Cardiac Magnetic Resonance in the Assessment of Atrial Cardiomyopathy and Pulmonary Vein Isolation Planning for Atrial Fibrillation
Abstract
1. Introduction
2. Methods
3. Discussion
3.1. Atrial Cardiomyopathy
3.2. CMR in AF-Related Atrial Cardiomyopathy
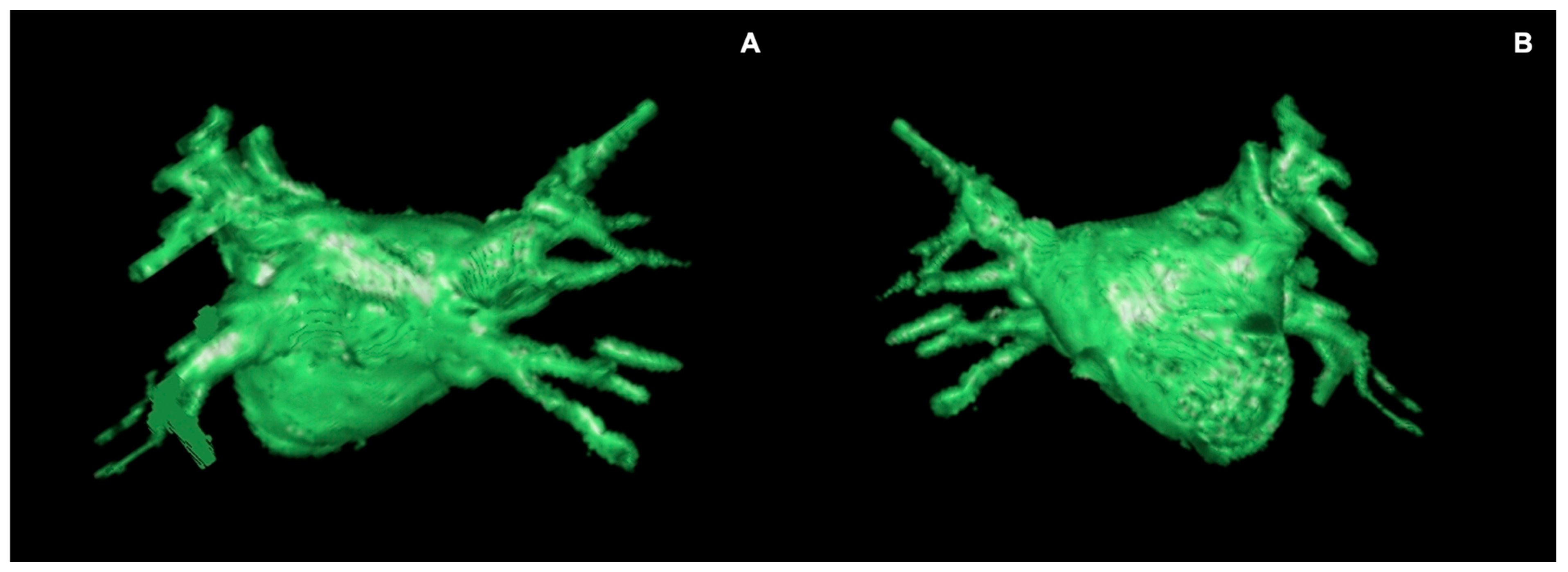
3.2.1. Left Atrial Volume and Morphology
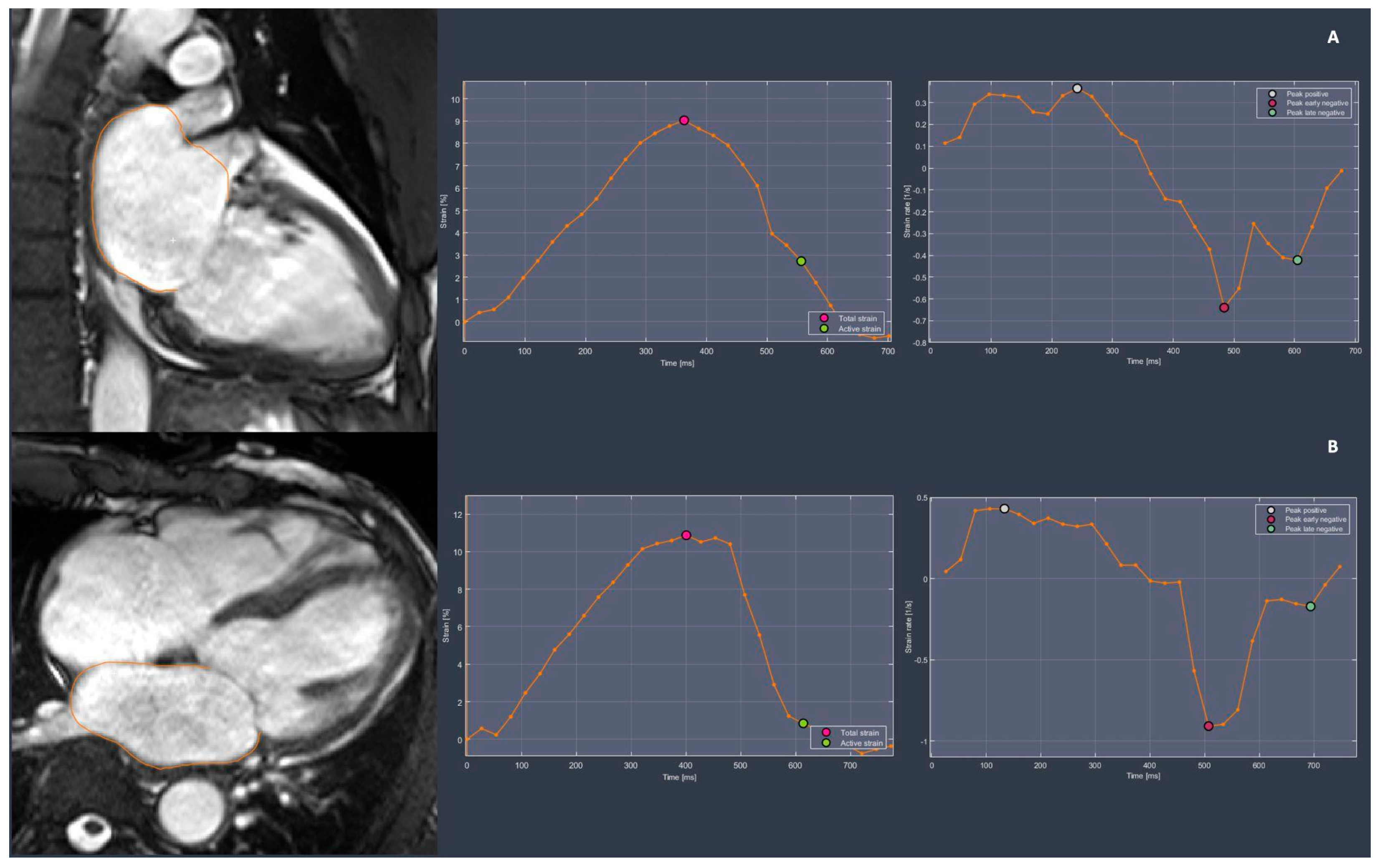
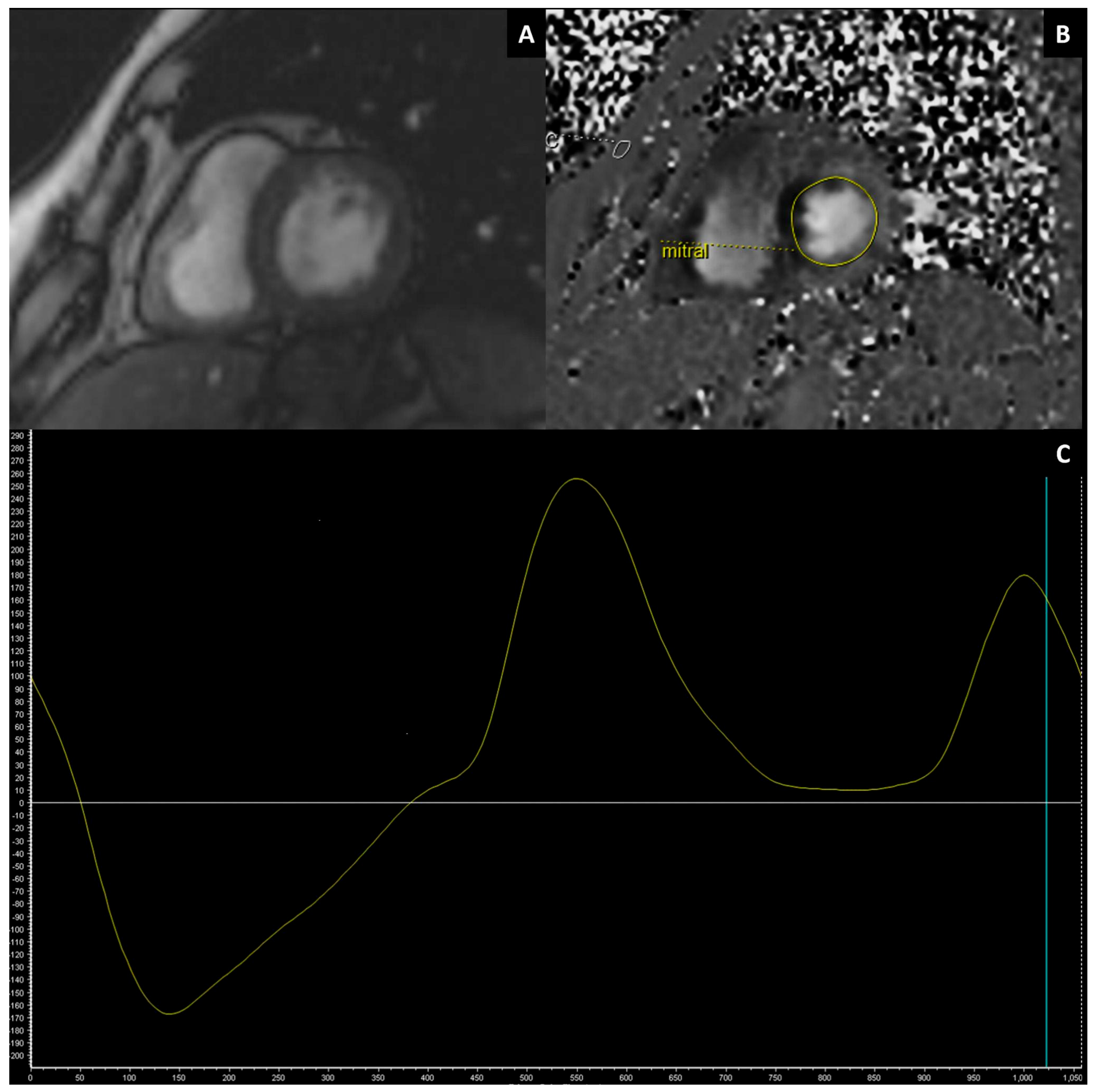
3.2.2. Left Atrial Function
3.2.3. Left Atrial Tissue Characterization
3.2.4. Left Ventricular Functional and Structural Assessment in AF
3.3. Pulmonary Vein Isolation (PVI)
3.4. CMR in the Planning of PVI
Pulmonary Vein Mapping
3.5. CMR-Guided Ablation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AtCM | atrial cardiomyopathy |
| AF | atrial fibrillation |
| CE-MRA | contrast-enhanced MR angiography |
| CTA | computed tomography angiography |
| TEE | transthoracic echocardiography |
| TOE | transesophageal echocardiography |
| EAT | epicardial adipose tissue |
| ETI | esophageal thermal injury |
| LA | left atrium |
| LAA | left atrial appendage |
| LAV | left atrial volume |
| LAVI | left atrial volume indexed |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| PVI | pulmonary vein isolation |
| RFA | radiofrequency ablation |
References
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the Europe. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterization, and Clinical Implication. Ep Eur. 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Baman, J.R.; Cox, J.L.; McCarthy, P.M.; Kim, D.; Patel, R.B.; Passman, R.S.; Wilcox, J.E. Atrial Fibrillation and Atrial Cardiomyopathies. J. Cardiovasc. Electrophysiol. 2021, 32, 2845–2853. [Google Scholar] [CrossRef]
- D’Alessandro, E.; Winters, J.; van Nieuwenhoven, F.A.; Schotten, U.; Verheule, S. The Complex Relation between Atrial Cardiomyopathy and Thrombogenesis. Cells 2022, 11, 2963. [Google Scholar] [CrossRef]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated with Presence and Persistency of Atrial Fibrillation but Not with Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Rapezzi, C.; Castiglione, V.; Fabiani, I.; Pucci, A.; Buda, G.; Passino, C.; Lupón, J.; Bayes-Genis, A.; et al. Atrial Amyloidosis: Mechanisms and Clinical Manifestations. Eur. J. Heart Fail. 2022, 24, 2019–2028. [Google Scholar] [CrossRef]
- Gagliardi, I.; Celico, M.; Gamberini, M.R.; Pontrelli, M.; Fortini, M.; Carnevale, A.; Napoli, N.; Zatelli, M.C.; Ambrosio, M.R. Efficacy and Safety of Teriparatide in Beta-Thalassemia Major Associated Osteoporosis: A Real-Life Experience. Calcif. Tissue Int. 2022, 111, 56–65. [Google Scholar] [CrossRef]
- Reant, P.; Lafitte, S.; Jaïs, P.; Serri, K.; Weerasooriya, R.; Hocini, M.; Pillois, X.; Clementy, J.; Haïssaguerre, M.; Roudaut, R. Reverse Remodeling of the Left Cardiac Chambers after Catheter Ablation after 1 Year in a Series of Patients with Isolated Atrial Fibrillation. Circulation 2005, 112, 2896–2903. [Google Scholar] [CrossRef]
- Mayyas, F.; Niebauer, M.; Zurick, A.; Barnard, J.; Gillinov, A.M.; Chung, M.K.; Van Wagoner, D.R. Association of Left Atrial Endothelin-1 with Atrial Rhythm, Size, and Fibrosis in Patients with Structural Heart Disease. Circ. Arrhythmia Electrophysiol. 2010, 3, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A.; Levy, D. Left Atrial Size and the Risk of Stroke and Death. The Framingham Heart Study. Circulation 1995, 92, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Fletcher, K.A.; Rothbart, R.M.; Halperin, J.L.; Hart, R.G. Clinical and Echocardiographic Features of Intermittent Atrial Fibrillation That Predict Recurrent Atrial Fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Am. J. Cardiol. 1995, 76, 355–358. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, H.M.; van der Zee, L.; Wijffels, M.C.; van Leuven, C.; Dorland, R.; Vos, M.A.; Jongsma, H.J.; Allessie, M.A. Atrial Fibrillation in the Goat Induces Changes in Monophasic Action Potential and mRNA Expression of Ion Channels Involved in Repolarization. J. Cardiovasc. Electrophysiol. 2000, 11, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Lima, J.A.C.; Khurram, I.M.; Zimmerman, S.L.; Zipunnikov, V.; Fukumoto, K.; Spragg, D.; Ashikaga, H.; Rickard, J.; Marine, J.E.; et al. Association of Left Atrial Function and Left Atrial Enhancement in Patients with Atrial Fibrillation: Cardiac Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2015, 8, e002769. [Google Scholar] [CrossRef]
- Kompella, R.; Amin, H.; Mather, J.F.; Hashim, S.W.; McKay, R.G.; McMahon, S.R. Impact of Persistent Versus Paroxysmal Preoperative Atrial Fibrillation on In-Hospital, One-Year and Late Clinical Outcomes Following Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2024, 225, 67–74. [Google Scholar] [CrossRef]
- Lemola, K.; Sneider, M.; Desjardins, B.; Case, I.; Chugh, A.; Hall, B.; Cheung, P.; Good, E.; Han, J.; Tamirisa, K.; et al. Effects of Left Atrial Ablation of Atrial Fibrillation on Size of the Left Atrium and Pulmonary Veins. Heart Rhythm 2004, 1, 576–581. [Google Scholar] [CrossRef]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left Atrial Volume Predicts Atrial Fibrillation Recurrence after Radiofrequency Ablation: A Meta-Analysis. Ep Eur. 2018, 20, 33–42. [Google Scholar] [CrossRef]
- Khan, M.A.; Yang, E.Y.; Zhan, Y.; Judd, R.M.; Chan, W.; Nabi, F.; Heitner, J.F.; Kim, R.J.; Klem, I.; Nagueh, S.F.; et al. Association of Left Atrial Volume Index and All-Cause Mortality in Patients Referred for Routine Cardiovascular Magnetic Resonance: A Multicenter Study. J. Cardiovasc. Magn. Reson. 2019, 21, 4. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiograp. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Khanji, M.Y.; Plein, S.; Lancellotti, P.; Bucciarelli-Ducci, C. European Association of Cardiovascular Imaging Expert Consensus Paper: A Comprehensive Review of Cardiovascular Magnetic Resonance Normal Values of Cardiac Chamber Size and Aortic Root in Adults and Recommendations for Grading Severity. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Andreini, D.; Mushtaq, S.; Conte, E.; Annoni, A.; Formenti, A.; Mancini, E.M.; et al. Multimodality Imaging of Left Atrium in Patients with Atrial Fibrillation. J. Cardiovasc. Comput. Tomogr. 2019, 13, 340–346. [Google Scholar] [CrossRef]
- Benito, E.M.; Carlosena-Remirez, A.; Guasch, E.; Prat-González, S.; Perea, R.J.; Figueras, R.; Borràs, R.; Andreu, D.; Arbelo, E.; Tolosana, J.M.; et al. Left Atrial Fibrosis Quantification by Late Gadolinium-Enhanced Magnetic Resonance: A New Method to Standardize the Thresholds for Reproducibility. Ep Eur. 2017, 19, 1272–1279. [Google Scholar] [CrossRef]
- Nakamori, S.; Ngo, L.H.; Tugal, D.; Manning, W.J.; Nezafat, R. Incremental Value of Left Atrial Geometric Remodeling in Predicting Late Atrial Fibrillation Recurrence After Pulmonary Vein Isolation: A Cardiovascular Magnetic Resonance Study. J. Am. Heart Assoc. 2018, 7, e009793. [Google Scholar] [CrossRef]
- den Uijl, D.W.; Cabanelas, N.; Benito, E.M.; Figueras, R.; Alarcón, F.; Borràs, R.; Prat, S.; Guasch, E.; Perea, R.; Sitges, M.; et al. Impact of Left Atrial Volume, Sphericity, and Fibrosis on the Outcome of Catheter Ablation for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 740–746. [Google Scholar] [CrossRef]
- Simon, J.; El Mahdiui, M.; Smit, J.M.; Száraz, L.; van Rosendael, A.R.; Herczeg, S.; Zsarnóczay, E.; Nagy, A.I.; Kolossváry, M.; Szilveszter, B.; et al. Left Atrial Appendage Size Is a Marker of Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation in Patients with Persistent Atrial Fibrillation. Clin. Cardiol. 2022, 45, 273–281. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Cardiovascular Magnetic Resonance in the Guidelines of the European Society of Cardiology: A Comprehensive Summary and Update. J. Cardiovasc. Magn. Reson. 2023, 25, 42. [Google Scholar] [CrossRef]
- Feletti, F.; Mucci, V.; Aliverti, A. Chest Ultrasonography in Modern Day Extreme Settings: From Military Setting and Natural Disasters to Space Flights and Extreme Sports. Can. Respir. J. 2018, 2018, 8739704. [Google Scholar] [CrossRef]
- Cau, R.; Bassareo, P.; Suri, J.S.; Pontone, G.; Saba, L. The Emerging Role of Atrial Strain Assessed by Cardiac MRI in Different Cardiovascular Settings: An up-to-Date Review. Eur. Radiol. 2022, 32, 4384–4394. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, E.; Sjögren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med. Imaging. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; Dibella, E.V.R.; et al. Left Atrial Strain and Strain Rate in Patients with Paroxysmal and Persistent Atrial Fibrillation: Relationship to Left Atrial Structural Remodeling Detected by Delayed-Enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Csécs, I.; Yamaguchi, T.; Kheirkhahan, M.; Czimbalmos, C.; Fochler, F.; Kholmovski, E.G.; Morris, A.K.; Kaur, G.; Vago, H.; Merkely, B.; et al. Left Atrial Functional and Structural Changes Associated with Ablation of Atrial Fibrillation—Cardiac Magnetic Resonance Study. Int. J. Cardiol. 2020, 305, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Seo, J.H.; Choi, K.H.; Lee, S.H.; Choi, J.-O.; Jeon, E.-S.; Yang, J.H. Prognostic Implications of Left Atrial Stiffness Index in Heart Failure Patients with Preserved Ejection Fraction. Cardiovasc. Imaging 2023, 16, 435–445. [Google Scholar] [CrossRef]
- Khurram, I.M.; Maqbool, F.; Berger, R.D.; Marine, J.E.; Spragg, D.D.; Ashikaga, H.; Zipunnikov, V.; Kass, D.A.; Calkins, H.; Nazarian, S.; et al. Association Between Left Atrial Stiffness Index and Atrial Fibrillation Recurrence in Patients Undergoing Left Atrial Ablation. Circ. Arrhythmia Electrophysiol. 2016, 9, e003163. [Google Scholar] [CrossRef]
- Correia, E.T.d.O.; Barbetta, L.M.D.S.; Silva, O.M.P.d.; Mesquita, E.T. Left Atrial Stiffness: A Predictor of Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation—A Systematic Review and Meta-Analysis. Arq. Bras. Cardiol. 2019, 112, 501–508. [Google Scholar] [CrossRef]
- Rabbat, M.G.; Kwong, R.Y.; Heitner, J.F.; Young, A.A.; Shanbhag, S.M.; Petersen, S.E.; Selvanayagam, J.B.; Berry, C.; Nagel, E.; Heydari, B.; et al. The Future of Cardiac Magnetic Resonance Clinical Trials. Cardiovasc. Imaging 2022, 15, 2127–2138. [Google Scholar] [CrossRef]
- Curta, A.; Fichtner, S.; Wakili, R.; Estner, H.; Kramer, H. Prospective Evaluation of Left Atrial Function and Late Gadolinium Enhancement with 3 T MRI in Patients with Atrial Fibrillation before and after Catheter Ablation. Int. J. Cardiovasc. Imaging 2019, 35, 499–504. [Google Scholar] [CrossRef]
- Kanagala, P.; Arnold, J.R.; Cheng, A.S.H.; Singh, A.; Khan, J.N.; Gulsin, G.S.; Yang, J.; Zhao, L.; Gupta, P.; Squire, I.B.; et al. Left Atrial Ejection Fraction and Outcomes in Heart Failure with Preserved Ejection Fraction. Int. J. Cardiovasc. Imaging 2020, 36, 101–110. [Google Scholar] [CrossRef]
- Spartera, M.; Pessoa-Amorim, G.; Stracquadanio, A.; Von Ende, A.; Fletcher, A.; Manley, P.; Neubauer, S.; Ferreira, V.M.; Casadei, B.; Hess, A.T.; et al. Left Atrial 4D Flow Cardiovascular Magnetic Resonance: A Reproducibility Study in Sinus Rhythm and Atrial Fibrillation. J. Cardiovasc. Magn. Reson. 2021, 23, 29. [Google Scholar] [CrossRef]
- Demirkiran, A.; Amier, R.P.; Hofman, M.B.M.; van der Geest, R.J.; Robbers, L.F.H.J.; Hopman, L.H.G.A.; Mulder, M.J.; van de Ven, P.; Allaart, C.P.; van Rossum, A.C.; et al. Altered Left Atrial 4D Flow Characteristics in Patients with Paroxysmal Atrial Fibrillation in the Absence of Apparent Remodeling. Sci. Rep. 2021, 11, 5965. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.; Akoum, N.; Patel, A.; Kholmovski, E.; Revelo, P.; Damal, K.; Wilson, B.; Cates, J.; Harrison, A.; Ranjan, R.; et al. Atrial Fibrillation Ablation Outcome Is Predicted by Left Atrial Remodeling on MRI. Circ. Arrhythmia Electrophysiol. 2014, 7, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Badger, T.J.; Kholmovski, E.G.; Akoum, N.; Burgon, N.S.; Fish, E.N.; Blauer, J.J.E.; Rao, S.N.; DiBella, E.V.R.; Segerson, N.M.; et al. Detection and Quantification of Left Atrial Structural Remodeling with Delayed-Enhancement Magnetic Resonance Imaging in Patients with Atrial Fibrillation. Circulation 2009, 119, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, K.; Franke, K.B.; Foo, F.S.; Stiles, M.K. Clinical Utility of Cardiac Magnetic Resonance Imaging to Assess the Left Atrium before Catheter Ablation for Atrial Fibrillation—A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2021, 339, 192–202. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Chelu, M.G.; King, J.B.; Kholmovski, E.G.; Ma, J.; Gal, P.; Marashly, Q.; AlJuaid, M.A.; Kaur, G.; Silver, M.A.; Johnson, K.A.; et al. Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging and Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up Data. J. Am. Heart Assoc. 2018, 7, e006313. [Google Scholar] [CrossRef]
- Gal, P.; Marrouche, N.F. Magnetic Resonance Imaging of Atrial Fibrosis: Redefining Atrial Fibrillation to a Syndrome. Eur. Heart J. 2017, 38, 14–19. [Google Scholar] [CrossRef]
- Al-Rawahi, M.; Proietti, R.; Thanassoulis, G. Pericardial Fat and Atrial Fibrillation: Epidemiology, Mechanisms and Interventions. Int. J. Cardiol. 2015, 195, 98–103. [Google Scholar] [CrossRef]
- Wong, C.X.; Abed, H.S.; Molaee, P.; Nelson, A.J.; Brooks, A.G.; Sharma, G.; Leong, D.P.; Lau, D.H.; Middeldorp, M.E.; Roberts-Thomson, K.C.; et al. Pericardial Fat Is Associated with Atrial Fibrillation Severity and Ablation Outcome. J. Am. Coll. Cardiol. 2011, 57, 1745–1751. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, J.; Park, J.-K.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Pak, H.-N. Pericardial Fat Volume Is Associated with Clinical Recurrence after Catheter Ablation for Persistent Atrial Fibrillation, but Not Paroxysmal Atrial Fibrillation: An Analysis of over 600-Patients. Int. J. Cardiol. 2014, 176, 841–846. [Google Scholar] [CrossRef]
- Malagù, M.; Tonet, E.; Orazio, G.; Longo, F.; De Raffele, M.; Sirugo, P.; Capanni, A.; Clò, S.; Berloni, M.L.; Marchini, F.; et al. Association between Epicardial Adipose Tissue and Atrial Fibrillation in Patients with Transfusion-Dependent β-Thalassemia. J. Clin. Med. 2024, 13, 3471. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Rizzi, P.; Mewton, N.; Volpe, G.J.; Murthy, S.; Strauss, D.G.; Liu, C.Y.; Marchlinski, F.E.; Spooner, P.; Berger, R.D.; et al. Infiltrated Atrial Fat Characterizes Underlying Atrial Fibrillation Substrate in Patients at Risk as Defined by the ARIC Atrial Fibrillation Risk Score. Int. J. Cardiol. 2014, 172, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Armstrong, A.C.; Liu, C.-Y.; Donekal, S.; Yoneyama, K.; Wu, C.O.; Gomes, A.S.; Hundley, G.W.; Bluemke, D.A.; Lima, J.A. Diastolic Function Assessed from Tagged MRI Predicts Heart Failure and Atrial Fibrillation over an 8-Year Follow-up Period: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, G.C.; Duarte, R.; Corral de la Calle, M.; Calatayud, J.; Sánchez González, J. Analysis of left ventricular diastolic function using magnetic resonance imaging. Radiologia 2012, 54, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.; Fernandez, G. Assessment of Left Ventricular Diastolic Function by MR: Why, How and When. Insights Imaging 2010, 1, 183–192. [Google Scholar] [CrossRef][Green Version]
- Kawaji, K.; Codella, N.C.F.; Prince, M.R.; Chu, C.W.; Shakoor, A.; LaBounty, T.M.; Min, J.K.; Swaminathan, R.V.; Devereux, R.B.; Wang, Y.; et al. Automated Segmentation of Routine Clinical Cardiac Magnetic Resonance Imaging for Assessment of Left Ventricular Diastolic Dysfunction. Circ. Cardiovasc. Imaging 2009, 2, 476–484. [Google Scholar] [CrossRef]
- Wu, V.; Chyou, J.Y.; Chung, S.; Bhagavatula, S.; Axel, L. Evaluation of Diastolic Function by Three-Dimensional Volume Tracking of the Mitral Annulus with Cardiovascular Magnetic Resonance: Comparison with Tissue Doppler Imaging. J. Cardiovasc. Magn. Reson. 2014, 16, 71. [Google Scholar] [CrossRef]
- Garg, P.; Gosling, R.; Swoboda, P.; Jones, R.; Rothman, A.; Wild, J.M.; Kiely, D.G.; Condliffe, R.; Alabed, S.; Swift, A.J. Cardiac Magnetic Resonance Identifies Raised Left Ventricular Filling Pressure: Prognostic Implications. Eur. Heart J. 2022, 43, 2511–2522. [Google Scholar] [CrossRef]
- Mascherbauer, J.; Zotter-Tufaro, C.; Duca, F.; Binder, C.; Koschutnik, M.; Kammerlander, A.A.; Aschauer, S.; Bonderman, D. Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure Predicts Outcome in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2017, 5, 795–801. [Google Scholar] [CrossRef]
- De Jong, A.M.; Van Gelder, I.C.; Vreeswijk-Baudoin, I.; Cannon, M.V.; Van Gilst, W.H.; Maass, A.H. Atrial Remodeling Is Directly Related to End-Diastolic Left Ventricular Pressure in a Mouse Model of Ventricular Pressure Overload. PLoS ONE 2013, 8, e72651. [Google Scholar] [CrossRef]
- Krezowski, J.T.; Wilson, B.D.; McGann, C.J.; Marrouche, N.F.; Akoum, N. Changes in Left Ventricular Filling Parameters Following Catheter Ablation of Atrial Fibrillation. J. Interv. Card. Electrophysiol. 2016, 47, 83–89. [Google Scholar] [CrossRef]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M.; Matta, M.; D’Ascenzo, F.; Bunch, T.J.; Schilling, R.J.; Hunter, R.J.; Pappone, C.; Neumann, T.; Noelker, G.; Fiala, M.; et al. Catheter Ablation of Atrial Fibrillation in Patients with Left Ventricular Systolic Dysfunction: A Systematic Review and Meta-Analysis. Circ. Arrhythmia Electrophysiol. 2014, 7, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Suksaranjit, P.; McGann, C.J.; Akoum, N.; Biskupiak, J.; Stoddard, G.J.; Kholmovski, E.G.; Navaravong, L.; Rassa, A.; Bieging, E.; Chang, L.; et al. Prognostic Implications of Left Ventricular Scar Determined by Late Gadolinium Enhanced Cardiac Magnetic Resonance in Patients with Atrial Fibrillation. Am. J. Cardiol. 2016, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Nance, J.W.J.; Khurram, I.M.; Nazarian, S.; DeWire, J.; Calkins, H.; Zimmerman, S.L. Prevalence, Patterns, and Clinical Predictors of Left Ventricular Late Gadolinium Enhancement in Patients Undergoing Cardiac Magnetic Resonance Prior to Pulmonary Vein Antral Isolation for Atrial Fibrillation: A Cross-Sectional Observational Study. Medicine 2015, 94, e1384. [Google Scholar] [CrossRef]
- Addison, D.; Farhad, H.; Shah, R.V.; Mayrhofer, T.; Abbasi, S.A.; John, R.M.; Michaud, G.F.; Jerosch-Herold, M.; Hoffmann, U.; Stevenson, W.G.; et al. Effect of Late Gadolinium Enhancement on the Recovery of Left Ventricular Systolic Function After Pulmonary Vein Isolation. J. Am. Heart Assoc. 2016, 5, e003570. [Google Scholar] [CrossRef]
- Serban, T.; Mannhart, D.; Abid, Q.; Höchli, A.; Lazar, S.; Krisai, P.; Bettelini, A.S.; Knecht, S.; Kühne, M.; Sticherling, C.; et al. Durability of Pulmonary Vein Isolation for Atrial Fibrillation: A Meta-Analysis and Systematic Review. EP Eur. 2023, 25, euad335. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Angileri, S.A.; Pellegrino, F.; Renzulli, M.; Golfieri, R.; Zhang, D.; Sun, H.; Giganti, M.; Dionigi, G.; et al. Outcomes Following Minimally Invasive Imagine-Guided Percutaneous Ablation of Adrenal Glands. Gland Surg. 2020, 9, 859–866. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Pellegrino, F.; Stefano, G.D.; Bonelli, C.; Renzulli, M.; Giganti, M.; Carrafiello, G. Uterine Myomas: Extravascular Treatment. Semin. Ultrasound CT MRI 2021, 42, 56–74. [Google Scholar] [CrossRef]
- De Greef, Y.; Ströker, E.; Schwagten, B.; Kupics, K.; De Cocker, J.; Chierchia, G.-B.; de Asmundis, C.; Stockman, D.; Buysschaert, I. Complications of Pulmonary Vein Isolation in Atrial Fibrillation: Predictors and Comparison between Four Different Ablation Techniques: Results from the MIddelheim PVI-Registry. Europace 2018, 20, 1279–1286. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.-A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.-H.; Klein, G.; Packer, D.; Skanes, A. Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circulation 2005, 111, 1100–1105. [Google Scholar] [CrossRef]
- Narayan, S.M.; Baykaner, T. Electroporation: The End of the Thermal Ablation Era? J. Am. Coll. Cardiol. 2019, 74, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Hassani, C.; Saremi, F. Comprehensive Cross-Sectional Imaging of the Pulmonary Veins. Radiographics 2017, 37, 1928–1954. [Google Scholar] [CrossRef] [PubMed]
- Marom, E.M.; Herndon, J.E.; Kim, Y.H.; McAdams, H.P. Variations in Pulmonary Venous Drainage to the Left Atrium: Implications for Radiofrequency Ablation. Radiology 2004, 230, 824–829. [Google Scholar] [CrossRef]
- Tonet, E.; Boccadoro, A.; Micillo, M.; Cocco, M.; Cossu, A.; Pompei, G.; Giganti, M.; Campo, G. Coronary Computed Tomography Angiography: Beyond Obstructive Coronary Artery Disease. Life 2023, 13, 1086. [Google Scholar] [CrossRef]
- Schonberger, M.; Usman, A.; Galizia, M.; Popescu, A.; Collins, J.; Carr, J.C. Time-Resolved MR Venography of the Pulmonary Veins Precatheter-Based Ablation for Atrial Fibrillation. J. Magn. Reson. Imaging 2013, 37, 127–137. [Google Scholar] [CrossRef]
- Potthast, S.; Mitsumori, L.; Stanescu, L.A.; Richardson, M.L.; Branch, K.; Dubinsky, T.J.; Maki, J.H. Measuring Aortic Diameter with Different MR Techniques: Comparison of Three-Dimensional (3D) Navigated Steady-State Free-Precession (SSFP), 3D Contrast-Enhanced Magnetic Resonance Angiography (CE-MRA), 2D T2 Black Blood, and 2D Cine SSFP. J. Magn. Reson. Imaging 2010, 31, 177–184. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Gruettner, H.; Trauzeddel, R.F.; Greiser, A.; Schulz-Menger, J. Comparison of Native High-Resolution 3D and Contrast-Enhanced MR Angiography for Assessing the Thoracic Aorta. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 651–658. [Google Scholar] [CrossRef]
- Diab, M.; Wazni, O.M.; Saliba, W.I.; Tarakji, K.G.; Ballout, J.A.; Hutt, E.; Rickard, J.; Baranowski, B.; Tchou, P.; Bhargava, M.; et al. Ablation of Atrial Fibrillation Without Left Atrial Appendage Imaging in Patients Treated with Direct Oral Anticoagulants. Circ. Arrhythmia Electrophysiol. 2020, 13, e008301. [Google Scholar] [CrossRef]
- Puwanant, S.; Varr, B.C.; Shrestha, K.; Hussain, S.K.; Tang, W.H.W.; Gabriel, R.S.; Wazni, O.M.; Bhargava, M.; Saliba, W.I.; Thomas, J.D.; et al. Role of the CHADS2Score in the Evaluation of Thromboembolic Risk in Patients with Atrial Fibrillation Undergoing Transesophageal Echocardiography Before Pulmonary Vein Isolation. J. Am. Coll. Cardiol. 2009, 54, 2032–2039. [Google Scholar] [CrossRef]
- Romero, J.; Husain, S.A.; Kelesidis, I.; Sanz, J.; Medina, H.M.; Garcia, M.J. Detection of Left Atrial Appendage Thrombus by Cardiac Computed Tomography in Patients with Atrial Fibrillation. Circ. Cardiovasc. Imaging 2013, 6, 185–194. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Rathi, V.K.; Reddy, S.T.; Anreddy, S.; Belden, W.; Yamrozik, J.A.; Williams, R.B.; Doyle, M.; Thompson, D.V.; Biederman, R.W.W. Contrast-Enhanced CMR Is Equally Effective as TEE in the Evaluation of Left Atrial Appendage Thrombus in Patients with Atrial Fibrillation Undergoing Pulmonary Vein Isolation Procedure. Heart Rhythm 2013, 10, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kitkungvan, D.; Nabi, F.; Ghosn, M.G.; Dave, A.S.; Quinones, M.; Zoghbi, W.A.; Valderrabano, M.; Shah, D.J. Detection of LA and LAA Thrombus by CMR in Patients Referred for Pulmonary Vein Isolation. Cardiovasc. Imaging 2016, 9, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Marashly, Q.; Gopinath, C.; Baher, A.; Acharya, M.; Kheirkhahan, M.; Hardisty, B.; Aljuaid, M.; Tawhari, I.; Ibrahim, M.; Morris, A.K.; et al. Late Gadolinium Enhancement Magnetic Resonance Imaging Evaluation of Post-Atrial Fibrillation Ablation Esophageal Thermal Injury Across the Spectrum of Severity. J. Am. Heart Assoc. 2021, 10, e018924. [Google Scholar] [CrossRef]
- Cossu, A.; Martin Rother, M.D.; Kusmirek, J.E.; Meyer, C.A.; Kanne, J.P. Imaging Early Postoperative Complications of Cardiothoracic Surgery. Radiol. Clin. N. Am. 2020, 58, 133–150. [Google Scholar] [CrossRef]
- Bourier, F.; Vukajlovic, D.; Brost, A.; Hornegger, J.; Strobel, N.; Kurzidim, K. Pulmonary Vein Isolation Supported by MRI-Derived 3D-Augmented Biplane Fluoroscopy: A Feasibility Study and a Quantitative Analysis of the Accuracy of the Technique. J. Cardiovasc. Electrophysiol. 2013, 24, 113–120. [Google Scholar] [CrossRef]
- Quinto, L.; Cozzari, J.; Benito, E.; Alarcón, F.; Bisbal, F.; Trotta, O.; Caixal, G.; San Antonio, R.; Garre, P.; Prat-Gonzalez, S.; et al. Magnetic Resonance-Guided Re-Ablation for Atrial Fibrillation Is Associated with a Lower Recurrence Rate: A Case-Control Study. Ep Eur. 2020, 22, 1805–1811. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Del Giudice, C.; Coppola, A.; Carnevale, A.; Giganti, M.; Renzulli, M.; Tacher, V.; Urbano, J.; Kobeiter, H.; Loffroy, R.; et al. Gastrointestinal Hemorrhages in Patients with COVID-19 Managed with Transarterial Embolization. Am. J. Gastroenterol. 2021, 116, 838–840. [Google Scholar] [CrossRef]
- Rogers, T.; Campbell-Washburn, A.E.; Ramasawmy, R.; Yildirim, D.K.; Bruce, C.G.; Grant, L.P.; Stine, A.M.; Kolandaivelu, A.; Herzka, D.A.; Ratnayaka, K.; et al. Interventional Cardiovascular Magnetic Resonance: State-of-the-Art. J. Cardiovasc. Magn. Reson. 2023, 25, 48. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wazni, O.; McGann, C.; Greene, T.; Dean, J.M.; Dagher, L.; Kholmovski, E.; Mansour, M.; Marchlinski, F.; Wilber, D.; et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients with Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022, 327, 2296. [Google Scholar] [CrossRef]
- Veeram Reddy, S.R.; Arar, Y.; Zahr, R.A.; Gooty, V.; Hernandez, J.; Potersnak, A.; Douglas, P.; Blair, Z.; Greer, J.S.; Roujol, S.; et al. Invasive Cardiovascular Magnetic Resonance (iCMR) for Diagnostic Right and Left Heart Catheterization Using an MR-Conditional Guidewire and Passive Visualization in Congenital Heart Disease. J. Cardiovasc. Magn. Reson. 2020, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Paetsch, I.; Sommer, P.; Jahnke, C.; Hilbert, S.; Loebe, S.; Schoene, K.; Oebel, S.; Krueger, S.; Weiss, S.; Smink, J.; et al. Clinical Workflow and Applicability of Electrophysiological Cardiovascular Magnetic Resonance-Guided Radiofrequency Ablation of Isthmus-Dependent Atrial Flutter. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Guttman, M.A.; Fink, S.; Elahi, H.; Patil, K.D.; Ashikaga, H.; Kolandaivelu, A.D.; Berger, R.D.; Halushka, M.K.; Schmidt, E.J.; et al. Ablation Lesion Characterization in Scarred Substrate Assessed Using Cardiac Magnetic Resonance. JACC Clin. Electrophysiol. 2019, 5, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Toupin, S.; Bour, P.; Lepetit-Coiffé, M.; Ozenne, V.; Denis de Senneville, B.; Schneider, R.; Vaussy, A.; Chaumeil, A.; Cochet, H.; Sacher, F.; et al. Feasibility of Real-Time MR Thermal Dose Mapping for Predicting Radiofrequency Ablation Outcome in the Myocardium in vivo. J. Cardiovasc. Magn. Reson. 2017, 19, 14. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegoraro, N.; Chiarello, S.; Bisi, R.; Muscogiuri, G.; Bertini, M.; Carnevale, A.; Giganti, M.; Cossu, A. Cardiac Magnetic Resonance in the Assessment of Atrial Cardiomyopathy and Pulmonary Vein Isolation Planning for Atrial Fibrillation. J. Imaging 2025, 11, 143. https://doi.org/10.3390/jimaging11050143
Pegoraro N, Chiarello S, Bisi R, Muscogiuri G, Bertini M, Carnevale A, Giganti M, Cossu A. Cardiac Magnetic Resonance in the Assessment of Atrial Cardiomyopathy and Pulmonary Vein Isolation Planning for Atrial Fibrillation. Journal of Imaging. 2025; 11(5):143. https://doi.org/10.3390/jimaging11050143
Chicago/Turabian StylePegoraro, Nicola, Serena Chiarello, Riccardo Bisi, Giuseppe Muscogiuri, Matteo Bertini, Aldo Carnevale, Melchiore Giganti, and Alberto Cossu. 2025. "Cardiac Magnetic Resonance in the Assessment of Atrial Cardiomyopathy and Pulmonary Vein Isolation Planning for Atrial Fibrillation" Journal of Imaging 11, no. 5: 143. https://doi.org/10.3390/jimaging11050143
APA StylePegoraro, N., Chiarello, S., Bisi, R., Muscogiuri, G., Bertini, M., Carnevale, A., Giganti, M., & Cossu, A. (2025). Cardiac Magnetic Resonance in the Assessment of Atrial Cardiomyopathy and Pulmonary Vein Isolation Planning for Atrial Fibrillation. Journal of Imaging, 11(5), 143. https://doi.org/10.3390/jimaging11050143








