Sludge-Based Superparamagnetic Nano-Sorbent Functionalized by Lanthanum Silicate Nanorods for Phosphorus Adsorption and Fertilization
Abstract
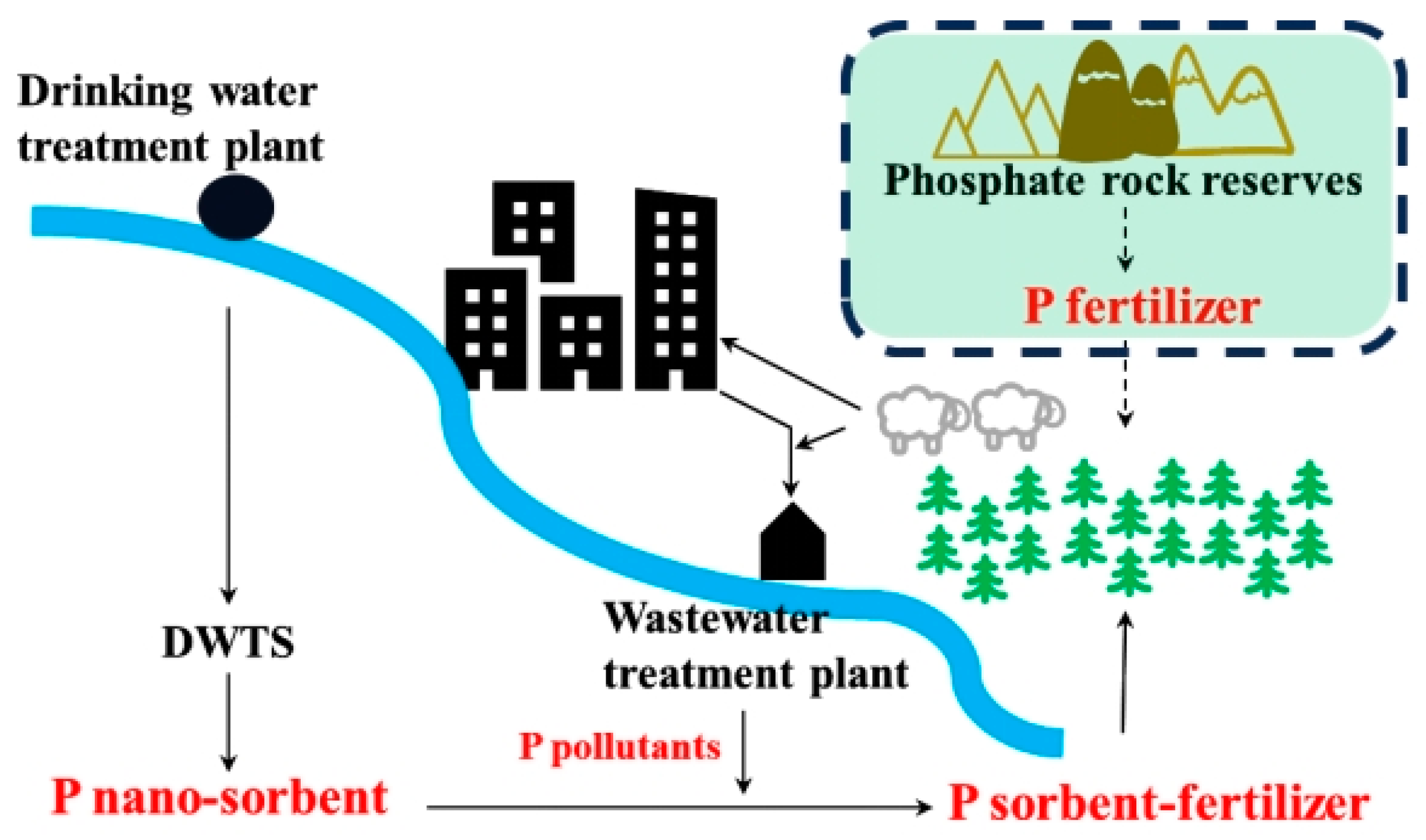
1. Introduction
2. Results and Discussion
2.1. Phosphate Adsorption by DWTS-Based Sorbent
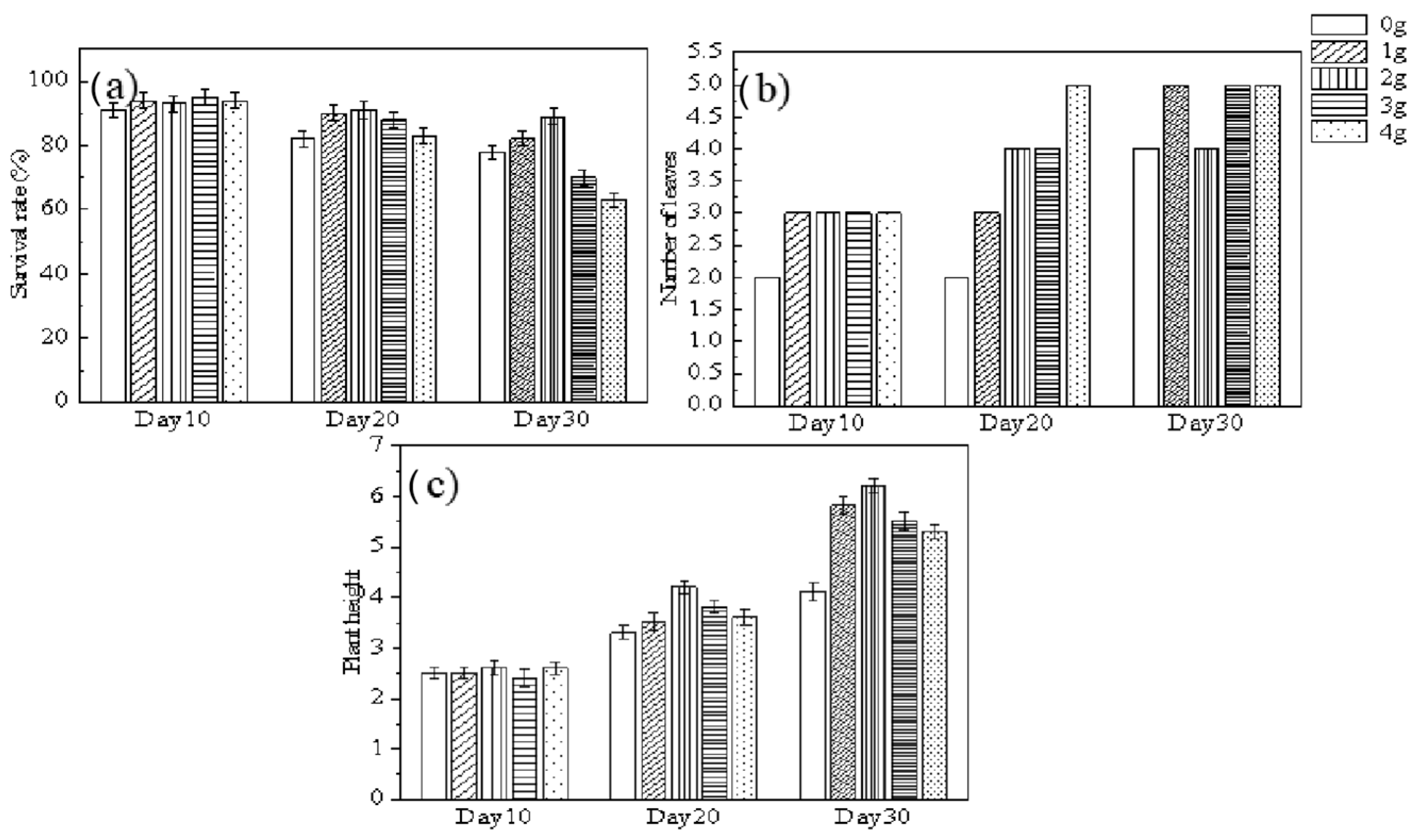
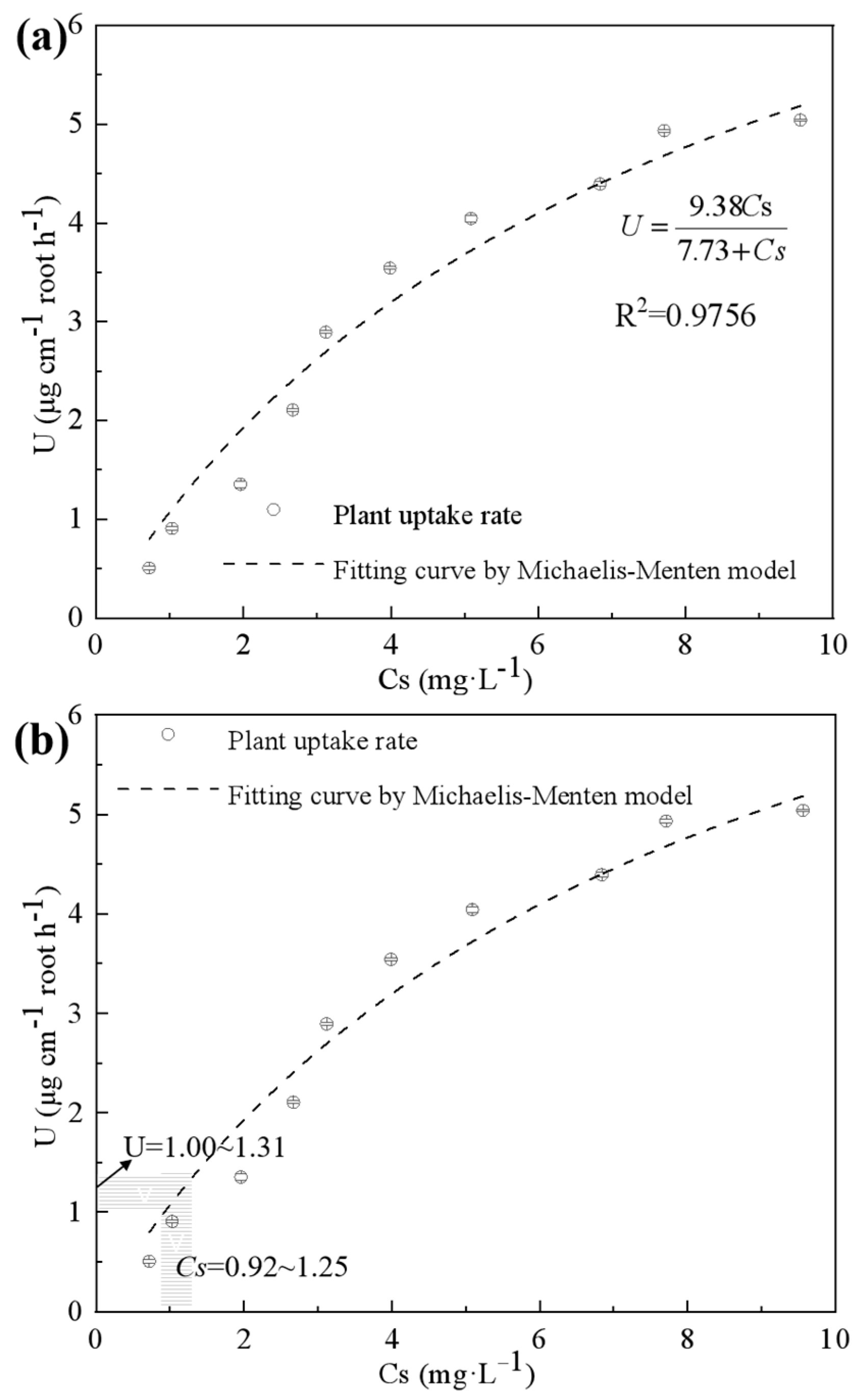
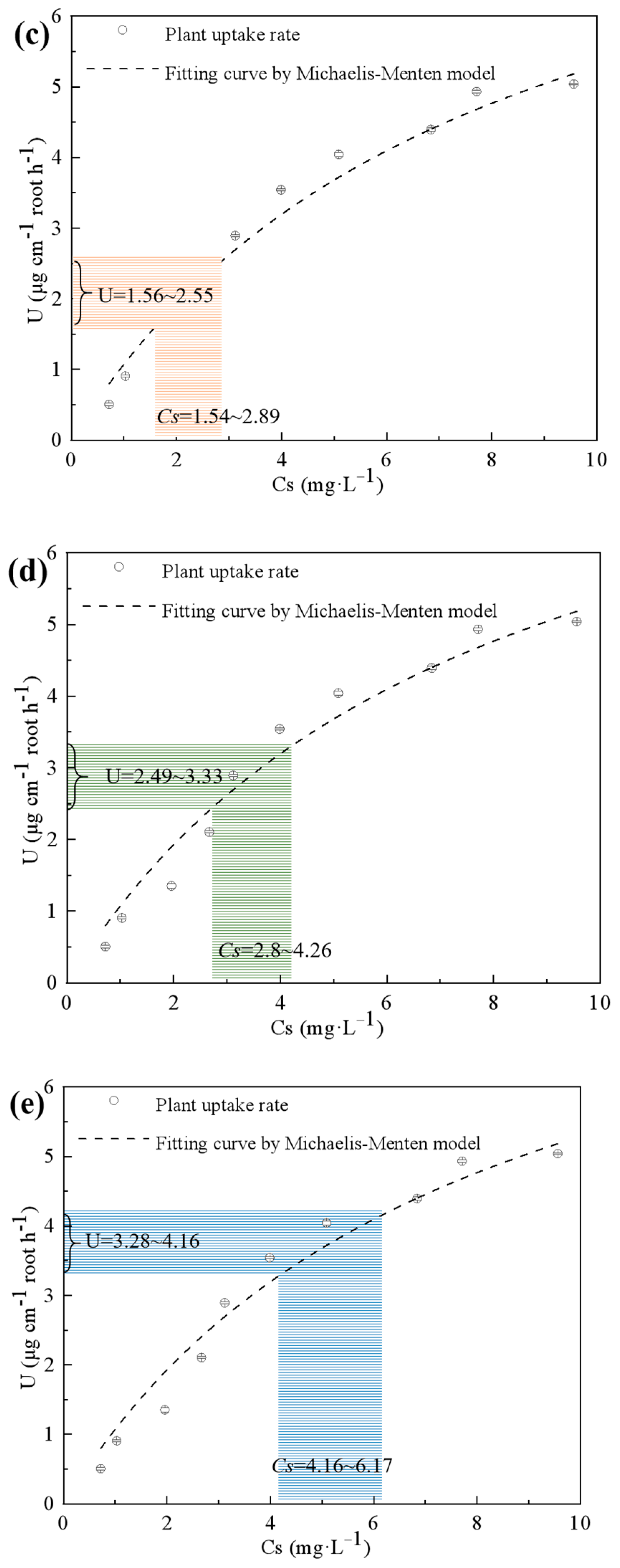
2.2. Fertilization Effect of Exhausted P-Sorbent D in Hydroponic Culture
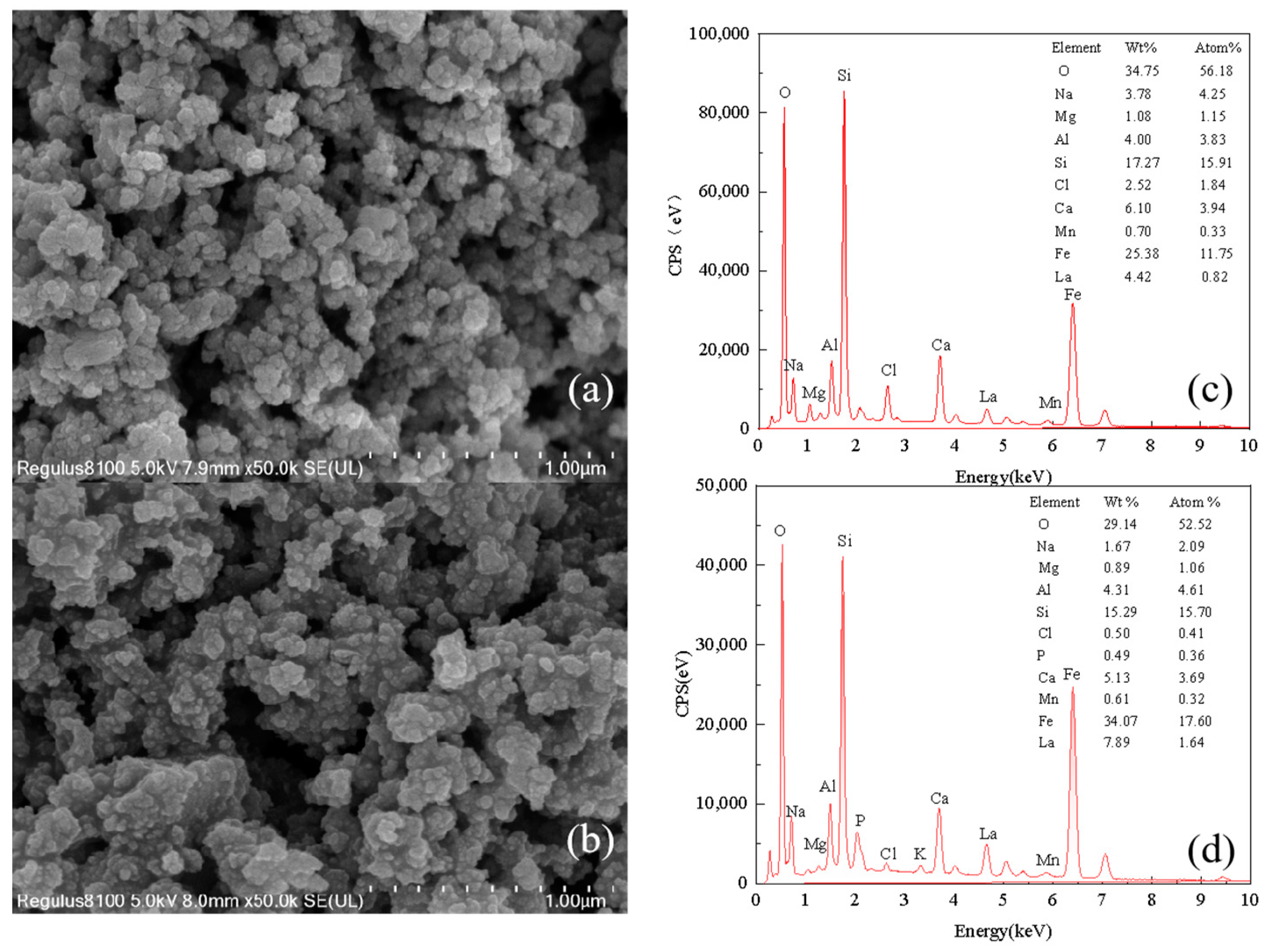
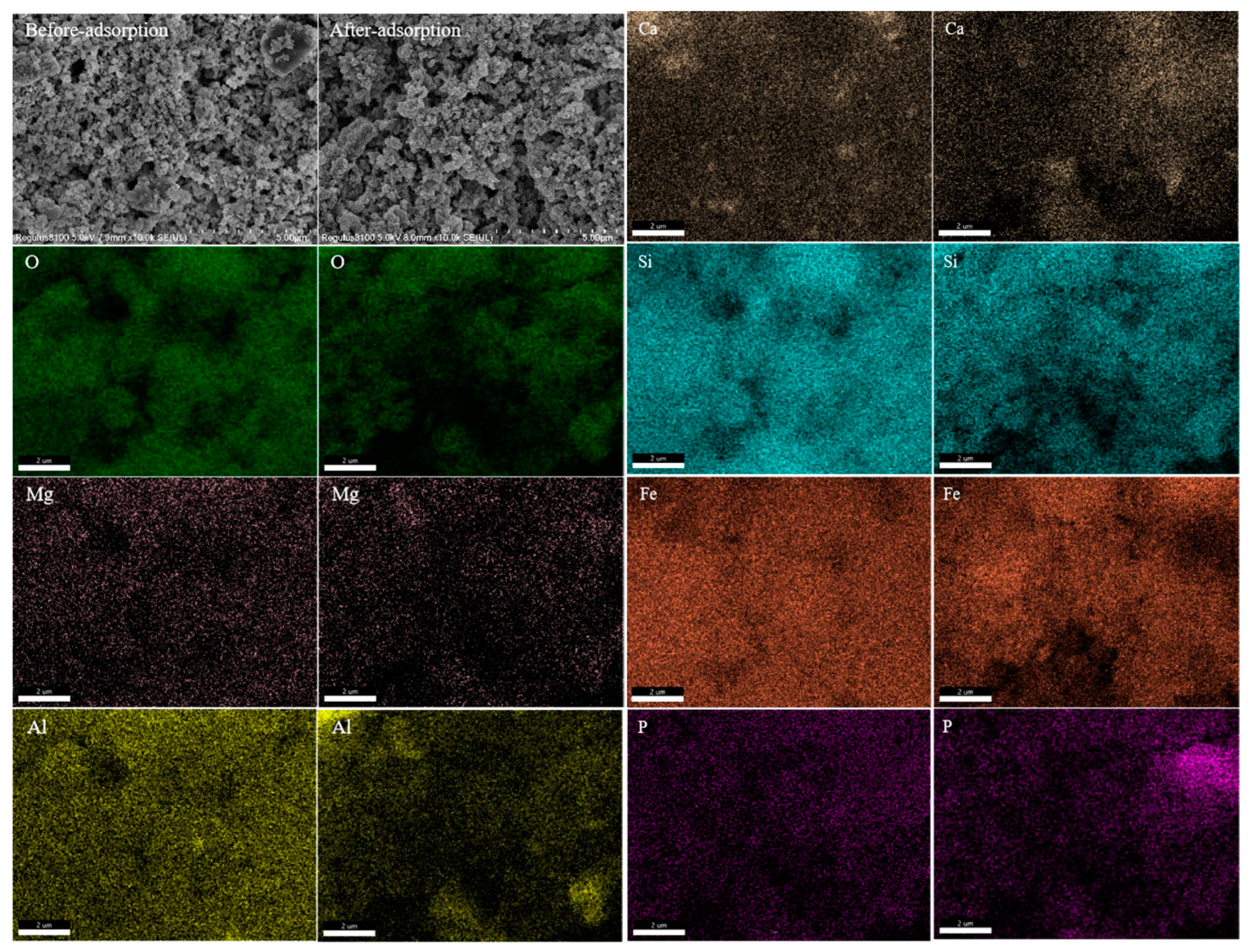
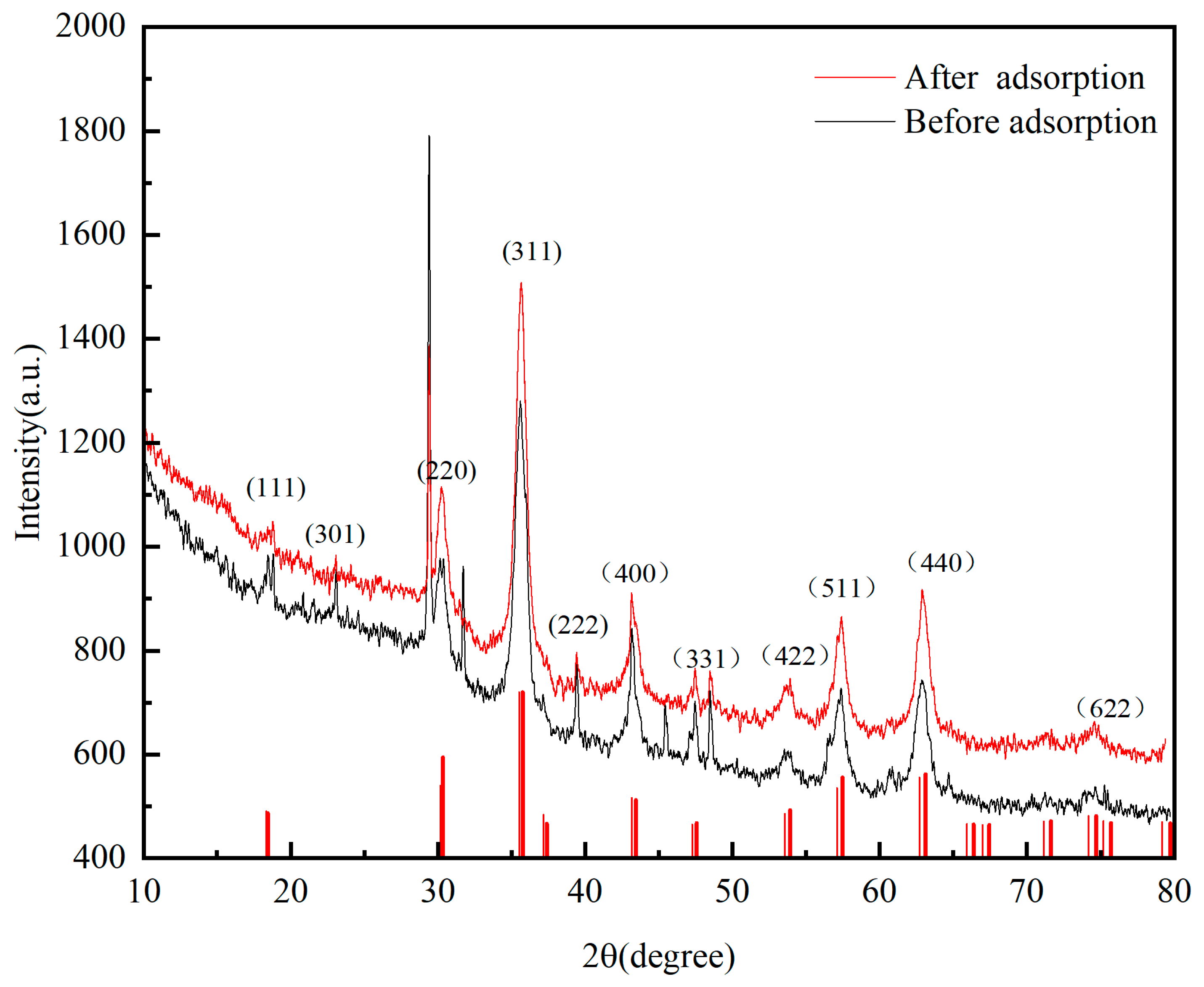
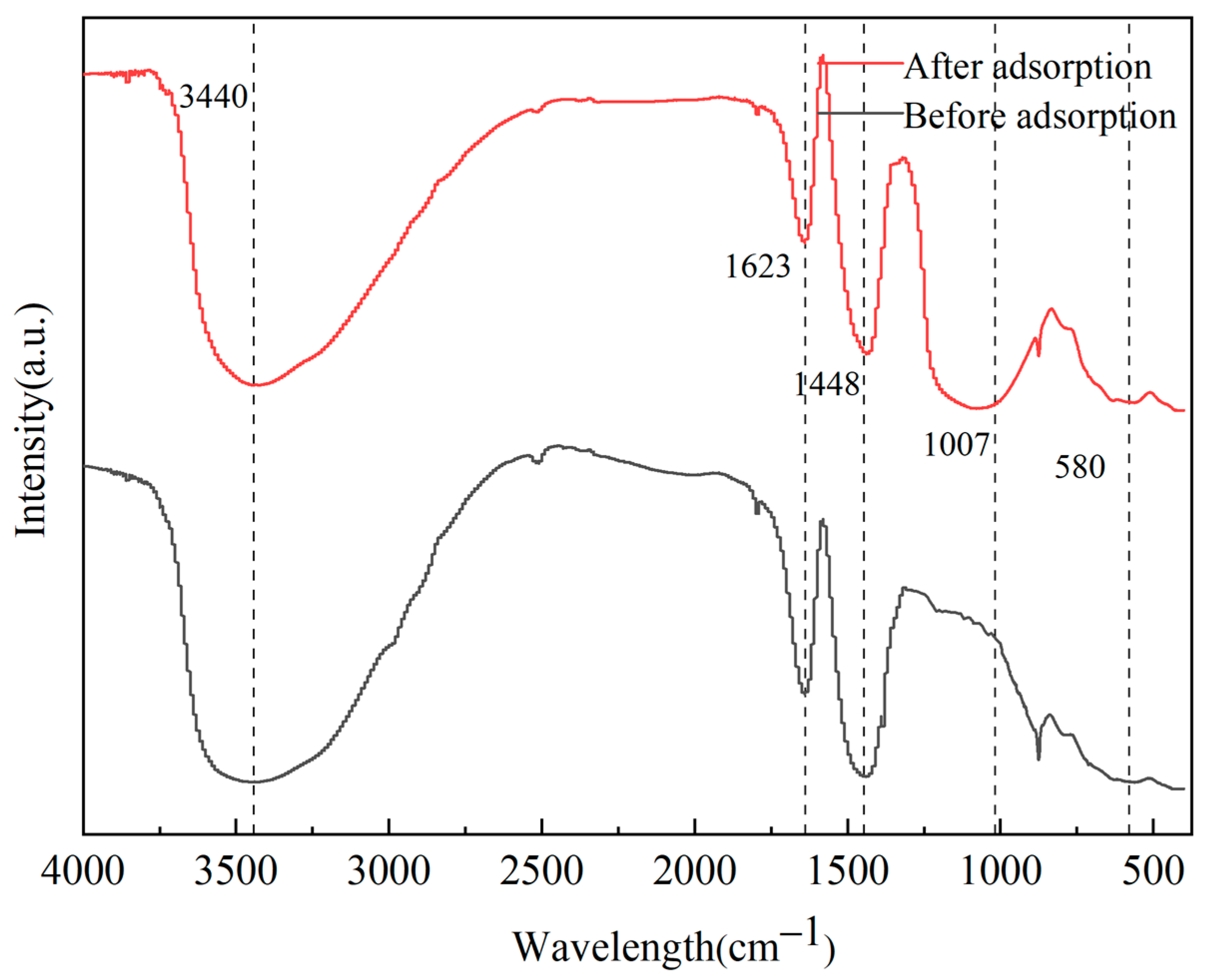
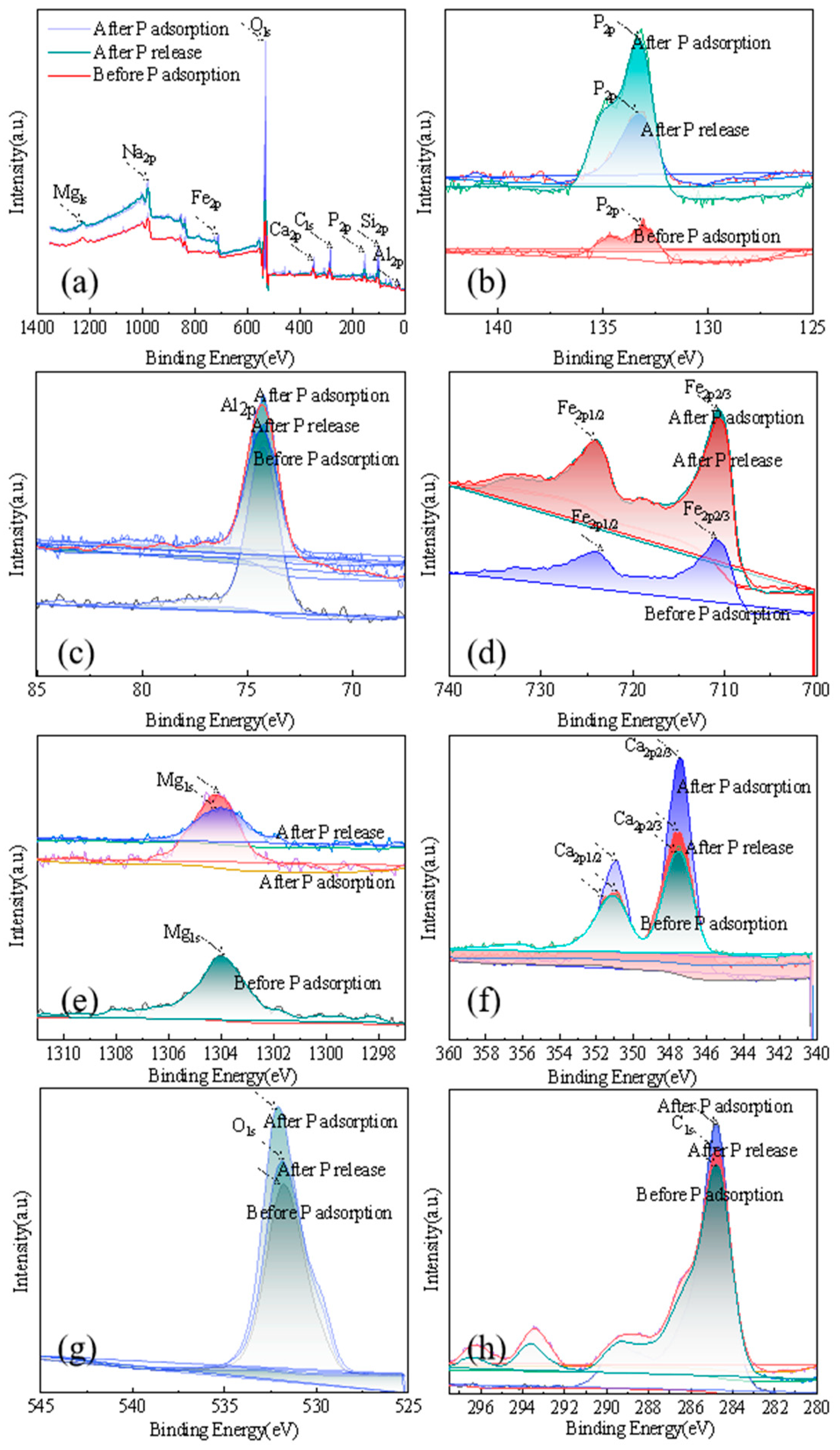
2.3. Characterization of P-Sorbent D and Property Variation during Adsorption and Fertilization
3. Materials and Methods
3.1. Re-Synthesis of P-Sorbents with DWTS
3.2. Adsorption Experiments
3.3. Hydroponic Experiments
3.4. Characterization for the P-Sorbent D
3.5. Modeling for the P Uptake in P-Sorbent D-Fertilized Hydroponic Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayeri, D.; Mousavi, S.A. A comprehensive review on the coagulant recovery and reuse from drinking water treatment sludge. J. Environ. Manag. 2022, 319, 115649. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.K.; Keegan, A.; Pham, P.N.; Li, D.; Qian, G.; Wang, L. Recycling drinking water treatment sludge into eco-concrete blocks with CO2 curing: Durability and leachability. Sci. Total Environ. 2020, 746, 141182. [Google Scholar] [PubMed]
- Yang, H.; Kang, J.K.; Park, S.J.; Lee, C.G. Phosphorus recovery from cattle manure bottom ash by extraction and precipitation methods. Environ. Sci. Pollut. Res. 2022, 29, 39567–39577. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, C.; Chen, Z.; Wang, X.; Zhang, Y.; Guo, L. Converting wastes to resource: Utilization of dewatered municipal sludge for calcium-based biochar adsorbent preparation and land application as a fertilizer. Chemosphere 2022, 298, 134302. [Google Scholar] [CrossRef]
- Sun, J.; Pikaar, I.; Sharma, K.R.; Keller, J.; Yuan, Z. Feasibility of sulfide control in sewers by reuse of iron rich drinking water treatment sludge. Water Res. 2015, 71, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, X.; Pham, C.U.; Nguyen, H.V.; Song, Y.; Chetty, K.; Kulandaivelu, J.; Wang, C.; Hai, F; Jiang, G. Co-digestion of primary sewage sludge with drinking water treatment sludge: A comprehensive evaluation of benefits. Bioresour. Technol. 2021, 330, 124994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, A.; Wang, H. Study on the feasibility and stability of drinking water treatment sludge (DWTS)@ zeolite to remove phosphorus from constructed wetlands. J Environ. Chem. Eng. 2022, 10, 108713. [Google Scholar] [CrossRef]
- Everaert, M.; Bergmans, J.; Broos, K.; Hermans, B.; Michielsen, B. Granulation and calcination of alum sludge for the development of a phosphorus adsorbent: From lab scale to pilot scale. J. Environ. Manag. 2021, 279, 111525. [Google Scholar] [CrossRef] [PubMed]
- Ashekuzzaman, S.M.; Jiang, J.-Q. Study on the sorption–desorption–regeneration performance of Ca-, Mg- and CaMg-based layered double hydroxides for removing phosphate from water. Chem. Eng. J. 2014, 246, 97–105. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Q.; Liu, J.; Feng, Y.; Shih, K. Phosphorus recovery through adsorption by layered double hydroxide nano-composites and transfer into a struvite-like fertilizer. Water Res. 2018, 145, 721–730. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, J.; Zhang, J.; Li, Q.; Gao, J.; Cai, M.; Zhang, J. Preparation of a new low-cost substrate prepared from drinking water treatment sludge (DWTS)/bentonite/zeolite/fly ash for rapid phosphorus removal in constructed wetlands. J. Clean. Prod. 2020, 261, 121110. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, J.; Zhang, K.; Wang, H.; Li, M.; Meng, S.; Mu, R.; Liu, L.; Yin, M.; Li, J.; et al. Phosphate recovery from the P-enriched brine of AnMBR-RO-IE treating municipal wastewater via an innovated phosphorus recovery batch reactor with nano-sorbents. Chemosphere 2021, 284, 131259. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Nie, L.; Ren, J.; Wang, W.; Xu, J.; Wang, N.; Zhao, Q. Complex Agent for Phosphate Sequestration from Digested Sludge Liquor: Performances and Economic Cost Analysis. Processes 2023, 11, 2050. [Google Scholar] [CrossRef]
- Cao, X.; Chen, X.; Liu, Y.; Wang, C.; Yue, L.; Elmer, W.H.; White, J.C.; Wang, Z.; Xing, B. Lanthanum Silicate Nanomaterials Enhance Sheath Blight Resistance in Rice: Mechanisms of Action and Soil Health Evaluation. ACS Nano 2023, 17, 15821–15835. [Google Scholar] [CrossRef]
- Belhaj, D.; Elloumi, N.; Jerbi, B.; Zouari, M.; Abdallah, F.B.; Ayadi, H.; Kallel, M. Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ. Sci. Pollut. Res. 2016, 23, 20168–20177. [Google Scholar] [CrossRef]
- Xiang, H.; Meng, J.; Shao, W.; Zeng, D.; Ji, J.; Wang, P.; Zhou, X.; Qi, P.; Liu, L.; Yang, S. Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, plant growth promotion, and plant resistance induction. Chem. Eng. J. 2023, 464, 142432. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Lee, J.I.; Jadamba, C.; Yoo, S.C.; Lee, C.G.; Shin, M.C.; Lee, J.; Park, S.J. Cycling of phosphorus from wastewater to fertilizer using wood ash after energy production. Chemosphere 2023, 336, 139191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Guo, X. Investigation on mechanism of phosphate removal on carbonized sludge adsorbent. J. Environ. Sci. 2018, 64, 335–344. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, C.; Xu, S.; Huang, Y.; Shi, J.; Liu, G. Adsorption of nitrogen and phosphorus from wastewater by modified sludge/biomass ash ceramsite: Preparation, adsorption mechanism and sustainable analysis. Water Environ. Res. 2023, 95, e10905. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, G.S.; Cazacliu, B.G.; Correa, C.R.; Ovsyannikova, E.; Kruse, A.; Sampaio, C.H.; Lima, E.C.; Dotto, G.L. Adsorption and recovery of phosphate from aqueous solution by the construction and demolition wastes sludge 489 and its potential use as phosphate-based fertiliser. J. Environ. Chem. Eng. 2020, 8, 103605. [Google Scholar] [CrossRef]
- Lian, F.; Gao, S.; Fu, Q.; Wu, Y.; Wang, J.; Huang, Q.; Hu, S. A comprehensive study of phosphorus removal and recovery with a Fe-loaded sulfoaluminate cement (FSC) adsorbent. J. Water Process Eng. 2021, 39, 101744. [Google Scholar] [CrossRef]
- Liu, M.; Li, R.; Wang, J.; Liu, X.; Li, S.; Shen, W. Recovery of phosphate from aqueous solution by dewatered dry sludge biochar and its feasibility in fertilizer use. Sci. Total Environ. 2022, 814, 152752. [Google Scholar] [CrossRef] [PubMed]
- Satyaprakash, M.; Sadhana, E.U.B.; Vani, S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M.R.; Ghodszad, L.; Asgari Lajayer, B. Dilemma of hydroxyapatite nanoparticles as phosphorus fertilizer: Potentials, challenges and effects on plants. Environ. Technol. Innov. 2020, 19, 100869. [Google Scholar] [CrossRef]
- Shabnam, N.; Ahn, Y.; Maksachev, A.; Lee, J.H.; Huang, C.-P.; Kim, H. Application of red-mud based ceramic media for phosphate uptake from water and evaluation of their effects on growth of Iris latifolia seedling. Sci. Total Environ. 2019, 688, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, J.G.; Esposti, L.D.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.-R.; Adamiano, A. Synergistic Release of Crop Nutrients and Stimulants from Hydroxyapatite Nanoparticles Functionalized with Humic Substances: Toward a Multifunctional Nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Q.; Jin, X.; Wang, H.; Zhang, K.; Li, M.; Wang, N.; Zhao, W.; Meng, S.; Mu, R. Preparation of a novel hafnium-loaded Fe3O4@ SiO2 superparamagnetic nanoparticles and its adsorption performance for phosphate in water. Desalin. Water Treat. 2021, 216, 188–198. [Google Scholar] [CrossRef]
- Yan, J.; Kirk, D.W.; Jia, C.Q.; Liu, X. Sorption of aqueous phosphorus onto bituminous and lignitous coal ashes. J. Hazard. Mater. 2007, 148, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Qiu, Z.; Yuan, L.; Tariq, M.; Lu, Y.; Yang, J.; Li, Z.; Lyu, S. Adsorption and mechanistic study for phosphate removal by magnetic Fe3O4-doped spent FCC catalysts adsorbent. Chemosphere 2019, 219, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Bai, H.; Tang, T. Recovery and utilization of phosphorus in wastewater by magnetic Fe3O4/Zn-Al-Fe-La layered double hydroxides(LDHs). Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 118–128. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C. Activation of peroxymonosulfate by magnetic catalysts derived from drinking water treatment residuals for the degradation of atrazine. J. Hazard. Mater. 2019, 366, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Jiang, S.; Wang, Y. Synthesis of Mn/Al double oxygen biochar from dewatered sludge for enhancing phosphate removal. J. Clean. Prod. 2020, 251, 119725. [Google Scholar] [CrossRef]
- Tang, S.; Tan, G.; Liang, J.; Li, O.; Xuan, W.; Li, Z. Key element course-tracked copyrolysis of sewage sludge and biomass for resource recovery and pollution control through kinetic and thermodynamic insights. Energy Convers. Manag. 2023, 280, 116830. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y. Is anaerobic digestion a reliable barrier for deactivation of pathogens in biosludge? Sci. Total Environ. 2019, 668, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, M.; Zhang, K.; Wang, N.; Wang, K.; Wang, H.; Meng, S.; Mu, R. Effect of ultrasound irradiation combined with ozone pretreatment on the anaerobic digestion for the biosludge exposed to trace-level levofloxacin: Degradation, microbial community and ARGs analysis. J. Environ. Manag. 2020, 262, 110356. [Google Scholar] [CrossRef]
- Teo, Y.; Beyrouty, C.; Gbur, E. Nitrogen, phosphorus, and potassium influx kinetic parameters of three rice cultivars. J. Plant Nutr. 1992, 15, 435–444. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Chen, W.; Chang, A.C.; Wu, L. Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol. Environ. Saf. 2007, 67, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ying, H.; Liu, Y.; Wang, H.; Xu, J.; Wang, W.; Ren, J.; Meng, S.; Wang, N.; Mu, R.; et al. Towards low energy-carbon footprint: Current versus potential P recovery paths in domestic wastewater treatment plants. J. Environ. Manag. 2023, 344, 118653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, C.; Song, H.; Liu, Y.; Wang, H.; Tian, F.; Meng, S.; Zhang, K.; Wang, N.; Mu, R.; et al. Mechanism of phosphate adsorption on superparamagnetic microparticles modified with transitional elements: Experimental observation and computational modelling. Chemosphere 2020, 258, 127327. [Google Scholar] [CrossRef] [PubMed]
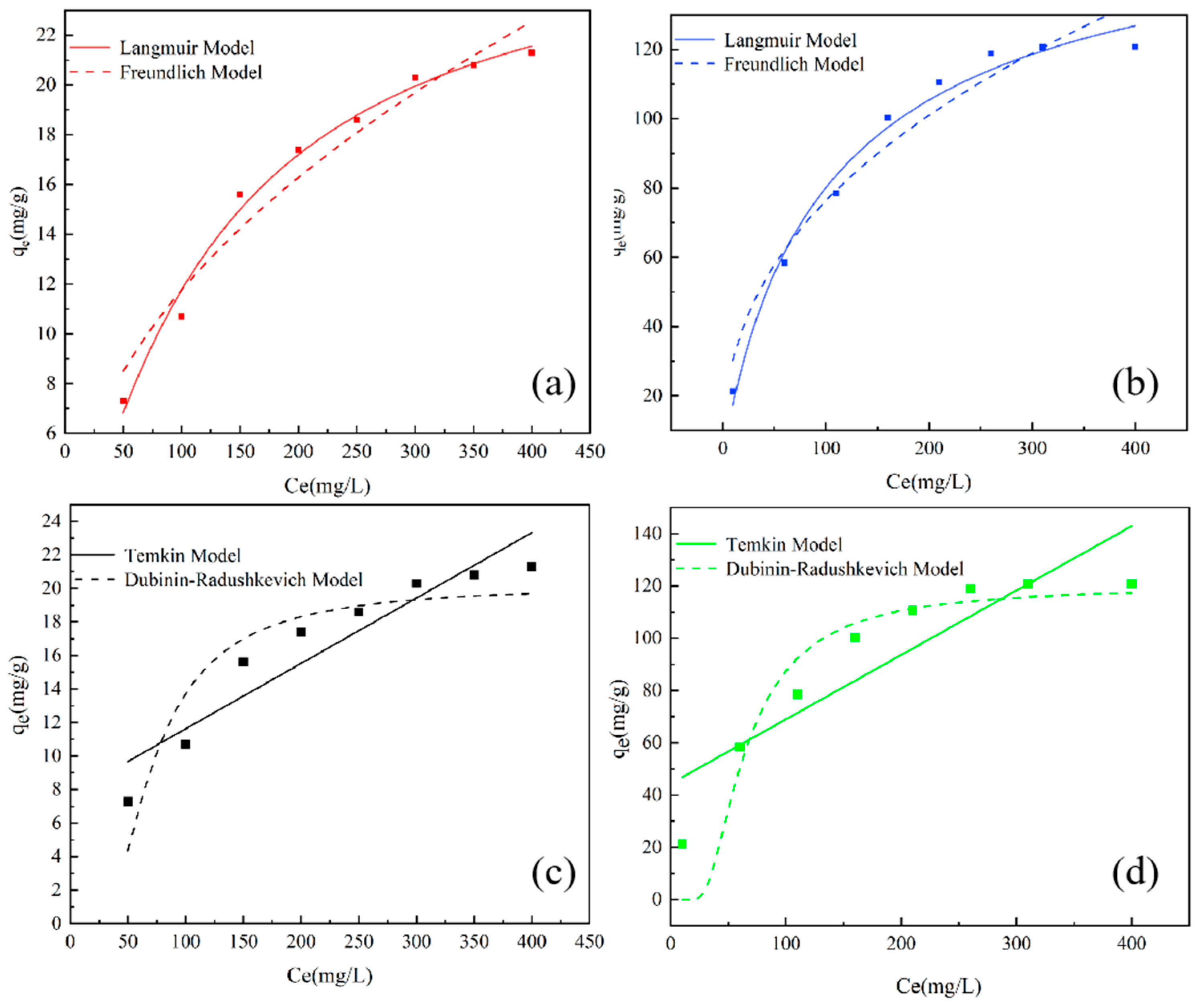

| Items | P-Sorbent D | P-Sorbent P | |
|---|---|---|---|
| Langmuir model | qm | 26.8 | 165.5 |
| KL | 0.54 | 0.67 | |
| R2 | 0.989 | 0.979 | |
| Freundlich model | Kf | 3.0 | 1.25 |
| n | 4.18 | 1.77 | |
| R2 | 0.955 | 0.945 | |
| Temkin model | KTlnA | 7.73 | 44.36 |
| KT | 0.039 | 0.25 | |
| R2 | 0.867 | 0.771 | |
| Dubinin–Radushkevich model | KD | 3905.39 | 3174.99 |
| qm | 20.17 | 119.64 | |
| R2 | 0.834 | 0.898 | |
| Absorbent | Adsorption Capacity (mg P/g) | Dosage (g/L) | C0 (mg/L) | pH | Reference |
|---|---|---|---|---|---|
| CSA | 2.99 | 2.5 | 35 | 2~9 | [19] |
| SBC | 4.29 | 1 | 5 | 3~11 | [20] |
| Fe-WAS | 8.5 | 2 | 20 | 2~8 | [21] |
| CSW-T | 15.7 | 7.5 | 130 | 3~9 | [22] |
| Fe-FSC | 46.8 | 1 | 40 | 3~8 | [23] |
| DSBC-700 °C | 51.793 | 0.5 | 200 | 3~9 | [24] |
| WAS-Ca900 °C | 83.95 | 0.3 | 80 | 2~11 | [3] |
| P-sorbent D | 7 | 1 | 50 | 7 | This study |
| Macro-Element | Concentration (μg·L−1) | Micro-Element | Concentration (μg·L−1) |
|---|---|---|---|
| Al | 42.9810 ± 0.23 | Cu | <3.17 |
| Ca | 21.2355 ± 0.13 | Pb | <0.63 |
| Fe | 1.1752 ± 0.04 | Cd | <0.16 |
| Mg | 31.8579 ± 0.25 | Cr | <0.55 |
| P | 43.4612 ± 0.33 | Zn | <7.62 |
| La | 10.6744 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wang, X.; Ren, J.; Wang, W.; Xu, J.; Meng, S.; Jin, J.; Li, X.; Fu, Y.; Han, K.; et al. Sludge-Based Superparamagnetic Nano-Sorbent Functionalized by Lanthanum Silicate Nanorods for Phosphorus Adsorption and Fertilization. Recycling 2024, 9, 53. https://doi.org/10.3390/recycling9040053
Zhao Q, Wang X, Ren J, Wang W, Xu J, Meng S, Jin J, Li X, Fu Y, Han K, et al. Sludge-Based Superparamagnetic Nano-Sorbent Functionalized by Lanthanum Silicate Nanorods for Phosphorus Adsorption and Fertilization. Recycling. 2024; 9(4):53. https://doi.org/10.3390/recycling9040053
Chicago/Turabian StyleZhao, Qian, Xiaole Wang, Juan Ren, Wei Wang, Jingtao Xu, Shujuan Meng, Jiarou Jin, Xiaochen Li, Yuyang Fu, Kechao Han, and et al. 2024. "Sludge-Based Superparamagnetic Nano-Sorbent Functionalized by Lanthanum Silicate Nanorods for Phosphorus Adsorption and Fertilization" Recycling 9, no. 4: 53. https://doi.org/10.3390/recycling9040053
APA StyleZhao, Q., Wang, X., Ren, J., Wang, W., Xu, J., Meng, S., Jin, J., Li, X., Fu, Y., Han, K., Mu, R., Li, X., Zhao, R., Wang, H., & Chen, F. (2024). Sludge-Based Superparamagnetic Nano-Sorbent Functionalized by Lanthanum Silicate Nanorods for Phosphorus Adsorption and Fertilization. Recycling, 9(4), 53. https://doi.org/10.3390/recycling9040053








