Sustainability in the Generation of Household Waste from Dishwasher Sponges for the Purpose of a New Adsorbent Material and Its Operating Costs
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Development
2.2. Material Preparation
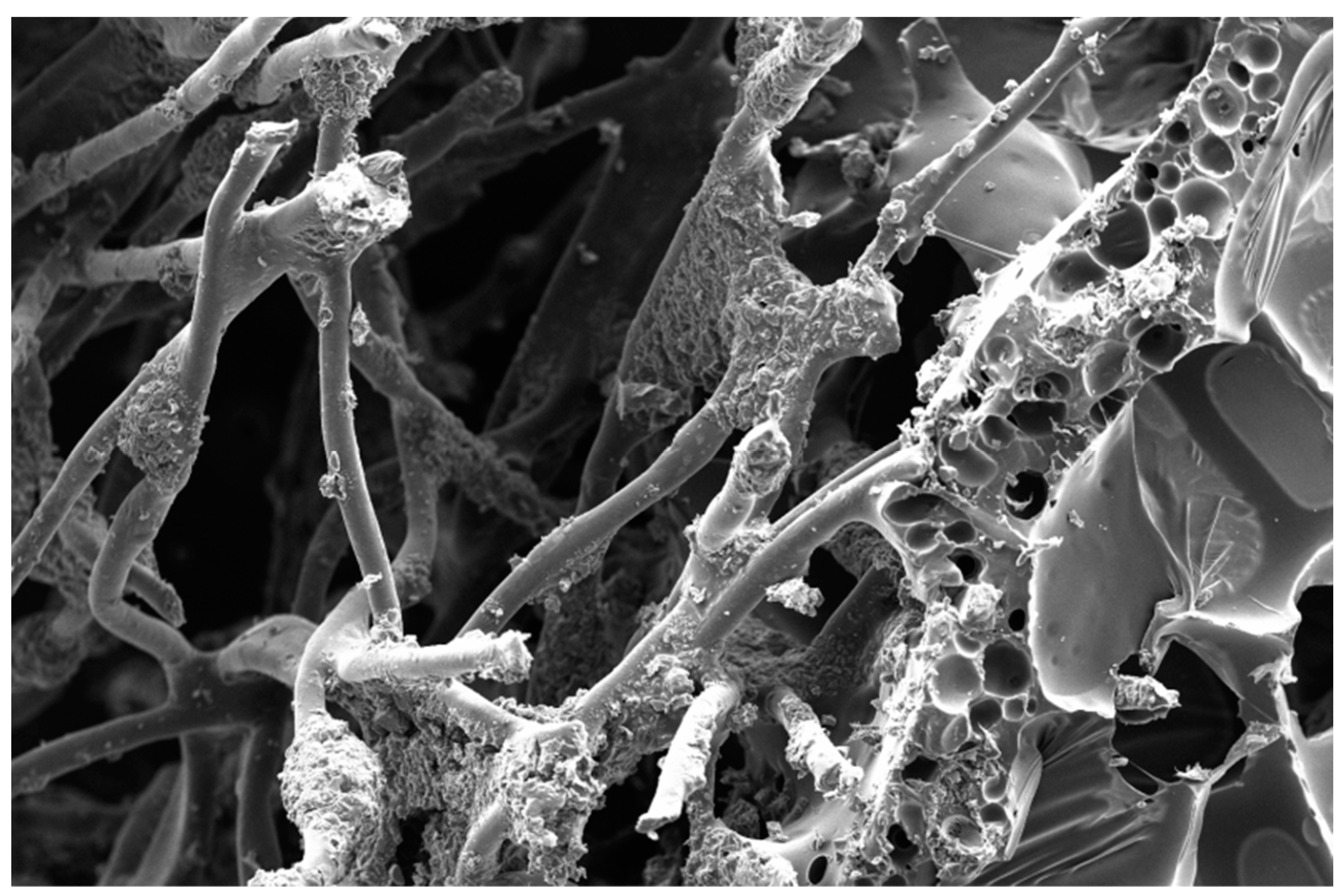
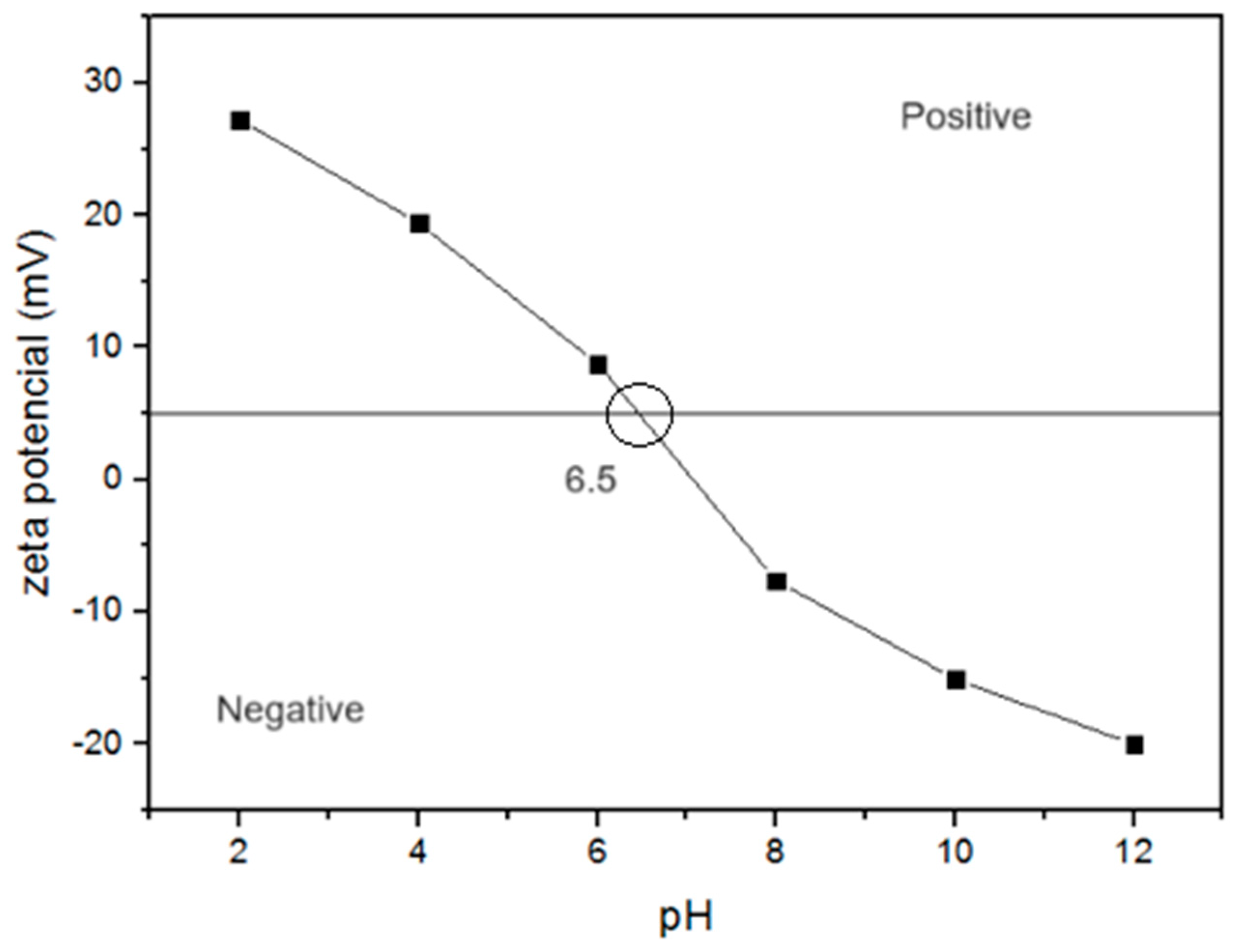
2.3. Material Characterization
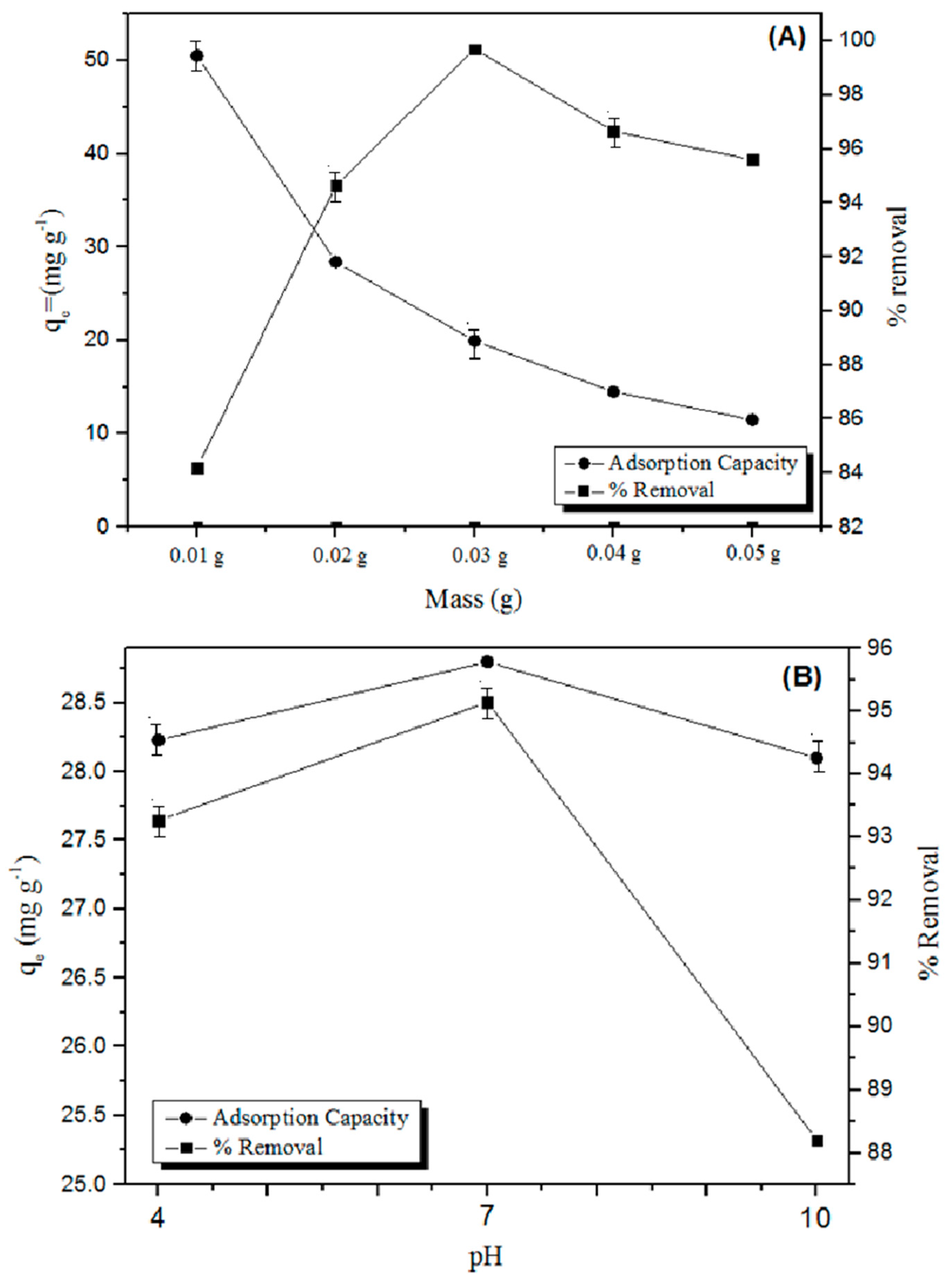
2.4. Effect of Mass and pH
2.5. Kinetic and Equilibrium Study
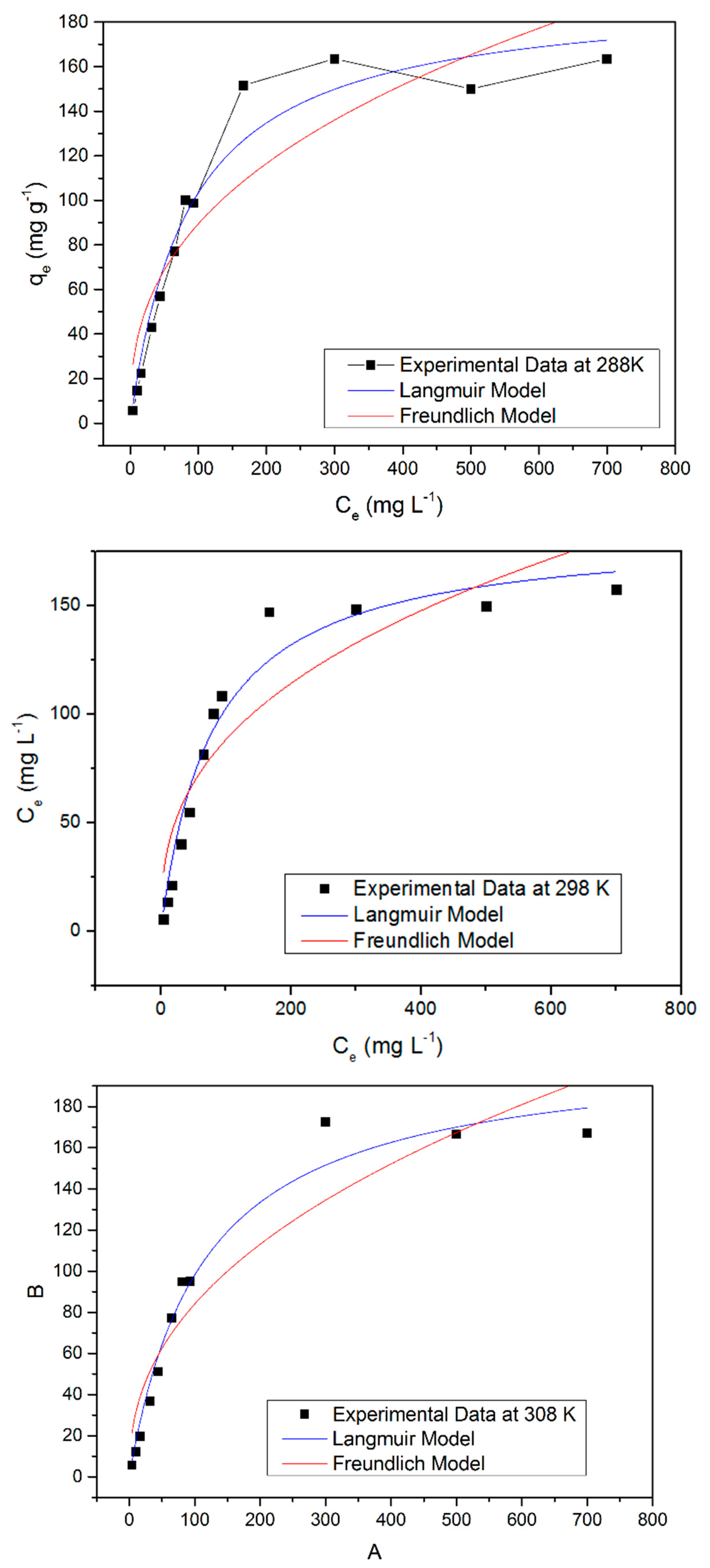
2.6. Adsorption Isotherms
2.7. Cost Analysis Involving the Use of Dish Sponge
3. Results and Discussion
3.1. Material Characterization
3.2. Study of Adsorption
Effect of Mass and pH
3.3. Study Kinetic
3.4. Adsorption Isotherm
Thermodynamic Parameters
3.5. Costs Attributed to Adsorbent Collection and Production Management
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Møretrø, T.; Moen, B.; Almli, V.L.; Teixeira, P.; Ferreira, V.B.; Åsli, A.W.; Nilsen, C.; Langsrud, S. Dishwashing Sponges and Brushes: Consumer Practices and Bacterial Growth and Survival. Int. J. Food Microbiol. 2021, 337, 108928. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M. Upaya World Wide Fund for Nature (Wwf) Dalam Menangani Kerusakan Lingkungan Akibat Sampah Plastik Di Pantai Bali. J. Online Mhs. (JOM) Bid. Ilmu Sos. Dan Ilmu Polit. 2019, 6, 1–15. [Google Scholar]
- Møretrø, T.; Ferreira, V.B.; Moen, B.; Almli, V.L.; Teixeira, P.; Kasbo, I.M.; Langsrud, S. Bacterial Levels and Diversity in Kitchen Sponges and Dishwashing Brushes Used by Consumers. J. Appl. Microbiol. 2022, 133, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Struchtrup, S.S.; Von Esmarch-Rummler, B.; Stamminger, R. Hygiene in Commercial Dishwashing—A Review about the State of Knowledge in Research, Standardization, Regulation and Market Information. Tenside Surfactants Deterg. 2021, 58, 320–333. [Google Scholar] [CrossRef]
- Sabharwal, J. Health Issues and Environmental Impact of Cleaning Agents. Int. J. Nov. Res. Life Sci. 2015, 2, 31–38. [Google Scholar]
- Ramli, R.P.; Syakira Salman, H.; Nursuria Sariza Sazari, L.; Azmi, A. Evaluating Microbiological Quality of Kitchen Equipment in School Canteens. Heal. Off. Res. Book Fac. Health Sci. UiTM 2020, 3, 49–53. [Google Scholar]
- Tamura, T.; Iihara, T.; Nishida, S.; Ohta, S. Cleaning Performance and Foaming Properties of Lauroylamidopropylbetaine/Nonionics Mixed Systems. J. Surfactants Deterg. 1999, 2, 207–211. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus Helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.T.B.; Estrela, F.N.; Pereira, P.S.; de Andrade Vieira, J.E.; de Lima Rodrigues, A.S.; Silva, F.G.; Malafaia, G. Toxicity of Polystyrene Nanoplastics in Ctenopharyngodon Idella Juveniles: A Genotoxic, Mutagenic and Cytotoxic Perspective. Sci. Total Environ. 2021, 752, 141937. [Google Scholar] [CrossRef]
- Singh, I.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancement in Plant Oil Derived Polyol-Based Polyurethane Foam for Future Perspective: A Review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900225. [Google Scholar] [CrossRef]
- Kloss, J.; Munaro, M.; De Souza, G.P.; Gulmine, J.V.; Wang, S.H.; Zawadzki, S.; Akcelrud, L. Poly(Ester Urethane)s with Polycaprolactone Soft Segments: A Morphological Study. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4117–4130. [Google Scholar] [CrossRef]
- Magalhães, E.A.; de Jesus, H.E.; Pereira, P.H.F.; Gomes, A.S.; Santos, H.F. dos Beach Sand Plastispheres Are Hotspots for Antibiotic Resistance Genes and Potentially Pathogenic Bacteria Even in Beaches with Good Water Quality. Environ. Pollut. 2024, 344, 123237. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G. Marine Plastics: What’s Wrong with Them? In Plastic Pollution and Marine Conservation: Approaches to Protect Biodiversity and Marine Life; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780128224717. [Google Scholar]
- Adler, R.W.; Wells, C.E. Plastics and the Limits of U.S. Environmental Law. Harvard Environ. Law Rev. 2023, 47, 1. [Google Scholar] [CrossRef]
- Zhang, W.; Sik Ok, Y.; Bank, M.S.; Sonne, C. Macro- and Microplastics as Complex Threats to Coral Reef Ecosystems. Environ. Int. 2023, 174, 107914. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Gu, J.D. Microbial Colonization of Polymeric Materials for Space Applications and Mechanisms of Biodeterioration: A Review. Int. Biodeterior. Biodegrad. 2007, 59, 170–179. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; Faga, M.G.; García-López, E.; Loddo, V.; Marcì, G.; Martra, G.; Palmisano, L. Photocatalytic Oxidation of Acetonitrile in Gas-Solid and Liquid-Solid Regimes. J. Catal. 2005, 235, 209–220. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Zhang, Y.; Hu, J.; Huang, J.; Ning, P.; Tian, S. Catalytic Decomposition of HCN on Copper Manganese Oxide at Low Temperatures: Performance and Mechanism. Chem. Eng. J. 2018, 346, 621–629. [Google Scholar] [CrossRef]
- Silva Spinacé, M.A.; Lucato, M.U.; Ferrão, M.F.; Davanzo, C.U.; De Paoli, M.A. Determination of Intrinsic Viscosity of Poly(Ethylene Terephthalate) Using Infrared Spectroscopy and Multivariate Calibration Method. Talanta 2006, 69, 643–649. [Google Scholar] [CrossRef]
- Kumar, B.; Dwivedy, D. Examining Legal Difficulties and Potential Benefits of Social Businesses. J. Law Sustain. Dev. 2023, 11, e1184. [Google Scholar] [CrossRef]
- Romero-Lopez, A.; Ramos, F.; Ochoa, C.Y.; Mataran, A.; Olmo, R.M.; Lopez, J.C.F.M.; Fuentes-Guerra, R.; Givens, G.; Dunning, R.; Michel-Villarreal, R.; et al. Market Research about Agriso Mobile Application for Farmers. Sci. Pap. Ser. Agron. 2020, 53, 284–288. [Google Scholar]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.F.; Wu, C.M.; Song, J.; Yu, J.J.; Li, Y.R. Characterizing the Thermal Transport and Kinetics of Droplet Evaporation on a Solid Surface with Hybrid Wettability. Int. Commun. Heat Mass Transf. 2023, 143, 106714. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Bezerra, C.d.O.; Quesada, H.B.; Alves Baptista, A.T.; Nishi, L.; Vieira, M.F.; Bergamasco, R. Modified Moringa oleifera Lam. Seed Husks as Low-Cost Biosorbent for Atrazine Removal. Environ. Technol. 2019, 42, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Cusioli, L.F.; Mantovani, D.; Quesada, H.B.; Gomes, R.G.; Bergamasco, R. Adsorption of COVID-19-Related Drug from Contaminated Water Using Activated Carbon. Desalin. Water Treat. 2022, 277, 85–89. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária: Fortaleza, Brazil, 2014; ISBN 978-85-7485-186-0. [Google Scholar]
- Kramar, A.; Luxbacher, T.; Moshfeghi Far, N.; González-Benito, J. Active Cellulose Acetate/Chitosan Composite Films Prepared Using Solution Blow Spinning: Structure and Electrokinetic Properties. Polymers 2023, 15, 3276. [Google Scholar] [CrossRef]
- Istchuk, R.N.; Ghisi, E. Financial Feasibility Analysis of Residential Rainwater Harvesting in Maringá, Brazil. Sustainability 2022, 14, 12859. [Google Scholar] [CrossRef]
- Libório, M.P.; Martinuci, O.d.S.; Bernardes, P.; Krohling, N.C.A.C.C.; Castro, G.; Guerra, H.L.; Ribeiro, E.A.; Fonzar, U.J.V.; Francisco, Í.d.C. Social Vulnerability and COVID-19 in Maringá, Brazil. Spat. Inf. Res. 2023, 31, 51–59. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Mantovani, D.; Bergamasco, R.; Tusset, A.M.; Lenzi, G.G. Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal. Processes 2023, 11, 2478. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. [Google Scholar] [CrossRef]
- Baptista, A.T.A.; Coldebella, P.F.; Cardines, P.H.F.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Coagulation-Flocculation Process with Ultrafiltered Saline Extract of Moringa Oleifera for the Treatment of Surface Water. Chem. Eng. J. 2015, 276, 166–173. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Fagundes-klen, R.; Valverde, K.C.; Cavalcanti, E.B.; Aparecida, O. Potential Effect of Chemical and Thermal Treatment on the Kinetics, Equilibrium, and Thermodynamic Studies for Atrazine Biosorption by the Moringa Oleifera Pods. Can. J. Chem. Eng. 2017, 95, 961–973. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, Impacts and General Aspects of Pesticides in Surface Water: A Review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Januário, E.F.D.; Araújo, M.F.; Bergamasco, R.; Vieira, A.M.S. Efficient Performance of CuO Nanoparticles Synthesized with Pomegranate Leaf Extract for Neutral Red Dye Adsorption. Environ. Prog. Sustain. Energy 2022, 41, e13864. [Google Scholar] [CrossRef]
- Abate, G.; Masini, J.C. Sorption of Atrazine, Propazine, Deethylatrazine, Deisopropylatrazine and Hydroxyatrazine onto Organovermiculite. J. Braz. Chem. Soc. 2005, 16, 936–943. [Google Scholar] [CrossRef]
- Guari, N.M.C.; Silva, A.S.; Diaz de Tuesta, J.L.; Pottker, W.E.; Cordeiro, P.Y.; Gomes, H.T. Magnetic CoFe2O4@carbon Yolk-Shell Nanoparticles as Catalysts for the Catalytic Wet Peroxide Oxidation of Paracetamol: Kinetic Insights. Glob. Nest J. 2023, 25, 57–66. [Google Scholar] [CrossRef]
- Haro, N.K.; Dávila, I.V.J.; Nunes, K.G.P.; de Franco, M.A.E.; Marcilio, N.R.; Féris, L.A. Kinetic, Equilibrium and Thermodynamic Studies of the Adsorption of Paracetamol in Activated Carbon in Batch Model and Fixed-Bed Column. Appl. Water Sci. 2021, 11, 38. [Google Scholar] [CrossRef]
- Spaltro, A.; Pila, M.N.; Colasurdo, D.D.; Noseda Grau, E.; Román, G.; Simonetti, S.; Ruiz, D.L. Removal of Paracetamol from Aqueous Solution by Activated Carbon and Silica. Experimental and Computational Study. J. Contam. Hydrol. 2021, 236, 103739. [Google Scholar] [CrossRef]
- Sajid, M.; Bari, S.; Saif Ur Rehman, M.; Ashfaq, M.; Guoliang, Y.; Mustafa, G. Adsorption Characteristics of Paracetamol Removal onto Activated Carbon Prepared from Cannabis Sativum Hemp. Alexandria Eng. J. 2022, 61, 7203–7212. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazar, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 Nanocomposite for Ibuprofen and Paracetamol Removal from Aqueous Solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef] [PubMed]
- Maneewong, Y.; Chaemchuen, S.; Verpoort, F.; Klomkliang, N. Paracetamol Removal from Water Using N-Doped Activated Carbon Derived from Coconut Shell: Kinetics, Equilibrium, Cost Analysis, Heat Contributions, and Molecular-Level Insight. Chem. Eng. Res. Des. 2022, 185, 163–175. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites. Nanomaterials 2020, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.H.; Cherif, E.K.; Ouzzine, M.; Touijer, A.; Coren, F.; Saidi, M. Promising Porous Carbon Material Derived from Argan Paste Cake by KOH Activation, for Paracetamol Removal. Processes 2023, 11, 2078. [Google Scholar] [CrossRef]
- Ogunmodede, J.; Akanji, S.B.; Bello, O.S. Moringa oleifera Seed Pod-Based Adsorbent for the Removal of Paracetamol from Aqueous Solution: A Novel Approach toward Diversification. Environ. Prog. Sustain. Energy 2021, 40, e13615. [Google Scholar] [CrossRef]
- Tunç, M.S.; Yıldız, B.; Taşar, Ş. Removal of Paracetamol from Aqueous Solution by Wood Sawdust-Derived Activated Carbon: Process Optimization Using Response Surface Methodology. Chem. Eng. Commun. 2022, 209, 1130–1150. [Google Scholar] [CrossRef]
- Cheblaoui, R.; Mohellebi, F.; Mameri, N. Acetaminophen Removal Using Activated Bentonite Characterized by (BET, XRF, SEM) and Bipolar Electrocoagulation: A Comparative Study. Application of Coupling Process on a Pharmaceutical Effluent. Chem. Data Collect. 2023, 44, 100998. [Google Scholar] [CrossRef]
- Issabayeva, G.; Wong, S.H.; Pang, C.Y.; Wong, M.C.; Aroua, M.K. Fluoride Removal by Low-Cost Palm Shell Activated Carbon Modified with Prawn Shell Chitosan Adsorbents. Int. J. Environ. Sci. Technol. 2022, 19, 3731–3740. [Google Scholar] [CrossRef]
- Tcheka, C.; Conradie, M.M.; Assinale, V.A.; Conradie, J. Mesoporous Biochar Derived from Egyptian Doum Palm (Hyphaene Thebaica) Shells as Low-Cost and Biodegradable Adsorbent for the Removal of Methyl Orange Dye: Characterization, Kinetic and Adsorption Mechanism. Chem. Phys. Impact 2024, 8, 100446. [Google Scholar] [CrossRef]
- CONAMA—Conselho Nacional do Meio Ambiente Brasil. Ministério do Meio Ambiente. Resolução N° 430, de 13 de Maio de 2011. Diário of. da União. 2011. Available online: https://conama.mma.gov.br/images/conteudo/LivroConama.pdf (accessed on 21 April 2024).


| Models | Parameters | Dish Sponge |
|---|---|---|
| Pseudo first order | qe (mg g−1) | 37.02 |
| k1 (min−1) | 0.096 | |
| R2 | 0.846 | |
| χ2 | 9.985 | |
| Pseudo second order | qe (mg g−1) | 38.18 |
| k2 (g mg−1 min−1) | 0.004 | |
| R2 | 0.940 | |
| χ2 | 3.43 |
| Type of Adsorbent Material | Capacity Adsorption (mg g−1) | pH | Temperature (K) | Reference |
|---|---|---|---|---|
| Activated Carbon and Silica | 167.00 | 3 | 298 | [41] |
| Activated carbon prepared from Cannabis sativum Hemp | 16.18 | 5.5 | 308 | [42] |
| CNT-COOH/MnO2/Fe3O4 nanocomposite | 114.2 | 7.2 | 298 | [43] |
| N-doped activated carbon derived from coconut shell | 39.9 | 6.4 | 298 | [44] |
| Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites | 24.7 | 7.0 | 323 | [45] |
| Argan Paste Cake by KOH Activation | 20.28 | 5.6 | 298 | [46] |
| Moringa oleifera Seed Pod-based Adsorbent | 21.34 | 3 | 298 | [47] |
| Present Study | 208.06 | 6.5 | 318 | _ |
| Models | Parameters | 288 K | 298 K | 308 K |
|---|---|---|---|---|
| Langmuir | qmáx (mg g−1) | 173.22 | 184.59 | 208.06 |
| KL (L mg−1) | 0.011 | 0.009 | 0.006 | |
| R2 | 0.960 | 0.962 | 0.979 | |
| Freundlich | KF (mg g−1)/(mg L−1)nF | 15.43 | 14.91 | 11.69 |
| nF | 1.432 | 1.010 | 0.635 | |
| R2 | 0.841 | 0.834 | 0.890 |
| T (°C) | T (K) | (ΔG) (KJ mol−1) | (ΔH) (KJ mol−1) | (ΔS) (KJ mol−1) |
|---|---|---|---|---|
| 15 | 288 | −31.28 | −22.72 | 0.07 |
| 25 | 298 | −33.54 | ||
| 25 | 308 | −35.87 |
| VARIABLE Expenses | US$ | FIXED Expenses | US$ |
|---|---|---|---|
| Transport of dish sponge (raw material) | 14,150.34 | Rent of the administrative building and factory | 2264.15 |
| Costs of other raw materials for adsorbent production | 94.33 | Electric power factory | 377.35 |
| Laboratory equipment maintenance | 93.80 | Insurance and surveillance | 56.60 |
| Employee salaries | 17,688.67 | ||
| Total | 32,027.14 | 2698.10 | |
| Total Expenses | 34,725.24 |
| Adsorption Costing | US$ |
|---|---|
| Total costs | 34,725.24 |
| Monthly production (units) | 376,234 |
| Cost per units-(adsorption) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovani, D.; Cusioli, L.F.; Gomes, D.A.; Bergamasco, R.; Tusset, A.M.; Gonçalves Lenzi, G. Sustainability in the Generation of Household Waste from Dishwasher Sponges for the Purpose of a New Adsorbent Material and Its Operating Costs. Recycling 2024, 9, 52. https://doi.org/10.3390/recycling9040052
Mantovani D, Cusioli LF, Gomes DA, Bergamasco R, Tusset AM, Gonçalves Lenzi G. Sustainability in the Generation of Household Waste from Dishwasher Sponges for the Purpose of a New Adsorbent Material and Its Operating Costs. Recycling. 2024; 9(4):52. https://doi.org/10.3390/recycling9040052
Chicago/Turabian StyleMantovani, Daniel, Luís Fernando Cusioli, Diana Aline Gomes, Rosângela Bergamasco, Angelo Marcelo Tusset, and Giane Gonçalves Lenzi. 2024. "Sustainability in the Generation of Household Waste from Dishwasher Sponges for the Purpose of a New Adsorbent Material and Its Operating Costs" Recycling 9, no. 4: 52. https://doi.org/10.3390/recycling9040052
APA StyleMantovani, D., Cusioli, L. F., Gomes, D. A., Bergamasco, R., Tusset, A. M., & Gonçalves Lenzi, G. (2024). Sustainability in the Generation of Household Waste from Dishwasher Sponges for the Purpose of a New Adsorbent Material and Its Operating Costs. Recycling, 9(4), 52. https://doi.org/10.3390/recycling9040052








