Point Projection Mapping System for Tracking, Registering, Labeling, and Validating Optical Tissue Measurements
Abstract
1. Introduction
- Developing a Point Projection Mapping (PPM) system, which allows for tracking of Point-based optical measurements performed on tissue specimens for the validation of optical tissue-sensing technologies.
- Introducing a newly developed framework for the registration, validation, and labeling of optical data with histopathology.
- Validating the proposed framework on a use-case scenario, namely Point-based optical tissue measurements performed on breast cancer lumpectomy specimens.
2. Material and Methods
2.1. Point Projection Mapping
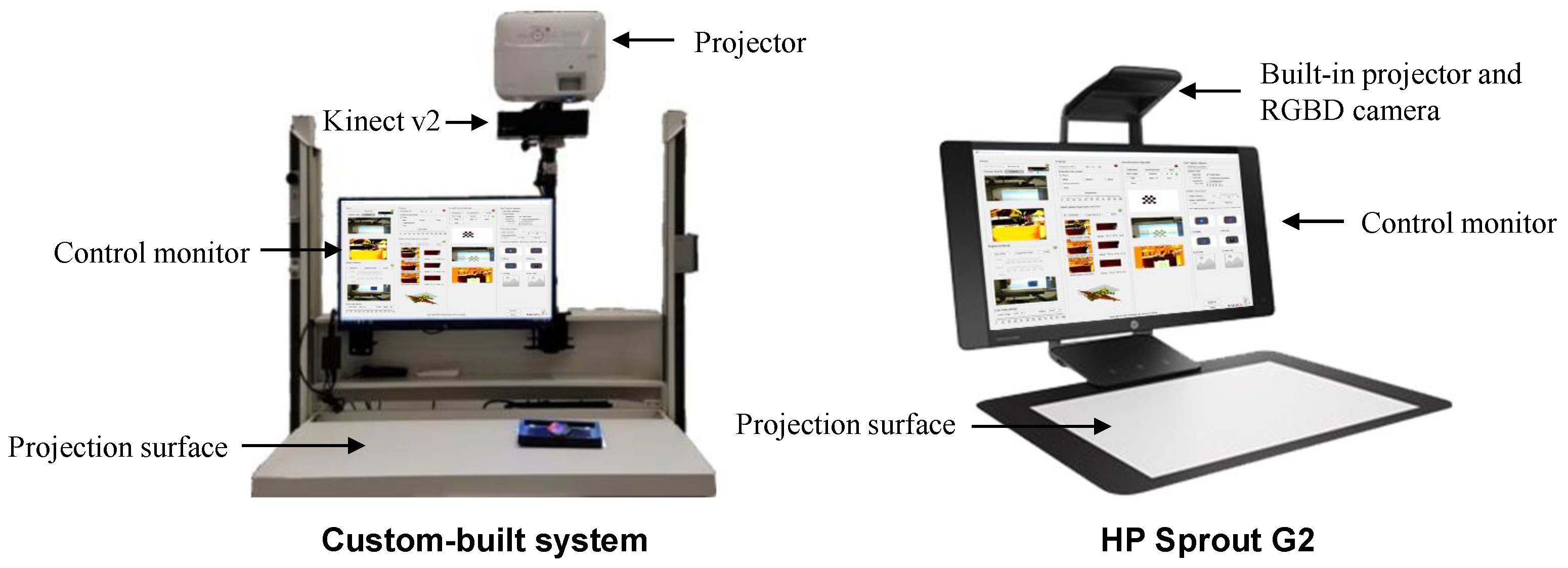
2.1.1. Hardware
Custom-Built Setup
HP Sprout
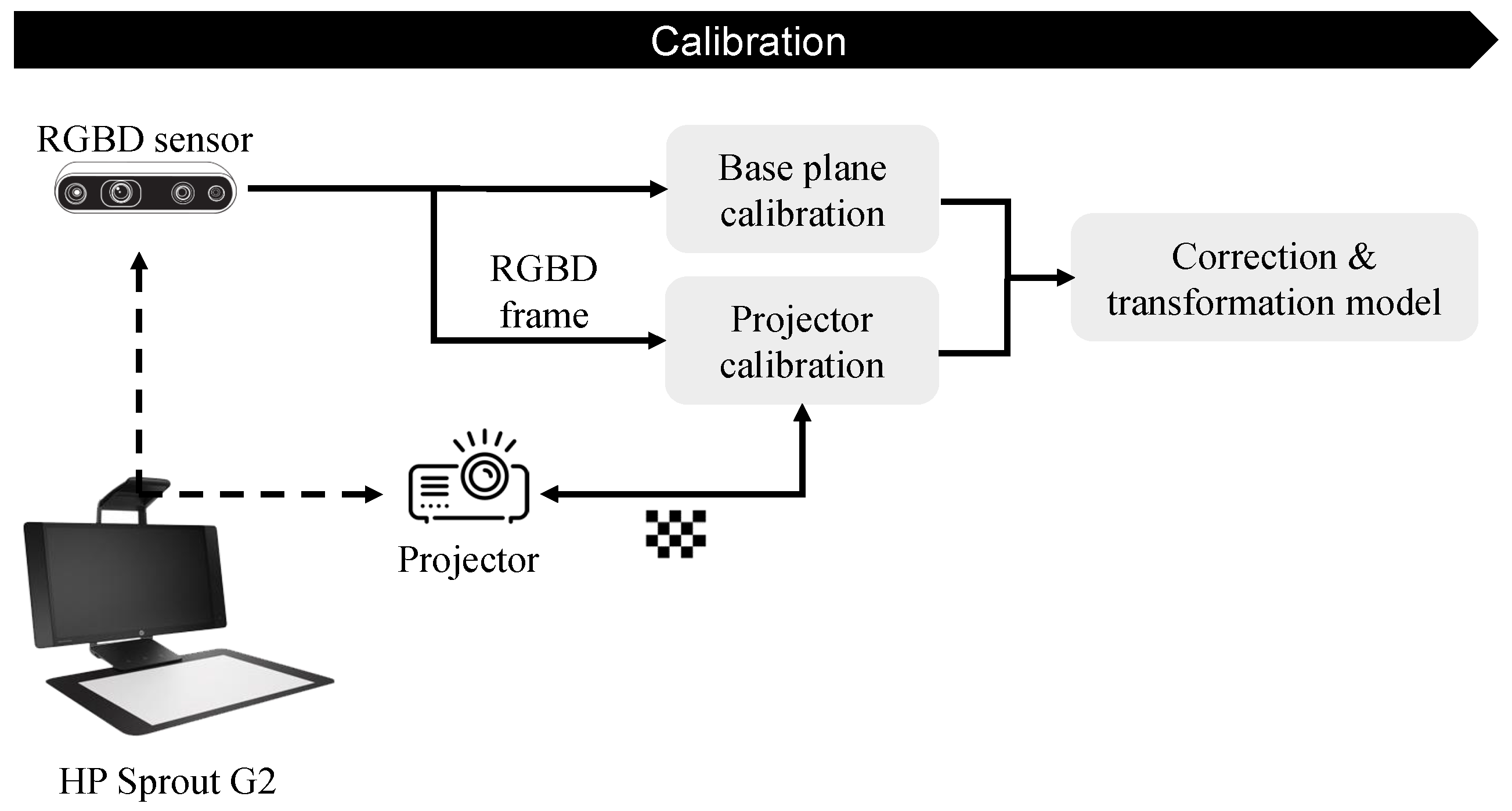
2.1.2. PPM Calibration
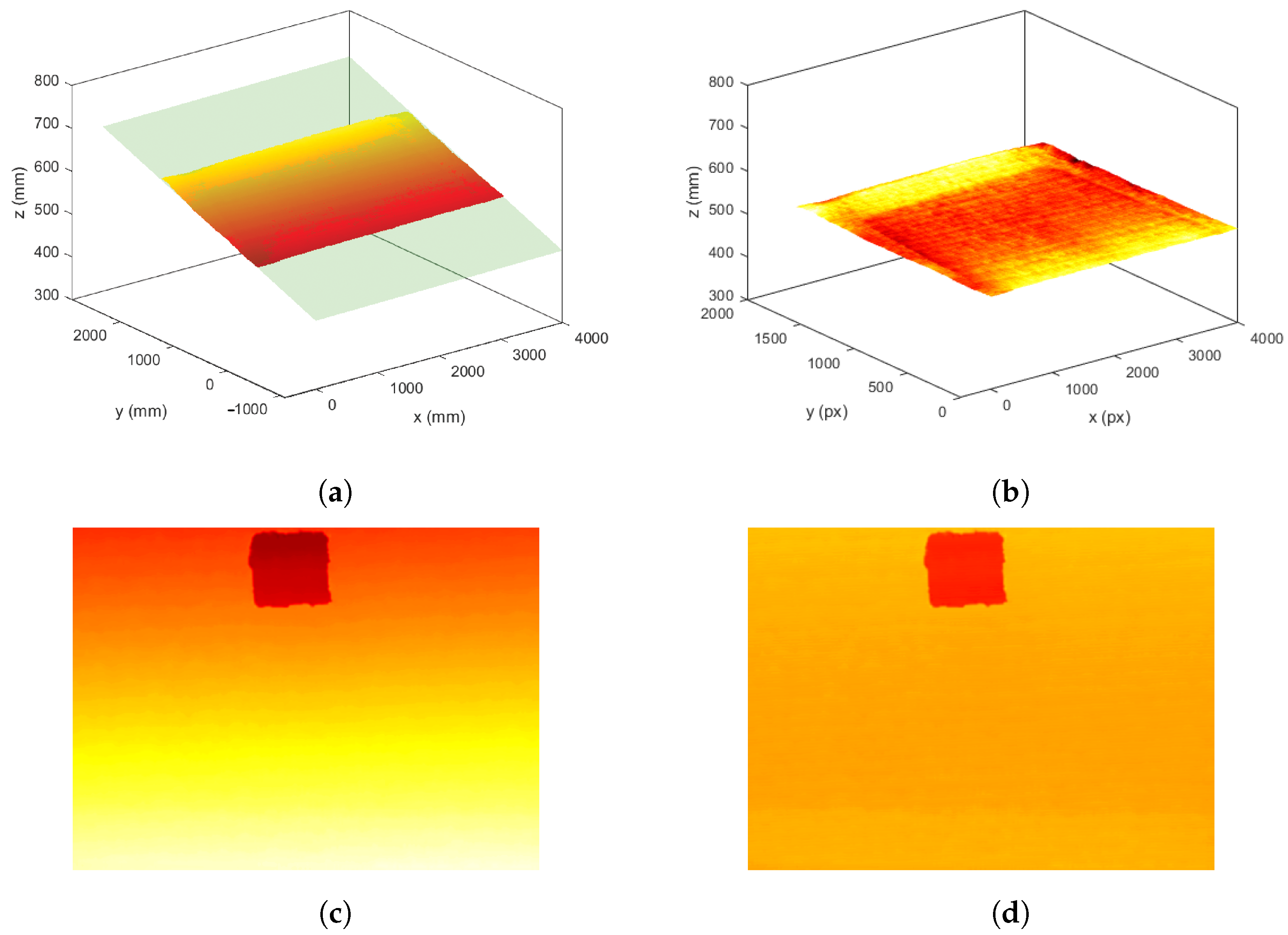
2.1.3. Base-Plane Calibration
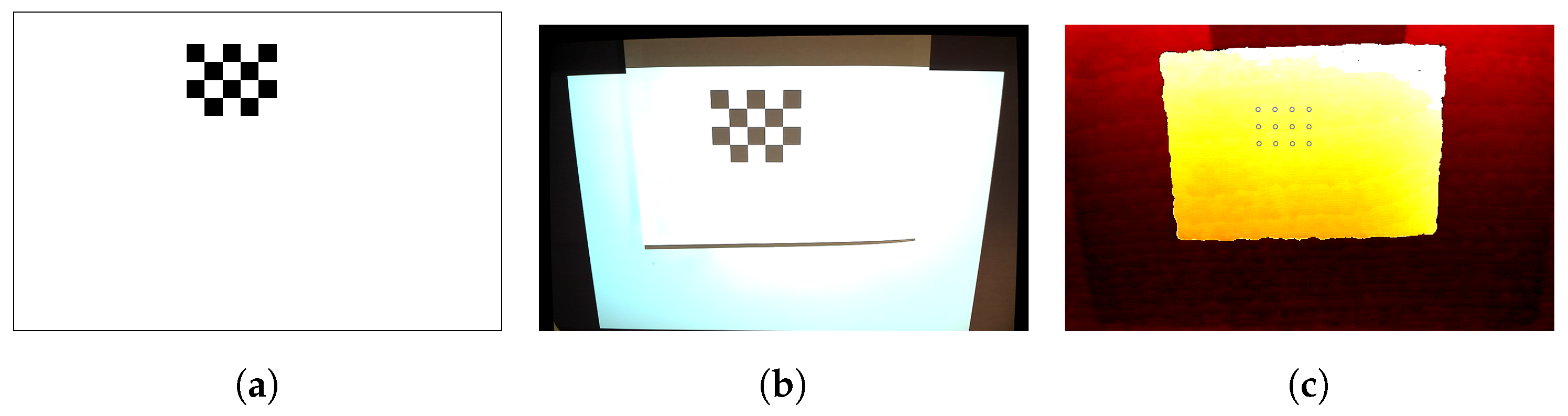
2.1.4. Projector Calibration
2.2. Framework for the Validation of Optical Tissue Sensing Technologies
2.2.1. Measurement Pipeline
2.2.2. Specimen Collection
2.2.3. Selecting, Tracking, and Performing Optical Tissue Measurements
2.2.4. H&E Processing
2.2.5. Correlation with Histopathology
2.2.6. Automatic Deformable Image Registration
2.2.7. Label Extraction for Tissue Classification
3. Results
3.1. Evaluation of PPM System
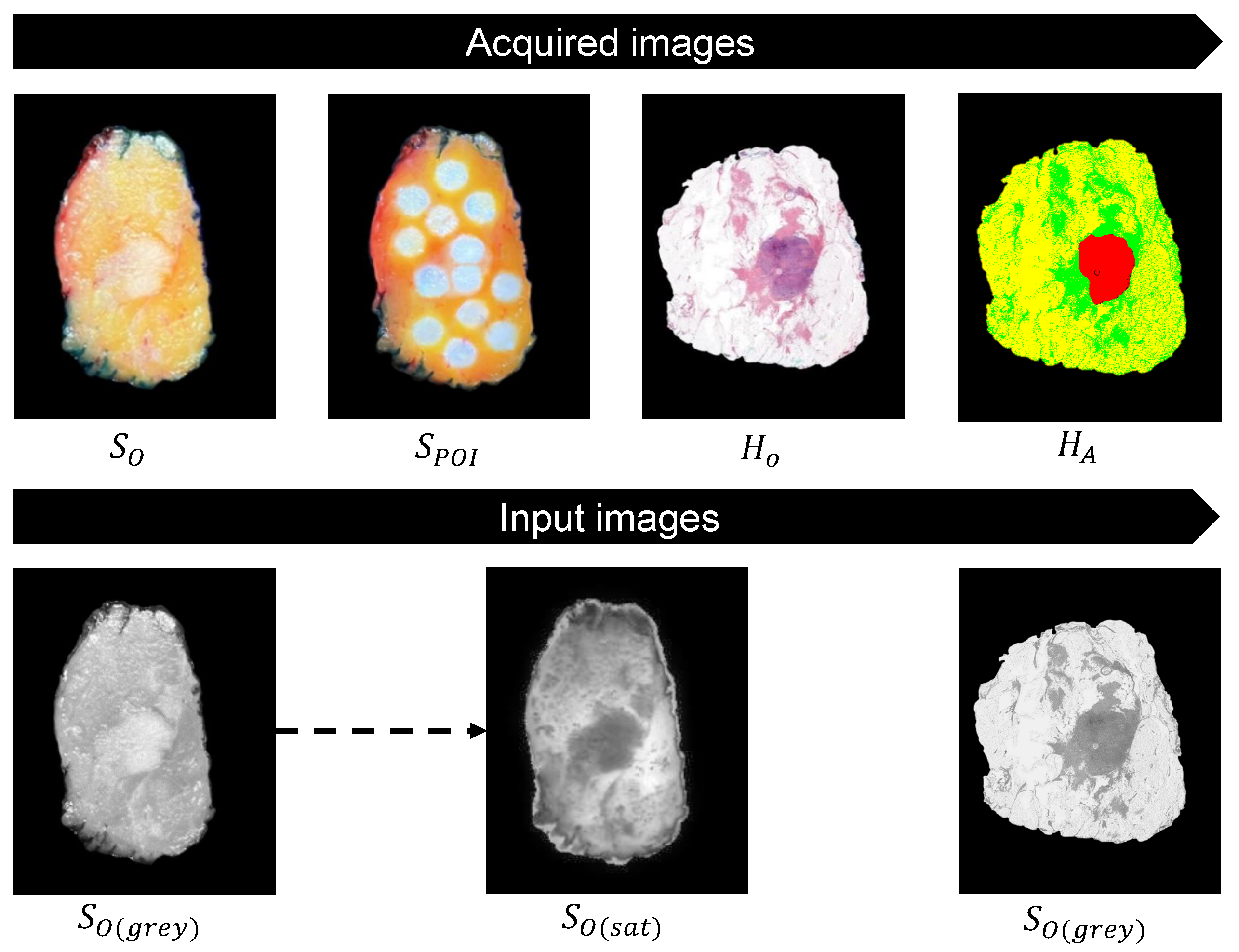
3.2. Acquired Images and Input Images
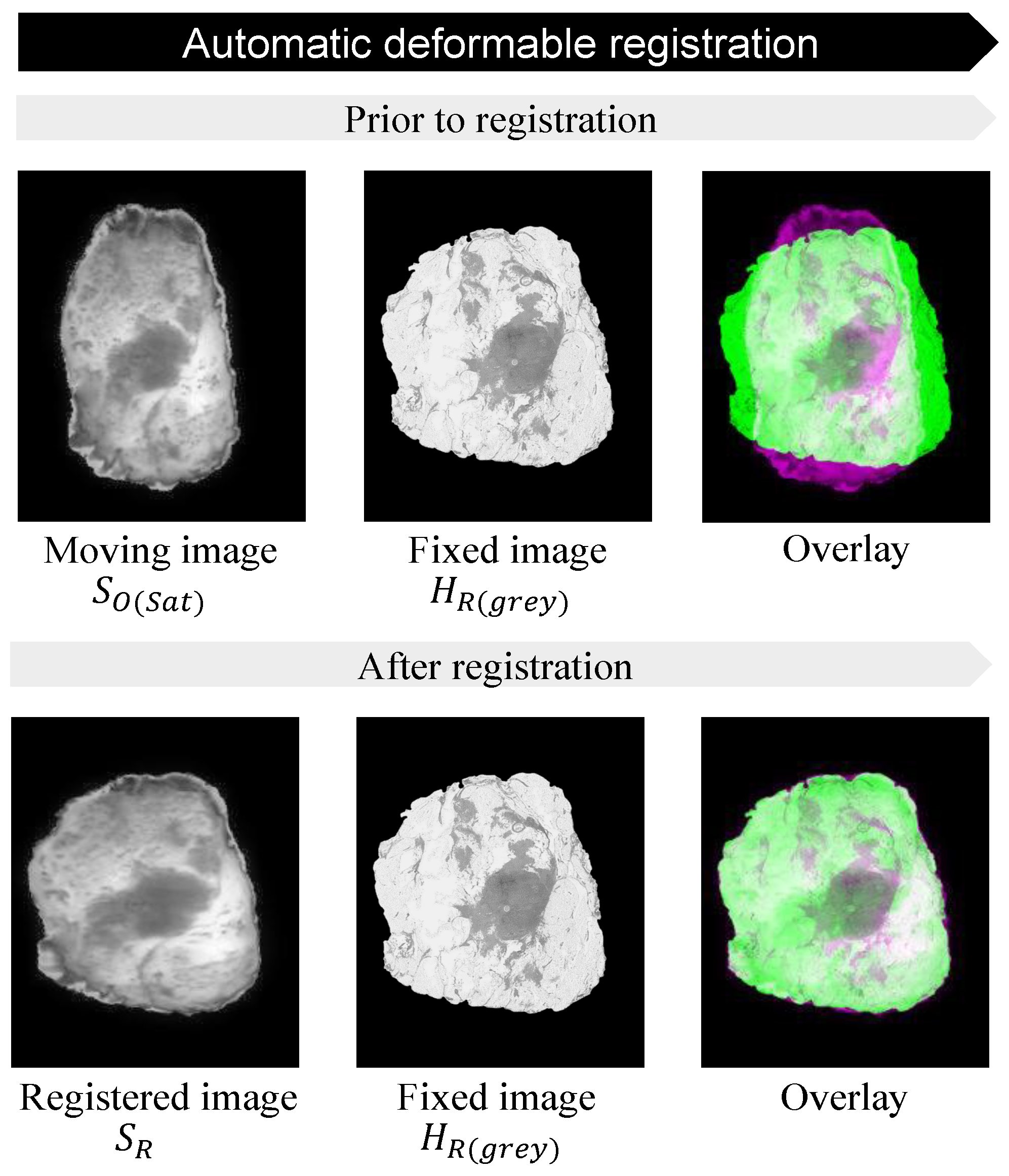
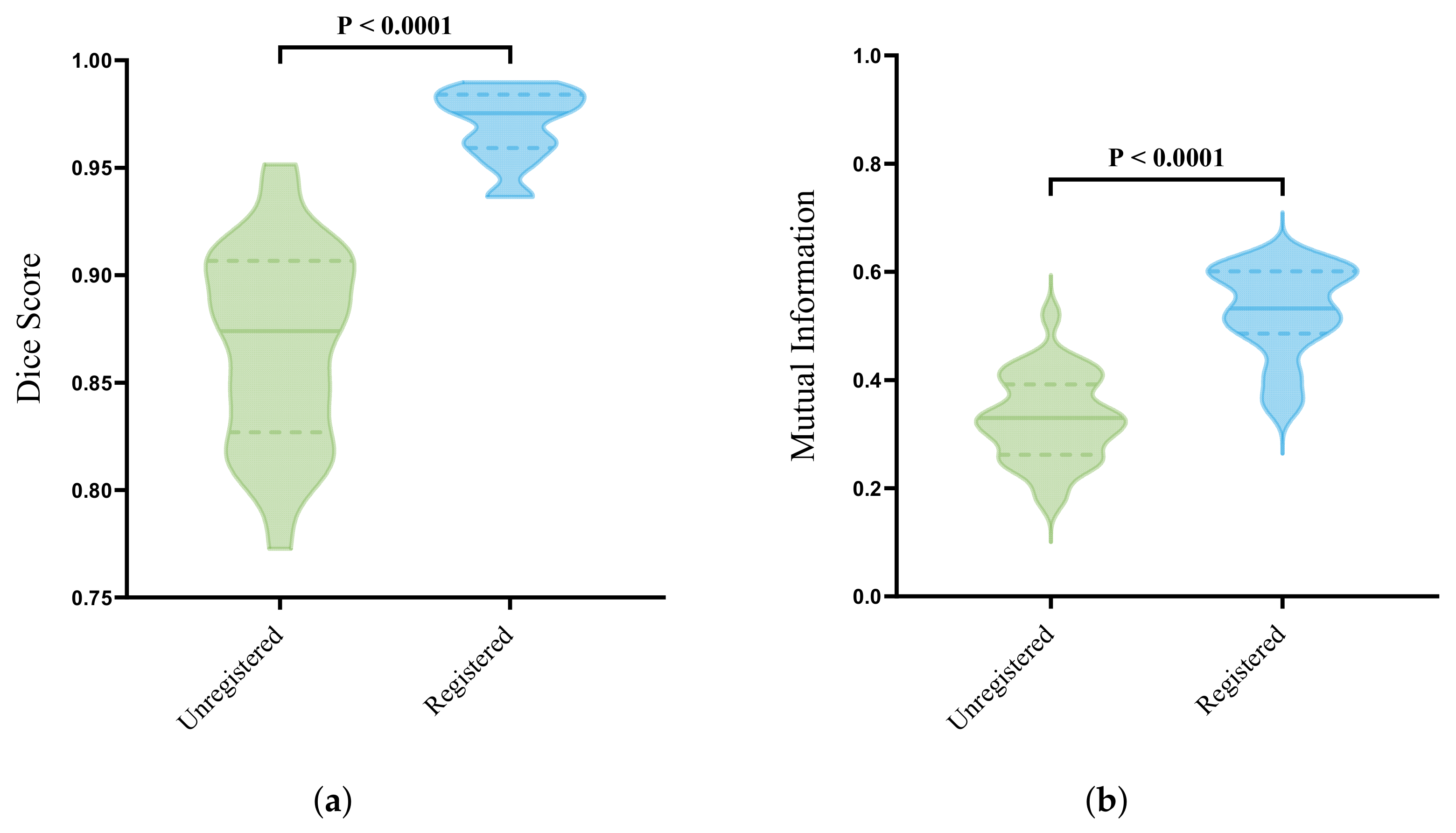
3.3. Automatic Deformable Image Registration
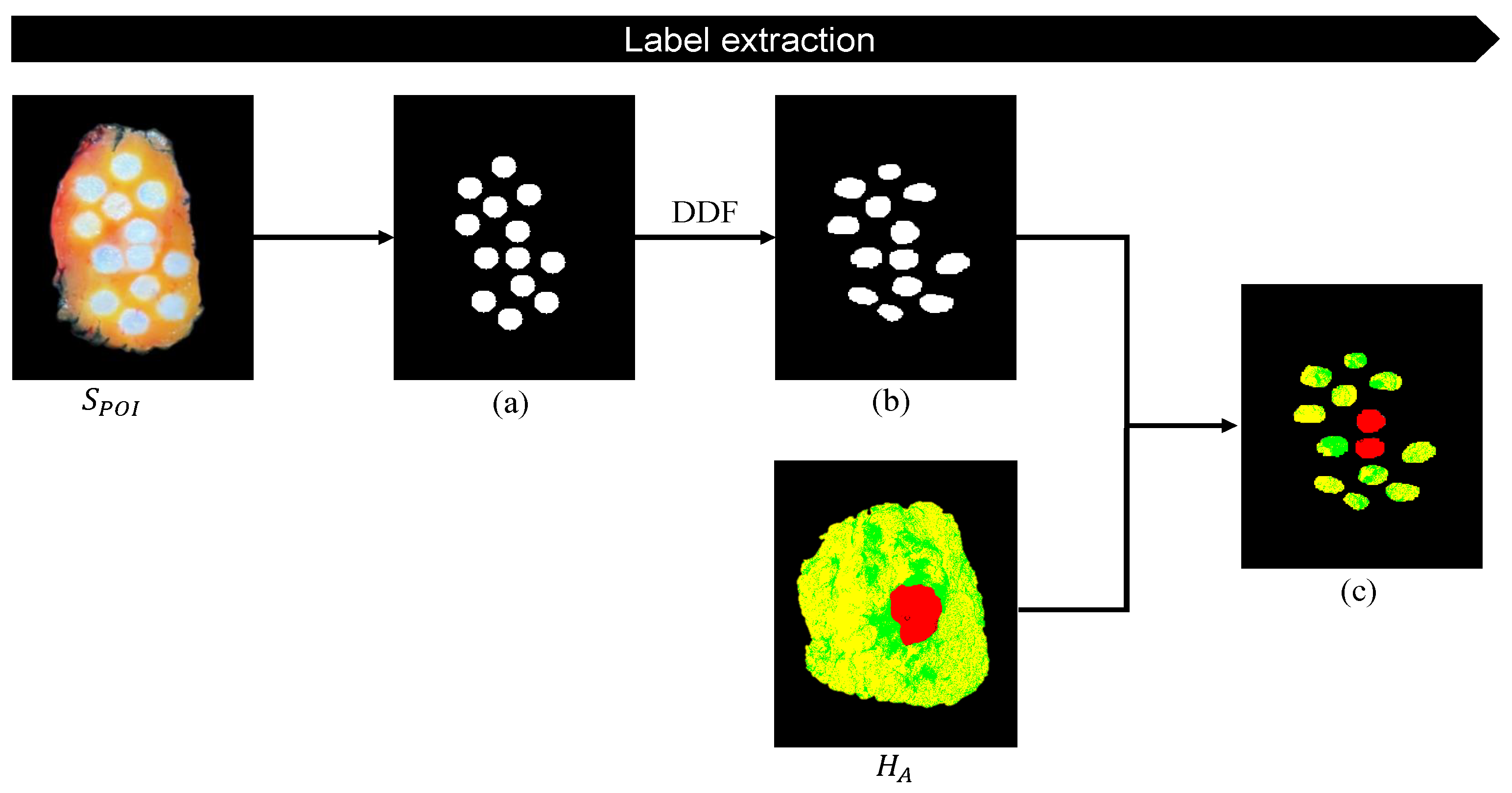
3.4. Label Extraction for Tissue Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusters, M.; Marijnen, C.A.; van de Velde, C.J.; Rutten, H.J.; Lahaye, M.J.; Kim, J.H.; La, M.J. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. J. Surg. Oncol. 2010, 36, 470. [Google Scholar] [CrossRef]
- Orosco, R.K.; Tapia, V.J.; Califano, J.A.; Clary, B.; Cohen, E.E.; Kane, C.; Lippman, S.M.; Messer, K.; Molinolo, A.; Murphy, J.D.; et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci. Rep. 2018, 8, 5686. [Google Scholar] [CrossRef]
- Hau, E.; Browne, L.; Capp, A.; Delaney, G.P.; Fox, C.; Kearsley, J.H.; Millar, E.; Nasser, E.H.; Papadatos, G.; Graham, P.H. The impact of breast cosmetic and functional outcomes on quality of life: Long-term results from the St. George and Wollongong randomized breast boost trial. Breast Cancer Res. Treat. 2013, 139, 115–123. [Google Scholar] [CrossRef]
- Valejo, F.A.M.; Tiezzi, D.G.; Mandarano, L.R.M.; Sousa, C.B.D.; Andrade, J.M.D. Volume of breast tissue excised during breast-conserving surgery in patients undergoing preoperative systemic therapy. Rev. Bras. Ginecol. Obstet. 2013, 35, 221–225. [Google Scholar] [CrossRef]
- Krekel, N.; Zonderhuis, B.; Muller, S.; Bril, H.; Van Slooten, H.J.; De Lange De Klerk, E.; Van Den Tol, P.; Meijer, S. Excessive resections in breast-conserving surgery: A retrospective multicentre study. Breast J. 2011, 17, 602–609. [Google Scholar] [CrossRef]
- de Boer, L.L.; Molenkamp, B.G.; Bydlon, T.M.; Hendriks, B.H.W.; Wesseling, J.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Fat/water ratios measured with diffuse reflectance Spectroscopy to detect breast tumor boundaries. Breast Cancer Res. Treat. 2015, 152, 509–518. [Google Scholar] [CrossRef]
- Baltussen, E.J.; Brouwer de Koning, S.G.; Sanders, J.; Aalbers, A.G.; Kok, N.F.; Beets, G.L.; Hendriks, B.H.; Sterenborg, H.J.; Kuhlmann, K.F.; Ruers, T.J. Using Diffuse Reflectance Spectroscopy to Distinguish Tumor Tissue From Fibrosis in Rectal Cancer Patients as a Guide to Surgery. Lasers Surg. Med. 2020, 52, 604–611. [Google Scholar] [CrossRef]
- Langhout, G.C.; Kuhlmann, K.F.D.; Schreuder, P.; Bydlon, T.; Smeele, L.E.; Van Den Brekel, M.W.M.; Sterenborg, H.J.C.M.; Hendriks, B.H.W.; Ruers, T.J.M. In Vivo Nerve Identification in Head and Neck Surgery Using Diffuse Reflectance Spectroscopy. Laryngoscope Investig. Otolaryngol. 2018, 3, 349–355. [Google Scholar] [CrossRef]
- Evers, D.; Hendriks, B.; Lucassen, G.; Ruers, T. Optical Spectroscopy: Current advances and future applications in cancer diagnostics and therapy. Future Oncol. 2012, 8, 307–320. [Google Scholar] [CrossRef]
- de Boer, L.L.; Bydlon, T.M.; van Duijnhoven, F.; Vranken Peeters, M.J.T.F.D.; Loo, C.E.; Winter-Warnars, G.A.O.; Sanders, J.; Sterenborg, H.J.C.M.; Hendriks, B.H.W.; Ruers, T.J.M. Towards the use of diffuse reflectance Spectroscopy for real-time in vivo detection of breast cancer during surgery. J. Transl. Med. 2018, 16, 367. [Google Scholar] [CrossRef]
- Brouwer De Koning, S.; Baltussen, E.; Karakullukcu, M.; Dashtbozorg, B.; Smit, L.; Dirven, R.; Hendriks, B.; Sterenborg, H.; Ruers, T. Toward complete oral cavity cancer resection using a handheld diffuse reflectance Spectroscopy probe. J. Biomed. Opt. 2018, 23, 121611. [Google Scholar] [CrossRef]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Diagnosing breast cancer by using Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 12371–12376. [Google Scholar] [CrossRef]
- Alfonso-Garcia, A.; Bec, J.; Weyers, B.; Marsden, M.; Zhou, X.; Li, C.; Marcu, L.; Abstract, G. Mesoscopic fluorescence lifetime imaging: Fundamental principles, clinical applications and future directions HHS Public Access. J. Biophotonics 2021, 14, 202000472. [Google Scholar] [CrossRef]
- Gurjarpadhye, A.A.; Parekh, M.B.; Dubnika, A.; Rajadas, J.; Inayathullah, M. Infrared Imaging Tools for Diagnostic Applications in Dermatology. SM J. Clin. Med. Imaging 2015, 1, 1–5. [Google Scholar] [PubMed]
- Kho, E.; Dashtbozorg, B.; Sanders, J.; Vrancken Peeters, M.J.T.; van Duijnhoven, F.; Sterenborg, H.J.; Ruers, T.J. Feasibility of ex vivo margin assessment with hyperspectral imaging during breast-conserving surgery: From imaging tissue slices to imaging lumpectomy specimen. Appl. Sci. 2021, 11, 8881. [Google Scholar] [CrossRef]
- Jong, L.J.S.; de Kruif, N.; Geldof, F.; Veluponnar, D.; Sanders, J.; Peeters, M.J.T.V.; van Duijnhoven, F.; Sterenborg, H.J.; Dashtbozorg, B.; Ruers, T.J. Discriminating healthy from tumor tissue in breast lumpectomy specimens using deep learning-based hyperspectral imaging. Biomed. Opt. Express 2022, 13, 2581. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jermyn, M.; Leblond, F. Challenges and opportunities in clinical translation of biomedical optical Spectroscopy and imaging. J. Biomed. Opt. 2018, 23, 030901. [Google Scholar] [CrossRef]
- Wells, W.A.; Barker, P.E.; MacAulay, C.; Novelli, M.; Levenson, R.M.; Crawford, J.M. Validation of novel optical imaging technologies: The pathologists’ view. J. Biomed. Opt. 2007, 12, 051801. [Google Scholar] [CrossRef]
- Keller, A.; Bialecki, P.; Wilhelm, T.J.; Vetter, M.K. Diffuse reflectance Spectroscopy of human liver tumor specimens—Towards a tissue differentiating optical biopsy needle using light emitting diodes. Biomed. Opt. Express 2018, 9, 1069. [Google Scholar] [CrossRef]
- Sircan-Kuçuksayan, A.; Denkceken, T.; Canpolat, M. Differentiating cancerous tissues from noncancerous tissues using single-fiber reflectance Spectroscopy with different fiber diameters. J. Biomed. Opt. 2015, 20, 115007. [Google Scholar] [CrossRef]
- Skyrman, S.; Skyrman, S.; Burström, G.; Burström, G.; Lai, M.; Lai, M.; Manni, F.; Hendriks, B.; Hendriks, B.; Frostell, A.; et al. Diffuse reflectance Spectroscopy sensor to differentiate between glial tumor and healthy brain tissue: A proof-of-concept study. Biomed. Opt. Express 2022, 13, 6470–6483. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Maryam, S.; Amissah, M.; Lu, H.; Lynch, N.; Killeen, S.; O’Riordain, M.; Andersson-Engels, S. Evaluation of wavelength ranges and tissue depth probed by diffuse reflectance Spectroscopy for colorectal cancer detection. Sci. Rep. 2021, 11, 798. [Google Scholar] [CrossRef]
- Lay, A.H.; Wang, X.; Morgan, M.S.; Kapur, P.; Liu, H.; Roehrborn, C.G.; Cadeddu, J.A. Detecting positive surgical margins: Utilisation of light-reflectance Spectroscopy on ex vivo prostate specimens. BJU Int. 2016, 118, 885–889. [Google Scholar] [CrossRef]
- Horgan, C.C.; Bergholt, M.S.; Thin, M.Z.; Nagelkerke, A.; Kennedy, R.; Kalber, T.L.; Stuckey, D.J.; Stevens, M.M. Image-guided Raman Spectroscopy probe-tracking for tumor margin delineation. J. Biomed. Opt. 2021, 26, 036002. [Google Scholar] [CrossRef]
- Gkouzionis, I.; Nazarian, S.; Kawka, M.; Darzi, A.; Patel, N.; Peters, C.J.; Elson, D.S. Real-time tracking of a diffuse reflectance Spectroscopy probe used to aid histological validation of margin assessment in upper gastrointestinal cancer resection surgery. J. Biomed. Opt. 2022, 27, 025001. [Google Scholar] [CrossRef]
- Unger, J.; Sun, T.; Chen, Y.L.; Phipps, J.E.; Bold, R.J.; Darrow, M.A.; Ma, K.L.; Marcu, L. Method for accurate registration of tissue autofluorescence imaging data with corresponding histology: A means for enhanced tumor margin assessment. J. Biomed. Opt. 2018, 23, 015001. [Google Scholar] [CrossRef]
- Lu, G.; Halig, L.; Wang, D.; Chen, Z.G.; Fei, B. Hyperspectral Imaging for Cancer Surgical Margin Delineation: Registration of Hyperspectral and Histological Images NIH Public Access. In Medical Imaging 2014: Image-Guided Procedures, Robotic Interventions, and Modeling; SPIE: Bellingham, DC, USA, 2014; Volume 9036, p. 90360. [Google Scholar] [CrossRef]
- Halicek, M.; Little, J.V.; Wang, X.; Chen, Z.G.; Patel, M.; Griffith, C.C.; El-Deiry, M.W.; Saba, N.F.; Chen, A.Y.; Fei, B. Deformable Registration of Histological Cancer Margins to Gross Hyperspectral Images using Demons. Proc. Spie Int. Soc. Opt. Eng. 2018, 10581, 22. [Google Scholar] [CrossRef]
- De Boer, L.L.; Kho, E.; Nijkamp, J.; Van De Vijver, K.K.; Sterenborg, H.J.C.M.; Ter, L.C.; Theo, B.; Ruers, J.M.; Ter Beek, L.C.; Ruers, T.J.M. Method for coregistration of optical measurements of breast tissue with histopathology: The importance of accounting for tissue deformations. J. Biomed. Opt. 2019, 24, 075002. [Google Scholar] [CrossRef]
- HP® Sprout Pro Desktop PC (1MU73UA#ABA). 2023. Available online: https://www.hp.com/us-en/shop/pdp/sprout-pro-by-hp-g2 (accessed on 27 November 2023).
- Sprout Pro by HP G2-Specifications|HP® Customer Support. 2023. Available online: https://support.hp.com/bg-en/document/c05415564 (accessed on 27 November 2023).
- Feenstra, L.; Lambregts, M.; Ruers, T.J.M.; Dashtbozorg, B. Deformable Multi-Modal Image Registration for the Correlation Between Optical Measurements and Histology Images. 2023. Available online: https://arxiv.org/abs/2311.14414v1 (accessed on 27 November 2023).
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Dalca, A.V.; Guttag, J. An Unsupervised Learning Model for Deformable Medical Image Registration. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 9252–9260. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Guttag, J.; Dalca, A.V. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging 2019, 38, 1788–1800. [Google Scholar] [CrossRef]
- Baltussen, E.J.; Brouwer De Koning, S.G.; Hendriks, B.H.; Jóźwiak, K.; Sterenborg, H.J.; Ruers, T.J. Comparing in vivo and ex vivo fiberoptic diffuse reflectance Spectroscopy in colorectal cancer. Transl. Biophotonics 2019, 1, e201900008. [Google Scholar] [CrossRef]
- Sharma, V.; Shivalingaiah, S.; Liu, H.; Euhus, D.; Gryczynski, Z.; Peng, Y. Auto-fluorescence lifetime and light reflectance Spectroscopy for breast cancer diagnosis: Potential tools for intraoperative margin detection. Biomed. Opt. Express 2012, 3, 1825–1840. [Google Scholar] [CrossRef]
- Laughney, A.M.; Krishnaswamy, V.; Rizzo, E.J.; Schwab, M.C.; Barth, R.J.; Pogue, B.W.; Paulsen, K.D.; Wells, W.A. Scatter spectroscopic imaging distinguishes between breast pathologies in tissues relevant to surgical margin assessment. Clin. Cancer Res. 2012, 18, 6315–6325. [Google Scholar] [CrossRef]
- Marsden, M.; Weyers, B.W.; Bec, J.; Sun, T.; Gandour-Edwards, R.F.; Birkeland, A.C.; Abouyared, M.; Bewley, A.F.; Farwell, D.G.; Marcu, L. Intraoperative Margin Assessment in Oral and Oropharyngeal Cancer Using Label-Free Fluorescence Lifetime Imaging and Machine Learning. IEEE Trans. Biomed. Eng. 2020, 68, 857–868. [Google Scholar] [CrossRef]
- Gorpas, D.; Ma, D.; Bec, J.; Yankelevich, D.R.; Marcu, L. Real-Time Visualization of Tissue Surface Biochemical Features Derived from Fluorescence Lifetime Measurements HHS Public Access. IEEE Trans. Med. Imaging 2016, 35, 1802–1811. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feenstra, L.; van der Stel, S.D.; Da Silva Guimaraes, M.; Dashtbozorg, B.; Ruers, T.J.M. Point Projection Mapping System for Tracking, Registering, Labeling, and Validating Optical Tissue Measurements. J. Imaging 2024, 10, 37. https://doi.org/10.3390/jimaging10020037
Feenstra L, van der Stel SD, Da Silva Guimaraes M, Dashtbozorg B, Ruers TJM. Point Projection Mapping System for Tracking, Registering, Labeling, and Validating Optical Tissue Measurements. Journal of Imaging. 2024; 10(2):37. https://doi.org/10.3390/jimaging10020037
Chicago/Turabian StyleFeenstra, Lianne, Stefan D. van der Stel, Marcos Da Silva Guimaraes, Behdad Dashtbozorg, and Theo J. M. Ruers. 2024. "Point Projection Mapping System for Tracking, Registering, Labeling, and Validating Optical Tissue Measurements" Journal of Imaging 10, no. 2: 37. https://doi.org/10.3390/jimaging10020037
APA StyleFeenstra, L., van der Stel, S. D., Da Silva Guimaraes, M., Dashtbozorg, B., & Ruers, T. J. M. (2024). Point Projection Mapping System for Tracking, Registering, Labeling, and Validating Optical Tissue Measurements. Journal of Imaging, 10(2), 37. https://doi.org/10.3390/jimaging10020037






