Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures
Abstract
1. Introduction
2. Anatomy
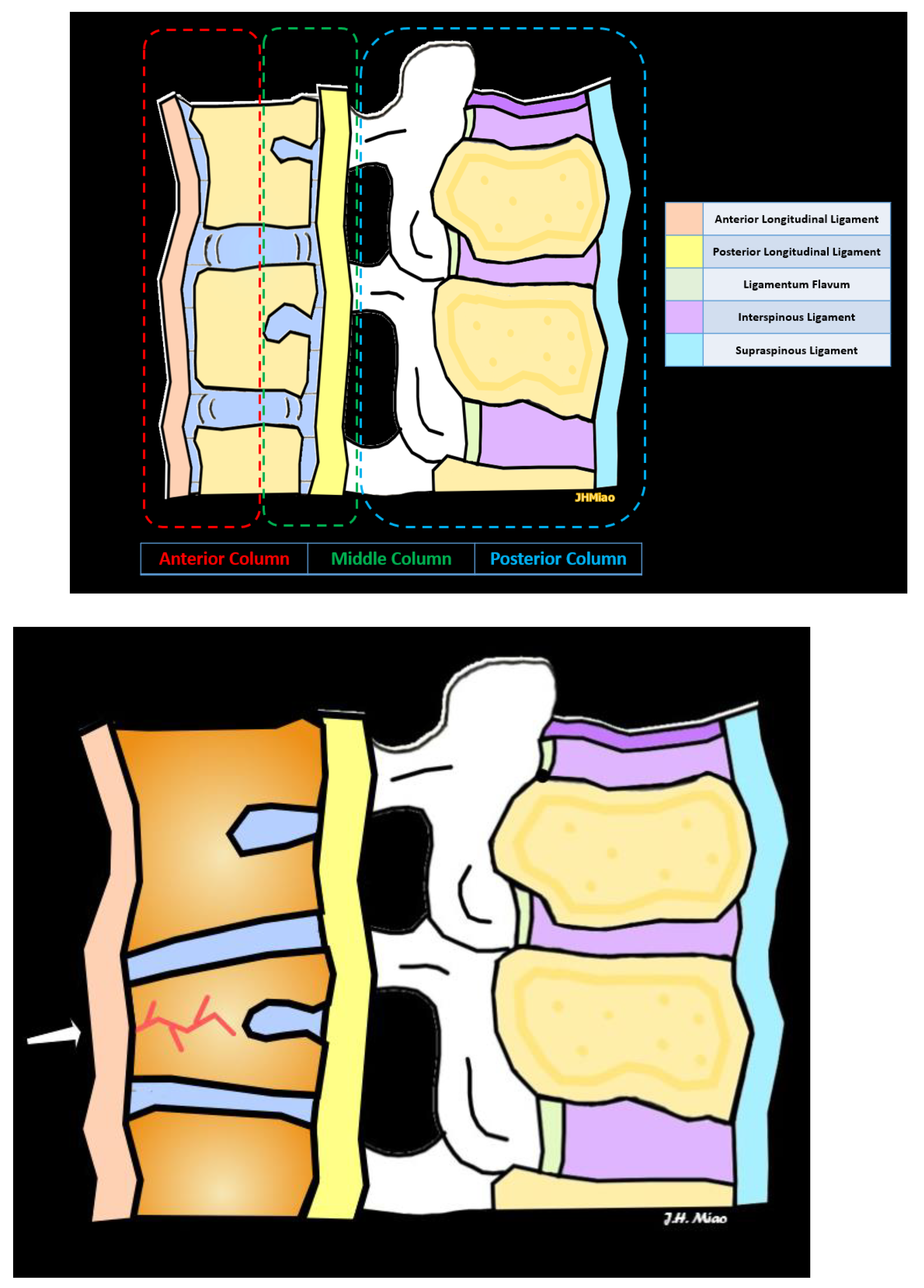
2.1. Three-Column Spinal Concept of Spinal Fractures
2.2. Types of Vertebral Compression Fractures
3. Classification of Fractures
3.1. The Genant Classification System
3.2. The Denis Classification System
3.3. Association for the Study of Internal Fixation/Arbeitsgemeinschaft für Osteosynthesefragen (AO) Spine Classification System
3.4. Thoracolumbar Injury Classification and Severity Score (TLICS) System
4. Imaging Modalities and Features
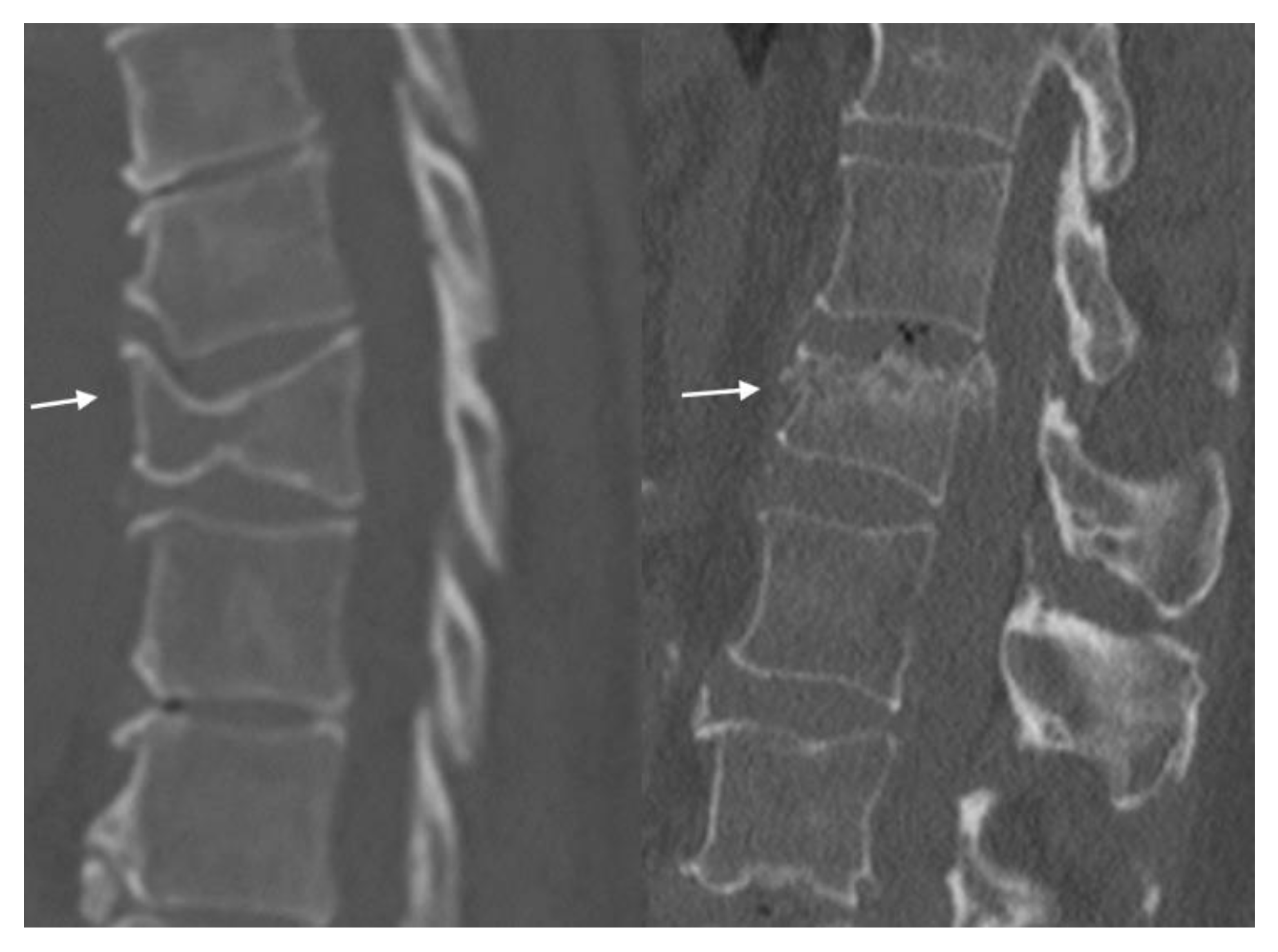
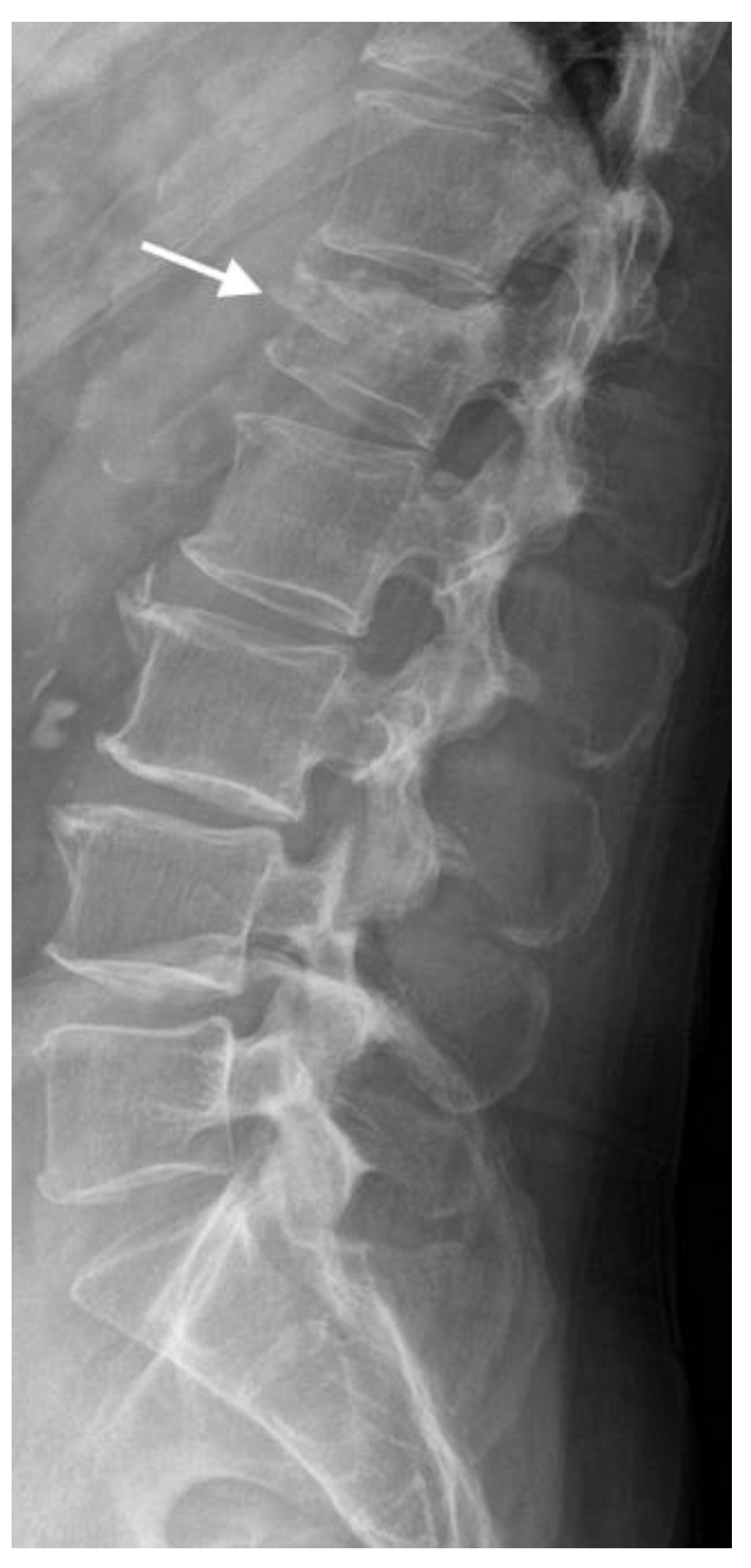
4.1. Radiographs
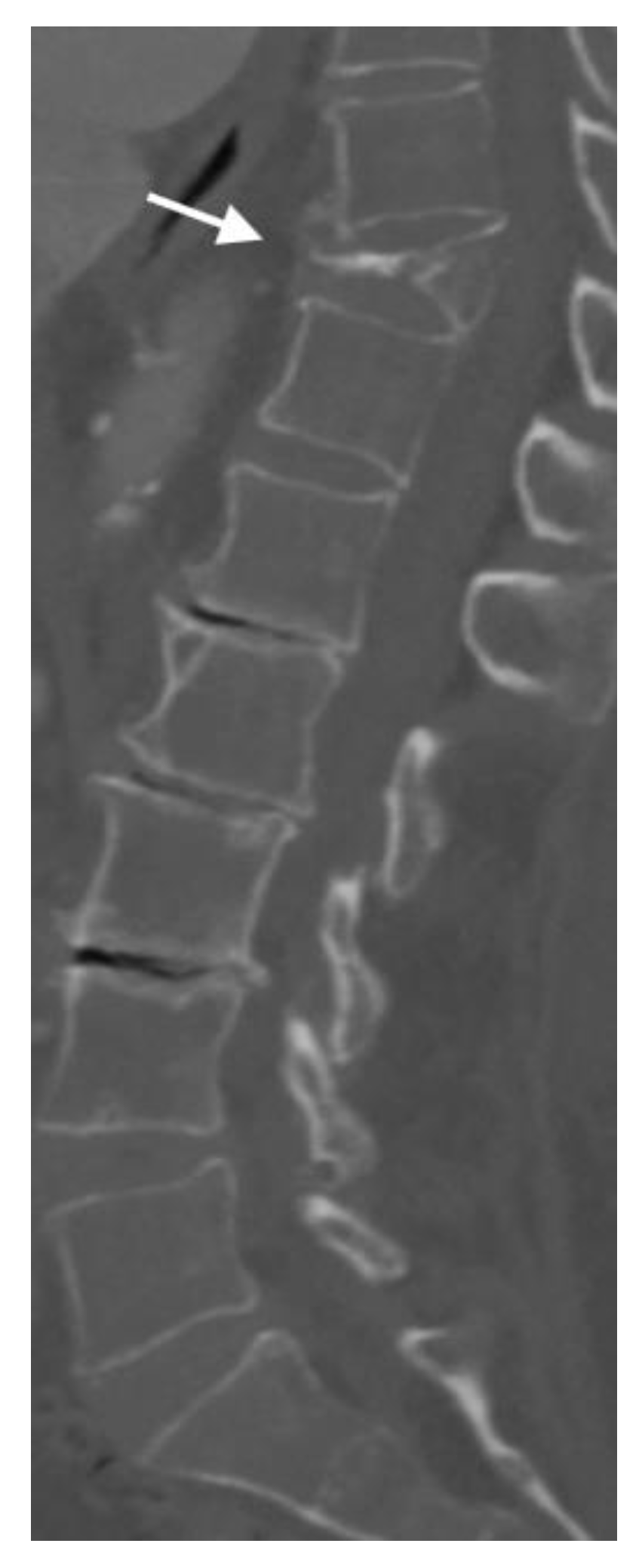
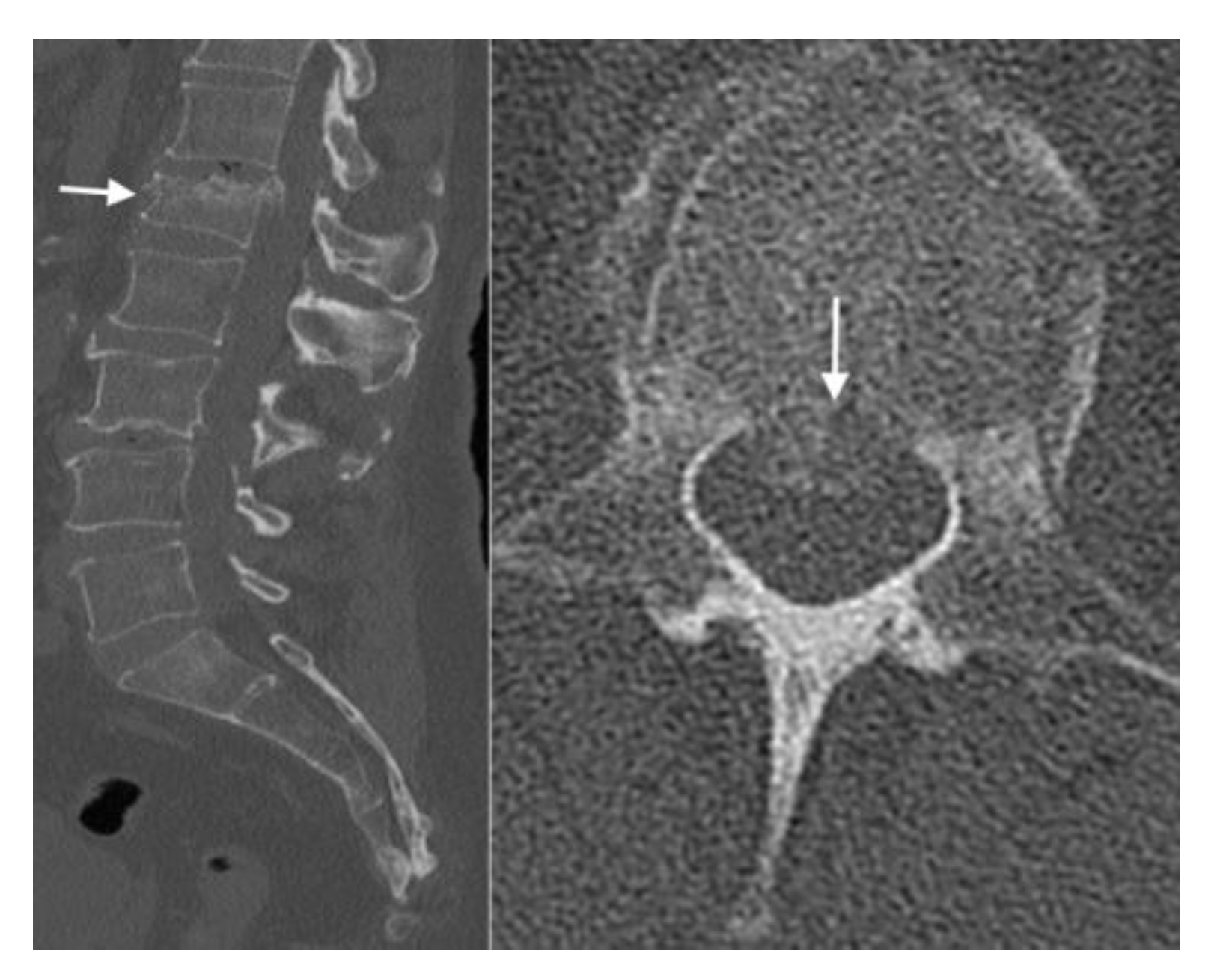
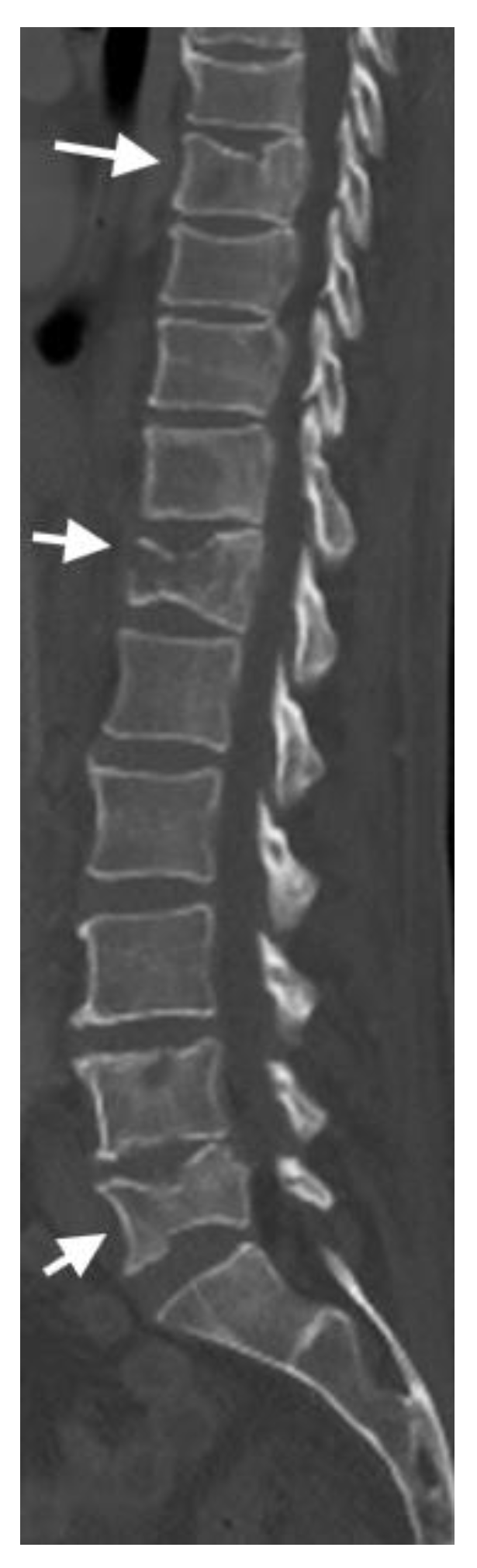
4.2. CT Scans
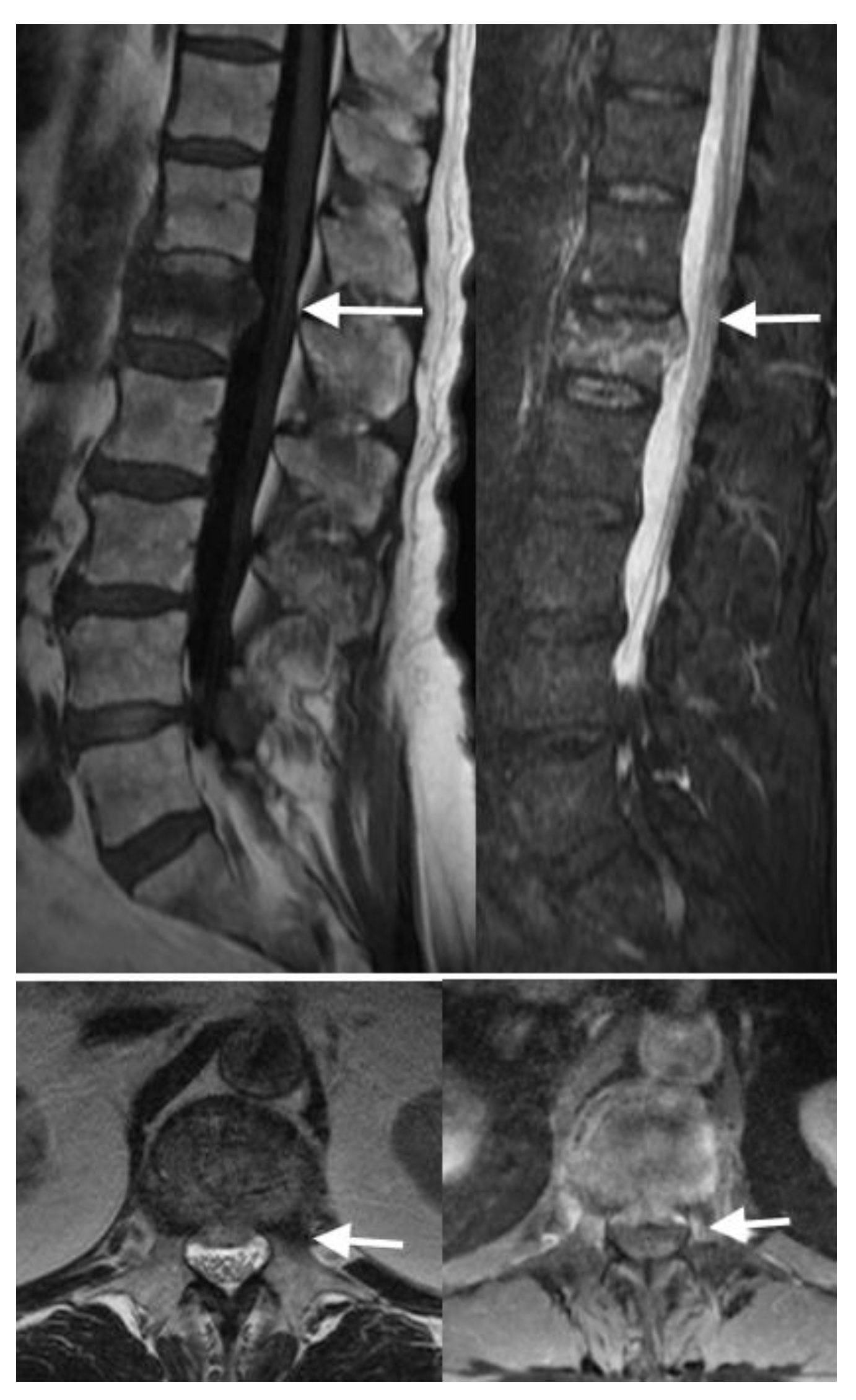
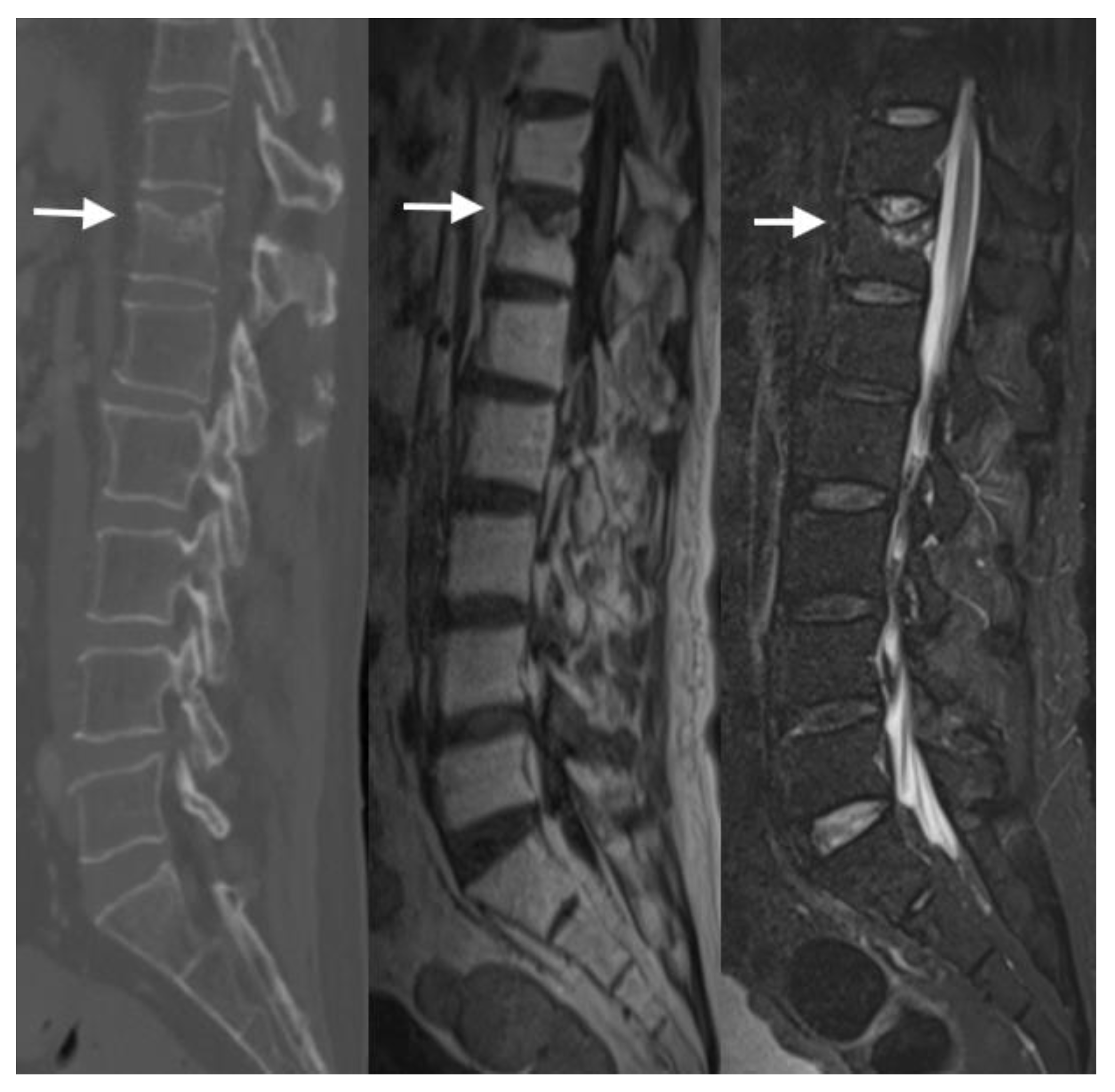
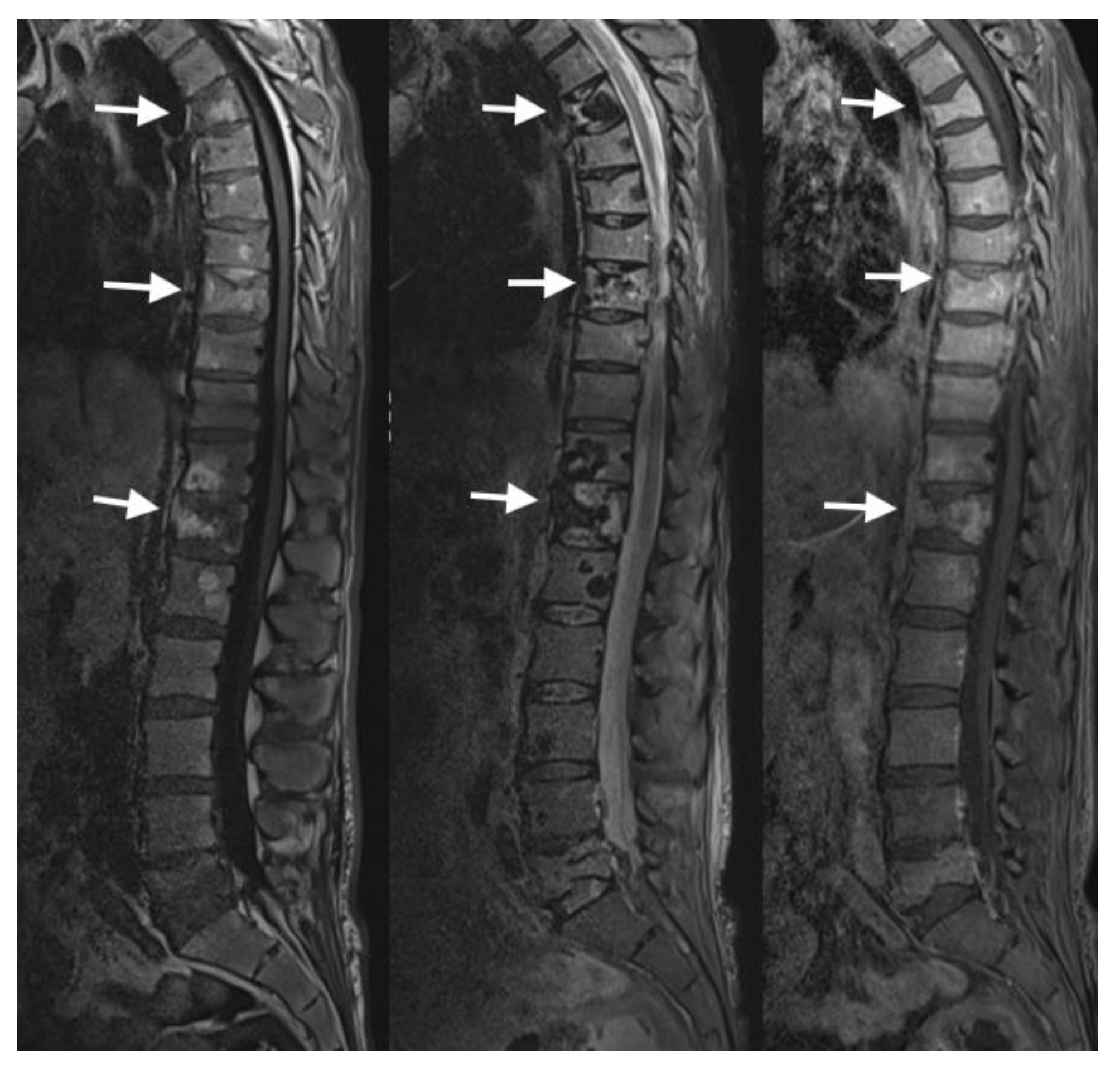
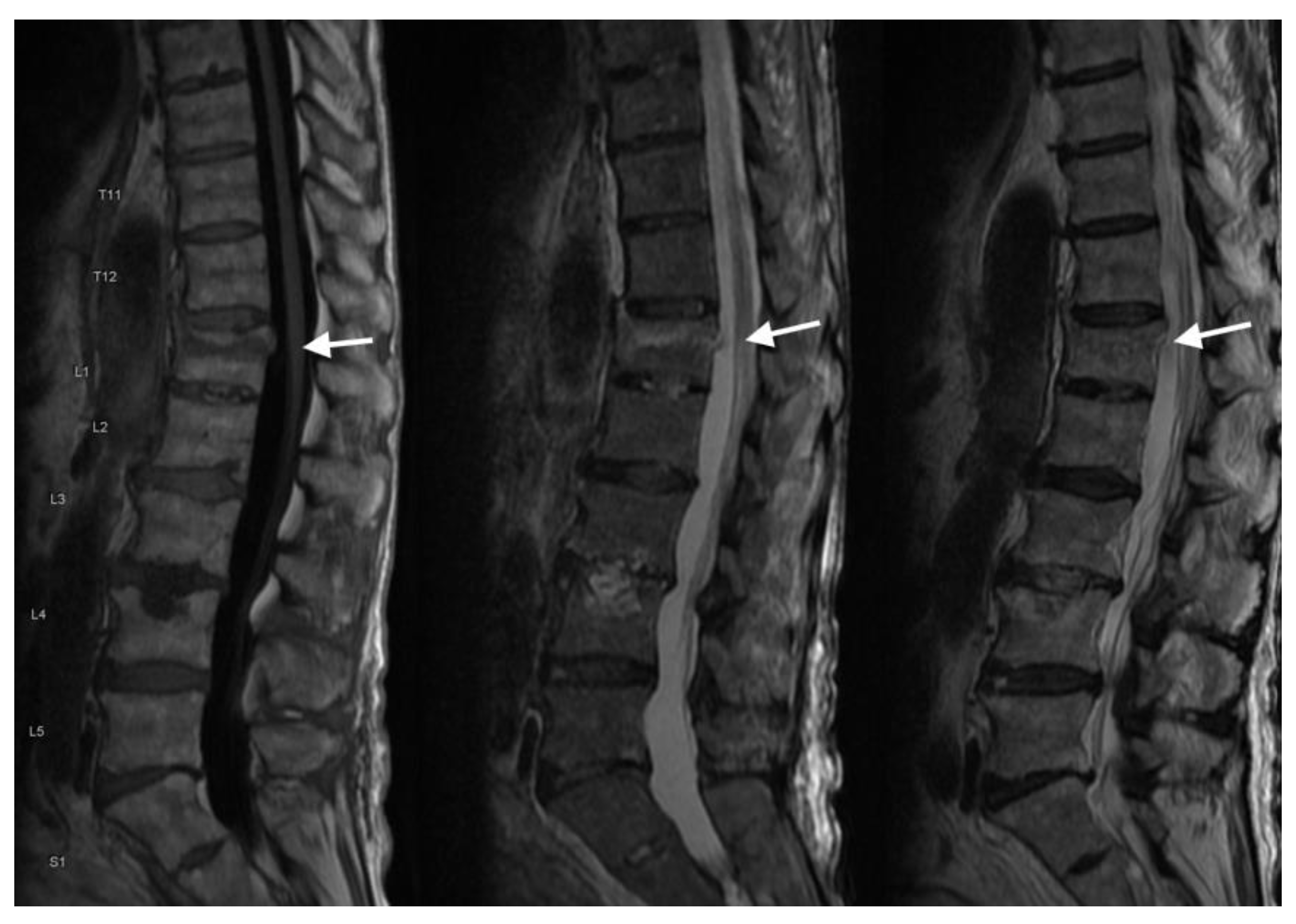
4.3. MRI Scans
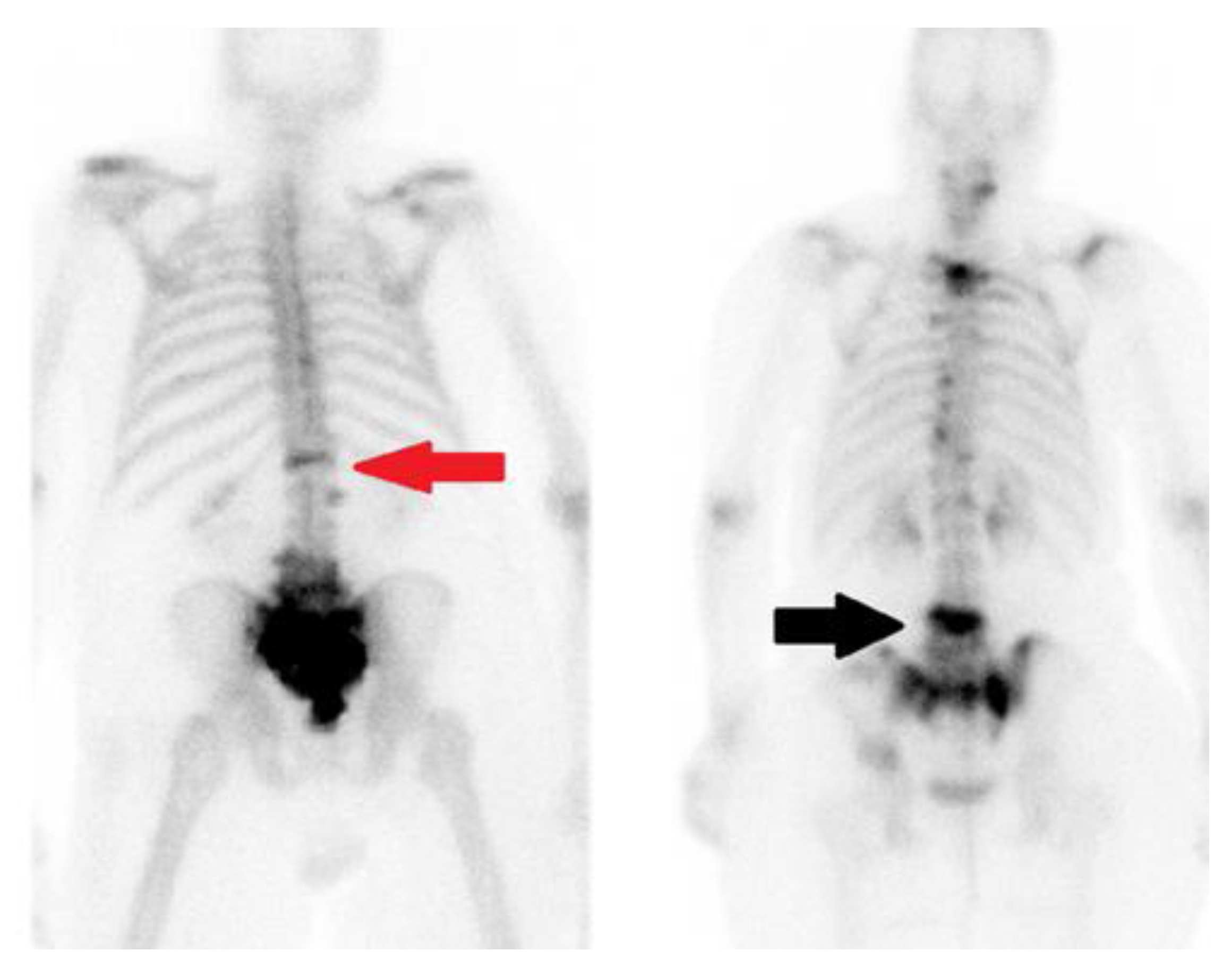
4.4. Nuclear Medicine
5. Prognosis
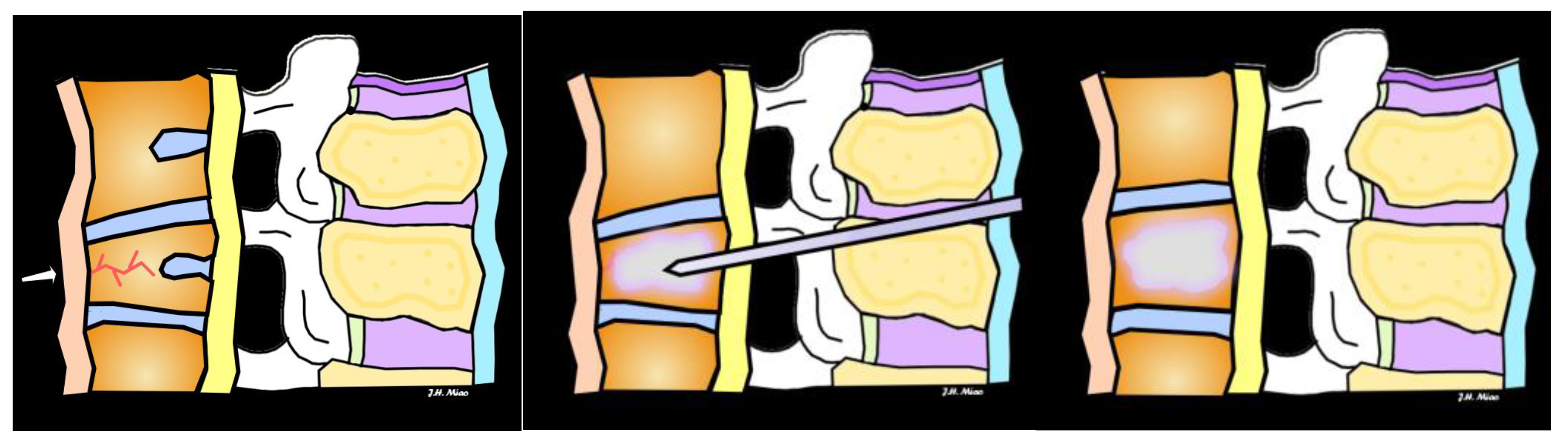
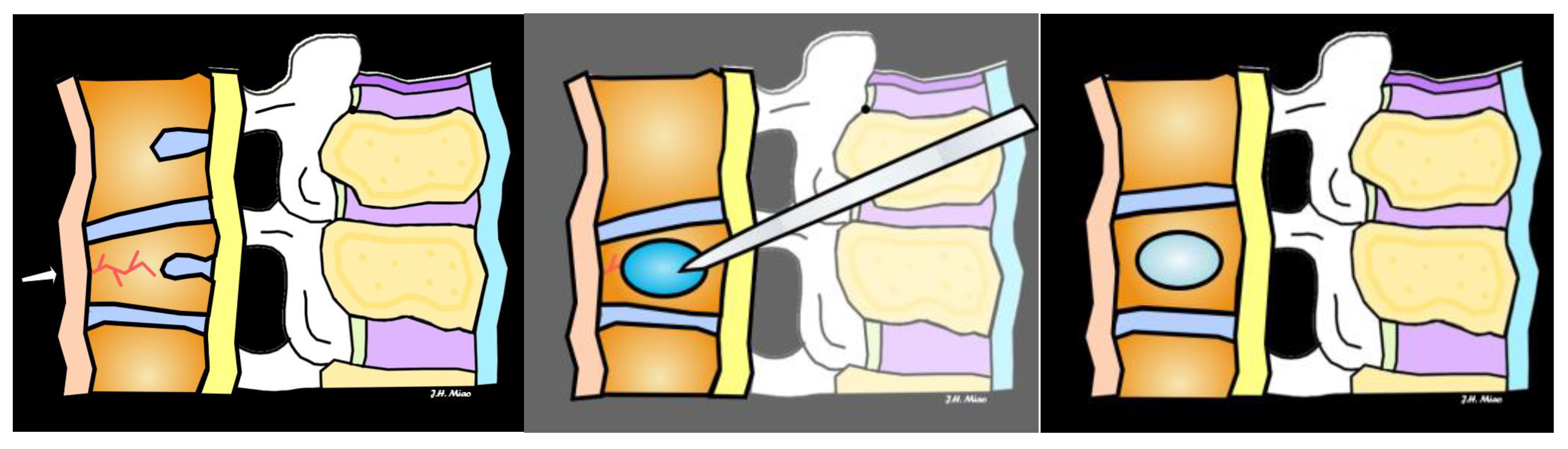
6. Treatment and Management
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J. Assessment of Osteoporosis at the Primary Health-Care Level. WHO Scientific Group Technical Report. 2007. Available online: https://frax.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf (accessed on 22 February 2019).
- Willers, C.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Borgström, F.; Kanis, J.A.; SCOPE Review Panel of the IOF. Osteoporosis in Europe: A compendium of country-specific reports. Arch. Osteoporos. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McArthur, C.; Lee, A.; Alrob, H.A.; Adachi, J.D.; Giangregorio, L.; Griffith, L.E.; Morin, S.; Thabane, L.; Ioannidis, G.; Lee, J.; et al. An update of the prevalence of osteoporosis, fracture risk factors, and medication use among community-dwelling older adults: Results from the Canadian Longitudinal Study on Aging (CLSA). Arch. Osteoporos. 2022, 17, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kado, D.M.; Browner, W.S.; Palermo, L.; Nevitt, M.C.; Genant, H.K.; Cummings, S.R.; Study of Osteoporotic Fractures Research Group. Vertebral fractures and mortality in older women: A prospective study. Arch. Intern. Med. 1999, 159, 1215–1220. [Google Scholar] [CrossRef]
- Backhauß, J.C.; Jansen, O.; Kauczor, H.U.; Sedaghat, S. Fatty Degeneration of the Autochthonous Muscles Is Significantly Associated with Incidental Non-Traumatic Vertebral Body Fractures of the Lower Thoracic Spine in Elderly Patients. J. Clin. Med. 2023, 12, 4565. [Google Scholar] [CrossRef]
- Wood, K.B.; Li, W.; Lebl, D.R.; Ploumis, A. Management of thoracolumbar spine fractures. Spine J. 2014, 14, 145–164, Erratum in Spine J. 2014, 14, A18. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; McCloskey, E.V.; Liu, E.; Åkesson, K.E.; Anderson, F.A.; Azagra, R.; Bager, C.L.; Beaudart, C.; Bischoff-Ferrari, H.A.; et al. Previous fracture and subsequent fracture risk: A meta-analysis to update FRAX. Osteoporos. Int. 2023, 34, 2027–2045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donnally, C.J., III; DiPompeo, C.M.; Varacallo, M. Vertebral Compression Fractures. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448171/ (accessed on 7 July 2024).
- Zhang, A.; Chauvin, B.J. Denis Classification. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Burns, J.E.; Yao, J.; Summers, R.M. Vertebral Body Compression Fractures and Bone Density: Automated Detection and Classification on CT Images. Radiology 2017, 284, 788–797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vu, C.; Gendelberg, D. Classifications in Brief: AO Thoracolumbar Classification System. Clin. Orthop. Relat. Res. 2020, 478, 434–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strickland, C.D.; DeWitt, P.E.; Jesse, M.K.; Durst, M.J.; Korf, J.A. Radiographic assessment of acute vs chronic vertebral compression fractures. Emerg. Radiol. 2023, 30, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, G.; Congia, S.; Verona, M.; Lombardo, M.; Podda, D.; Capone, A. The impact of magnetic resonance imaging in the diagnostic and classification process of osteoporotic vertebral fractures. Injury 2018, 49 (Suppl. S3), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Cicala, D.; Briganti, F.; Casale, L.; Rossi, C.; Cagini, L.; Cesarano, E.; Brunese, L.; Giganti, M. Atraumatic vertebral compression fractures: Differential diagnosis between benign osteoporotic and malignant fractures by MRI. Musculoskelet. Surg. 2013, 97 (Suppl. S2), S169–S179. [Google Scholar] [CrossRef] [PubMed]
- Mauch, J.T.; Carr, C.M.; Cloft, H.; Diehn, F.E. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. AJNR Am. J. Neuroradiol. 2018, 39, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Parker, S.L.; Wolinsky, J.P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: An evidenced-based review of the literature. Spine J. 2009, 9, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ogikubo, O.; Hansson, T. The prognosis for pain, disability, activities of daily living and quality of life after an acute osteoporotic vertebral body fracture: Its relation to fracture level, type of fracture and grade of fracture deformation. Eur. Spine J. 2009, 18, 77–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCarthy, J.; Davis, A. Diagnosis and Management of Vertebral Compression Fractures. Am. Fam. Physician 2016, 94, 44–50. [Google Scholar] [PubMed]
- Clerk-Lamalice, O.; Beall, D.P.; Ong, K.; Lorio, M.P. ISASS Policy 2018-Vertebral Augmentation: Coverage Indications, Limitations, and/or Medical Necessity. Int. J. Spine Surg. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Griffith, J. Identifying osteoporotic vertebral fracture. Quant. Imaging Med. And. Surg. 2015, 5, 592–602. [Google Scholar] [CrossRef]
- Dong, Q.; Luo, G.; Lane, N.E.; Lui, L.Y.; Marshall, L.M.; Kado, D.M.; Cawthon, P.; Perry, J.; Johnston, S.K.; Haynor, D.; et al. Deep Learning Classification of Spinal Osteoporotic Compression Fractures on Radiographs using an Adaptation of the Genant Semiquantitative Criteria. Acad. Radiol. 2022, 29, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Polzer, C.; Yilmaz, E.; Meyer, C.; Jang, H.; Jansen, O.; Lorenz, C.; Bürger, C.; Glüer, C.C.; Sedaghat, S. AI-based automated detection and stability analysis of traumatic vertebral body fractures on computed tomography. Eur. J. Radiol. 2024, 173, 111364. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xiong, Y.; Ye, G.; Liang, Y.; Guo, W.; Deng, Q.; Wu, L.; Jia, W.; Wu, D.; Chen, S.; et al. Deep learning-based artificial intelligence model for classification of vertebral compression fractures: A multicenter diagnostic study. Front. Endocrinol. 2023, 14, 1025749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, M.; Zhou, Z.; Tang, Q.; Xu, J.; Huang, Q.; Lu, L.; Duan, S.; Zhu, J.; Li, H. Differentiation of usual vertebral compression fractures using CT histogram analysis as quantitative biomarkers: A proof-of-principle study. Eur. J. Radiol. 2020, 131, 109264. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, H.; Chong, Y.; Porter, D.A.; Chan, L.L. Benign versus metastatic vertebral compression fractures: Combined diffusion-weighted MRI and MR spectroscopy aids differentiation. Eur. Radiol. 2013, 23, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, A.; Yoshiura, T.; Noguchi, T.; Togao, O.; Yamashita, K.; Kamano, H.; Honda, H. Usefulness of cone-beam CT before and after percutaneous vertebroplasty. AJR Am. J. Roentgenol. 2008, 191, 1401–1405. [Google Scholar] [CrossRef] [PubMed]



| Grade | Description |
|---|---|
| Grade 0 | Normal vertebral body. |
| Grade 1 | Mild height reduction (<25%) or mild endplate deformity. |
| Grade 2 | Moderate height reduction (25–40%) or moderate endplate deformity. |
| Grade 3 | Severe height reduction (>40%) or severe endplate deformity. |
| Type | Description |
|---|---|
| Type A | Anterior column only. |
| Type B | Middle column (including the posterior cortex of the vertebral body). |
| Type C | Posterior column (including the neural arch). |
| Type | Description |
|---|---|
| Type A | Simple fractures without much fragmentation. |
| Type A1 | Minimal displacement, indicating minimal instability. |
| Type A2 | More displacement than type A1 fractures, indicating a moderate level of instability. |
| Type A3 | Extensive displacement and are the most unstable among type A fractures. |
| Type B | Partial articular fractures that involve the joint surface but do not extend through it completely. |
| Type B1 | Involve a single articular surface and have a low level of instability. |
| Type B2 | Affect two articular surfaces and exhibit moderate instability. |
| Type B3 | Involve multiple articular surfaces and are highly unstable. |
| Type C | Complete articular fractures that extend through the joint surface. Highly unstable and often require surgical intervention. |
| Type C1 | Extra-articular, and occur away from the joint itself. |
| Type C2 | Involve a metaphyseal region and extend into the joint. |
| Type C3 | Extend into the joint and affect both the metaphysis and diaphysis. |
| Severity Score | Description |
|---|---|
| Score 1–3 | Nonoperative treatment recommended. |
| Score 4–7 | Operative or nonoperative treatment based on neurological status and injury characteristics. |
| Score ≥8 | Operative treatment recommended. |
| Description | Indications | Benefits | |
|---|---|---|---|
| Conservative Management | Rest, bracing, pain management with analgesics, physiotherapy | Mild to moderate fractures, no neurological deficits, stable fractures | Non-invasive, avoids surgical risks, allows natural healing |
| Vertebroplasty | Injection of bone cement into fractured vertebra to stabilize | Painful fractures not responding to conservative treatment, no neurological symptoms | Provides rapid pain relief, improves vertebral stability |
| Kyphoplasty | Balloon inserted to restore vertebral height, followed by cement injection | Fractures causing significant vertebral height loss, pain, or deformity | Restores vertebral height, reduces pain, improves mobility |
| Surgical Intervention | Spinal fusion, implants, decompression surgery for nerve involvement | Severe fractures, instability, neurological deficits, failure of other treatments | Corrects deformity, stabilizes spine, relieves nerve compression |
| Modality | Characteristics | Benefits | Limitations |
|---|---|---|---|
| Radiograph | Standard imaging technique, often first-line for detecting fractures and alignment |
|
|
| CT including DECT | Detailed cross-sectional images of bone and vertebral structures |
|
|
| MRI | Superior soft tissue contrast, gold standard for detecting bone marrow edema |
|
|
| PET and Bone Scan | Detects metabolic activity and useful in identifying metastatic disease or infection, detects increased bone activity |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, K.H.; Miao, J.H.; Belani, P.; Dayan, E.; Carlon, T.A.; Cengiz, T.B.; Finkelstein, M. Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures. J. Imaging 2024, 10, 244. https://doi.org/10.3390/jimaging10100244
Miao KH, Miao JH, Belani P, Dayan E, Carlon TA, Cengiz TB, Finkelstein M. Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures. Journal of Imaging. 2024; 10(10):244. https://doi.org/10.3390/jimaging10100244
Chicago/Turabian StyleMiao, Kathleen H., Julia H. Miao, Puneet Belani, Etan Dayan, Timothy A. Carlon, Turgut Bora Cengiz, and Mark Finkelstein. 2024. "Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures" Journal of Imaging 10, no. 10: 244. https://doi.org/10.3390/jimaging10100244
APA StyleMiao, K. H., Miao, J. H., Belani, P., Dayan, E., Carlon, T. A., Cengiz, T. B., & Finkelstein, M. (2024). Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures. Journal of Imaging, 10(10), 244. https://doi.org/10.3390/jimaging10100244






