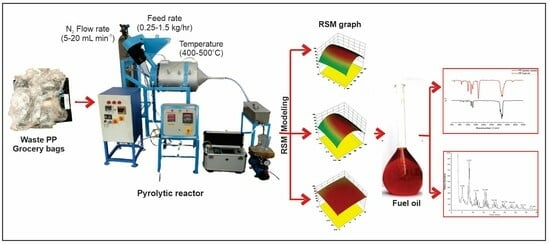
Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design
Abstract
1. Introduction
2. Results and Discussion
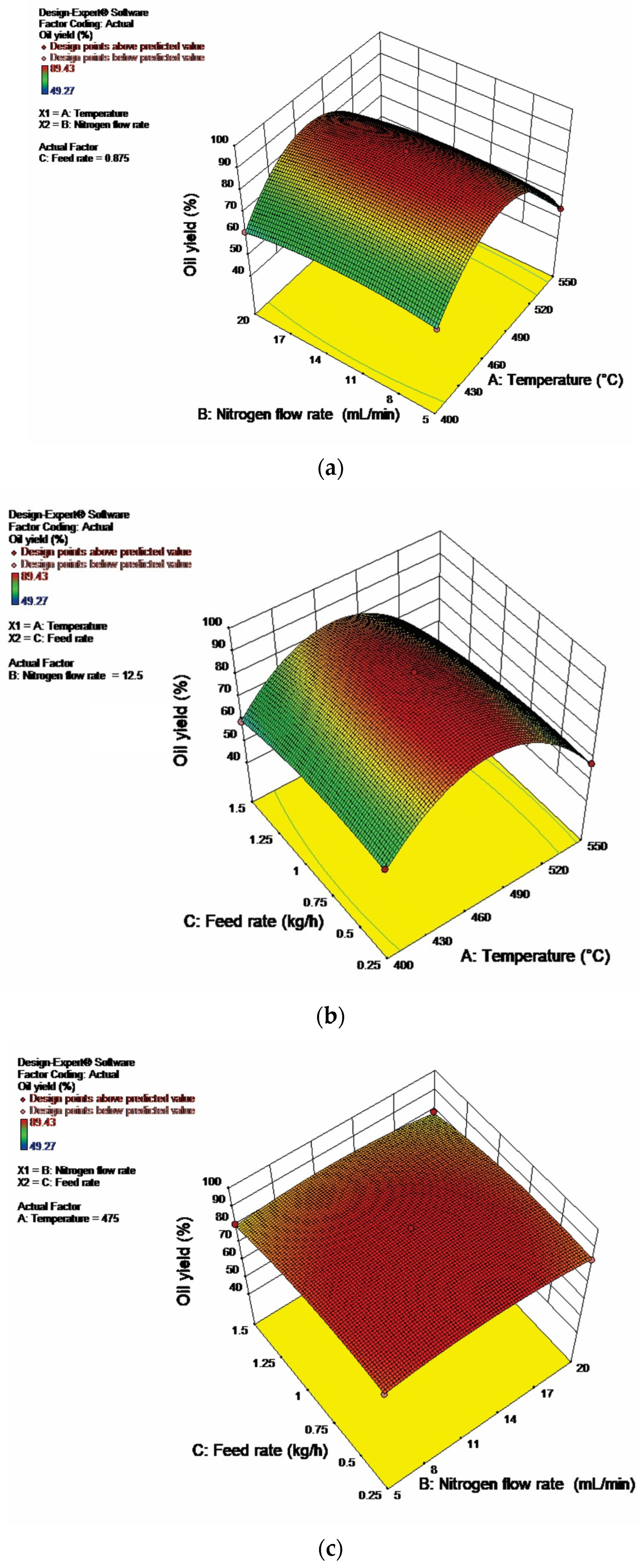
2.1. Process Optimization Using the RSM
2.2. Properties of the Fuel Oil
2.2.1. FT-IR Analysis
2.2.2. GC-MS Analysis of Fuel Oil
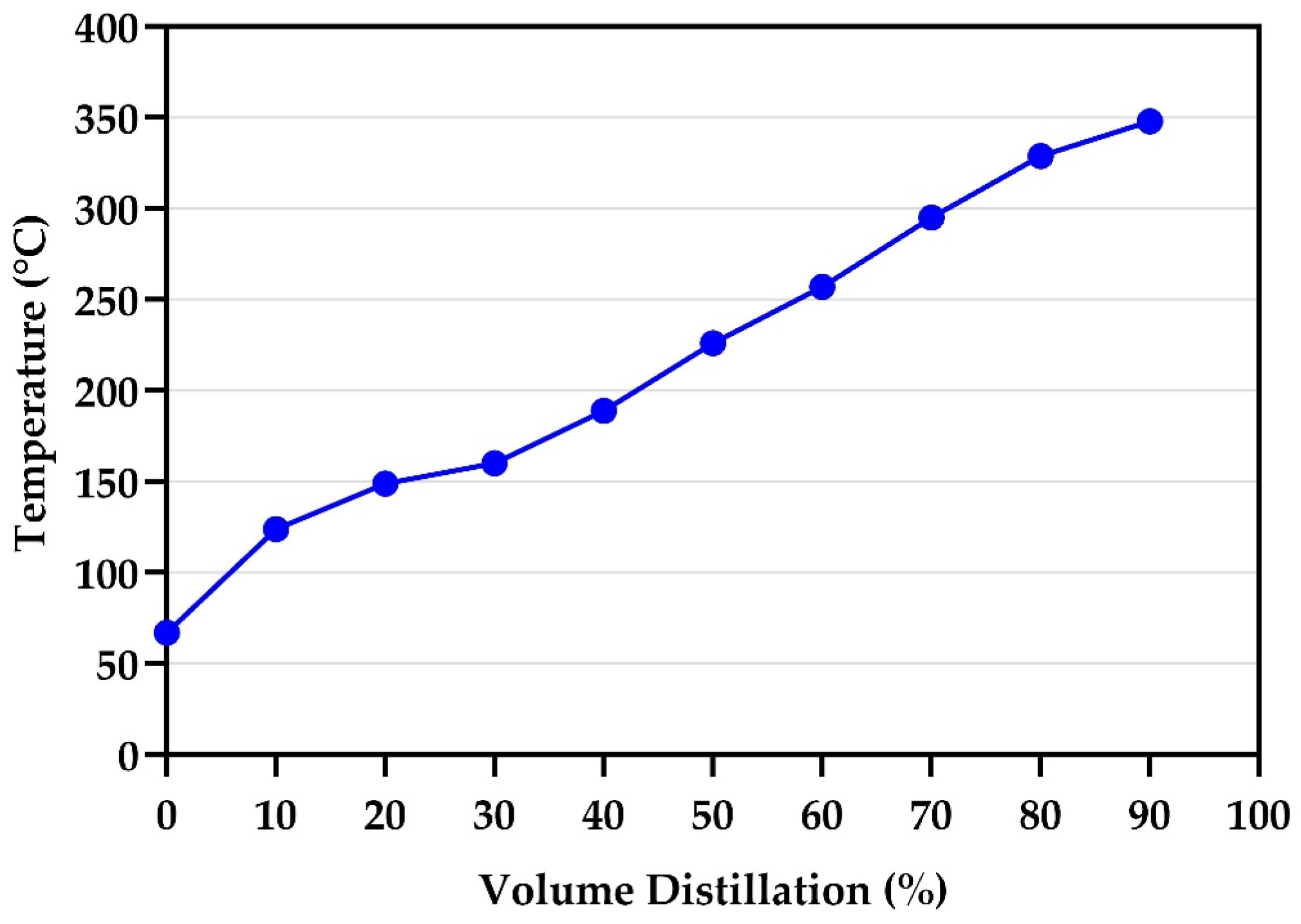
2.3. Fractional Distillation of the Waste PP Fuel Oil
3. Materials and Methods
3.1. Sample Preparation and Characterization
3.2. Description of the Pyrolytic Reactor
3.3. Operating Procedure
3.4. Process Optimization Using the Box–Behnken Design
3.5. Mathematical Modeling
3.6. Fuel Oil Properties
3.7. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.8. Gas Chromatography–Mass Spectrophotometry (GC-MS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basha, S.A.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 628–1634. [Google Scholar] [CrossRef]
- Arbab, M.I.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Imtenan, S.; Sajjad, H. Fuel properties, engine performance and emission characteristic of common biodiesels as a renewable and sustainable source of fuel. Renew. Sustain. Energy Rev. 2013, 22, 133–147. [Google Scholar] [CrossRef]
- Callegari, B. Changes in the Global Flow of Plastic Waste and Their Effects on Sustainable Development. In Proceedings of the World Human Rights Cities Forum, Gwangju, Republic of Korea, 7–10 October 2021; p. 69. [Google Scholar]
- Noorimotlagh, Z.; Mirzaee, S.A.; Kalantar, M.; Barati, B.; Fard, M.E.; Fard, N.K. The SARS-CoV-2 (COVID-19) pandemic in hospital: An insight into environmental surfaces contamination, disinfectants’ efficiency, and estimation of plastic waste production. Environ. Res. 2021, 202, 111809. [Google Scholar]
- Plastics Europe. Plastics—The Facts. An analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2019. [Google Scholar]
- CPCB. Annual Report for the Year 2018-19 on Implementation of Plastic Waste Management Rules; Ministry of Environment, Forest and Climate Change, Govt. of India: New Delhi, India, 2019; Available online: https://cpcb.nic.in/uploads/plasticwaste/Annual_Report_2018-19_PWM.pdf (accessed on 26 May 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Producción, uso y destino de todos los plásticos jamás fabricados. Sci. Adv. 2017, 3, 1207–1221. [Google Scholar]
- Al-Jarallah, R.; Aleisa, E. A baseline study characterizing the municipal solid waste in the State of Kuwait. Waste Manag. 2014, 34, 952–960. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.P. GHG emission and carbon sequestration potential from MSW of Indian metro cities. Urban Clim. 2014, 8, 30–41. [Google Scholar] [CrossRef]
- CIWM; Wasteaid UK. From the Land to the Sea. Available online: https://wasteaid.org/wp-content/uploads/2018/08/From-the-Land-to-the-Sea-1.pdf (accessed on 15 October 2023).
- Grigore, M.E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E. An overview of the challenges and trade-offs in closing the loop of post-consumer plastic waste (PCPW): Focus on recycling. J. Hazard. Mater. 2019, 380, 120887. [Google Scholar] [CrossRef]
- Delva, L.; Hubo, S.; Cardon, L.; Ragaert, K. On the role of flame retardants inmechanical recycling of solid plastic waste. Waste Manag. 2018, 82, 198–206. [Google Scholar] [CrossRef]
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene Terephthalate Deconstruction Catalyzed by a Carbon-Supported Single-Site Molybdenum-Dioxo Complex. Angew. Chem. Int. Ed. 2020, 59, 19857–19861. [Google Scholar] [CrossRef] [PubMed]
- Schyns, Z.O.; Shaver, M.P. Mechanical recycling of packaging plastics: A review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Maitlo, G.; Ali, I.; Maitlo, H.A.; Ali, S.; Unar, I.N.; Ahmad, M.B.; Bhutto, D.K.; Karmani, R.K.; Naich, S.U.R.; Sajjad, R.U.; et al. Plastic Waste Recycling, Applications, and Future Prospects for a Sustainable Environment. Sustainability 2022, 14, 11637. [Google Scholar] [CrossRef]
- Rahimi, A.; Garcia, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Nyashina, G.S.; Vershinina, K.Y.; Shlegel, N.E.; Strizhak, P.A. Effective incineration of fuel-waste slurries from several related industries. Environ. Res. 2019, 176, 108559. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.; Stevanovic, S.; Rahman, M.M.; Pourkhesalian, A.M.; Morawska, L.; Thai, P.K. Emissions of particulate matter, carbon monoxide and nitrogen oxides from the residential burning of waste paper briquettes and other fuels. Environ. Res. 2018, 167, 536–543. [Google Scholar] [CrossRef]
- Reyna-Bensusan, N.; Wilson, D.C.; Smithb, S.R. Uncontrolled burning of solid waste by households in Mexico is a significant contributor to climate change in the country. Environ. Res. 2018, 163, 280–288. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste-a review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-Operation and Trade; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N. Study on the effect of Kankara zeolite-Y-based catalyst on the chemical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym. Bull. 2021, 78, 377–398. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N.; Obasi, H.C. The effect of Kankara zeolite-Y-based catalyst on some physical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym. Bull. 2020, 77, 1399–1415. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Yuan, X.; Attanayake, C.P.; Wang, S.; You, S.; Tsang, D.C.; Nzihou, A.; Ok, Y.S. Sustainable management of plastic wastes in COVID-19 pandemic: The biochar solution. Environ. Res. 2022, 212, 113495. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Murugan, S.; Singh, R.K. Performance and emission analysis of blends of waste plastic oil obtained by catalytic pyrolysis of waste HDPE with diesel in a CI engine. Energy Convers. Manag. 2013, 74, 323–331. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Yin, F.; Zhuang, Q.; Chang, T.; Zhang, C.; Sun, H.; Sun, Q.; Wang, C.; Li, L. Study on pyrolysis characteristics and kinetics of mixed plastic waste. J. Mater. Cycles Waste Manag. 2021, 23, 1984–1994. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Fakhr, H.S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 487676. [Google Scholar]
- Uthpalani, P.G.I.; De Silva, D.S.M.; Weerasinghe, V.P.A.; Premachandra, J.K.; Sarathchandra, T.V. Pyrolysis of waste LDPE and waste PP plastics into fuel oil in a low-cost, lab-scale pyrolyzing unit. J. Sci. Univ. Kelaniya 2023, 16, 47–55. [Google Scholar] [CrossRef]
- Thahir, R.; Altway, A.; Juliastuti, S.R. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy Rep. 2019, 5, 70–77. [Google Scholar] [CrossRef]
- Martynis, M.; Winanda, E.; Harahap, A.N. Thermal pyrolysis of polypropylene plastic waste into liquid fuel: Reactor performance evaluation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 543, p. 012047. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Laouge, Z.B.; Cığgın, A.S.; Merdun, H. Optimization and characterization of bio-oil from fast pyrolysis of Pearl Millet and Sida cordifolia L. by using response surface methodology. Fuel 2020, 274, 117842. [Google Scholar] [CrossRef]
- Jung, K.A.; Nam, C.W.; Woo, S.H.; Park, J.M. Response surface method for optimization of phenolic compounds production by lignin pyrolysis. J. Anal. Appl. Pyrolysis 2016, 120, 409–415. [Google Scholar] [CrossRef]
- Yiga, V.A.; Lubwama, M.; Pagel, S. Optimization of tensile strength of PLA/clay/rice husk composites using Box-Behnken design. Biomass Convers. Biorefinery 2021, 13, 11727–11753. [Google Scholar] [CrossRef]
- Raheem, A.; Ding, L.; He, Q. Effective pretreatment of corn straw biomass using hydrothermal carbonization for co-gasification with coal: Response surface methodology–Box Behnken design. Fuel 2022, 324, 124544. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yu, N.; Yin, H. Catalytic upgrading ofpyrolyticvapors from the vacuum pyrolysis of rape straw over nanocrystalline HZSM-5 zeolite in a two-stage fixed-bed reactor. J. Anal. Appl. Pyrolysis 2014, 108, 185–195. [Google Scholar] [CrossRef]
- Balasundram, V.; Zaman, K.K.; Ibrahim, N.; Kasmani, R.M.; Isha, R.; Hamid, M.K.A.; Hasbullah, H. Optimizing the catalytic performance of Ni-Ce/HZSM-5 catalyst for enriched C 6–C 8 hydrocarbons in pyrolysis oil via response surface methodology. Biomass Convers. Biorefinery 2020, 13, 8603–8613. [Google Scholar] [CrossRef]
- Jegan, J.; Praveen, S.; Kumar, B.M.; Pushpa, T.B.; Gokulan, R. Box-Behnken experimental design for the optimization of Basic Violet 03 dye removal by groundnut shell derived biochar. Desalin. Water Treat. 2021, 209, 379–391. [Google Scholar] [CrossRef]
- Hammani, H.; El Achaby, M.; El Harfi, K.; El Mhammedi, M.A.; Aboulkas, A. Optimization and characterization of bio-oil and biochar production from date stone pyrolysis using Box–Behnken experimental design. Comptes Rendus Chim. 2020, 23, 589–606. [Google Scholar] [CrossRef]
- Liu, D.; Teng, D.; Zhu, Y.; Wang, X.; Wang, H. Optimization of Process Parameters for Pellet Production from Corn Stalk Rinds Using Box–Behnken Design. Energies 2023, 16, 4796. [Google Scholar] [CrossRef]
- Bajad, G.; Vijayakumar, R.P.; Rakhunde, P.; Hete, A.; Bhade, M. Processing of mixed-plastic waste to fuel oil, carbon nanotubes and hydrogen using multi–core reactor. Chem. Eng. Process. Process Intensif. 2017, 121, 205–214. [Google Scholar] [CrossRef]
- Mariyam, S.; Alherbawi, M.; Pradhan, S.; Al-Ansari, T.; McKay, G. Biochar yield prediction using response surface methodology: Effect of fixed carbon and pyrolysis operating conditions. Biomass Convers. Biorefinery 2023, 1–14. [Google Scholar] [CrossRef]
- Mishra, A.; Meikap, B.C. Optimization of process parameters for waste motor oil pyrolysis towards sustainable waste-to-energy utilizing a combinatorial approach of response surface methodology and desirability criteria. Fuel 2023, 353, 129226. [Google Scholar] [CrossRef]
- Ekren, O.; Ekren, B.Y. Size optimization of a PV/wind hybrid energy conversion system with battery storage using response surface methodology. Appl. Energy 2008, 85, 1086–1101. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, L.; Dilbaghi, N. Optimization of process variables for decolorization of Disperse Yellow 211 by Bacillus subtilis using Box–Behnken design. J. Hazard. Mater. 2009, 164, 1024–1029. [Google Scholar] [CrossRef]
- Gopal, L.C.; Govindarajan, M.; Kavipriya, M.R.; Mahboob, S.; Al-Ghanim, K.A.; Virik, P.; Ahmed, Z.; Al-Mulhm, N.; Senthilkumaran, V.; Shankar, V. Optimization strategies for improved biogas production by recycling of waste through response surface methodology and artificial neural network: Sustainable energy perspective research. J. King Saud Univ. Sci. 2021, 33, 101241. [Google Scholar] [CrossRef]
- Hasan, S.H.; Ranjan, D.; Talat, M. “Rice Polish” for the removal of arsenic from aqueous solution: Optimization of process variables. Ind. Eng. Chem. Res. 2009, 48, 4194–4201. [Google Scholar] [CrossRef]
- Duong, T.L.; Nguyen, D.T.; Nguyen, H.H.M.; Phan, B.M.Q.; Nguyen, H.L.; Huynh, T.M. Fast pyrolysis of Vietnamese waste biomass: Relationship between biomass composition, reaction conditions, and pyrolysis products, and a strategy to use a biomass mixture as feedstock for bio-oil production. J. Mater. Cycles Waste Manag. 2019, 21, 624–632. [Google Scholar] [CrossRef]
- Gulab, H.; Jan, M.R.; Shah, J.; Manos, G. Plastic catalytic pyrolysis to fuels as tertiary polymer recycling method: Effect of process conditions. J. Environ. Sci. Health Part A 2010, 45, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Diebold, J.P.; Milne, T.A.; Czernik, S.; Oasmaa, A.; Bridgwater, A.V.; Cuevas, A.; Gust, S.; Huffman, D.; Piskorz, J. Proposed specifications for various grades of pyrolysis oils. In Developments in Thermochemical Biomass Conversion; Springer: Dordrecht, The Netherlands, 1997; pp. 433–447. [Google Scholar]
- Sarker, M.; Rashid, M.M.; Rahman, M.S.; Molla, M. Conversion of low density polyethylene (LDPE) and polypropylene (PP) waste plastics into liquid fuel using thermal cracking process. Br. J. Environ. Clim. Chang. 2012, 2, 1–11. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K. Experimental optimization of process for the thermo-catalytic degradation of waste polypropylene to liquid fuel. Adv. Energy Eng. 2013, 1, 74–84. [Google Scholar]
- Kalghatgi, G.T. Fuel anti-knock quality-Part II. Vehicle Studies-how relevant is Motor Octane Number (MON) in modern engines? SAE Trans. 2001, 110, 2005–2015. [Google Scholar]
- Sarker, M.; Rashid, M.M. Mixture of LDPE, PP and PS waste plastics into fuel by thermolysis process. Int. J. Eng. Technol. Res. 2013, 1, 1–16. [Google Scholar]
- Nisar, J.; Aziz, M.; Shah, A.; Shah, I.; Iqbal, M. Conversion of Polypropylene Waste into Value-Added Products: A Greener Approach. Molecules 2022, 27, 3015. [Google Scholar] [CrossRef]
- Sarker, M.; Rashid, M.M. Polypropylene waste plastic conversion into fuel oil by using thermal degradation with fractional process. Am. J. Environ. Energy Power Res. 2014, 2, 1–10. [Google Scholar]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Sonawane, Y.B.; Shindikar, M.R.; Khaladkar, M.Y. Use of catalyst in pyrolysis of polypropylene waste into liquid fuel. Int. Res. J. Environ. Sci. 2015, 4, 24–28. [Google Scholar]
- Panda, A.K.; Singh, R.K. Conversion of waste polypropylene to liquid fuel using acid-activated kaolin. Waste Manag. Res. 2014, 32, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.K.; Borkowski, J.J. New 3-level response surface designs constructed from incomplete block designs. J. Stat. Plan. Inference 2008, 138, 294–305. [Google Scholar] [CrossRef]
- Manohar, M.; Joseph, J.; Selvaraj, T.; Sivakumar, D. Application of Box Behnken design to optimize the parameters for turning Inconel 718 using coated carbide tools. Int. J. Sci. Eng. Res. 2013, 4, 620–644. [Google Scholar]
- Rakhmania; Kamyab, H.; Yuzir, M.A.; AI-Qaim, F.F.; Purba, L.D.A.; Riyadi, F.A. Application of Box-Behnken design to mineralization and color removal of palm oil mill effluent by electrocoagulation process. Environ. Sci. Pollut. Res. 2021, 30, 71741–71753. [Google Scholar] [CrossRef]


| Run Order | Factor | Response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature, °C | N2 Flow Rate, mL min−1 | Feed Rate, kg/h | Oil Yield, % | Solid Residue, % | Gas Yield, % | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| 1 | 400 | 12.5 | 0.250 | 64.71 | 63.99 | 4.49 | 4.66 | 30.8 | 31.36 |
| 2 | 475 | 12.5 | 0.875 | 89.43 | 89.43 | 2.94 | 2.94 | 7.63 | 7.63 |
| 3 | 400 | 5.0 | 0.875 | 62.58 | 62.765 | 5.73 | 5.63 | 31.69 | 31.60 |
| 4 | 550 | 12.5 | 0.250 | 57.82 | 57.29 | 1.80 | 1.84 | 40.38 | 40.88 |
| 5 | 475 | 5.0 | 0.250 | 85.36 | 85.895 | 3.92 | 3.85 | 10.72 | 10.26 |
| 6 | 550 | 20.0 | 0.875 | 52.95 | 52.77 | 1.97 | 2.07 | 45.08 | 45.17 |
| 7 | 475 | 12.5 | 0.875 | 89.43 | 89.43 | 2.94 | 2.94 | 7.63 | 7.63 |
| 8 | 475 | 12.5 | 0.875 | 89.43 | 89.43 | 2.94 | 2.94 | 7.63 | 7.63 |
| 9 | 550 | 5.0 | 0.875 | 54.66 | 54.66 | 2.63 | 2.66 | 42.71 | 42.68 |
| 10 | 400 | 20.0 | 0.875 | 61.35 | 61.355 | 4.86 | 4.83 | 33.79 | 33.82 |
| 11 | 475 | 5.0 | 1.500 | 80.85 | 80.14 | 4.18 | 4.32 | 14.97 | 15.56 |
| 12 | 550 | 12.5 | 1.500 | 49.27 | 49.99 | 2.48 | 2.31 | 48.25 | 47.70 |
| 13 | 475 | 12.5 | 0.875 | 89.43 | 89.43 | 2.94 | 2.94 | 7.63 | 7.63 |
| 14 | 475 | 20.0 | 0.250 | 83.42 | 84.14 | 3.26 | 3.12 | 13.38 | 12.79 |
| 15 | 475 | 20.0 | 1.500 | 79.13 | 78.60 | 3.59 | 3.66 | 17.28 | 17.74 |
| 16 | 400 | 12.5 | 1.500 | 59.46 | 59.99 | 5.25 | 5.21 | 35.29 | 34.79 |
| 17 | 475 | 12.5 | 0.875 | 89.43 | 89.43 | 2.94 | 2.94 | 7.63 | 7.63 |
| Response | Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|---|
| Fuel oil yield (%) | Model | 3734.96 | 9 | 415.00 | 890.55 | <0.0001 | Significant at a 5% level |
| A—Temperature | 139.45 | 1 | 139.45 | 299.24 | <0.0001 | ||
| B—Nitrogen flow rate | 5.45 | 1 | 5.45 | 11.68 | 0.0112 | ||
| C—Feed rate | 63.85 | 1 | 63.85 | 137.01 | <0.0001 | ||
| AB | 0.06 | 1 | 0.06 | 0.12 | 0.7355 | ||
| AC | 2.72 | 1 | 2.72 | 5.84 | 0.0463 | ||
| BC | 0.01 | 1 | 0.01 | 0.03 | 0.8765 | ||
| A2 | 3291.63 | 1 | 3291.63 | 7063.58 | <0.0001 | ||
| B2 | 54.11 | 1 | 54.11 | 116.13 | <0.0001 | ||
| C2 | 56.25 | 1 | 56.25 | 120.71 | <0.0001 | ||
| Residual | 3.26 | 7 | 0.47 | ||||
| Lack of Fit | 3.26 | 3 | 1.09 | ||||
| Pure Error | 0.00 | 4 | 0.00 | ||||
| Cor Total | 3738.22 | 16 | |||||
| Std. Dev. | 0.68 | R-Squared | 0.99 | ||||
| Mean | 72.87 | Adj R-Squared | 0.99 | ||||
| C.V. % | 0.94 | Pred R-Squared | 0.98 | ||||
| PRESS | 52.19 | Adeq Precision | 75.33 |
| S. No | Waste PP | Fuel Oil | ||||
|---|---|---|---|---|---|---|
| Wavenumber (cm−1) | Bond | Functional Group | Wave Number, (cm−1) | Bond | Functional Group | |
| 1 | 3394.10 | O-H stretch, H-bonded | Alcohols, Phenols | 3457.74 | O-H stretch, H-bonded | Alcohols, Phenols |
| 2 | 3188.72 | O-H stretch | Carboxylic acids | 3071.08 | =C-H stretch | Alkenes |
| 3 | 2952.48 | C-H stretch | Alkanes | 2955.38 | C-H stretch | Alkanes |
| 4 | 2915.84 | C-H stretch | Alkanes | 2956.34 | C-H stretch | Alkanes |
| 5 | 2873.42 | C-H stretch | Alkanes | 2918.73 | C-H stretch | Alkanes |
| 6 | 2194.60 | -C- stretch | Alkynes | 2913.91 | C-H stretch | Alkanes |
| 7 | 1455.90 | C-H bend | Alkanes | 2873.42 | C-H stretch | Alkanes |
| 8 | 972.91 | =C-H bend | Alkenes | 2203.27 | -CC- stretch | Alkynes |
| 9 | 839.85 | =C-H bend | Alkenes | 1741.41 | C=O stretch | Esters, Saturated aliphatics |
| 10 | - | - | - | 1648.84 | -C=C- stretch | Alkenes |
| 11 | - | - | - | 1456.96 | C-H bend | Alkanes |
| 12 | - | - | - | 1371.14 | C-H bend | Alkanes |
| 13 | - | - | - | 1221.68 | C-N stretch | Aliphatic amines |
| 14 | - | - | - | 1158.04 | C-N stretch | Aliphatic amines |
| 15 | - | - | - | 970.019 | =C-H bend | Alkenes |
| 16 | - | - | - | 886.131 | =C-H bend | Alkenes |
| 17 | - | - | - | 887.095 | =C-H bend | Alkenes |
| 18 | - | - | - | 738.603 | =C-H bend | Alkenes |
| 19 | - | - | - | 695.212 | -CC-H:C-H bend | Alkynes |
| Retention Time (min.) | Name of the Compound | Area (%) |
|---|---|---|
| 3.93 | Pentane, 3,3-dimethyl- | 1.90 |
| 5.08 | 1-Propene, 2-methyl- | 10.95 |
| 5.77 | Cyclopropane | 1.31 |
| 6.27 | 1-Propyne | 1.43 |
| 7.22 | (E)-6-Dodecene | 1.84 |
| 7.77 | 1,6-Octadiene, 2,5 dimethyl-, (E)- | 5.46 |
| 8.25 | 1-Undecene, 7-methyl | 24.26 |
| 8.87 | 3-Undecene | 2.57 |
| 9.34 | 2-Undecanethiol, 2-methyl- | 1.30 |
| 10.77 | 9-Octadecene | 1.94 |
| 11.43 | 1-Octadecene | 5.82 |
| 12.34 | 7-Octadecyne, 2-methyl- | 2.85 |
| 13.05 | 2-Methyl-octadecyne | 1.62 |
| 14.93 | 1-Dodecanol, 2-hexyl- | 2.53 |
| 15.69 | Decane, 2,3,5,8-tetramethyl | 6.37 |
| 16.28 | Naphthalene | 4.24 |
| 16.66 | Cyclopentane | 1.61 |
| 17.40 | Cyclododecanemethanol | 1.24 |
| 19.20 | Cyclotetracosane | 1.42 |
| 19.94 | 1-Hexadecanol, 3,7,11,15-tetramethyl- | 3.28 |
| 20.87 | Oxirane, tetradecyl- | 2.23 |
| 21.58 | 2,6,10,14-Tetramethyl-7-pentadecane | 1.36 |
| 23.18 | 1-Eicosene | 1.27 |
| 23.89 | 1-Dodecanol, 2-octyl- | 2.37 |
| 24.74 | Dodecane | 1.83 |
| 25.42 | 1-Heptacosanol | 1.74 |
| 27.74 | 11-Dodecen-1-ol, 2,4,6-trimethyl- | 1.56 |
| 28.33 | 11-Dodecen-1-ol difluoroacetate | 1.46 |
| 30.39 | 11,13-Dimethyl-12-tetradecen-1-ol acetate | 1.33 |
| 30.86 | 9-Hexacosene | 1.30 |
| Factors | Range and Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Reaction temperature (A) (°C) | 400 | 475 | 550 |
| Nitrogen flow rate (B) (mL min−1) | 5 | 12.5 | 20 |
| Feed rate (C) (kg h−1) | 0.25 | 0.875 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabha, B.; Ramesh, D.; Sriramajayam, S.; Uma, D. Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design. Recycling 2024, 9, 15. https://doi.org/10.3390/recycling9010015
Prabha B, Ramesh D, Sriramajayam S, Uma D. Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design. Recycling. 2024; 9(1):15. https://doi.org/10.3390/recycling9010015
Chicago/Turabian StylePrabha, Balasubramaniam, Desikan Ramesh, Srinivasan Sriramajayam, and Doraiswamy Uma. 2024. "Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design" Recycling 9, no. 1: 15. https://doi.org/10.3390/recycling9010015
APA StylePrabha, B., Ramesh, D., Sriramajayam, S., & Uma, D. (2024). Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design. Recycling, 9(1), 15. https://doi.org/10.3390/recycling9010015