Color Biomimetics in Textile Design: Reproduction of Natural Plant Colors through Instrumental Colorant Formulation
Abstract
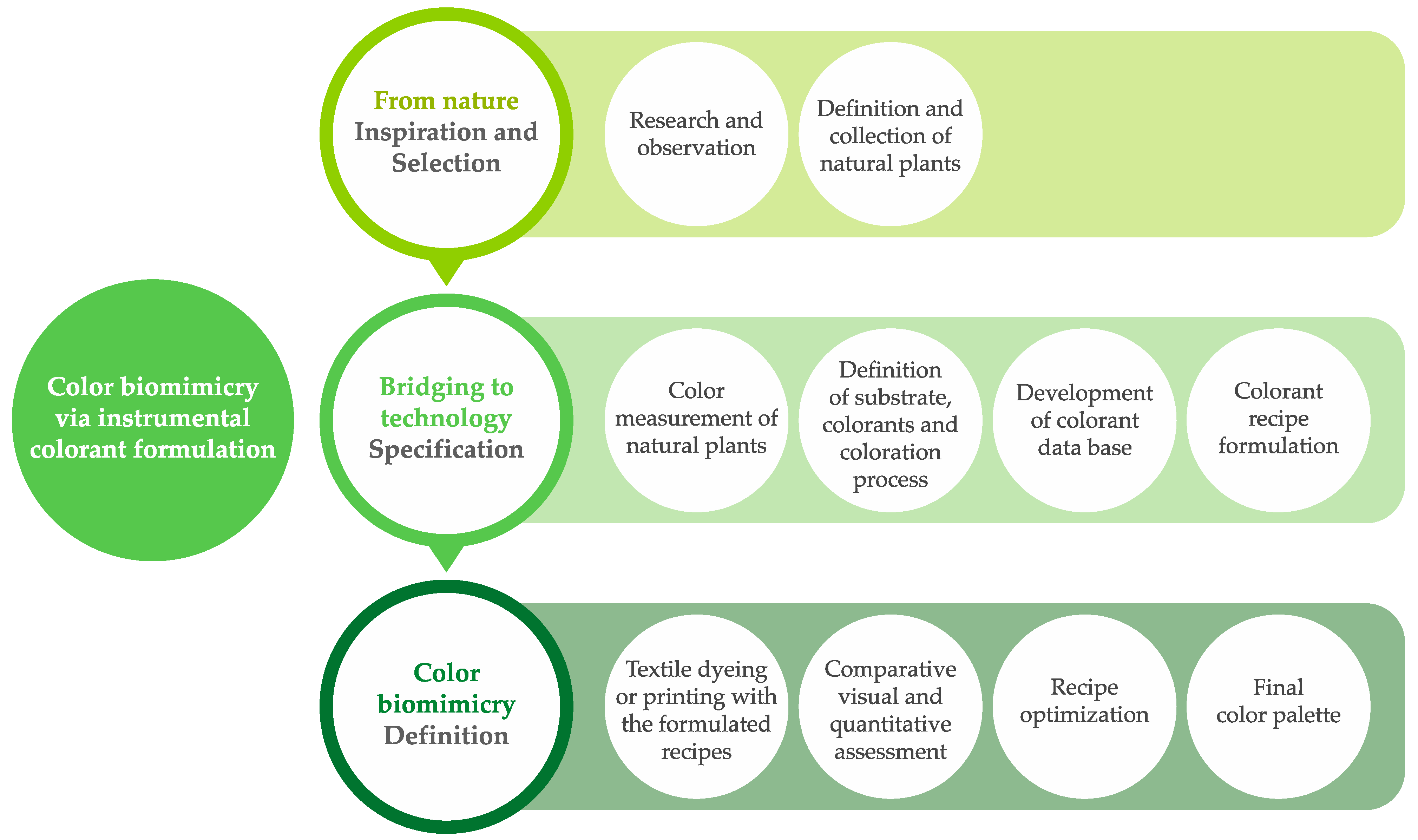
1. Introduction
2. Materials and Methods
2.1. Textile Dyeing
2.2. Spectral Reflectance
2.3. Instrumental Colorant Formulation
2.3.1. Spectrophotometric Calibration Data
2.3.2. Colorant Formulation Recipe
2.4. Qualitative and Quantitative Analysis
3. Results and Discussion
3.1. Textile Dyed Concentration Samples
3.1.1. Colorimetric Properties of the Concentration Samples
3.1.2. Color Strength (K/S)
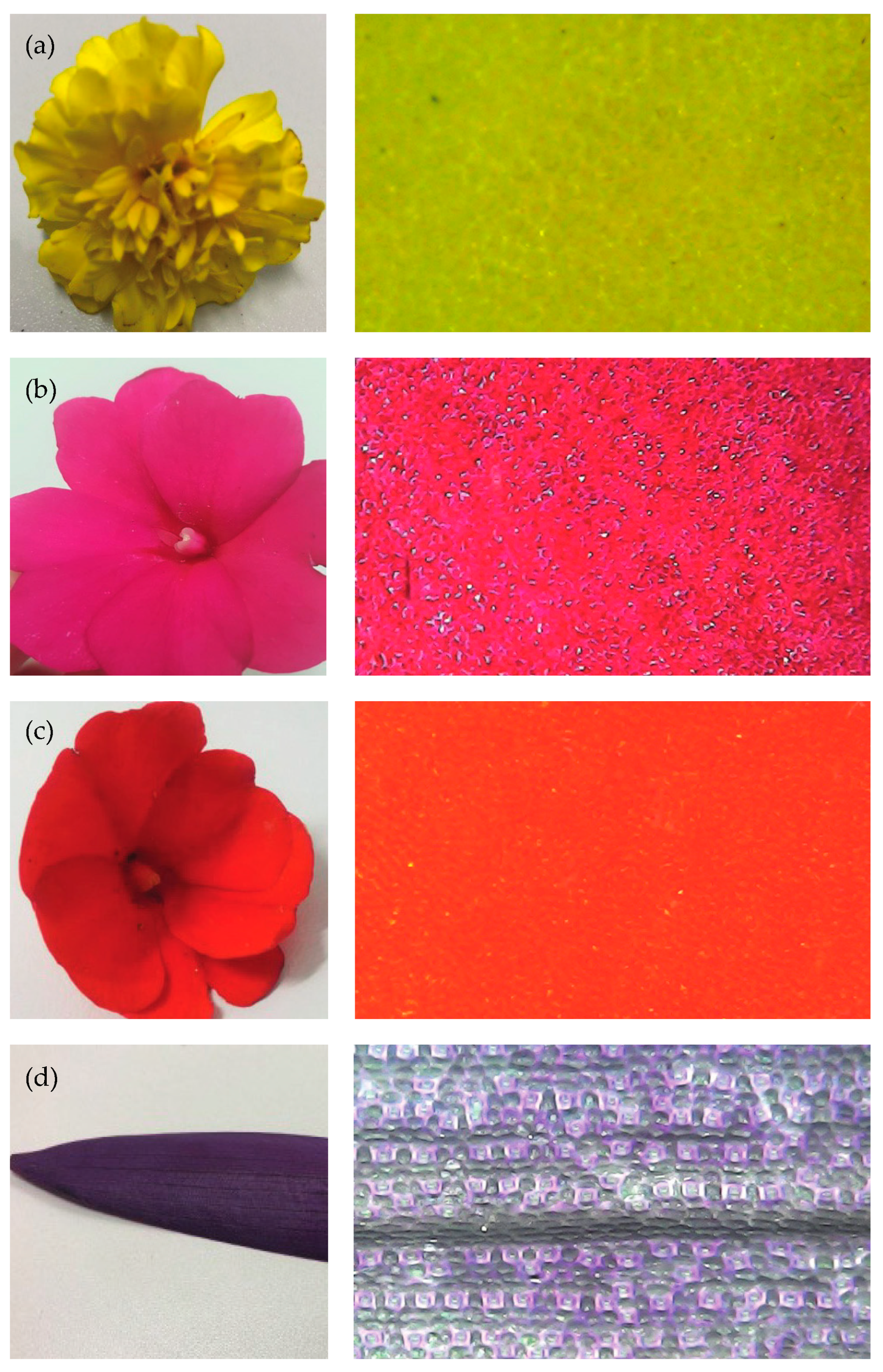
3.2. Biomimicry Color of Selected Flower Petals and Leaves
3.2.1. Instrumental Color Recipe Formulation
3.2.2. Visual Analysis
3.2.3. Spectral Reflectance Analysis
3.3. Scanning Electron Microscope and Optical Microscopy Analysis
3.4. White Light Interferometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, Y.H.; Reich, Y. The Biomimicry Discipline: Boundaries, Definitions, Drivers, PromisesandLimits. In Biomimetic Design Method for Innovation and Sustainability; Cohen, Y.H., Reich, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Salifu, A.A.; Mannoor, M.S.; Soboyejo, W. Bioinspired and Biomimetic Design of Multilayered and Multiscale Structures. In Bioinspired Structures and Design; Soboyejo, W.O., Daniel, L., Eds.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Drack, M.; Jansen, L. Biomimetics Analyzed: Examples from an Epistemological and Ontological Perspective. In Biomimetic and Biohybrid Systems; Springer: Cham, Switzerland, 2023; pp. 273–289. [Google Scholar]
- Ilieva, L.; Ursano, I.; Traista, L.; Hoffmann, B.; Dahy, H. Biomimicry as a Sustainable Design Methodology—Introducing the ‘Biomimicry for Sustainability’ Framework. Biomimetics 2022, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Kapsali, V. Biomimetics for Designers: Applying Nature’s Processes and Materials in the Real World; Thames & Hudson: London, UK, 2021. [Google Scholar]
- Basak, S.; Samanta, K.K.; Chattopadhyay, S.K.; Pandit, P. Sustainable Coloration and Value Addition to Textiles. In Handbook of Renewable Materials for Coloration and Finishing; Scrivener Publishing: Beverly, MA, USA, 2018; pp. 521–547. [Google Scholar]
- Das, S.K.; Bhowmick, M.; Chattopadhyay, S.K.; Basak, S. Application of biomimicry in textiles. Curr. Sci. 2015, 109, 893–901. [Google Scholar] [CrossRef]
- Krivenko, O.; Pylypchuk, O.; Avdieieva, N.; Avdieieva, M.; Bieber, S. Biomimetic approaches to color formation in ecological architecture. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1150, 012007. [Google Scholar] [CrossRef]
- Primrose, S.B. Biomimetics: Nature-Inspired Design and Innovation; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Moury, N.N.; Islam, M.T. Structural Coloration in Textiles. In Advanced Technology in Textiles: Fibre to Apparel; Rahman, M.M., Mashud, M., Rahman, M.M., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 257–278. [Google Scholar]
- Chatterjee, A. At the Intersection of Natural Structural Coloration and Bioengineering. Biomimetics 2022, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liu, G.; Zhou, L. Biomimetic nanocoatings for structural coloration of textiles. In Active Coatings for Smart Textiles; Hu, J., Ed.; Woodhead Publishing: Oxford, UK, 2016. [Google Scholar]
- Choudhury, A.K.R. Principles of Colour Appearance and Measurement Volume 1: Object Appearance, Colour Perception and Instrumental Measurement; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Tilley, R.J.D. Colour and Optical Properties of Materials: An Exploration of the Relationship between Light, the Optical Properties of Materials and Colour; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Fernandes, R.D.V.; Gomes, P.; Zille, A.; Souto, A.P. The influence of chemical reaction conditions upon poly(styrene-methyl methacrylate-acrylic acid) synthesis: Variations in nanoparticle size, colour and deposition methods. Color. Technol. 2019, 36, 101–109. [Google Scholar] [CrossRef]
- Yavuz, G.; Zille, A.; Seventekin, N.; Souto, A.P. Structural coloration of chitosan coated cellulose fabrics by electrostatic self-assembled poly(styrene-methyl methacrylate-acrylic acid) photonic crystals. Carbohydr. Polym. 2018, 193, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.D.V.; Pranovich, A.; Valyukh, S.; Zille, A.; Hallberg, T.; Järrendahl, K. Iridescence Mimicking in Fabrics: A Ultraviolet/Visible Spectroscopy Study. Biomimetics 2024, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Cabral, I.; Santiago, D.; Steffens, F. Chromic textiles: Colour fastness properties and irreversible colour change behaviour of textiles screen printed with thermochromic, photochromic and hydrochromic colourants. Color. Technol. 2023, 139, 200–208. [Google Scholar] [CrossRef]
- Cabral, I.D.; Souto, A.P.; Worbin, L. Experimental Work: Thermochromic Leuco Dyes. In Dynamic Light Filters: Smart Materials Applied to Textile Design; Cabral, I.D., Souto, A.P., Worbin, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 33–63. [Google Scholar]
- Gauche, H.; Oliveira, F.R.; Merlini, C.; Hiller, A.P.; Souto, A.P.G.V.; Cabral, I.D.; Steffens, F. Screen Printing of Cotton Fabric with Hydrochromic Paste: Evaluation of Color Uniformity, Reversibility and Fastness Properties. J. Nat. Fibers 2022, 19, 2694–2705. [Google Scholar] [CrossRef]
- Sakai, M.; Seki, T.; Takeoka, Y. Bioinspired Color Materials Combining Structural, Dye, and Background Colors. Small 2018, 14, 1800817. [Google Scholar] [CrossRef] [PubMed]
- Viková, M.; Pechová, M. Study of adaptive thermochromic camouflage for combat uniform. Text. Res. J. 2020, 90, 2070–2084. [Google Scholar] [CrossRef]
- Hossain, M.A. Adaptive Camouflage Textiles with Thermochromic Colorant and Liquid Crystal for Multidimensional Combat Background, a Technical Approach for Advancement in Defence Protection. Am. J. Mater. Eng. Technol. 2021, 9, 31–47. [Google Scholar] [CrossRef]
- Pimenta, C.; Pereira, C.C.; Fangueiro, R. Textile Pattern Design in Thermal Vision—A Study on Human Body Camouflage. Materials 2021, 14, 4364. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, F.; Luo, H.; Qiu, B. Review of recent advancements in the biomimicry of structural colors. Dye. Pigment. 2023, 210, 111019. [Google Scholar] [CrossRef]
- Militký, J.; Křemenáková, D. New Structures with Light Effects. In Fibrous Structures and Their Impact on Textile Design; Militký, J., Venkataraman, M., Periyasamy, A.P., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 149–186. [Google Scholar]
- Best, J. Colour specification and visual approval methods for textiles. In Colour Design: Theories and Applications, 2nd ed.; Best, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Steed, J.; Stevenson, F. Sourcing Ideas for Textile Design: Researching Colour, Surface, Structure, Texture and Pattern; Bloomsbury: London, UK, 2020. [Google Scholar]
- Bleicher, S. Contemporary Color: Theory and Use, 3rd ed.; Routledge: London, UK, 2023. [Google Scholar]
- King, J.A. Colour in fashion design. In Colour Design: Theories and Applications, 2nd ed.; Best, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Sherin, A. Design Elements Color Fundamentals; Rockport Publishers: Beverly, MA, USA, 2012. [Google Scholar]
- Hidefi, M. Understanding and forecasting colour trends in design. In Colour Design: Theories and Applications, 2nd ed.; Best, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology, 4th ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Setchell, J.S. Colour description and communication. In Colour Design: Theories and Applications, 2nd ed.; Best, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Cassidy, T. Color Knowledge. In Textile and Clothing Design Technology; Cassidy, T., Goswami, P., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 333–356. [Google Scholar]
- Goodman, T.M. International standards for colour. In Colour Design: Theories and Applications; Best, J., Ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Westland, S. Color communication. In Handbook of Visual Display Technology, 2nd ed.; Chen, J., Cranton, W., Fihn, M., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Choudhury, A.K.R. Principles of Colour and Appearance Measurement: Volume 2: Visual Measurement of Colour, Colour Comparison and Management; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Samanta, P. Basic Principles of Colour Measurement and Colour Matching of Textiles and Apparels. In Colorimetry; Samanta, A.K., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. Ch. 5. [Google Scholar]
- Gangakhedar, N. Colour measurement methods for textiles. In Colour Measurement: Principles, Advances and Industrial Applications, Gulrajani, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Wardman, R.H. An Introduction to Textile Coloration: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- AATCC. American Association of Textile Chemists and Colorists (AATCC) Technical Manual; AATCC: Durham, NC, USA, 2010. [Google Scholar]
- Schmit, J.; Pakuła, A. White Light Interferometry. In Handbook of Advanced Nondestructive Evaluation; Ida, N., Meyendorf, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 421–467. [Google Scholar]
- Chae, Y.; Moon, S. Color discrimination threshold of human vision for textiles under different illumination conditions. Text. Res. J. 2023, 93, 3158–3170. [Google Scholar] [CrossRef]





| Color | Concentration (%) | Substrate 1 (S1) | Substrate 2 (S1) | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| Yellow | 0.1 | 86.2 | −6.6 | 55.8 | 86.7 | −4.6 | 69.9 |
| 0.5 | 83.3 | −2.5 | 86.3 | 83.0 | 2.8 | 93.9 | |
| 1.0 | 81.4 | 0.8 | 95.0 | 80.8 | 6.4 | 100.1 | |
| 2.0 | 79.6 | 4.6 | 98.7 | 79.0 | 10.3 | 101.5 | |
| 3.0 | 75.4 | 9.3 | 95.3 | 74.6 | 17.4 | 96.9 | |
| 4.0 | 76.5 | 12.0 | 97.5 | 72.4 | 19.5 | 93.5 | |
| Blue 110% | 0.1 | 65.2 | 3.8 | −32.3 | 57.5 | 4.8 | −28.7 |
| 0.5 | 45.1 | 6.8 | −40.0 | 38.8 | 6.0 | −35.3 | |
| 1.0 | 35.2 | 9.7 | −41.1 | 30.9 | 8.8 | −36.1 | |
| 2.0 | 27.5 | 12.4 | −38.9 | 23.8 | 10.7 | −33.2 | |
| 3.0 | 23.6 | 13.4 | −36.1 | 20.2 | 11.1 | −29.6 | |
| 4.0 | 20.9 | 13.3 | −32.6 | 18.9 | 10.4 | −26.1 | |
| Blue 150% | 0.1 | 70.9 | −3.2 | −30.8 | 66.5 | −6.3 | −28.1 |
| 0.5 | 53.7 | −3.1 | −38.8 | 47.2 | −1.8 | −36.9 | |
| 1.0 | 44.6 | −1.0 | −40.9 | 39.0 | 0.7 | −38.5 | |
| 2.0 | 36.3 | 2.8 | −41.3 | 31.0 | 4.6 | −38.2 | |
| 3.0 | 31.5 | 5.9 | −40.8 | 26.9 | 7.0 | −36.8 | |
| 4.0 | 27.9 | 8.0 | −39.2 | 24.2 | 8.5 | −34.8 | |
| Yellowish brown | 0.1 | 77.5 | 12.7 | 25.1 | 75.4 | 18.9 | 36.2 |
| 0.5 | 65.0 | 24.6 | 43.5 | 61.2 | 29.7 | 46.9 | |
| 1.0 | 57.7 | 29.4 | 48.0 | 53.3 | 33.7 | 48.7 | |
| 2.0 | 50.3 | 33.4 | 48.1 | 45.2 | 35.5 | 45.3 | |
| 3.0 | 45.6 | 35.9 | 45.2 | 41.1 | 36.6 | 41.7 | |
| 4.0 | 42.5 | 35.8 | 41.5 | 38.5 | 36.7 | 37.8 | |
| Orange | 0.1 | 78.1 | 15.6 | 26.0 | 77.7 | 22.2 | 39.3 |
| 0.5 | 68.5 | 30.8 | 50.7 | 65.2 | 37.0 | 55.2 | |
| 1.0 | 62.2 | 38.5 | 58.3 | 61.2 | 44.2 | 62.6 | |
| 2.0 | 57.0 | 44.8 | 61.0 | 55.9 | 49.6 | 63.3 | |
| 3.0 | 55.1 | 48.8 | 61.8 | 52.4 | 51.7 | 60.6 | |
| 4.0 | 52.2 | 49.9 | 58.9 | 50.8 | 53.7 | 58.8 | |
| Marine | 0.1 | 62.6 | −0.9 | −23.6 | 56.1 | −2.2 | −19.7 |
| 0.5 | 39.4 | −1.0 | −25.7 | 34.9 | −0.8 | −22.0 | |
| 1.0 | 30.0 | 0.3 | −24.3 | 25.3 | 0.8 | −20.4 | |
| 2.0 | 22.4 | 2.3 | −19.8 | 19.2 | 2.1 | −15.8 | |
| 3.0 | 19.2 | 2.8 | −15.8 | 17.7 | 2.4 | −12.0 | |
| 4.0 | 17.8 | 2.8 | −12.7 | 16.1 | 2.3 | −8.6 | |
| Ruby | 0.1 | 62.8 | 38.6 | −8.5 | 59.3 | 43.8 | −1.7 |
| 0.5 | 46.2 | 50.9 | −0.2 | 42.3 | 52.0 | 4.3 | |
| 1.0 | 40.0 | 53.0 | 5.5 | 36.5 | 52.1 | 8.2 | |
| 2.0 | 34.1 | 50.9 | 10.2 | 31.1 | 48.8 | 12.2 | |
| 3.0 | 30.9 | 48.0 | 12.6 | 28.8 | 45.3 | 13.7 | |
| 4.0 | 28.9 | 44.4 | 12.6 | 26.9 | 42.4 | 14.1 | |
| Red | 0.1 | 64.0 | 44.8 | −11.1 | 60.7 | 49.5 | −4.3 |
| 0.5 | 48.6 | 59.2 | −1.4 | 45.7 | 60.3 | 3.7 | |
| 1.0 | 42.3 | 60.9 | 5.4 | 39.5 | 60.1 | 9.3 | |
| 2.0 | 37.3 | 58.7 | 11.8 | 34.7 | 56.5 | 14.7 | |
| 3.0 | 34.4 | 55.7 | 15.2 | 32.0 | 52.9 | 16.9 | |
| 4.0 | 33.2 | 52.7 | 16.2 | 30.7 | 50.3 | 17.7 | |
| Violet | 0.1 | 59.9 | 21.9 | −34.2 | 53.8 | 22.7 | −31.3 |
| 0.5 | 39.2 | 30.4 | −38.5 | 34.3 | 29.4 | −34.9 | |
| 1.0 | 30.3 | 32.1 | −37.2 | 26.8 | 29.4 | −32.6 | |
| 2.0 | 23.8 | 29.4 | −31.9 | 21.2 | 25.8 | −27.0 | |
| 3.0 | 21.2 | 26.2 | −27.5 | 19.5 | 21.7 | −22.1 | |
| 4.0 | 20.4 | 24.2 | −25.0 | 18.3 | 19.8 | −19.8 | |
| Dye Color | Pattern Color A: Yellow Marigold | Pattern Color B: Pink Impatiens | Pattern Color C: Orange Impatiens | Pattern Color D: Purple Trapoeraba | ||||
|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | |||||
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Yellow | 1.524 | 1.524 | 0.457 | 0.259 | 0.201 | 0.092 | ||
| Blue 110% | 0.591 | 0.424 | ||||||
| Blue 150% | ||||||||
| Yellowish brown | ||||||||
| Orange | ||||||||
| Marine | ||||||||
| Ruby | ||||||||
| Red | 1.463 | 0.984 | 0.832 | 0.610 | 0.355 | 0.238 | ||
| Violet | 0.013 | 0.008 | ||||||
| Predicted ΔE* | 2.4 | X | 0.6 | 0.9 | 0.1 | 0.0 | 0.0 | 0.0 |
| Natural Element | Sample | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| Yellow marigold | Pattern color A | 82.5 | 8.2 | 109.4 | _ |
| Sample A–S1 | 81.7 | 3.6 | 100.8 | 9.8 | |
| Sample A–S2 | 81.5 | 9.5 | 104.8 | 4.9 | |
| Pink impatiens | Pattern color B | 38.6 | 61.4 | 8.9 | _ |
| Sample B–S1 | 39.1 | 60.0 | 8.6 | 1.5 | |
| Sample B–S2 | 39.3 | 59.1 | 8.7 | 2.4 | |
| Orange impatiens | Pattern color C | 45.9 | 59.2 | 43.2 | _ |
| Sample C–S1 | 45.0 | 56.0 | 41.0 | 4.0 | |
| Sample C–S2 | 44.6 | 56.3 | 42.0 | 3.4 | |
| Purple trapoeraba | Pattern color D | 29.5 | 11.2 | −9.0 | _ |
| Sample D–S1 | 30.0 | 13.1 | −9.9 | 2.2 | |
| Sample D–S2 | 31.0 | 12.2 | −12.0 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, I.; Schuch, A.; Steffens, F. Color Biomimetics in Textile Design: Reproduction of Natural Plant Colors through Instrumental Colorant Formulation. J. Imaging 2024, 10, 150. https://doi.org/10.3390/jimaging10070150
Cabral I, Schuch A, Steffens F. Color Biomimetics in Textile Design: Reproduction of Natural Plant Colors through Instrumental Colorant Formulation. Journal of Imaging. 2024; 10(7):150. https://doi.org/10.3390/jimaging10070150
Chicago/Turabian StyleCabral, Isabel, Amanda Schuch, and Fernanda Steffens. 2024. "Color Biomimetics in Textile Design: Reproduction of Natural Plant Colors through Instrumental Colorant Formulation" Journal of Imaging 10, no. 7: 150. https://doi.org/10.3390/jimaging10070150
APA StyleCabral, I., Schuch, A., & Steffens, F. (2024). Color Biomimetics in Textile Design: Reproduction of Natural Plant Colors through Instrumental Colorant Formulation. Journal of Imaging, 10(7), 150. https://doi.org/10.3390/jimaging10070150





