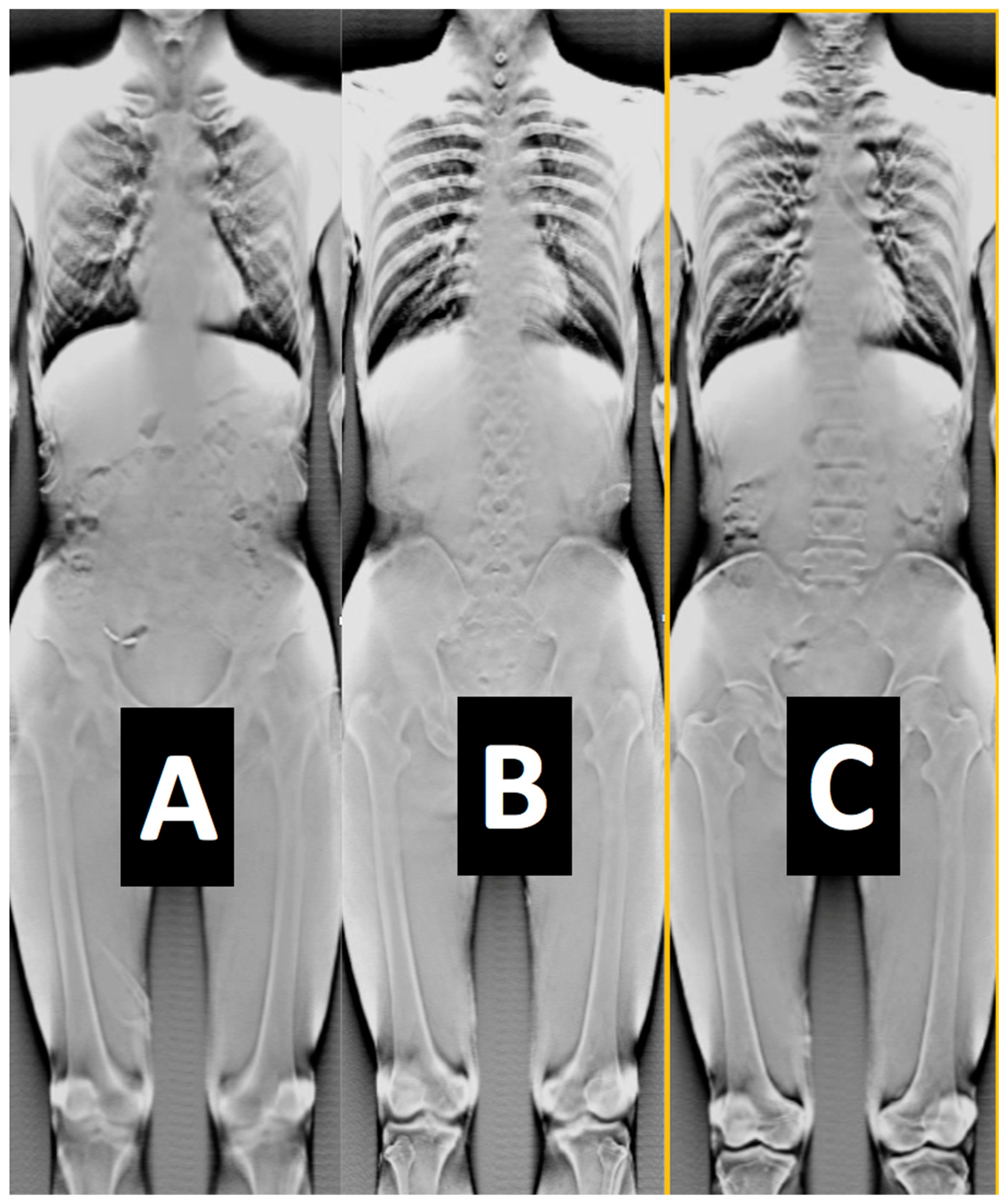
- Another set of calibration and radiology dose tests included a pediatric whole-body phantom (PBU-70, Kyoto Kagagu Ltd., Kyoto, Japan), which consists of tissue-equivalent materials for bone and soft tissues in a 5-year-old. This was located on a specially designed frame [52] to emulate the irradiation received and scatter caused by the patient’s body with respect to the X-ray source. This custom calibration frame allowed us to test for an unlimited choice of distances from the source to the phantom to the detector including the cross point of the mid-transverse, mid-coronal, and mid-sagittal planes. Several different source-to-detector configurations and distances were investigated.
- (d)
- To assess the output of the X-ray source in the fluoroscopy mode, the dose–area product (DAP) was also measured. A DAP meter (VacuTec, Dresden, Germany) and/or Accu-Dose+ (Radcal, Monrovia, CA, USA) was placed on the inner surface of the robotic trajectories and attached to the X-ray tube. A total of 5 min of radiation was applied and averaged into the final DAP measurements. The air kerma in the middle of the X-ray beam was also measured by placing a 5 cc ion chamber (Capintec, PM-05, Cambridge, MA, USA) at the center of the field of view along with the measurement of scatter radiation in the surrounding area of the patient at equally spaced polar coordinate grids. The air kerma rate was measured as a scatter radiation dose using an 1800 cc ionization chamber during 5 min of continuous use imaging. This measurement was performed at horizontal distances of 40, 60, 80, and 100 cm from the xyphoid process at angle positions of 0, 45, 90, 135, 180, 225, and 270°. The tube position was anterior posterior (AP), and the distances between the patient phantom and the tube in the robotic system were 65 cm.
- (e)
- A very simplified, one-source-only-each-time dosage experiment was also designed to demonstrate the fundamental characteristics of the burst progressive pulse fluoro (BPPF) mode. This mode was applied as a stereovideography mode with a minimum of two sources firing synchronously. The grid-control system was by default paired with the system’s grid-controlled X-ray tubes. When the system CPU commanded an X-ray exposure, a generator turned on the high voltage (kVp), but the tube current (and X-ray creation) was restricted by the presence of the previously applied negative high voltage (applied by the system CPU) upon the X-ray tube grid. Without this tube current, X-rays were not generated. This tube’s current modulation technology was utilized in this system to minimize radiation dose for patient safety. While the intention was to minimize patient dose, the technical objective was to create X-rays, when the imaging camera/detector was “looking”, and stop producing X-ray, when the camera was in “blinking-off status”. The physics of the energy involved dictated that high-speed X-ray/camera looking–blinking synchronization could only work with grid-control technology. This grid-control tube-current modulation technology was simply a way to control tube current. Tube current was commanded by the system CPU when it called for a specific length and quantity of X-ray signal releases (these releases came from the switching of the grid potential, from its previously applied negative voltage to zero volts, relative to the cathode). Each release lasted, at full X-ray potential, no more than 2 μs after the CPU’s command for said pulse was initiated. This super-fast “up” time was one of the ways the grid-control system produced the remarkable dose savings. Thereafter, the time for actual X-rays to reach the receptor was even shorter. After the initiation of these X-ray pulses, each pulse began to stop when commanded to do so by the system’s CPU. Note that this happened when the system commanded the reapplication of a negative voltage to the grid. This same sequence of events happened for each and every pulse (each frame).
- (f)
- Additional dosage experiments were expanded to include the following: (Dose A): a 10 μs integration example with 40 mA/60 kV for 15 s in pulsed output fluoro mode using a 100% pulsed max dose, that is, a “non-gridded baseline” example; (Dose B): the same technique at 40 mA/60 kV/15 s run in pulsed fluoro mode at 30 fps, with 10 ms pulses (based on an assumed 10 ms integration grab time); (Dose C): the same technique, 40 mA/60 kV/15 s continuous output fluoro coupled with a high-data-acquisition-speed camera at 1 kHz and a 100% max dose; (Dose D): the same technique, 40 mA/60 kV with 15 s runs of pulsed fluoro at 1 kHz-Fps, 500 μs pulses (based on a 400 μs camera integration grab time). The same techniques were repeated for 30, 60, and 120 fps. Dosimetry was obtained in all these experiments.
- (g)
- In this calibration tests, the pediatric phantom was used in over-and-under-exposure settings and the images were evaluated by a certified radiologist to systematize the correspondence between the tests’ gray-level histogram (calculated using ImageJ -ImageJ NIH, Version 1.54p 2024) and the diagnostic quality of the image.
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI/A | Magnetic Resonance imaging/Angiography |
| FoV | Field of view |
| HMI | Human–machine interface |
| EoAT | End-of-arm toolkits |
| SOD | Source-to-object distance |
| SID | Source-to-imager distance |
| OID | Object-to-imager distance |
| PLC | Programmable Logic Controllers |
| SNR | Signal-to-noise ratio |
| CNR | Contrast-to-noise ration |
| IGZO | Indium gallium zinc oxide |
| CMOS | Complementary metal–oxide–semiconductor |
| DC-TDI | Direct conversion, Time Delay Integration |
| PAN | Panoramic |
| CATHLAB | Catheterization laboratory |
| AIS | Acute Ischemic Stroke |
| CHD | Congenital heart disease |
| ESPR | European Society of Pediatric Radiology |
| CAD | Computer aided design |
| AI | Artificial intelligence |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 4D | Four-dimensional |
| VH | Volume height (of cone) |
| VD | Volume diameter (of cone) |
| FS | Focal spot |
| LDDR | Lowest detectable dose rate |
| BPPF | Burst progressive pulse fluoro mode |
| EDR | Extreme dynamic range |
| PA | Posterior-anterior |
| SC | Scatter correction |
| BHC | Beam-hardening correction |
| ACR | American College of Radiology |
| CTDI | Computed Tomography Dose Index |
| DAP | Dose–area product |
| ALARA | As low as reasonably achievable |
| DOF | Degree of freedom |
References
- O-am Surgical Imaging System. Available online: https://europe.medtronic.com (accessed on 3 April 2025).
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; Berrington de Gonzalez, A.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- National Cancer Institute. Radiation Risks and Pediatric Computed Tomography (CT): A Guide for Health Care Providers. 2025. Available online: https://www.cancer.gov/about-cancer/causesprevention/risk/radiation/pediatric-ct-scans (accessed on 3 April 2025).
- Park, M.S.; Lee, K.M.; Lee, B.; Min, E.; Kim, Y.; Jeon, S.; Huh, Y.; Lee, K. Comparison of operator radiation exposure between c-arm and o-arm fluoroscopy for orthopaedic surgery. Radiat. Prot. Dosim. 2012, 148, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Miksys, N.; Gordon, C.L.; Thomas, K.; Connolly, B.L. Estimating Effective Dose to Pediatric Patients Undergoing Interventional Radiology Procedures Using Anthropomorphic Phantoms and MOSFET Dosimeters. Med. Phys. Inform. 2010, 194, 1315–1322. [Google Scholar] [CrossRef]
- Teubner, U.; Brückner, H.J. Characterization of imaging systems. In Optical Imaging and Photography; De Gruyter: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- American Association of Physicists in Medicine. Specification and Acceptance Testing of Computed, Tomography Scanners; Report No. 39; AAPM: New York, NY, USA, 1993. [Google Scholar]
- Ng, C.K.C. Artificial Intelligence for Radiation Dose Optimization in Pediatric Radiology: A Systematic Review. Children 2022, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.P.; Naik, S.; Andrabi, S.A.U.H.; Luharia, A.; Tivaskar, S. Enhancing Radiological Diagnosis: A Comprehensive Review of Image Quality Assessment and Optimization Strategies. Cureus 2024, 16, e63016. [Google Scholar] [CrossRef] [PubMed]
- Zarembo, A. Cedars-Sinai Radiation Overdoses Went Unseen at Several Points. LA Times, 14 October 2010. Available online: http://articles.latimes.com/2009/oct/14/local/me-cedars-sinai14 (accessed on 3 April 2025).
- Kanal, K.M. U.S. Diagnostic Reference Levels and Achievable Doses for 10 Pediatric CT Examinations. Radiology 2022, 302, 164–174. [Google Scholar] [CrossRef]
- Saver, J.Y.L.; Smith, E.E.; Fonarow, G.C.; Reeves, M.J.; Zhao, X.; Olson, D.M.; Schwamm, L.H. The “Golden Hour” and Acute Brain Ischemia Presenting Features and Lytic Therapy in >30 000 Patients Arriving Within 60 Minutes of Stroke Onset. Stroke 2010, 41, 1431–1439. [Google Scholar] [CrossRef]
- Tsze, D.S.; Vale, J.H. Pediatric Stroke: A Review. Emerg. Med. Int. 2011, 1, 734506. [Google Scholar] [CrossRef]
- Tabarki, B. Inherited Metabolic Causes of Stroke in Children: Mechanisms, Types, and Management. Front. Neurol. 2021, 12, 633119. [Google Scholar] [CrossRef]
- Kirton, A.; deVeber, G. Pediatric stroke: Pressing issues and promising directions. Lancet Neurol. 2015, 14, 92–102. [Google Scholar] [CrossRef]
- Roa, A.M.; Andersen, H.K.; Martinsen, A.C. CT image quality over time: Comparison of image quality for six different CT scanners over a six-year period. J. Appl. Clin. Med. Phys. 2015, 16, 4972. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Bakalyar, D.; Boedeker, K.L.; Brady, S.; Fan, J.; Leng, S.; Myers, K.J.; Popescu, L.M.; Ramirez Giraldo, J.C.; Ranallo, F.; et al. Performance evaluation of computed tomography systems: Summary of AAPM Task Group 233. Med. Phys. 2019, 46, e735–e756. [Google Scholar] [CrossRef]
- Ogbole, G.I. Radiation dose in pediatric computed tomography: Risks and benefits. Ann. Ib. Postgrad. Med. 2010, 8, 118–126. [Google Scholar]
- Feldmann, M.; Hagmann, C.; de Vries, L.; Disselhoff, V.; Pushparajah, K.; Logeswaran, T.; Jansen, N.J.G.; Breur, J.M.P.J.; Knirsch, W.; Benders, M.; et al. Neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices in neonatal congenital heart disease: A European survey. Pediatr. Res. 2023, 93, 168–175. [Google Scholar] [CrossRef]
- Dirrichs, T.; Tietz, E.; Rüffer, A.; Hanten, J.; Nguyen, T.D.; Dethlefsen, E.; Kuhl, C.K. Photon-counting versus Dual-Source CT of Congenital Heart Defects in Neonates and Infants: Initial Experience. Radiology 2023, 307, e223088. [Google Scholar] [CrossRef]
- Frush, D.P.; Donnelly, L.F.; Rosen, N.S. Computed tomography and radiation risks: What pediatric health care providers should know. Pediatrics 2003, 112, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Thukral, B.B. Problems and preferences in pediatric imaging. Indian J. Radiol. Imaging 2015, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology, ACR Appropriateness Criteria. Radiation Dose Assessment Introduction. Available online: https://www.acr.org (accessed on 28 December 2018).
- World Health Organisation, The WHO Child Growth Standards. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 9 August 2024).
- Ng, C.K.C. Generative Adversarial Network (Generative Artificial Intelligence) in Pediatric Radiology: A Systematic Review. Children 2023, 10, 1372. [Google Scholar] [CrossRef]
- Vañó, E.; Miller, D.L.; Martin, C.J.; Rehani, M.M.; Kang, K.; Rosenstein, M.; Ortiz-López, P.; Mattsson, S.; Padovani, R.; Rogers, A.; et al. ICRP Publication 135: Diagnostic Reference Levels in Medical Imaging. Ann. ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, Z.; Xu, Y.; Xia, L.; Zhan, Z.; Xu, T.; Cao, Y.; Han, Z. Associations of Clinical Characteristics and Etiology With Death in Hospitalized Chinese Children After Spontaneous Intracerebral Hemorrhage: A Single-Center, Retrospective Cohort Study. Front. Pediatr. 2021, 8, 576077. [Google Scholar] [CrossRef]
- Von Ungern-Sternberg, B.S.; Habre, W. Pediatric anesthesia—Potential risks and their assessment: Part I. Paediatr. Anaesth. 2007, 17, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Matthes, K.; Laubach, A.E.; Wang, E.; Anderson, T.A. Pediatric Anesthesiology: A Comprehensive Board Review; Oxford University Press: Oxford, UK, 2015; ISBN -13. [Google Scholar]
- Dolk, H.; Loane, M.; Garne, E. European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnson, W.H., Jr.; et al. American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation 2012, 126, 1143–1172. [Google Scholar] [CrossRef]
- Atal, B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin. Perinatol. 2016, 43, 173–185. [Google Scholar]
- Mastrangelo, M.; Giordo, L.; Ricciardi, G.; De Michele, M.; Toni, D.; Leuzzi, V. Acute ischemic stroke in childhood: A comprehensive review. Eur. J. Pediatr. 2022, 181, 45–58. [Google Scholar] [CrossRef]
- Sakhatskyi, K.; Turedi, B.; Matt, G.J. Stable perovskite single-crystal X-ray imaging detectors with single-photon sensitivity. Nat. Photon. 2023, 17, 510–517. [Google Scholar] [CrossRef]
- Lu, X.; Xin, D.; Lei, L.; Fan, Z.; Dong, S.; Tie, S.; Yuan, R.; Lin, P.; Zhu, J.; Zheng, X. High-Performance Flat-Panel Perovskite X-ray Detectors Enabled by Defect Passivation in Ruddlesden-Popper Perovskites. ACS Appl. Mater. Interfaces 2024, 16, 14006–14014. [Google Scholar] [CrossRef] [PubMed]
- You, B.M.; Siy, P.; Anderst, W.; Tashman, S. In vivo measurement of 3-D skeletal kinematics from sequences of biplane radiographs: Application to knee kinematics. IEEE Trans. Med. Imaging 2001, 20, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Dilmanian, F.A.; Krishnan, S.; McLaughlin, W.E.; Lukaniec, B.; Baker, J.T.; Ailawadi, S.; Hirsch, K.N.; Cattell, R.F.; Roy, R.; Helfer, J.; et al. Merging Orthovoltage X-Ray Minibeams spare the proximal tissues while producing a solid beam at the target. Sci. Rep. 2019, 9, 1198. [Google Scholar] [CrossRef]
- Papaioannou, G.; Nianios, G.; Mitrogiannis, C.; Fyhrie, D.; Tashman, S.; Yang, K.H. Patient-specific knee joint finite element model validation with high-accuracy kinematics from biplane dynamic Roentgen stereogrammetric analysis. J. Biomech. 2008, 41, 2633–2638. [Google Scholar] [CrossRef]
- Papaioannou, G.; Mitrogiannis, C.; Nianios, G. An Improved Tracking Technique for assessment of high-Resolution Dynamic Radiography kinematics. Comput. Model. Eng. Sci. (CMES) 2008, 8, 41–46. [Google Scholar]
- Trivizakis, E.; Papadakis, G.Z.; Souglakos, I.; Papanikolaou, N.; Koumakis, L.; Spandidos, D.A.; Tsatsakis, A.; Karantanas, A.H.; Marias, K. Artificial intelligence radiogenomics for advancing precision and effectiveness in oncologic care (Review). Int. J. Oncol. 2020, 57, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Baka, N.; Kaptein, B.L.; Giphart, J.E.; Staring, M.; de Bruijne, M.; Lelieveldt, B.P.; Valstar, E. Evaluation of automated statistical shape model based knee kinematics from biplane fluoroscopy. J. Biomech. 2014, 47, 122–129. [Google Scholar] [CrossRef]
- Torres, J.J.C.; Mitrogiannis, C.; Nianios, G.; Papaioannou, G. Comparison between Computed Tomography-based amputee geometry and standard geometry measures of socket fit. In Proceedings of the Annual Meeting of the Orthopedic Research Society, San Antonio, TX, USA, 26–29 January 2013. [Google Scholar]
- Papaioannou, G.; Demetropoulos, C.K.; King, Y.H. Predicting the effects of knee focal articular surface injury with a patient- specific finite element model. Knee 2010, 17, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Tsiokos, D.; Fiedler, G.; Mitrogiannis, C.; Avdeev, I.; Wood, J.; McKinney, R. Dynamic radiography imaging as a tool in the design and validation of a novel intelligent amputee socket. In Computational Vision & Medical Image, 1st ed.; Tavares, M.R.S., Natal Jorge, R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 91–112. ISBN 978-94-007-3420-3. [Google Scholar]
- Papaioannou, G.; Mitrogiannis, C.; Nianios, G.; Fiedler, G. Assessment of amputee socket-stump-residual bone kinematics during strenuous activities using Dynamic Roentgen Stereogrammetric Analysis. J. Biomech. 2010, 43, 871–878. [Google Scholar] [CrossRef]
- Papaioannou, G. Method for the Control of Collimation, Penumbra, Dynamic Exposure, and Dosage in a Number of Robotics-driven 2D Radiology and 3D Computer Tomography and Other Multimodality Imaging. Patent #2013,010,0822, 15 July 2024. [Google Scholar]
- Papaioannou, G.; Bafas, C. Robotics-driven two, three and four dimensional imaging enhances dosage and image resolution for a number of diagnostic modalities. In Proceedings of the Invited Paper at 12th Scientific Conference of the Medical School of Aristotle University of Thessaloniki, Thessaloniki, Greece, 15–18 March 2023. [Google Scholar]
- Papaioannou, G.; Mitrogiannis, C.; Nianios, G.; Fiedler, G. Tracking high speed arthrokinematics using a new and high resolution Biplane Dynamic Roentgen Stereogrammetric method. Int. J. Imaging Robot. 2009, 2, 66–85. [Google Scholar]
- Papaioannou, G. A Robotics-driven Multimodal Imaging System for Quality Inspection. In Proceedings of the Plenary Technical Invited Talk and Paper at the 73rd College International pour la Recherche en Productique (CIRP) General Assembly in Thessaloniki, Thessaloniki, Greece, 18–24 August 2024. [Google Scholar]
- ANSA BETA CAE Systems, Lucerne, Switzerland. 2024. Available online: https://www.beta-cae.com/ (accessed on 5 August 2018).
- KINEMAGINE-Achilles Robotic Imaging System, Brochure and KINEDOSE Operating Manual; ATLAS Robotics: New York, NY, USA; Thessaloniki, Greece, 2024; pp. 1–1967. Available online: https://atlasimaging.eu/ (accessed on 7 December 2022).
- 3DNet Medical Cloud. Biotronics 3D Inc.: Boston, MA, USA. 2025. Available online: https://www.3dnetmedical.com/public/ (accessed on 21 January 2019).
- Baek, J.; Pineda, A.R.; Pelc, N.J. To bin or not to bin? The effect of CT system limiting resolution on noise and detectability. Phys. Med. Biol. 2013, 58, 1433–1446. [Google Scholar] [CrossRef]


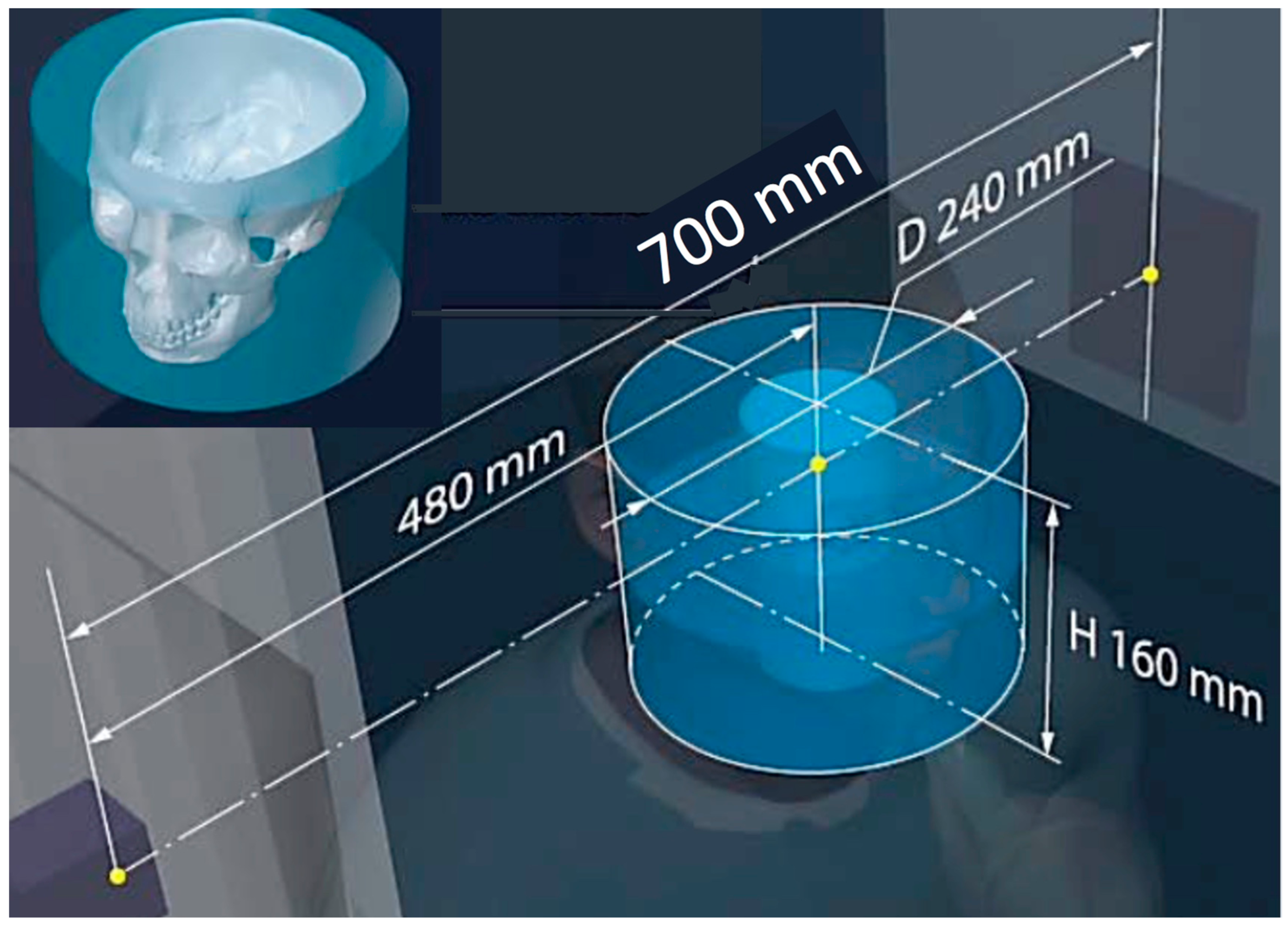
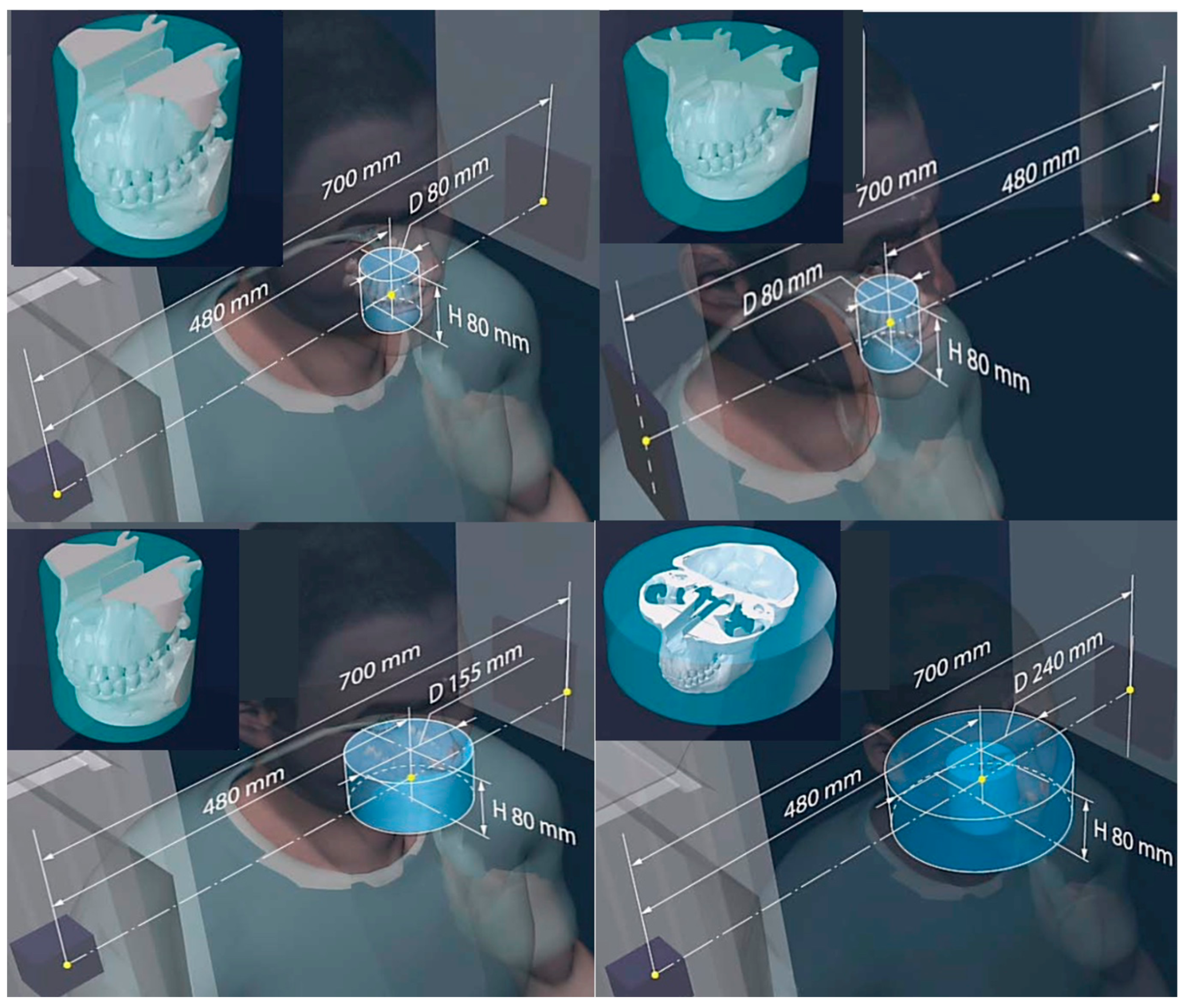





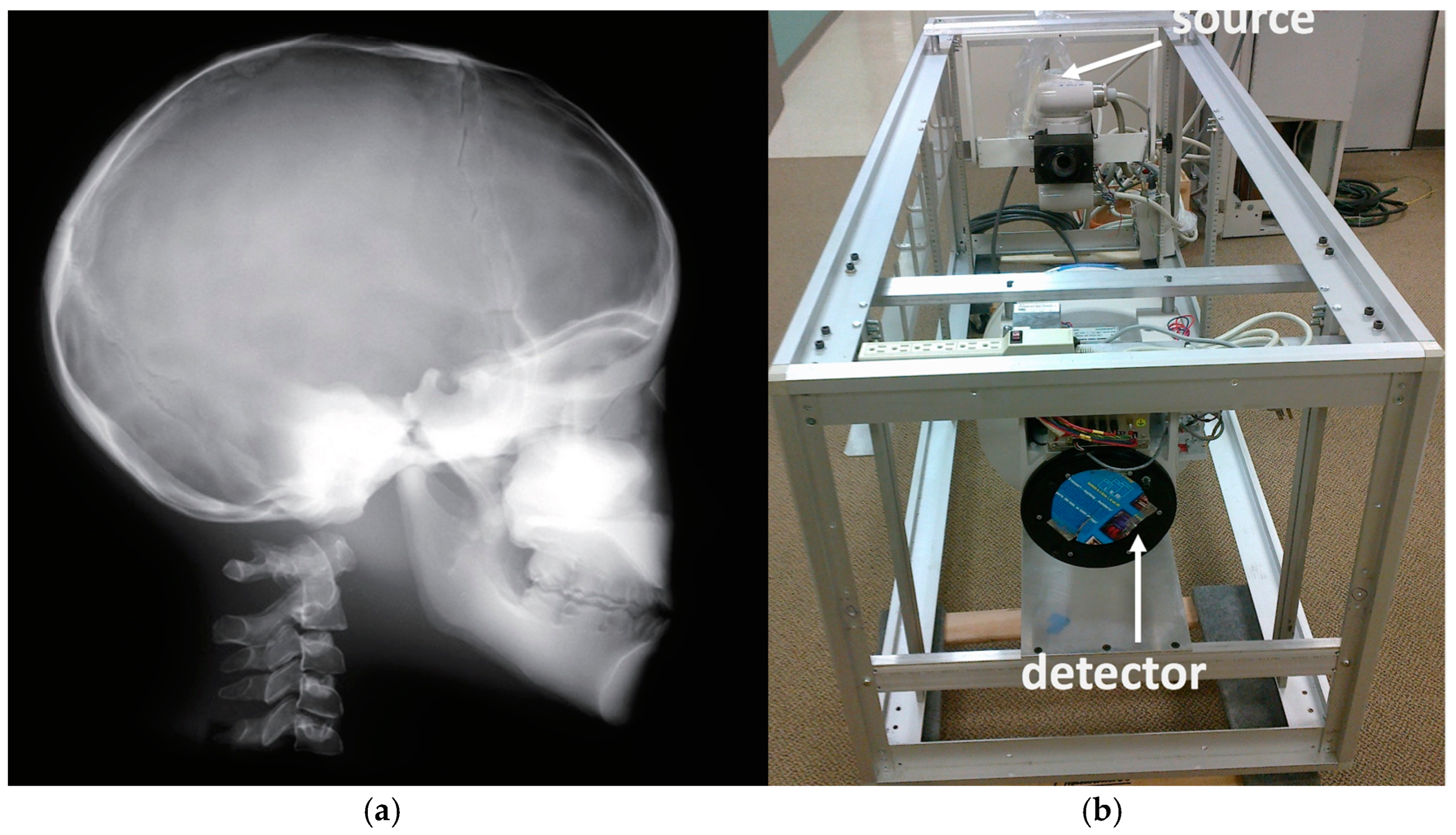
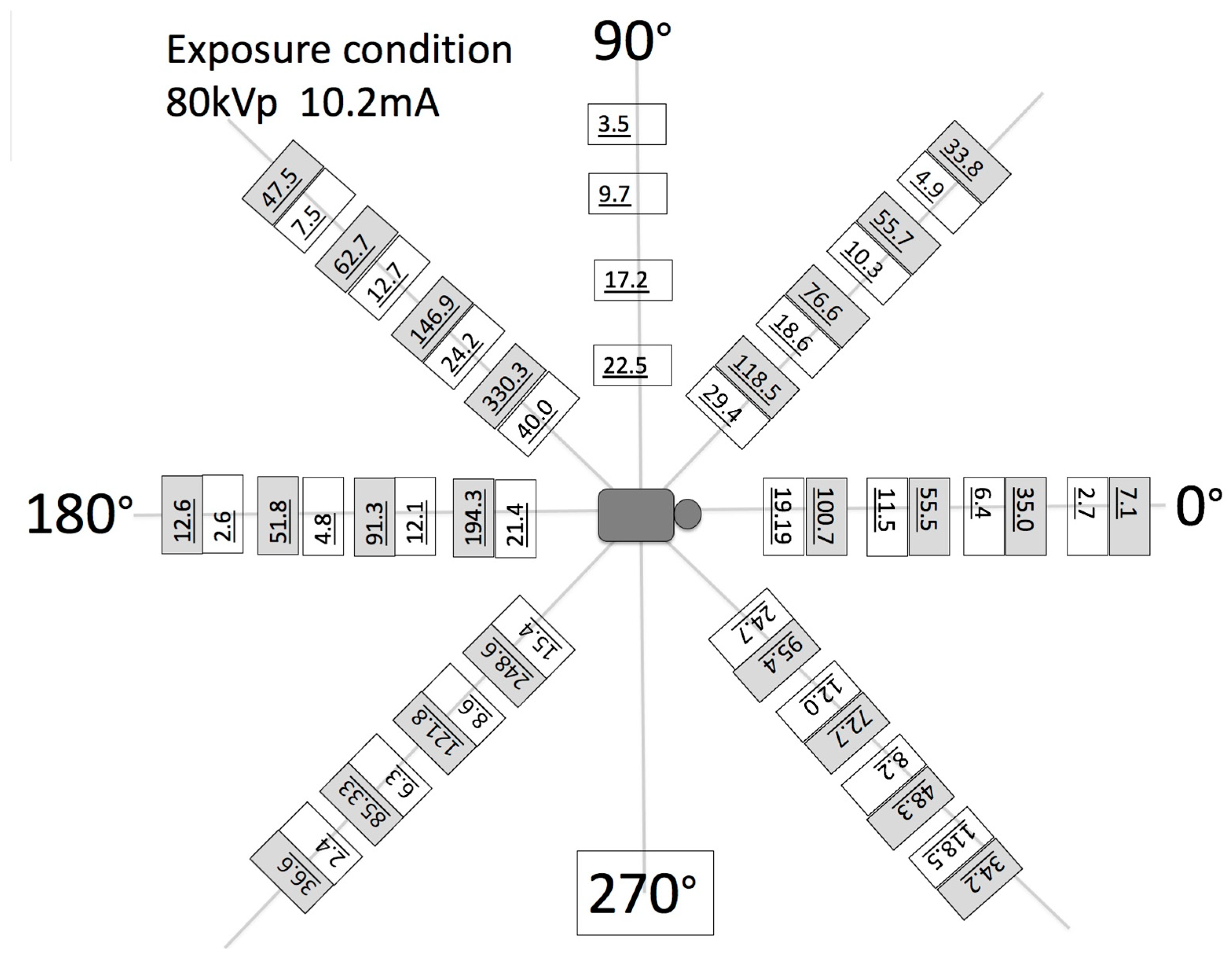
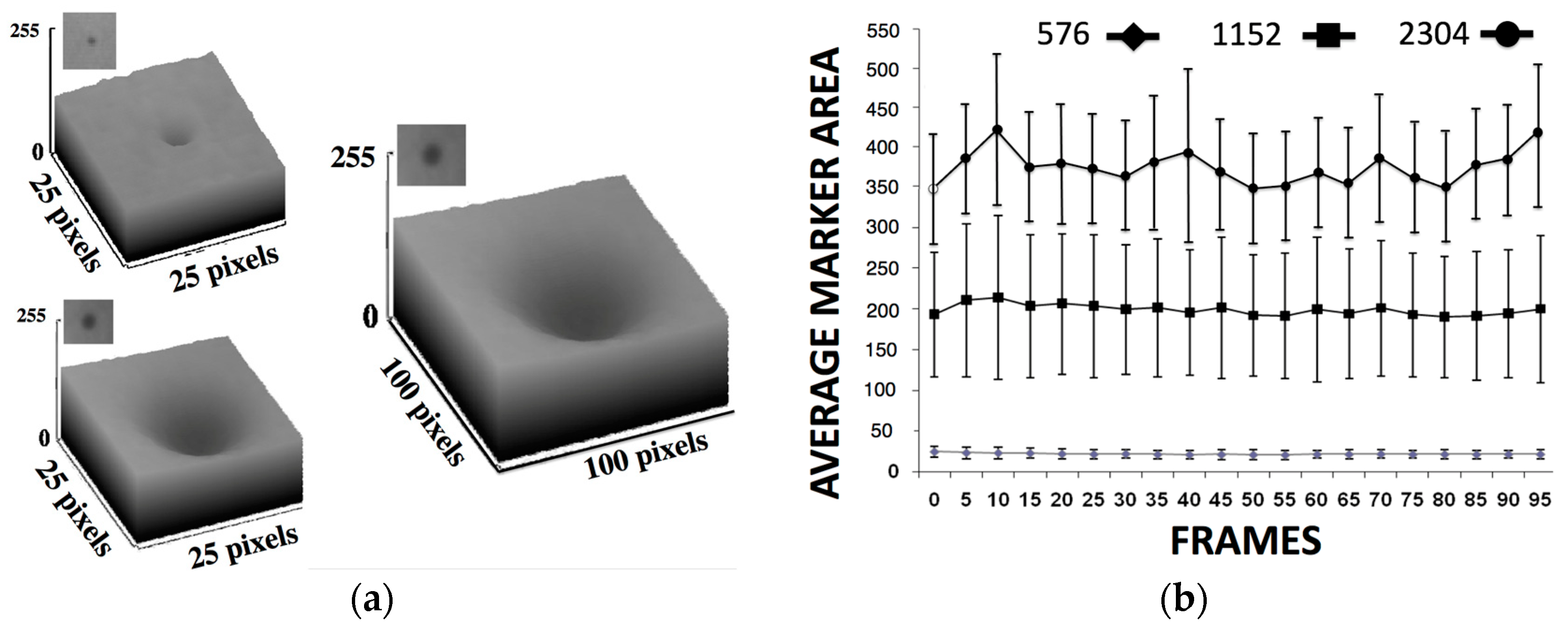
| Parameters Modality | Resolution /Type of Reconstruction | Min–Max Scan Duration/Min- Max Frame acq. Rate | Dose Chest/Gonad/Head Imaging (μGy min−1) | SID/SOD/VD/VH/FS (mm) |
|---|---|---|---|---|
| 2D radiography (see Figure 5, Figure 6, Figure 11 and Figure 16) | See detector a, b, c resolution | 1 s to 2 min/1–360 | ~50/50/50 | SID 50–1100/ everything else is variable |
| 3D CT (Figure 2 and Figure 12) | 80–200 μm full CT, 3x mode CT, half-beam mode, stacked-volume mode | 6 s to 4 min/40–5000 | ~50–500/16.4–200/62–120 Total dose in full in vivo high-res. CT: 6.2 mSv | 700/480/240/0.06 |
| 3D tomosynthesis (see Figure 2 and Figure 11). Optical mag., x4 | 10–40 μm fixed detector, moving emitter | 6 s to 2 min/5–5000 | ~30/16.4/34 | 50–1100/variable SOD/variable VD and VH/0.02–0.2 |
| 3D dynamic imaging stereovideography (Figure 5, Figure 6 and Figure 9) | 20–200 μm Static or moving image intensifiers with high-speed cameras | 1 s, as required by IR/10–10000 | 0.02–1.1 mSv min−1/0.08–0.9 mSv min−1/0.02–0.08 mSv min−1 | 50–1100 /10–500/variable VD and VH/0.02–0.2 |
| 3D “microCT” optical and geometrical magnification x4 (Figure 2 and Figure 13) | 0.02 mm Emitter/detector rotation only | 1 min to 5 min or as required/100–5000 | 0.2–5 mSv min−1/not available/0.2–5 mSv min−1 | 50–100 /10–50/variable VD and VH/0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, G.; Mitrogiannis, C.; Schweitzer, M.; Michailidis, N.; Pappa, M.; Khosravi, P.; Karantanas, A.; Starling, S.; Ruberg, C. Towards the Performance Characterization of a Robotic Multimodal Diagnostic Imaging System. J. Imaging 2025, 11, 147. https://doi.org/10.3390/jimaging11050147
Papaioannou G, Mitrogiannis C, Schweitzer M, Michailidis N, Pappa M, Khosravi P, Karantanas A, Starling S, Ruberg C. Towards the Performance Characterization of a Robotic Multimodal Diagnostic Imaging System. Journal of Imaging. 2025; 11(5):147. https://doi.org/10.3390/jimaging11050147
Chicago/Turabian StylePapaioannou, George, Christos Mitrogiannis, Mark Schweitzer, Nikolaos Michailidis, Maria Pappa, Pegah Khosravi, Apostolos Karantanas, Sean Starling, and Christian Ruberg. 2025. "Towards the Performance Characterization of a Robotic Multimodal Diagnostic Imaging System" Journal of Imaging 11, no. 5: 147. https://doi.org/10.3390/jimaging11050147
APA StylePapaioannou, G., Mitrogiannis, C., Schweitzer, M., Michailidis, N., Pappa, M., Khosravi, P., Karantanas, A., Starling, S., & Ruberg, C. (2025). Towards the Performance Characterization of a Robotic Multimodal Diagnostic Imaging System. Journal of Imaging, 11(5), 147. https://doi.org/10.3390/jimaging11050147









