Upcycling Mill Scale and Aluminum Dross for Sustainable Materials Processing: Synthesis of Hercynite via Fe2O3-Al2O3-C Combustion
Abstract
1. Introduction
2. Results and Discussion
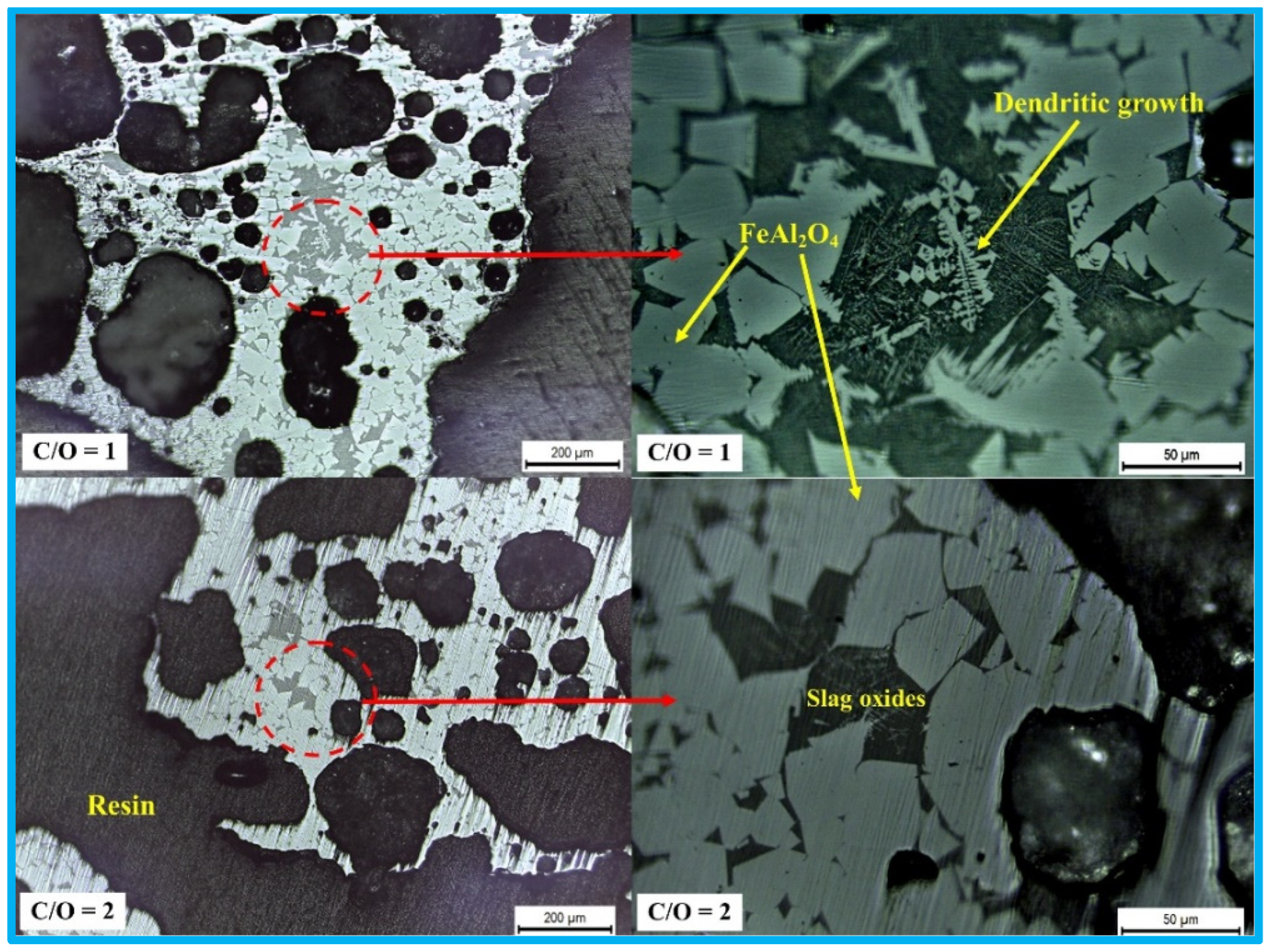
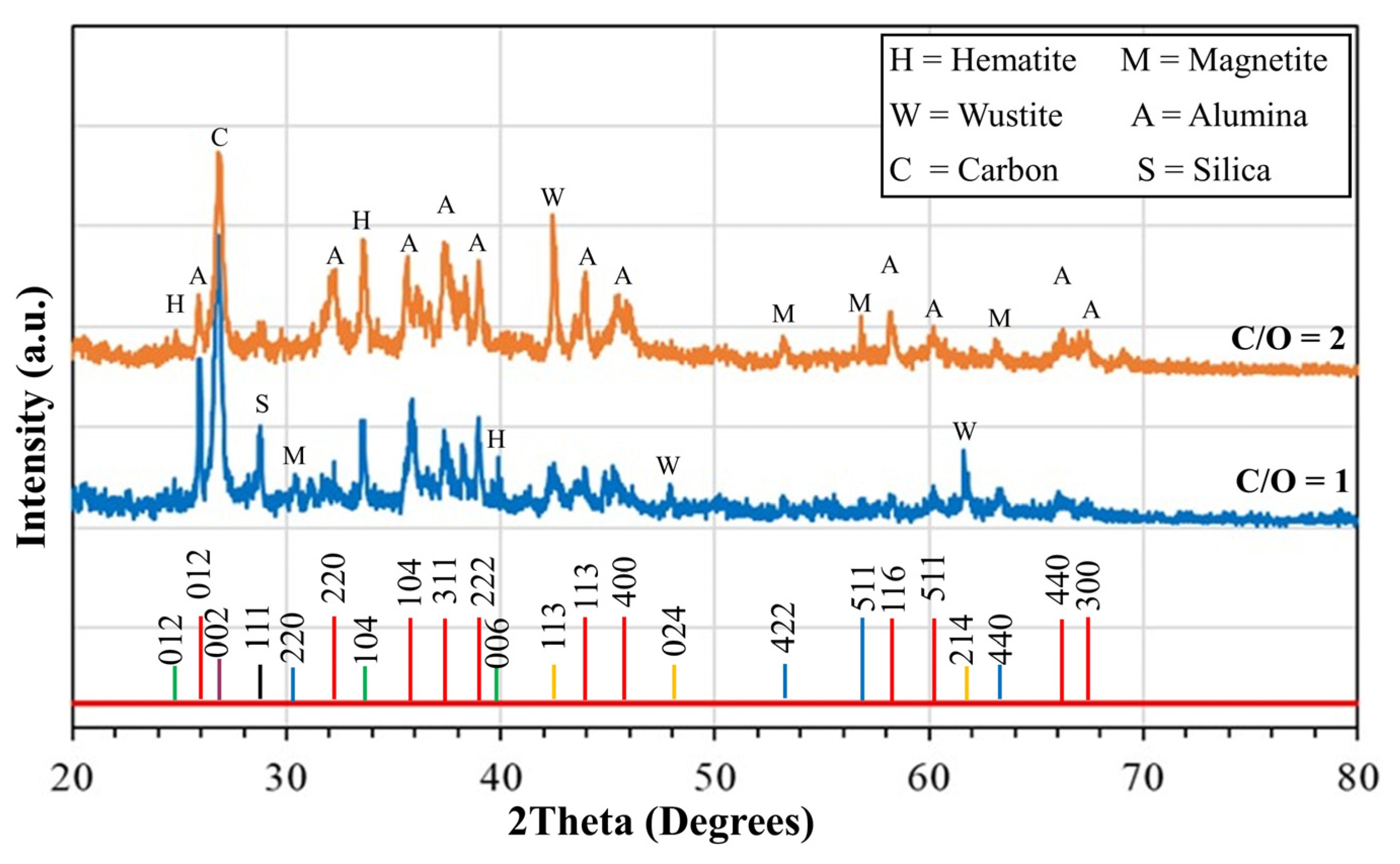
2.1. High-Temperature Products and Phase Analysis
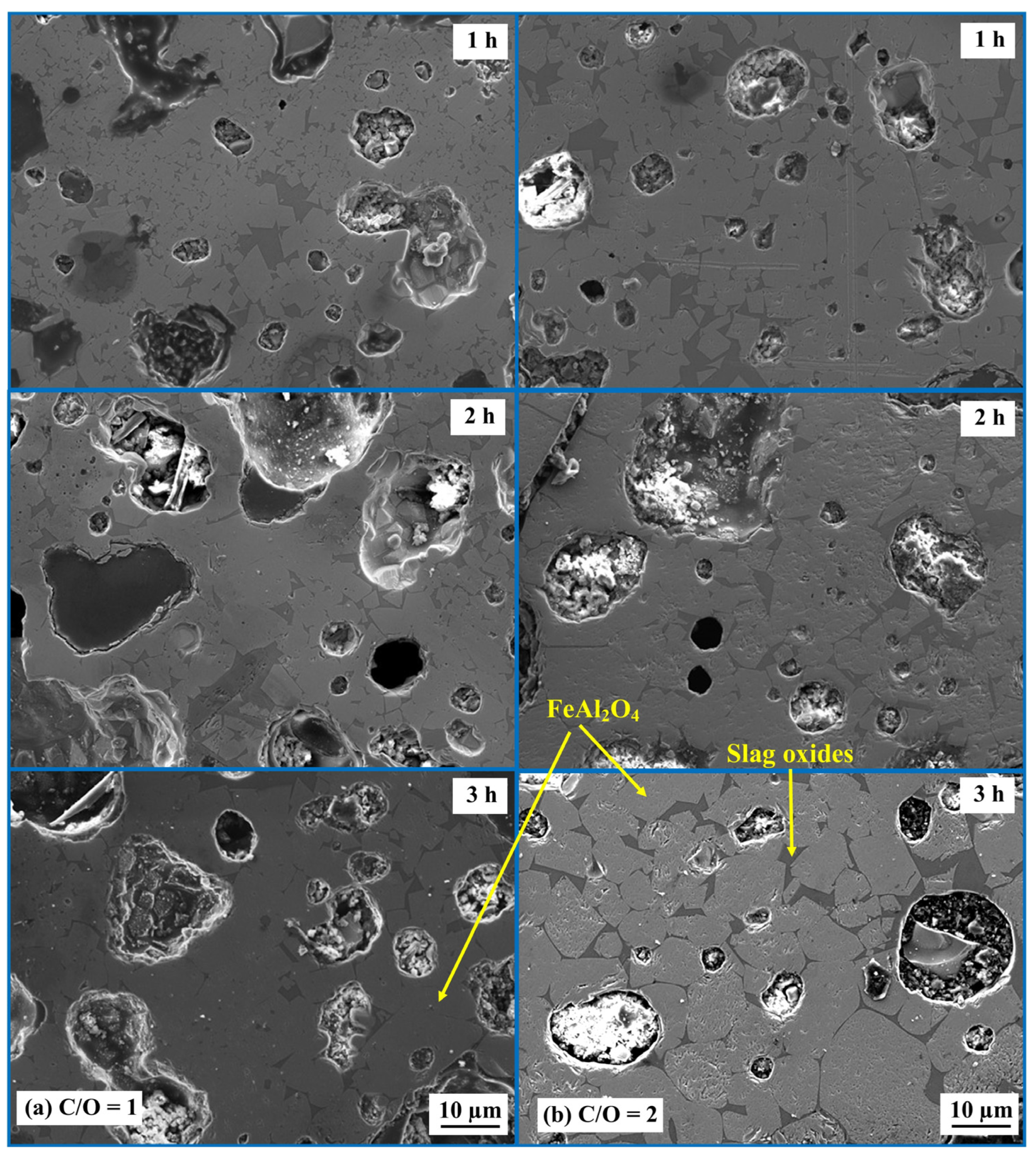
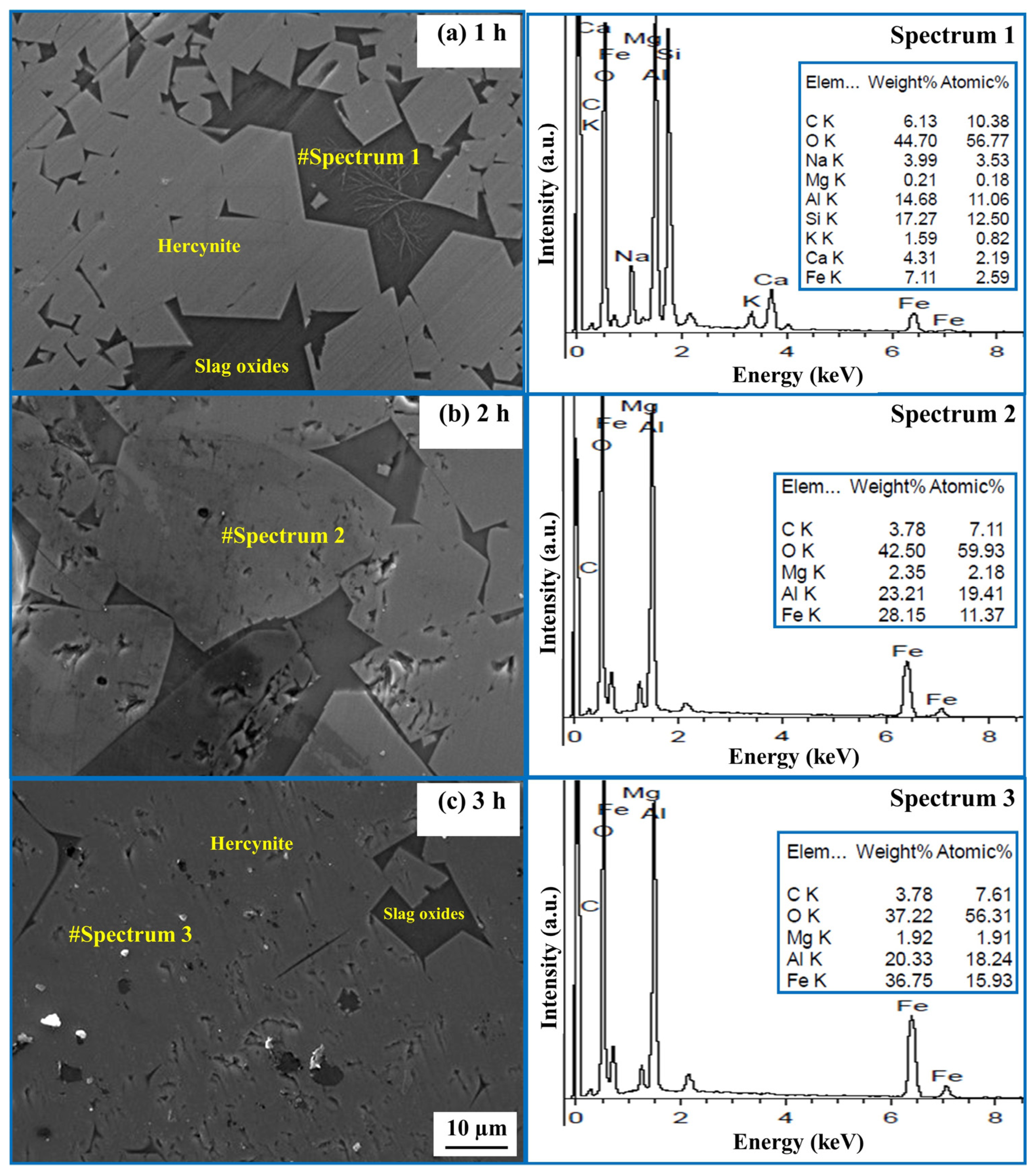
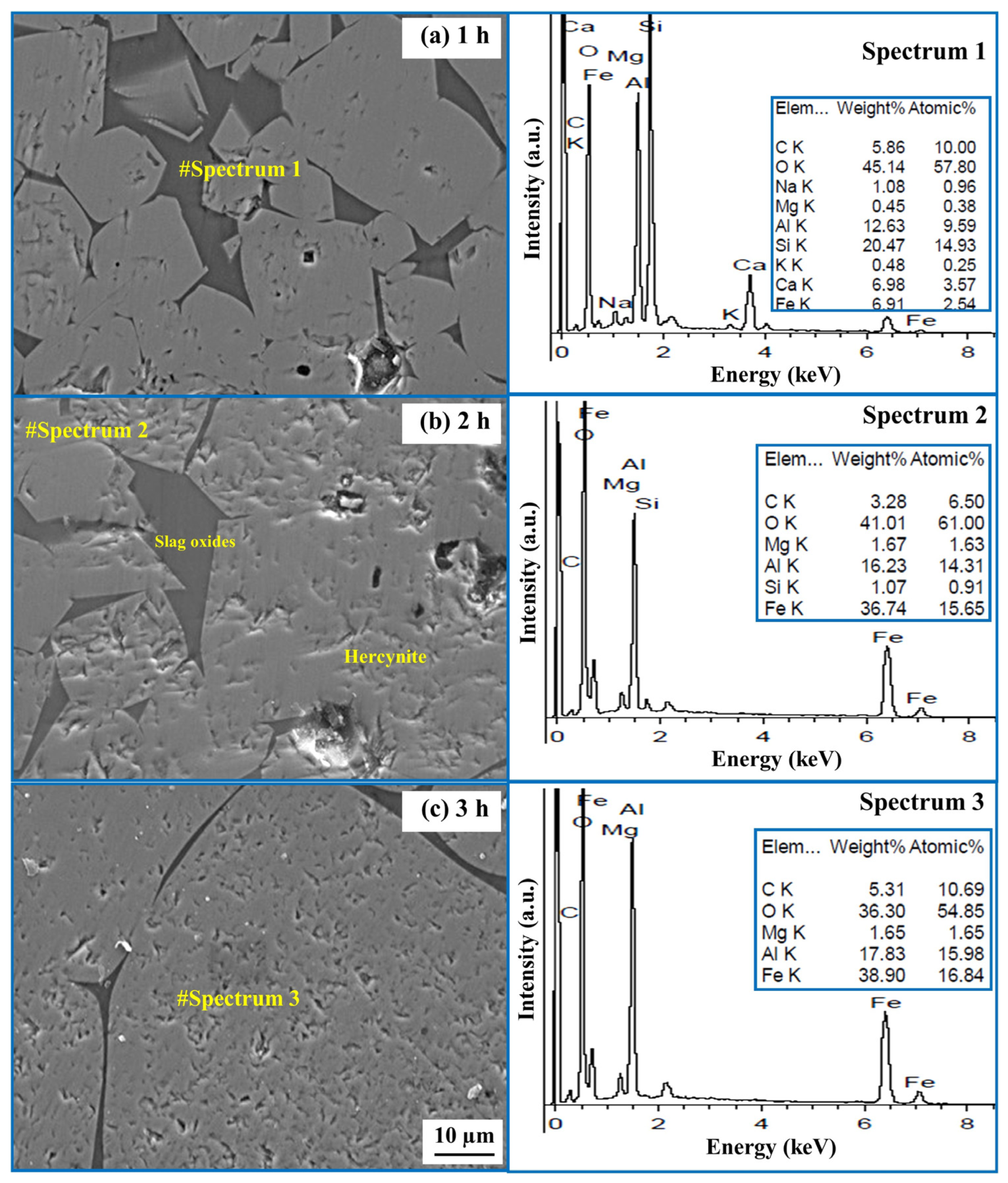
2.2. Effect of Time on the Formation of Hercynite
2.3. Effect of Carbon on the Formation of Hercynite
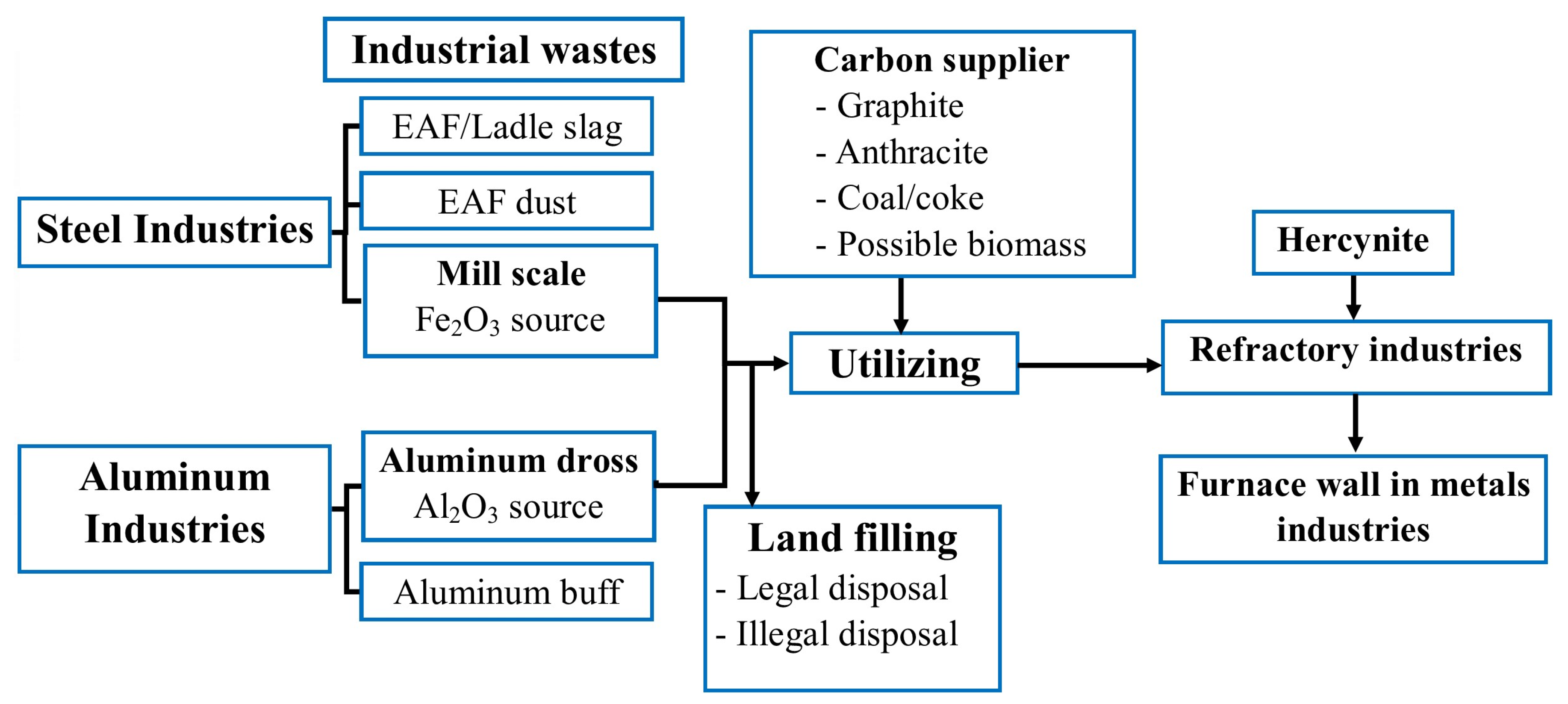
2.4. Preliminary Evaluation of Worthiness for the Production of Hercynite from the Industrial Wastes
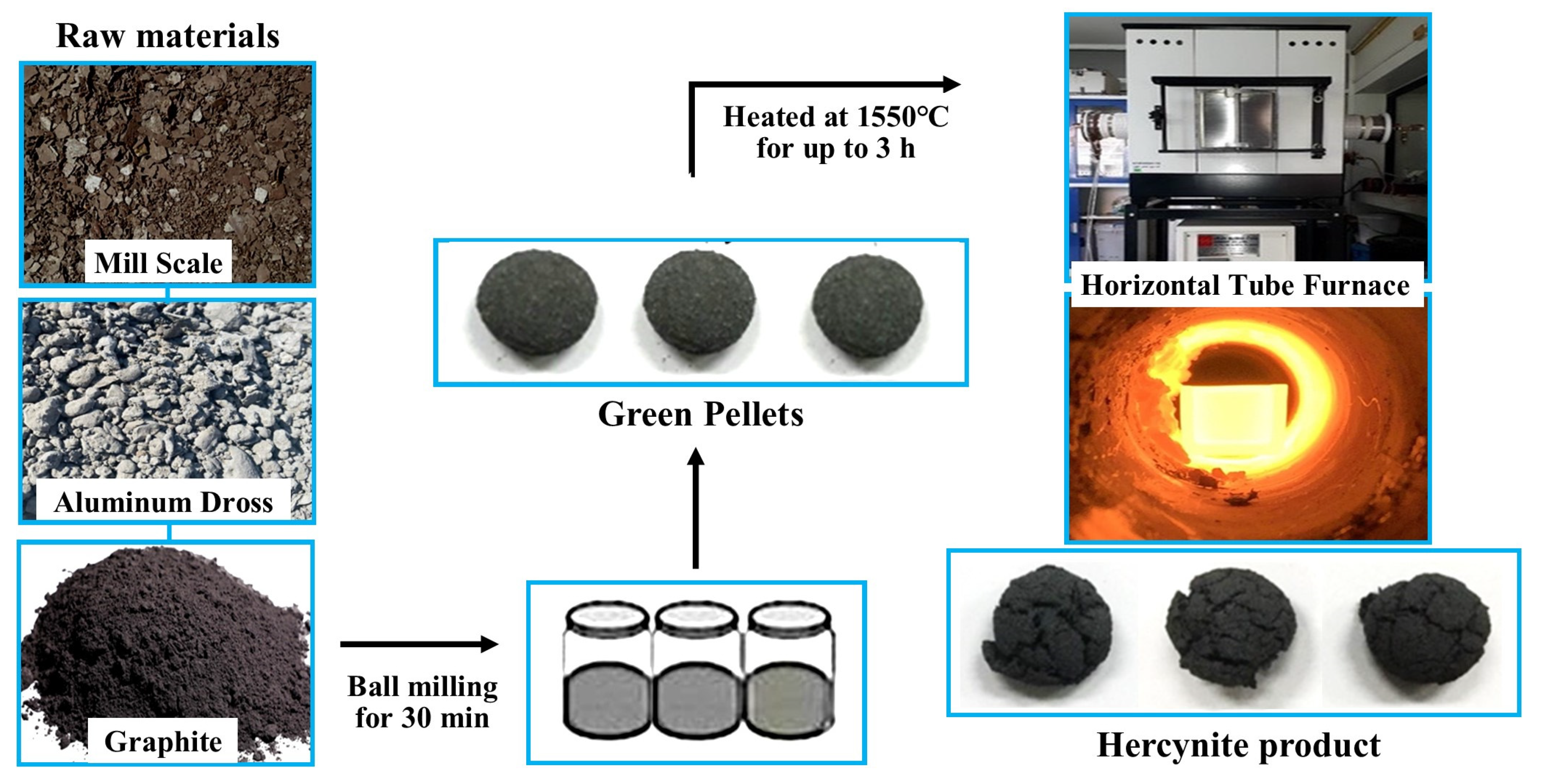
3. Materials and Methods
3.1. Materials Preparation
3.2. High-Temperature Experiment
3.3. Analysis
4. Conclusions
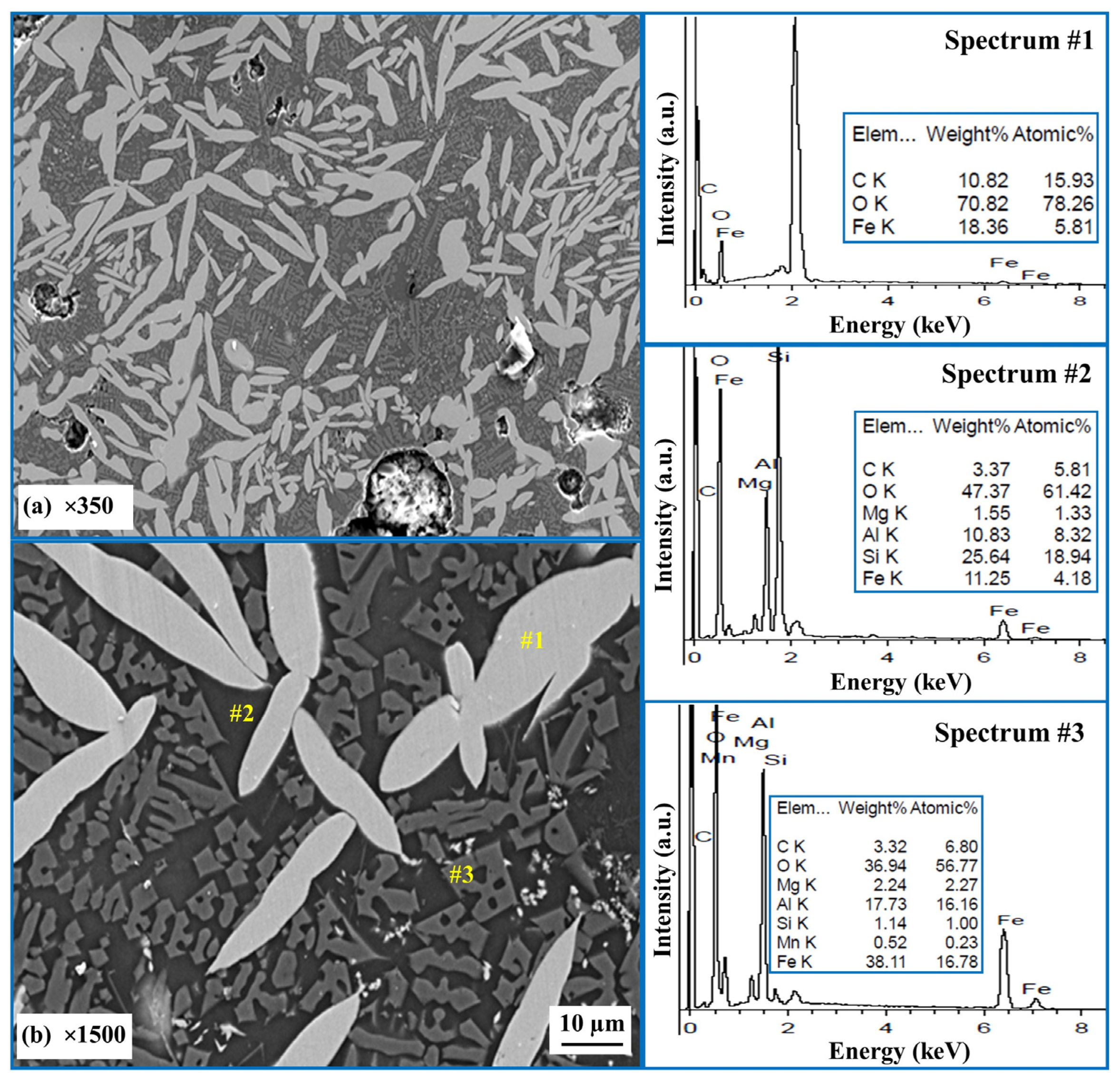
- The utilization of AD and MS in the production of hercynite is indeed feasible. The formation mechanism of hercynite in the AD-MS-graphite system involves the formation of a dendritic shape, and subsequent fusing to form grain shapes, followed by further grain growth.
- The formation of the hercynite phase is influenced by the heating duration from 1 to 3 h. Visual observations indicate that, with longer heating times, the grain size of hercynite increases.
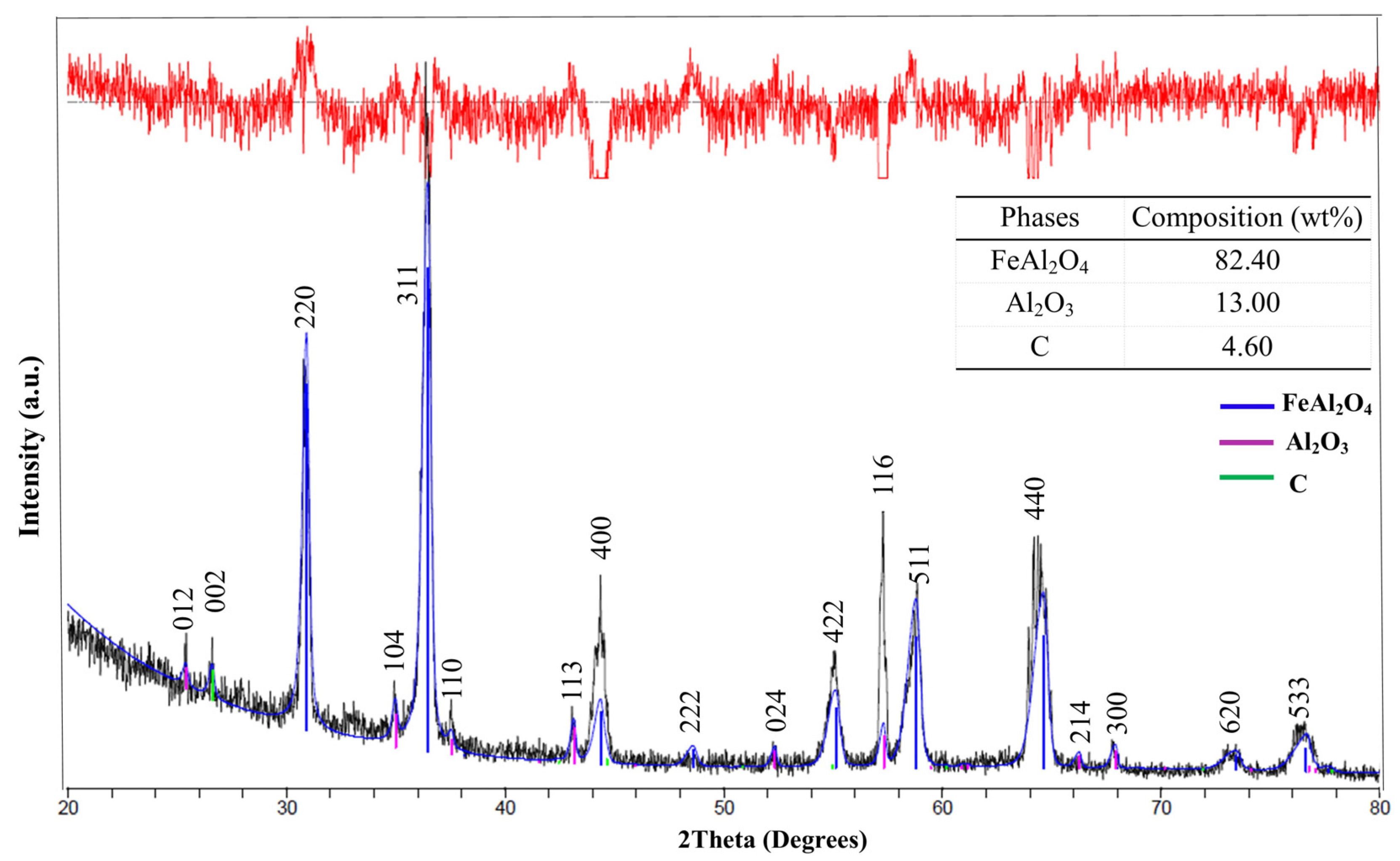
- XRD phase analysis using the Rietveld method revealed that the major components of the product were FeAl2O4, Al2O3, and C. For the product with a C/O ratio of 1, the amounts were 85.11% FeAl2O4, 10.99% Al2O3, and 3.9% C. For the product with a C/O ratio of 2, the amounts were 82.4% FeAl2O4, 13.0% Al2O3, and 4.6% C. The combustion of raw pellets with a C/O ratio of 1 at 1550 °C for 1 h in a normal air atmosphere is economically viable for producing hercynite, with a yield of 85.11 wt%.
- Carbon is essential for the formation of hercynite within the AD-MS-graphite system. Its crucial role lies in reducing Fe2O3 present in the MS, which facilitates subsequent reactions leading to the formation of hercynite. The increase in carbon content in the system from C/O ratios of 1 to 2 can slightly decrease the yield of hercynite products. Visual observations indicated that this increase in carbon content impacts the development of the microstructure of the product, with hercynite grain size being larger in pellets with a C/O ratio of 2.
- AD and MS can serve as sources of Al2O3 and Fe2O3, respectively, for producing hercynite, potentially eliminating the need for commercial alumina and iron ore entirely. This utilization presents a sustainable, cost-effective, and eco-friendly alternative to using virgin raw materials.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, M.I.; Lopez, F.A.; Torralba, J.M. Recycling of steel plant mill scale via iron ore palletisation Process. Ironmak. Steelmak. 2009, 36, 409–415. [Google Scholar]
- Bagatini, M.C.; Zymla, V.; Osorio, E.; Vilela, A.C.F. Characterization and reduction behavior of mill scale. ISIJ Int. 2011, 51, 1072. [Google Scholar] [CrossRef]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of mill scale waste into valuable products via carbothermic reduction. J. Metall. 2015, 4, 1–9. [Google Scholar] [CrossRef][Green Version]
- Martin, M.I.; Lopez, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef]
- Sen, R.; Dehiya, S.; Pandel, U.; Banerjee, M.K. Utilization of low-grade coal for direct reduction of mill scale to obtain sponge iron: Effect of reduction time and particle size. Procedia Earth Planet. Sci. 2015, 11, 8–14. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, H.; Zhang, L.; Ma, J.; Zhou, L.; Liu, P.; Chen, J.; Chen, G.; Peng, J. Preparation of reduced iron powder using combined distribution of wood-charcoal by microwave heating. J. Alloys Compd. 2014, 613, 102–106. [Google Scholar] [CrossRef]
- Khaerudini, D.S.; Chanif, I.; Insiyanda, D.R.; Destyorini, F.; Alva, S.; Premono, A. Preparation and characterization of mill scale industrial waste reduced by biomass-based carbon. J. Sustain. Metall. 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Baganiti, M.C.; Kan, T.; Evans, T.J.; Strezov, V. Iron ore reduction by biomass volatiles. J. Metall. 2021, 7, 215–226. [Google Scholar]
- Shi, J.; Wang, D.R.; He, Y.D.; Qi, H.B.; Wei, G. Reduction of oxide scale on hot-rolled strip steels by carbon monoxide. Mater. Lett. 2018, 62, 3500–3502. [Google Scholar]
- Sista, K.S.; Dwarapudi, S.; Nerune, V.P. Direct reduction recycling of mill scale through iron powder synthesis. ISIJ Int. 2019, 5, 787–794. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, Q.; Ye, Z. Life cycle environmental and economic assessment of alumina recovery from secondary aluminum dross in China. J. Clean. Prod. 2020, 277, 123291. [Google Scholar] [CrossRef]
- Huang, K.; Yi, X. Resource utilization and high-value targeted conversion for secondary aluminum dross: A review. JOM 2023, 75, 279–290. [Google Scholar] [CrossRef]
- Xie, H.; Guo, Z.; Xu, R.; Zhang, Y. Particle sorting to improve the removal of fluoride and aluminum nitride from secondary aluminum dross by roasting. Environ. Sci. Pollut. Res. 2023, 30, 54536–54546. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tsai, C.H.; Zhang, D.N.; Syu, S.S.; Kuo, Y.M. Recycling of aluminum dross for producing calcinated alumina by microwave plasma. Sustain. Environ. Res. 2022, 32, 50. [Google Scholar] [CrossRef]
- Daghetta, M.A.A.; Dapiaggib, M.; Pellegrinoa, L.; Pastorea, B.; Pagliarib, L.; Mazzocchiaa, C.V. Synthesis of Hercynite at very Mild Condition. Chem. Eng. Trans. 2015, 43, 1741–1746. [Google Scholar]
- Zhang, X.; Yu, R.; Yu, X. Characteristics of hercynite and its application: In refractories. China’s Refract. 2012, 21, 17–22. [Google Scholar]
- Chen, J.; Su, J.; Yan, M.; Yang, S. Two methods of synthesizing hercynite. Appl. Mech. Mater. 2014, 543–547, 3830–3833. [Google Scholar] [CrossRef]
- Botta, P.M.; Aglietti, E.F.; Porto López, J.M. Mechanochemical synthesis of hercynite. Mater. Chem. Phys. 2022, 76, 104–109. [Google Scholar] [CrossRef]
- Chen, J.; Yu, L.; Sun, J.; Li, Y.; Xue, W. Synthesis of hercynite by reaction sintering. Eur. Ceram. Soc. 2011, 31, 259–263. [Google Scholar] [CrossRef]
- Ma, S.; Li, Y.; Sun, J.; Wang, Z. Synthesis of hercynites under N2 atmosphere. Kuei Suan Jen Hsueh J. Chin. Ceram. Soc. 2011, 39, 424–429. [Google Scholar]
- Dutta, D.P.; Sharma, G. Synthesis and magnetic behavior of spinel FeAl2O4 nanoparticles. Mater. Sci. Eng. B 2011, 176, 177–180. [Google Scholar] [CrossRef]
- Fukushima, J.; Hayashi, Y.; Takizawa, H. Structure and magnetic properties of FeAl2O4 synthesized by microwave magnetic feld irradiation. J. Asian Ceram. Soc. 2013, 1, 41–45. [Google Scholar] [CrossRef]
- Moura, J.; Loreto, R.; de Araujo, J.F.F.; Solórzano, G. Magnetic mapping of hercynite produced by combustion synthesis. Microsc. Microanal. Microstruct. 2021, 27, 3312–3314. [Google Scholar] [CrossRef]
- Tan, W.; Guo, X.; She, Y.; Li, H.; Lei, Y.; You, J.; Zhang, X.; Luo, X. Efects of molten salt temperature and holding time on synthesis of hercynite by molten salt method. Ceram. Int. 2022, 48, 11555–11560. [Google Scholar] [CrossRef]
- Perdomo-Gonzales, L.; Quintana-Puchol, R.; Alujas-Diaz, A.; Perdomo-Gomez, L.A.; Ruiz-Perez, R.; Cruz-Crespo, A. Aluminothermic synthesis of ceramics from the hercynite-alumina system, using mill scale and aluminium Chips. DYNA 2023, 90, 106–113. [Google Scholar] [CrossRef]
- Wongsawan, P.; Srichaisiriwech, W.; Kongkarat, S. Synthesis of Ferroalloys via Mill Scale-Dross-Graphite Interaction: Implication for Industrial Wastes Upcycling. Metals 2022, 12, 1909. [Google Scholar] [CrossRef]
- Halmann, M.; Frei, A.; Steinfeld, A. Carbothermal reduction of alumina: Thermochemical equilibrium calculations and experimental investigation. Energy 2007, 32, 2420–2427. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; Saha-Chaudhury, N.; O’Kane, P.; Skidmore, C.; Knights, D.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by metallurgical coke and waste plastics blends. ISIJ Int. 2011, 51, 498–507. [Google Scholar] [CrossRef]
- Kim, I.T.; Lee, J.; An, J.C.; Jung, E.; Lee, H.K.; Morita, M.; Shim, J. Capacity improvement of Tin-on carbon-coated graphite anode for rechargeable lithium ion batteries. J. Electrochem. Sci. 2016, 11, 5807–5818. [Google Scholar] [CrossRef]
- Jayashree, M.; Parthibavarman, M.; Prabhakaran, S. Hydrothermal-induced ɑ-Fe2O3/graphene nanocomposite with ultrahigh capacitance for stabilized and enhanced supercapacitor electrodes. Ionics 2019, 25, 3309–3319. [Google Scholar] [CrossRef]
- Alumina Price: Charts, Forecasts & News, FocusEconomics. Available online: https://www.focus-economics.com/commodities/base-metals/alumina/ (accessed on 18 May 2024).
- Industrial Metals, Markets Insider. Available online: https://markets.busines sinsider.com/commodities/iron-ore-price (accessed on 18 May 2024).
- Current Metal Prices, iScrap App. Available online: https://iscrapapp.com/prices/ (accessed on 4 May 2024).
- The Environmental Protection Cost Facing a Huge Increase after Aluminum Dross as Hazardouswaste, Aluminum Machinery Solution Provider. Available online: https://www.machine4aluminium.com/ (accessed on 18 May 2024).
- Mill Scale Latest Price from Manufacturers, Indian Suppliers-Mill Scales. Available online: https://www.exportersindia.com/indian-suppliers/mill-scales.htm (accessed on 18 May 2024).



| C/O Ratios | Times (h) | Oxides (wt%) | |||||
|---|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | MgO | SiO2 | CaO | Other | ||
| 1 | 1 | 55.64 | 26.43 | 2.38 | 10.49 | 1.34 | 3.72 |
| 1 | 2 | 55.15 | 26.68 | 2.38 | 10.95 | 1.34 | 3.62 |
| 1 | 3 | 56.13 | 26.90 | 2.62 | 10.25 | 1.22 | 2.88 |
| 2 | 1 | 54.77 | 26.74 | 2.25 | 11.35 | 1.65 | 3.24 |
| 2 | 2 | 55.61 | 25.91 | 2.35 | 11.27 | 1.65 | 3.21 |
| 2 | 3 | 55.14 | 26.64 | 2.53 | 11.02 | 1.61 | 3.06 |
| Raw Materials | Cost (US$/ton) | Types of Supplier | Worthiness | ||
|---|---|---|---|---|---|
| Aluminum Sources | Iron Sources | Economy | Environmental | ||
| Alumina [31] | - | 340 | Commercial Mining/ Commercial Recycler/ Commercial | Higher productions cost/ Natural resources consumption | Wastes generation/ More landfill/ Underground water and air pollutions |
| - | Iron ore [32] | 117 | |||
| - | Scrap iron [33] | 213.31 | |||
| AD [34] | - | 145 | Aluminum Smelter waste Steel mill byproduct | Lower productions cost/ Reduce disposal cost/ Waste valorization/ Resources consumption reduction | Waste reduction/ Landfill reduction/ Reduced pollutions/ Resource conservation/ Energy Savings |
| - | MS [35] | 36–108 | |||
| Oxides (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Fe2O3 | CaO | K2O | MgO | MnO | Na2O | SO3 | CuO | TiO2 | ZnO | Others |
| 69.94 | 5.01 | 0.54 | 1.0 | 0.76 | 4.91 | 0.15 | 10.65 | 2.46 | 0.37 | 0.17 | 0.25 | 3.79 |
| Oxides (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Al2O3 | CaO | SO3 | TiO2 | K2O | P2O5 | other |
| 93.66 | 1.42 | 0.82 | 0.17 | 0.08 | 0.04 | 0.02 | 0.04 | 3.75 |
| Blend | AD (wt%) | MS (wt%) | Graphite (wt%) | C/O Ratios |
|---|---|---|---|---|
| A | 52.5 | 39.2 | 8.3 | 1 |
| B | 48.5 | 36.2 | 15.3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongkajun, N.; Cherdhirunkorn, B.; Kongkarat, S. Upcycling Mill Scale and Aluminum Dross for Sustainable Materials Processing: Synthesis of Hercynite via Fe2O3-Al2O3-C Combustion. Recycling 2024, 9, 80. https://doi.org/10.3390/recycling9050080
Kongkajun N, Cherdhirunkorn B, Kongkarat S. Upcycling Mill Scale and Aluminum Dross for Sustainable Materials Processing: Synthesis of Hercynite via Fe2O3-Al2O3-C Combustion. Recycling. 2024; 9(5):80. https://doi.org/10.3390/recycling9050080
Chicago/Turabian StyleKongkajun, Nuntaporn, Benya Cherdhirunkorn, and Somyote Kongkarat. 2024. "Upcycling Mill Scale and Aluminum Dross for Sustainable Materials Processing: Synthesis of Hercynite via Fe2O3-Al2O3-C Combustion" Recycling 9, no. 5: 80. https://doi.org/10.3390/recycling9050080
APA StyleKongkajun, N., Cherdhirunkorn, B., & Kongkarat, S. (2024). Upcycling Mill Scale and Aluminum Dross for Sustainable Materials Processing: Synthesis of Hercynite via Fe2O3-Al2O3-C Combustion. Recycling, 9(5), 80. https://doi.org/10.3390/recycling9050080









