Development of A Micro-CT Scanner with Dual-Energy Option and Endovascular Contrast Agent Administration Protocol for Fetal and Neonatal Virtual Autopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Scanner Design and Choice of Dimensions, Resolution, and Components
- The option of cooling the scanner chamber down to around 5 °C. This serves to preserve the specimen (reduce skin maceration and body deformation) and keep it stiffer to minimize motion artifacts during scans. This aspect is especially relevant for sequential scans in dual-energy CT;
- X-ray beam prefiltering (dual-energy CT);
- An isotropic voxel size between 30 and 150 μm should be sufficient to resolve vascular features that are relevant for diagnosis considering the limitations of current post-mortem fetal imaging for different relevant organs and organ groups if the vascular structures are contrasted well;
- Maximal specimen length 600 mm;
- Maximal specimen diameter 200 mm.
- 40–80 kVp, 100 W power X-ray tube with 150 µm focal spot;
- Complementary metal-oxide-semiconductor (CMOS) detector with columnar CsI converter, 5600 × 2400 pixel matrix, and 50 µm pixel size;
- Isotropic voxel size range of 35–210 µm;
- Resolution of ~70 µm;
- Automatic filter changer with 3 filter options;
- Sample chamber and specimen cooler;
- Whole-body stack scan option by combined movement of the gantry and the specimen holder over the maximal specimen length.
2.2. Development of Protocol for Endovascular Infusion of the Contrast Agent and Choice of Contrast Agent
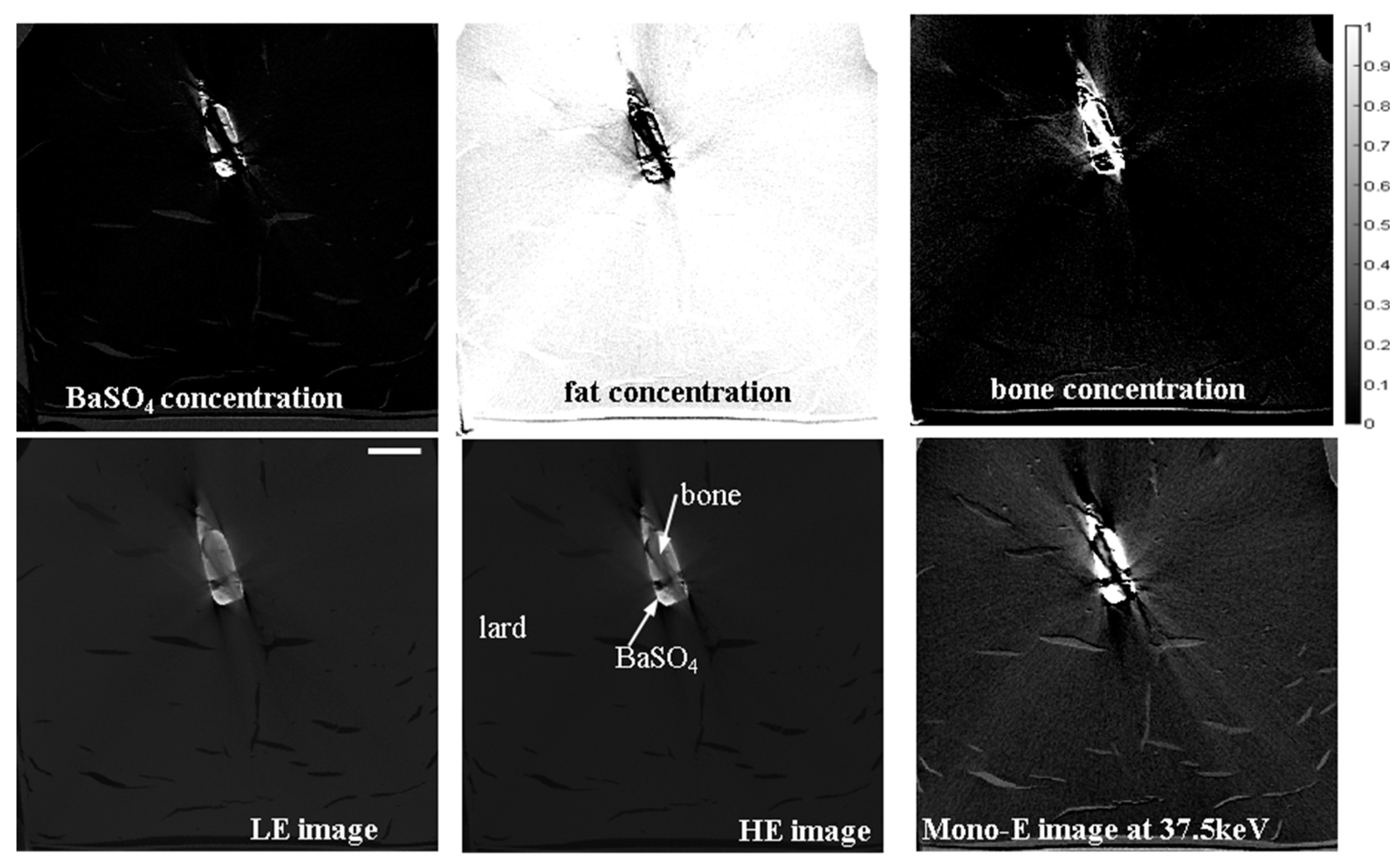
2.3. Optimization of the Dual-E CT Method for PMCTA Using BaSO4 Contrast Agent
2.4. Specimen
3. Results
3.1. Validation and Proof-of-Concept on a Phantom
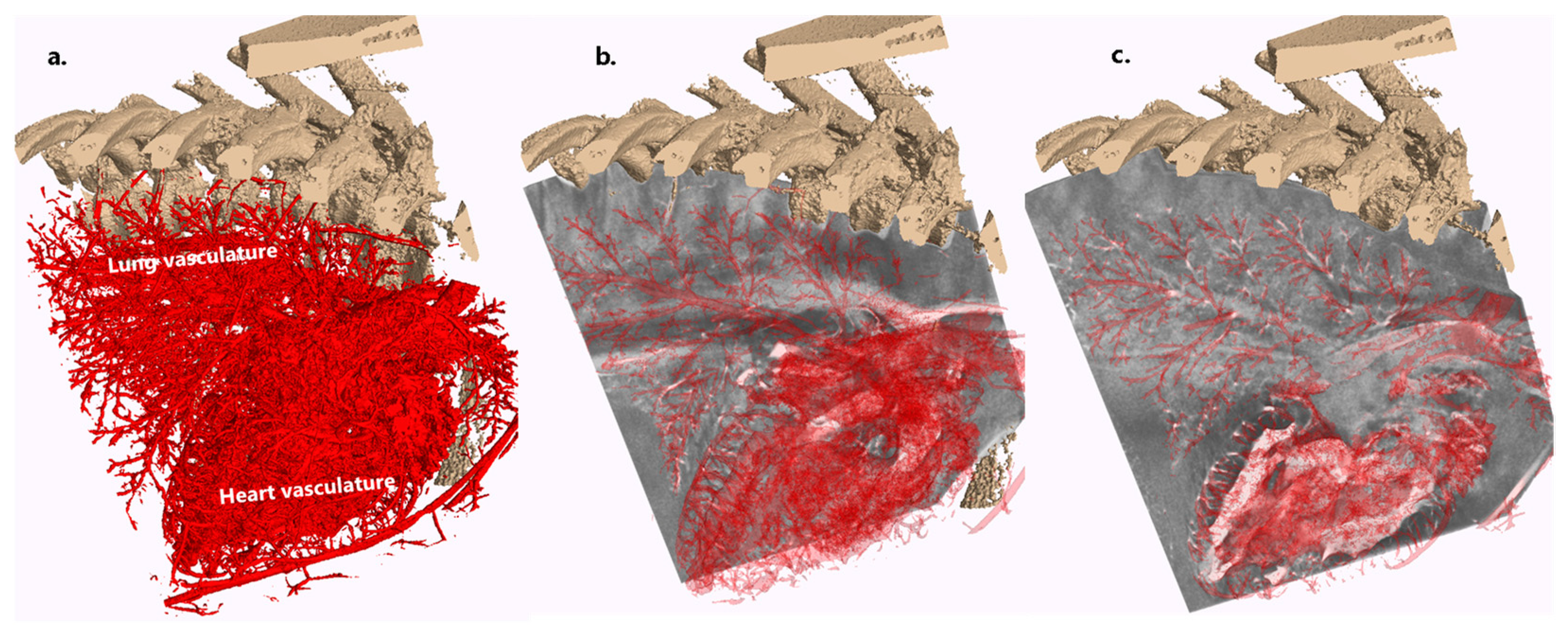
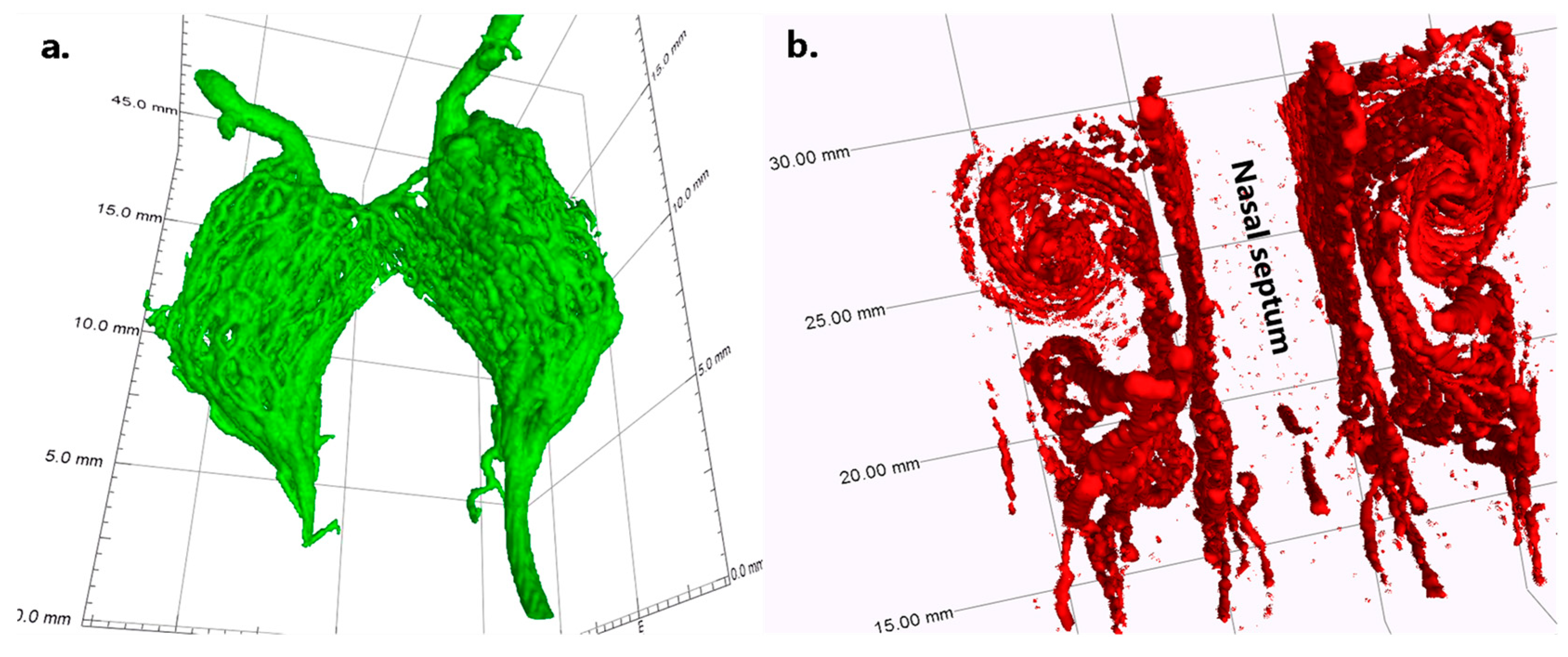
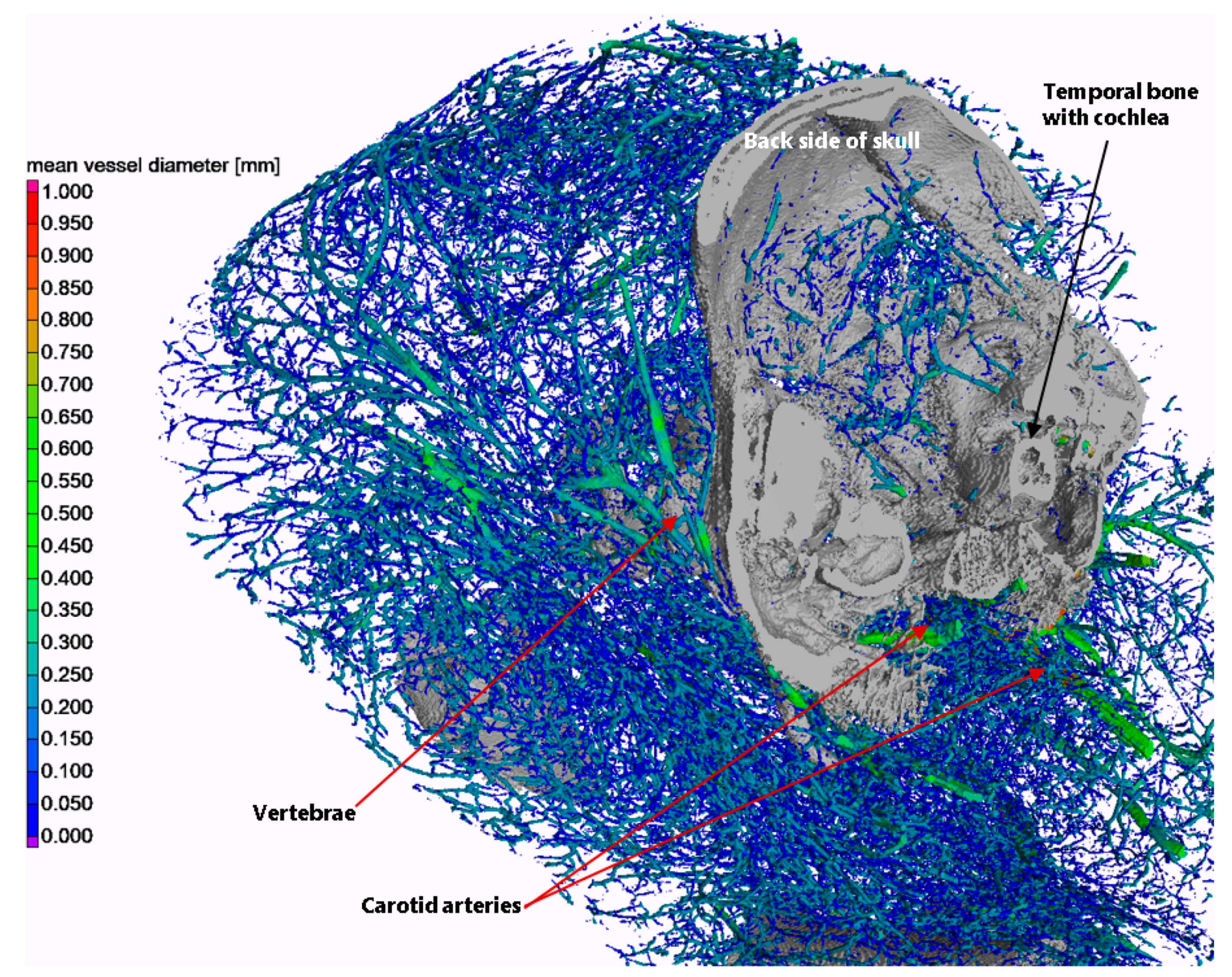
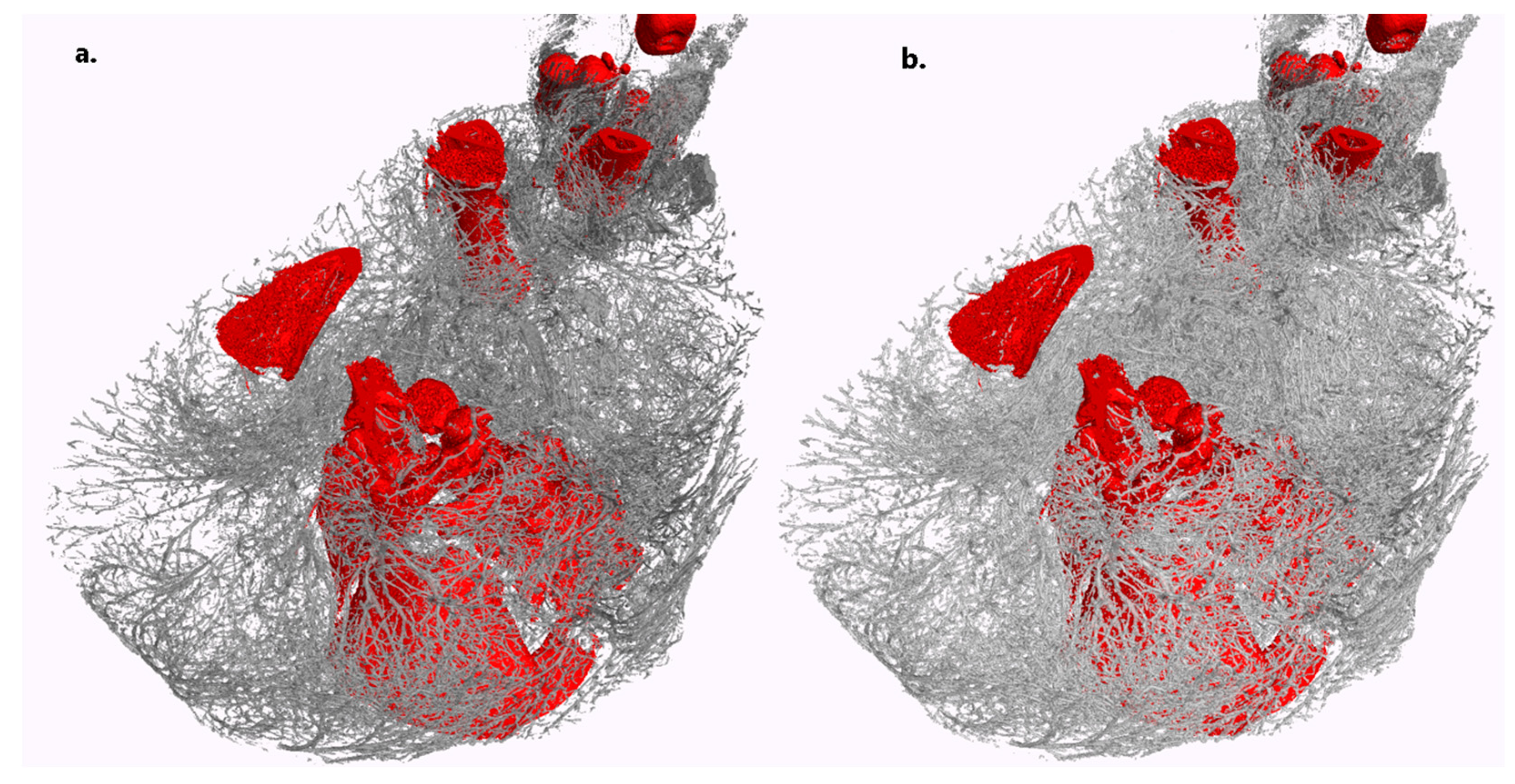
3.2. Validation and Proof-of-Concept on the Bodies of Piglets
- Threshold segmentation at a gray value of 10,000 (16-bit images), including only thick vessels above the G gray level of the temporal bones;
- Seeded region growing with a local dynamic tolerance level to segment the temporal bones;
- Threshold segmentation at a gray value of 6700, including both thin vessels and temporal bones;
- Subtraction of regions of the temporal bones from the above.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Optimization of the Dual-E CT Option for the Scanner

Appendix B. A Case with Lung Rupture
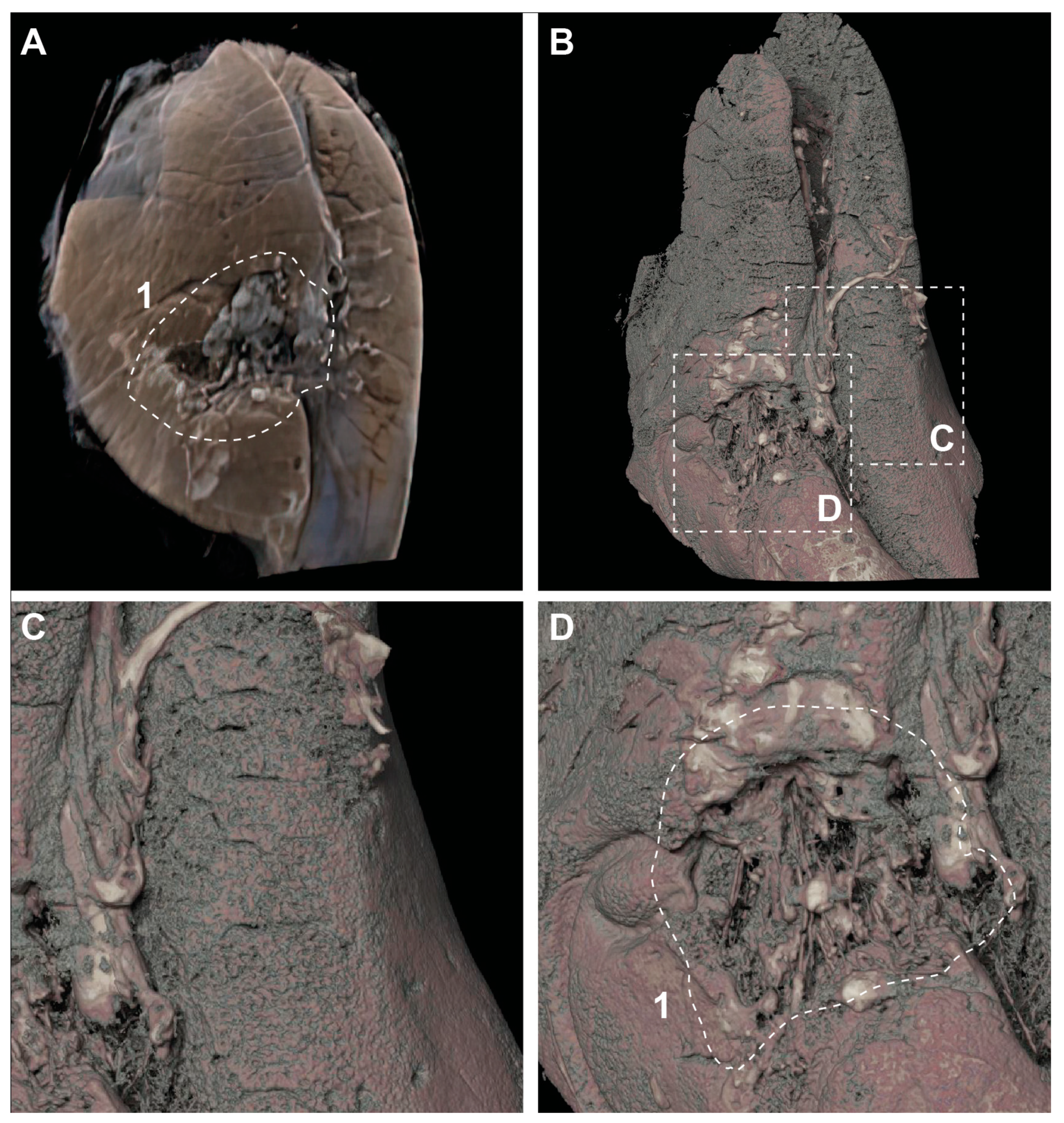
Appendix C. A Case for Lung and Heart Delineation with No Apparent Traumatic Cause of Death
References
- Shojania, K.G.; Burton, E.C. The Vanishing Nonforensic Autopsy. N. Engl. J. Med. 2008, 358, 873–875. [Google Scholar] [CrossRef]
- Lewis, C.; Hill, M.; Arthurs, O.J.; Hutchinson, C.; Chitty, L.S.; Sebire, N.J. Factors Affecting Uptake of Postmortem Examination in the Prenatal, Perinatal and Paediatric Setting. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 172–181. [Google Scholar] [CrossRef]
- Judge-Kronis, L.; Hutchinson, J.C.; Sebire, N.J.; Arthurs, O.J. Consent for Paediatric and Perinatal Postmortem Investigations: Implications of Less Invasive Autopsy. J. Forensic Radiol. Imaging 2016, 4, 7–11. [Google Scholar] [CrossRef]
- Dawood, Y.; Buijtendijk, M.F.J.; Shah, H.; Smit, J.A.; Jacobs, K.; Hagoort, J.; Oostra, R.J.; Bourne, T.; van den Hoff, M.J.B.; de Bakker, B.S. Imaging Fetal Anatomy. Semin. Cell Dev. Biol. 2022, 131, 78–92. [Google Scholar] [CrossRef]
- De Bakker, B.S.; De Jong, K.H.; Hagoort, J.; De Bree, K.; Besselink, C.T.; De Kanter, F.E.C.; Veldhuis, T.; Bais, B.; Schildmeijer, R.; Ruijter, J.M.; et al. An Interactive Three-Dimensional Digital Atlas and Quantitative Database of Human Development. Science 2016, 354, aag0053. [Google Scholar] [CrossRef]
- Docter, D.; Dawood, Y.; Jacobs, K.; Hagoort, J.; Oostra, R.-J.; van den Hoff, M.J.B.; Arthurs, O.J.; de Bakker, B.S. Microfocus Computed Tomography for Fetal Postmortem Imaging: An Overview. Pediatr. Radiol. 2022, 53, 632–639. [Google Scholar] [CrossRef]
- Simcock, I.C.; Shelmerdine, S.C.; Langan, D.; Anna, G.; Sebire, N.J.; Arthurs, O.J. Micro-CT Yields High Image Quality in Human Fetal Post-Mortem Imaging despite Maceration. BMC Med. Imaging 2021, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Dawood, Y.; Strijkers, G.J.; Limpens, J.; Oostra, R.J.; de Bakker, B.S. Novel Imaging Techniques to Study Postmortem Human Fetal Anatomy: A Systematic Review on Microfocus-CT and Ultra-High-Field MRI. Eur. Radiol. 2020, 30, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, O.J.; Hutchinson, J.C.; Sebire, N.J. Current Issues in Postmortem Imaging of Perinatal and Forensic Childhood Deaths. Forensic Sci. Med. Pathol. 2017, 13, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Alderliesten, M.E.; Peringa, J.; Van Der Hulst, V.P.M.; Blaauwgeers, H.L.G.; Van Lith, J.M.M. Perinatal Mortality: Clinical Value of Postmortem Magnetic Resonance Imaging Compared with Autopsy in Routine Obstetric Practice. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 378–382. [Google Scholar] [CrossRef]
- Arthurs, O.J.; Thayyil, S.; Olsen, O.E.; Addison, S.; Wade, A.; Jones, R.; Norman, W.; Scott, R.J.; Robertson, N.J.; Taylor, A.M.; et al. Diagnostic Accuracy of Post-Mortem MRI for Thoracic Abnormalities in Fetuses and Children. Eur. Radiol. 2014, 24, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Thayyil, S.; Cleary, J.O.; Sebire, N.J.; Scott, R.J.; Chong, K.; Gunny, R.; Owens, C.M.; Olsen, O.E.; Offiah, A.C.; Parks, H.G.; et al. Post-Mortem Examination of Human Fetuses: A Comparison of Whole-Body High-Field MRI at 9·4 T with Conventional MRI and Invasive Autopsy. Lancet 2009, 374, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.C.; Kang, X.; Shelmerdine, S.C.; Segers, V.; Lombardi, C.M.; Cannie, M.M.; Sebire, N.J.; Jani, J.C.; Arthurs, O.J. Postmortem Microfocus Computed Tomography for Early Gestation Fetuses: A Validation Study against Conventional Autopsy. Am. J. Obstet. Gynecol. 2018, 218, 445.e1–445.e12. [Google Scholar] [CrossRef]
- Hutchinson, J.C.; Arthurs, O.J.; Ashworth, M.T.; Ramsey, A.T.; Mifsud, W.; Lombardi, C.M.; Sebire, N.J. Clinical Utility of Postmortem Microcomputed Tomography of the Fetal Heart: Diagnostic Imaging vs Macroscopic Dissection. Ultrasound Obstet. Gynecol. 2016, 47, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.C.; Barrett, H.; Ramsey, A.T.; Haig, I.G.; Guy, A.; Sebire, N.J.; Arthurs, O.J. Virtual Pathological Examination of the Human Fetal Kidney Using Micro-CT. Ultrasound Obstet. Gynecol. 2016, 48, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Simcock, I.C.; Shelmerdine, S.C.; Hutchinson, J.C.; Sebire, N.J.; Arthurs, O.J. Human Fetal Whole-Body Postmortem Microfocus Computed Tomographic Imaging. Nat. Protoc. 2021, 16, 2594–2614. [Google Scholar] [CrossRef]
- Kang, X.; Carlin, A.; Cannie, M.M.; Sanchez, T.C.; Jani, J.C. Fetal Postmortem Imaging: An Overview of Current Techniques and Future Perspectives. Am. J. Obstet. Gynecol. 2020, 223, 493–515. [Google Scholar] [CrossRef]
- De Marco, E.; Vacchiano, G.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Guglielmi, G.; Fineschi, V. Evolution of Post-Mortem Coronary Imaging: From Selective Coronary Arteriography to Post-Mortem CT-Angiography and Beyond. La Radiol. Medica 2018, 123, 351–358. [Google Scholar] [CrossRef]
- Grabherr, S.; Grimm, J.; Dominguez, A.; Vanhaebost, J.; Mangin, P. Advances in Post-Mortem CT-Angiography. Br. J. Radiol. 2014, 87, 20130488. [Google Scholar] [CrossRef]
- Ross, S.G.; Bolliger, S.A.; Ampanozi, G.; Oesterhelweg, L.; Thali, M.J.; Flach, P.M. Postmortem CT Angiography: Capabilities and Limitations in Traumatic and Natural Causes of Death. Radiographics 2014, 34, 830–846. [Google Scholar] [CrossRef]
- Jackowski, C.; Sonnenschein, M.; Thali, M.J.; Aghayev, E.; von Allmen, G.; Yen, K.; Dirnhofer, R.; Vock, P. Virtopsy: Postmortem Minimally Invasive Angiography Using Cross Section Techniques--Implementation and Preliminary Results. J. Forensic Sci. 2005, 50, 1175–1186. [Google Scholar] [CrossRef]
- Ashby, C.; Razzak, A.N.; Kogler, A.; Amireh, A.; Dempsey, J.; Lin, K.K.; Waller, J.; Jha, P. The Practicality of Post-Mortem Imaging in Prenatal, Perinatal, and Pediatric Cases. Cureus 2022, 14, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, O.J.; Taylor, A.M.; Sebire, N.J. Indications, Advantages and Limitations of Perinatal Postmortem Imaging in Clinical Practice. Pediatr. Radiol. 2014, 45, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Proisy, M.; Jérôme, A.; Loget, P.; Bouvet, R.; Roussey, M.; Pelé, F.; Rozel, C.; Treguier, C.; Darnault, P.; Bruneau, B.; et al. Whole-Body Post-Mortem Computed Tomography Compared with Autopsy in the Investigation of Unexpected Death in Infants and Children. Eur. Radiol. 2013, 23, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, W.; Thali, M.; Martinez, R.M.; Ebert, L. Post Mortem CT Angiography in Fetuses or Newborn: Very Affordable Pump and Catheter Solution. J. Forensic Radiol. Imaging 2018, 13, 17–22. [Google Scholar] [CrossRef]
- Alvarez, R.E.; Macovski, A. Energy-Selective Reconstructions in X-ray Computerised Tomography. Phys. Med. Biol. 1976, 21, 002. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.A.; Alvarez, R.E.; Macovski, A.; Brody, W.R.; Pelc, N.J.; Riederer, S.J.; Hall, A.L. Generalized Image Combinations in Dual KVP Digital Radiography. Med. Phys. 1981, 8, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Leng, S.; McCollough, C.H. Dual-Energy CT–Based Monochromatic Imaging. Am. J. Roentgenol. 2012, 199, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Christner, J.A.; Leng, S.; Wang, J.; Fletcher, J.G.; McCollough, C.H. Virtual Monochromatic Imaging in Dual-Source Dual-Energy CT: Radiation Dose and Image Quality. Med. Phys. 2011, 38, 6371–6379. [Google Scholar] [CrossRef]
- Heismann, B.J.; Leppert, J.; Stierstorfer, K. Density and Atomic Number Measurements with Spectral X-ray Attenuation Method. J. Appl. Phys. 2003, 94, 2073–2079. [Google Scholar] [CrossRef]
- Sinkovskaya, E.; Klassen, A.; Abuhamad, A. A Novel Systematic Approach to the Evaluation of the Fetal Venous System. Semin. Fetal Neonatal Med. 2013, 18, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yagel, S.; Kivilevitch, Z.; Cohen, S.M.; Valsky, D.V.; Messing, B.; Shen, O.; Achiron, R. The Fetal Venous System, Part II: Ultrasound Evaluation of the Fetus with Congenital Venous System Malformation or Developing Circulatory Compromise. Ultrasound Obs. Gynecol. 2010, 36, 93–111. [Google Scholar] [CrossRef]
- Johnson, T.R.C. Dual-Energy CT: General Principles. AJR. Am. J. Roentgenol. 2012, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.A. A Method of Injecting the Blood-Vessels for Roentgenological Studies and Simultaneously Embalming. Anat. Rec. 1920, 18, 193–203. [Google Scholar] [CrossRef]
- Kingston, M.J.; Perriman, D.M.; Neeman, T.; Smith, P.N.; Webb, A.L. Contrast Agent Comparison for Three-Dimensional Micro-CT Angiography: A Cadaveric Study. Contrast Media Mol. Imaging 2016, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, D.; Ott, D. Barium Sulfate Suspensions: An Evaluation of Available Products. Am. J. Roentgenol. 1982, 138, 935–941. [Google Scholar] [CrossRef]
- Yu, L.; Ren, L.; Li, Z.; Leng, S.; McCollough, C.H. Dual-Source Multienergy CT with Triple or Quadruple X-ray Beams. J. Med. Imaging 2018, 5, 033502. [Google Scholar] [CrossRef]
- Cong, W.; Xi, Y.; Fitzgerald, P.; De Man, B.; Wang, G. Virtual Monoenergetic CT Imaging via Deep Learning. Patterns 2020, 1, 100128. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Rindal, O.M.H.; D’Hooge, J.; Masoy, S.E.; Austeng, A.; Lediju Bell, M.A.; Torp, H. The Generalized Contrast-to-Noise Ratio: A Formal Definition for Lesion Detectability. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 745–759. [Google Scholar] [CrossRef]
- Kuchenbecker, S.; Faby, S.; Sawall, S.; Lell, M.; Kachelrieß, M. Dual Energy CT: How Well Can Pseudo-Monochromatic Imaging Reduce Metal Artifacts? Med. Phys. 2015, 42, 1023–1036. [Google Scholar] [CrossRef]
- Schweitzer, W.; Flach, P.M.; Thali, M.; Laberke, P.; Gascho, D. Very Economical Immersion Pump Feasibility for Postmortem CT Angiography. J. Forensic Radiol. Imaging 2016, 5, 8–14. [Google Scholar] [CrossRef]
- Wolf, S.; Bruno, K.; Stefan, H.; Stephan, W.; Robert, Z.; Thali Michael, E.L. Fetal and Newborn Angiography Using Micro-CT and Electric Immersion Pump—Updated Technical Note. In Proceedings of the ISRI 2022, Tokyo, Japan, 21–24 March 2022. [Google Scholar]
- Schweitzer, W.; Thali, M.; Martinez, R.M.; Ebert, L. Technical Requirements and Goals for Forensic Post Mortem Computed Tomography Angiography Scanning of Human Fetuses and Neonates. Ph.D. Thesis, The University of Potsdam, Potsdam, Germany, 2018. [Google Scholar]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative Imaging of Element Composition and Mass Fraction Using Dual-Energy CT: Three-Material Decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Deresch, A. Modellierung von Röntgenspektren für Technische Anwendungen; Universität Potsdam: Potsdam, Germany, 2015. [Google Scholar]


| BaSO4-fat | BaSO4-fat | ||||||
| LE | HE | Mono-E | LE | HE | Mono-E | ||
| CNR | 2.17 | 3.24 | 4.14 | gCNR | 0.9938 | 0.997 | 1.0000 |
| BaSO4-bone | BaSO4-bone | ||||||
| LE | HE | Mono-E | LE | HE | Mono-E | ||
| CNR | 1.12 | 2.16 | 3.28 | gCNR | 0.8312 | 0.9509 | 0.9846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zboray, R.; Schweitzer, W.; Ebert, L.; Wolf, M.; Guglielmini, S.; Haemmerle, S.; Weiss, S.; Koller, B. Development of A Micro-CT Scanner with Dual-Energy Option and Endovascular Contrast Agent Administration Protocol for Fetal and Neonatal Virtual Autopsy. J. Imaging 2024, 10, 60. https://doi.org/10.3390/jimaging10030060
Zboray R, Schweitzer W, Ebert L, Wolf M, Guglielmini S, Haemmerle S, Weiss S, Koller B. Development of A Micro-CT Scanner with Dual-Energy Option and Endovascular Contrast Agent Administration Protocol for Fetal and Neonatal Virtual Autopsy. Journal of Imaging. 2024; 10(3):60. https://doi.org/10.3390/jimaging10030060
Chicago/Turabian StyleZboray, Robert, Wolf Schweitzer, Lars Ebert, Martin Wolf, Sabino Guglielmini, Stefan Haemmerle, Stephan Weiss, and Bruno Koller. 2024. "Development of A Micro-CT Scanner with Dual-Energy Option and Endovascular Contrast Agent Administration Protocol for Fetal and Neonatal Virtual Autopsy" Journal of Imaging 10, no. 3: 60. https://doi.org/10.3390/jimaging10030060
APA StyleZboray, R., Schweitzer, W., Ebert, L., Wolf, M., Guglielmini, S., Haemmerle, S., Weiss, S., & Koller, B. (2024). Development of A Micro-CT Scanner with Dual-Energy Option and Endovascular Contrast Agent Administration Protocol for Fetal and Neonatal Virtual Autopsy. Journal of Imaging, 10(3), 60. https://doi.org/10.3390/jimaging10030060







