Abstract
Fish processing produces large amounts of fish waste. Instead of disposing of it, it is wiser to recover the valuable resource for high-value-added products. Our study proposed a process using carbon dioxide-acidified water as a green solvent under supercritical conditions to successfully recover collagen/gelatin from the skin and bone of striped catfish. The optimum extraction conditions were obtained at 75 bar, 37 °C, and 24 h. The yields from the dry skin and bone mass were around 37% and 8%, respectively. The extracted products were characterized by Fourier-transformed infrared spectroscopy to study the functional groups, scanning electron microscopy to evaluate the morphology, sodium dodecyl-sulfate polyacrylamide gel electrophoresis to study the protein pattern, UV–vis analysis to measure the absorption peak, and thermal gravimetric analysis to determine the denaturation temperature. The results show the viability of the proposed method on an industrial scale. The characteristics of the extracted product show promising results and potential for being developed further in many applications such as biomaterial engineering in healthcare or natural polymer-based absorbent material for efficient removal of heavy metals from water and wastewater.
1. Introduction
Fish processing produces large amounts of fish waste (approximately 50–80%), subject to the level of processing and species [1]. Improper disposal of these wastes causes sanitation and environmental problems. Attempting to recycle what is still valuable in such wastes is therefore a wiser waste management solution than disposal. Discards from fish processing are abundant sources of protein and many valuable compounds [2]. Fish waste is generally recycled for the production of fertilizers or applied directly as feed in aquaculture. Currently, pursuing the biobased-circular-green economy (BCG) model conversion of fish waste to high-commercial value products at the same time plays a key role in acquiring economic growth and achieving sustainable development [1]. Freshwater and marine fishes have received much consideration as alternative sources for collagen/gelatin production. Many studies have valorized fish wastes to collagen, for example, from channel catfish [3], tilapia [4,5], snakehead [6], golden carp [7], sole fish [8,9], and silver carp [10]. Apart from tilapia, Pangasianodon hypophthalmus (striped catfish) is the most abundant and vital aquaculture fish in Thailand. It is also a viable source of protein for people in the Mekong subregion (i.e., Cambodia, Vietnam, and Loa) [11]. The leftovers from catfish processing can be used as a potential alternative raw material for the production of collagen/gelatin, which is a highly valued added product and can be applied in various applications [10,12,13,14,15]. Recently, one of the most interesting applications is to develop further gelatin-based composite absorbents for heavy metal removal from water and wastewater [14,16,17,18].
Collagen is one of the most essential structural proteins and constitutes approximately 30% of all proteins in animals. The signature feature of collagen is the unique amino acid sequence [Gly–X–Y]n, in which proline and hydroxyproline are mostly X and Y locations, respectively. A collagen triple-helical structure is formed from the same three α-polypeptide chains and is stabilized by intramolecular hydrogen bonds. The XXIX types of collagen have been studied and applied in various fields, such as pharmaceuticals, foods, biomedical products, and cosmetics [3,19,20]. Collagen of mammalian origin is supplied in amounts of more than a hundred thousand tons per year. However, there has been a concern about disease spread, such as foot and mouth disease, avian influenza, transmissible spongiform encephalopathy (TSEs), and bovine spongiform encephalopathy (BSE). In addition, products derived from bovine and porcine sources are restricted by the Jewish, Hindu, and Muslim religions [4,21,22].
Recovering collagen from fish waste can be performed by various extraction methods, namely acid extraction, pepsin extraction, pulsed electric field (PEF), carbon dioxide acidified water, and supercritical fluid CO2 (SF) [22,23]. In the literature, the classical method referring to acid extraction and pepsin extraction generally requires high acidity, large amounts of chemicals and solvents, and long-processing time, and requires complicated steps, i.e., extracting, purifying, and cleaning [23,24]. In particular, harmful extracting solvents can incorporate and remain in the extracted collagen, which causes both serious health and environmental problems. Before PEF technology can be fully utilized, a number of technical problems, financial obstacles, consumer acceptability challenges, regulatory concerns, and toxicity dangers must be resolved [25]. Therefore, many researchers have investigated innovative and sustainable extraction methods to acquire higher collagen yields faster and employ less toxic solvents. Many reports revealed positive findings on the use of a pressurized technique, i.e., supercritical fluids technology or the use of water acidified with carbon dioxide, to extract collagen/gelatin from various sources [12,15,22,26]. Beyond the critical point, the physical properties of solvents (i.e., water and CO2) are modified to have similar qualities to both gas and liquid, such as modest viscosity, modest density, high solvation, high diffusion, and high mass transfer.
Our work is the first to apply the carbon dioxide-acidified water technique to both the skins and bones of freshwater fish instead of marine sources. In addition, response surface methodology (RSM) was used for optimization. Extraction temperature, which is an important factor, was also studied. Our study proposed a collagen/gelatin extraction method that can possibly be further applied on an industrial scale. Complying with the principles of green chemistry, a combination of CO2 and water, i.e., carbon dioxide-acidified water, was used as the sole solvent in this study and was pressurized in an autoclave reactor model P2313 (Amar, Mumbai, India). At this stage, the objective of this study was to optimize the extraction process of gelatin/collagen from the skin and bone of striped catfish using RSM. The independent variables, namely, carbon dioxide pressure, extraction time, and extraction temperature, on the yields were investigated. The characterization of the obtained product was also studied to confirm its suitability for use in various applications.
2. Results
2.1. Effect of Extraction Conditions on the Yield
The yields of the extracts from the designed conditions are reported in Table 1. We found a significant difference in yields of collagen/gelatin from different operating conditions. At a pressure above 73.8 bars and a temperature of 31 °C, supercritical fluid CO2 can be obtained [27]. Our results revealed that the yields were maximized under a supercritical CO2 condition. From the same conditions of 75 bar, 37 °C, and 24 h, the highest yields of 36.85% and 8.10% were obtained from skin and bone, respectively. At this condition, CO2 has the density of liquid but the viscosity and diffusion of a gas. This condition promotes the diffusion of CO2 and water into the fish skin and bone matrix and enhances the dissolution process. An increase in pressure will increase the density of CO2, which subsequently increases the solubility of the solute. Extending the extraction time also showed an increase in the yield until it reaches equilibrium.

Table 1.
The 2k factorial design and the responses of collagen/gelatin from the skin and bone of striped catfish.
2.2. Optimization of Collagen Extraction Conditions on the Yield
The yields were correlated to the extraction conditions according to the following first-order polynomial Equations (1) and (2). The yields were predicted by substituting the given levels of each condition (only in a range of experimental levels).
where X and Y are the yields of the collagen/gelatin from the skin and bone of striped catfish, and A, B, C, and D are the variables of extraction time, temperature, pressure, and center point value, respectively.
Table 2 shows the analysis of variance (ANOVA) results of the yield from the skin of striped catfish. A smaller p-value and a larger F-value suggested a more significant effect on the yield [28]. The linear terms and 2-way and 3-way interactions were significant (p < 0.05). In addition, the coefficient of determination (R2 = 0.9978) of the model indicated that most variations were explained. The adjusted coefficients of determination (Adj R2 = 0.9959) showed a good fit for the linear regression. Center points were added to the design; however, the result suggested that no curvature was detected (p > 0.05) for the selected ranges of each variable. The ANOVA of the model for extraction yield from the bone is provided in Table 3. Similarly, the linear and 2-way and 3-way interaction terms were significant. The model also showed a coefficient (R2) of determination of 99.51% and an adjusted coefficient (Adj R2) of determination of 99.08%. In addition, no curvature resulted from each variable on the extracted yield from the bone.

Table 2.
Analysis of variance (ANOVA) of the yield of collagen/gelatin extraction from the skin.

Table 3.
ANOVA of the yield of collagen/gelatin extraction from the bone.
2.3. Interaction of Process Conditions
The interactions between variables were studied using surface plots between two conditions, while the other was kept constant at the center point. Figure 1 and Figure 2 show the graphical surface plots of the interaction effects of extraction conditions against the yields obtained from skin and bone samples, respectively. Similarly, the results showed a strong interaction between yields and extraction pressures. The yields rapidly increased with increased pressure because CO2 pressure plays an important role in improving the solubility of collagen in the extraction. The yields slowly increased with increasing temperature due to the small range of selected levels. It is quite certain that using temperatures above 37 °C may result in the degradation of the extracted collagen and lower yields. However, the longer extraction time rapidly increased the maximum yields, which were in good agreement with the previous work [15] and the ANOVA results as shown in Table 2 and Table 3. Therefore, the optimum extraction conditions were determined at supercritical fluid CO2 at a pressure of 75 bar, a temperature of 37 °C, and an extraction time of 24 h. In comparison to the yield of the extracted product from the skin of Pangasius, the values of our study (36.60 to 36.85%) are higher than the previous report using the conventional extraction methods (acidic and enzymatic extraction) [24], and are in the compatible range of 5 to 42.36% when using acids (pH 1.8 to 3.0, time 60 h, and temperature of 4 °C) [3,23]. In the previous work, 73% of the collagen was obtained by using conventional methods of acidic and enzymatic extraction, which have many disadvantages, as mentioned in the introduction section [29]. We did not compare them because the method and the purpose are different. Unlike our study, they used supercritical carbon dioxide for removing lipids from crude collagen. However, our study employed water-acidified CO2 for the extraction of collagen from the skin and bone of basa fish.
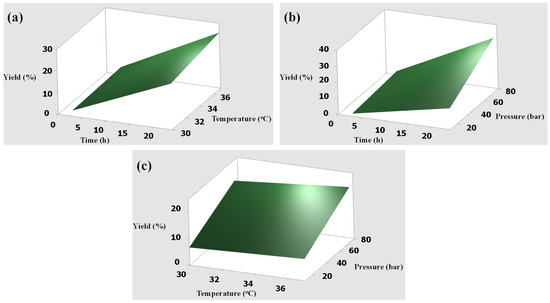
Figure 1.
Surface plots of the interaction effects on yield obtained from the skin: (a) time and temperature, (b) time and pressure, (c) temperature and pressure.
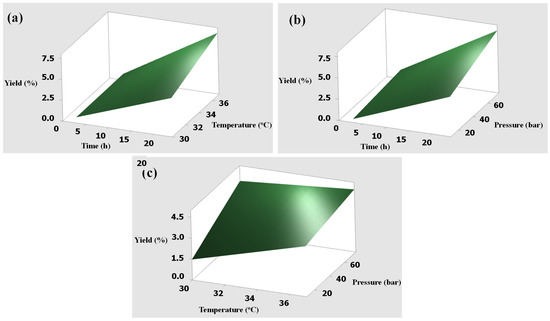
Figure 2.
Surface plots of the interaction effects on yield obtained from the bone: (a) time and temperature, (b) time and pressure, (c) temperature and pressure.
2.4. Assessment of Process Efficiency
The process efficiency of the proposed method was evaluated, as shown in Table 4. Our method required only three main steps, namely pretreatment, extraction, and drying steps. However, the conventional acidic/enzymatic method for the extraction of collagen from basa fish required more steps, namely pretreatment, extraction, salting out, dialysis, and drying steps [24,29]. Because the processing time is shortened, production time, energy, and chemical usage could be reduced, suggesting the greenness of the proposed method. Considering the chemicals used in the extraction step are only water and carbon dioxide, it can be said that no harmful substances remained in the product. This suggested that the product could be applied in a wide range of applications.

Table 4.
Process efficiency of the proposed method based on 20 g of raw material.
2.5. FTIR Spectra
FTIR spectroscopy was used to confirm the functional groups present in collagen expressed by absorbance and wavenumber. In general, the amide A band relates to the stretching vibration of the NH group in the range of 3400–3440 cm−1 [30]. This value will decrease to approximately 3300 cm−1 when the NH group incorporates the hydrogen bonds of the peptide chain. Amide B band (2924–2928 cm−1) relates to the asymmetric stretching vibration of =CH and –NH3+. The shifting of amide B to a higher frequency indicates an increase in free NH–NH3+ clusters in the N-terminal lysine residues. Additionally, a set of amide bands indicates the secondary structure of the polypeptide chain, and it could be used to recognize the presence of imino and amino acids (i.e., proline and hydroxyproline rings). Amide I is used as a marker of the secondary structure. The C=O stretching (1600–1700 cm−1) of the amide I band is associated with the formation of hydrogen bonds between adjacent chains [31]. The amide II associated with the CN elongation and NH deformation vibration (1550–1600 cm−1) is used to indicate the number of NH groups involved in hydrogen bonds adhered to α-chains. This bonding maintains the helical structure of the collagen. Amide III is used to indicate the presence of glycine CH2, which is one of the predominant amino acids in collagen. This band is associated with NH deformation and CN elongation in collagen. It was also suggested that the ratio between the amide III and 1440 cm−1 peaks equal to one indicates the preservation of the triple helix structure of collagen.
From Figure 3, our extracted products show a similar result to the previous report of acid-solubilized collagen (ASC) from the skin of striped catfish using the acid extraction method [24,31,32]. Five major peaks of amide were observed. The peak locations of the five major peaks of the extracted products obtained from the skin and bone were similar to the standard type I collagen. From Table 5, the peaks of amide A obtained from the skin and bone were 3282 and 3290 cm−1, respectively. According to previous reports [30,31,33], a shifting of amide A from 3400–3440 cm−1 to a lower frequency (near 3300 cm−1) denoted that the NH group incorporates hydrogen bonds in the peptide chain. The FTIR spectra showed amide A peaks at wavenumbers of 3290–3294 cm−1. The amide B bands were observed at wavenumbers of 2924–2927 cm−1, which is in the range of 2924–2928 cm−1 [30,31]. This indicated asymmetric stretching vibration of =CH and –NH3+ in the polypeptide chains. There was no indication of the presence of free NH–NH3+ clusters in the N-terminal lysine residues. The positions of amide I usually appear from 1600–1700 cm−1, which is attributed to the stretching vibrations of the carbonyl (C=O) group with the peptide backbone. Our study revealed an amide I peak at wavenumbers of 1631–1633 cm−1. This confirmed the forming of hydrogen bonds between N-H and the C=O of the adjacent polypeptide chains of our extracted product. A slightly lower frequency than ASC from the previous reports (1651.07 cm−1) is related to an increase in hydrogen bonds, which subsequently increase in the molecular organization [31,33]. The amide II band was located at 1537–1542 cm−1, representing CN stretching vibrations coupled with NH bending vibrations. A shift to a lower frequency when compared with the typical amide ΙΙ band position of 1550–1600 cm−1 indicated a greater number of hydrogen bonds in the polypeptide, which highlighted a high degree of maintaining the helical structure of the obtained collagen. Additionally, the vibration of glycine CH2 bending was observed at 1542 cm−1 (from the skin) and 1544 cm−1 (from the bone), which was close to that of ASC from the skin of Pangasius sp. reported earlier [31]. From Table 5, the absorption at 1337–1338 cm−1 for the skin and bone represents the CH2 wagging vibration of the proline side chains generally found in the type I collage. The absorption frequencies at 1238–1239 cm−1 represent the NH bending coupled with CH stretching from amide linkage as well as the absorption resulting from the wagging vibration of the glycine CH2 backbone and the proline side chains [21]. According to [10], for ASC from fish, the ratio of the absorption intensity between the amide III peak and of the 1450 cm−1 band equal to 1.0 can explain the degree of maintaining the original structure of the collagen molecule after the extraction step. As shown in Figure 4, the ratio for the extracted product from the skin (0.22/0.22) and bone (0.16/0.16) equal 1.0 proves that our extraction method can sustain the native collagen structure and confirm that the extracted products contain a triple helical structure of collagen.
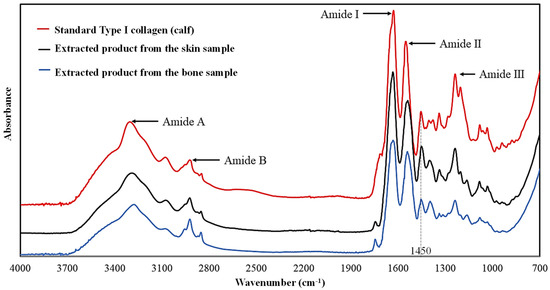
Figure 3.
FTIR spectra of the extracted product obtained from the skin and bone of striped catfish.

Table 5.
FTIR spectra range locations of Type I collagen standard and peak locations of the extracted product from the skin and bone of striped catfish (Pangasianodon hypophthalmus).
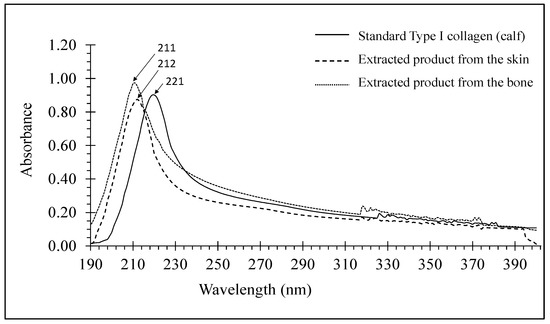
Figure 4.
UV spectra of extracts from the skin and bone of striped catfish.
In recent reports, one of the most effective eliminations of heavy metals from contaminated water is via hydrogel-based absorbents, and collagen is classified as a natural hydrogel because it is a natural source for the making of hydrogels. According to their three-dimensional porous networks and abundance of the inherent hydrophilic functional groups, such as –COOH, –NH2, –OH, etc., collagen-based hydrogels can be highly effective absorbents in water and wastewater treatment. According to [16], examples of the active functional groups capable of the removal of heavy metals from contaminated water include R-COOH, R-OH, R-CONH2, R-NH2, etc. At the alkaline pH, the H+ ions release, leaving the negative charge ions that readily absorb divalent metal cations (such as Pb2+, Co2+, Cu2+, Cd2+, and Ni2+, etc.) [14,16].
2.6. UV Spectra
UV spectra are one of the basic spectroscopic methods for collagen characterization. Most proteins have a maximum absorption wavelength of approximately 356 nm, while proteins with a high content of amino acids generally show a maximum absorption near 280 nm, resulting from the presence of aromatic groups, such as phenylalanine, tyrosine, and tryptophan. For the triple-helical proteins, the maximum absorbance wavelength usually appears near 230 nm [31].
Figure 4 shows the UV spectra of the standard type I calf collagen (calfskin) and the extracted products from the skin and bone of striped catfish. The maximum absorption wavelengths of the extracted product from the catfish skin and bone appeared at 212 nm and 211 nm, respectively, which were in a range of 210–240 nm for freshwater fish such as Labeo rohita and Catla catla. According to [34], a relatively lower value compared with the value obtained from the skin of southern catfish (220 nm) [35] suggests a low concentration of tyrosine in the extracted products.
2.7. Electrophoretic Patterns
The SDS-PAGE method was used as a qualitative tool to examine the protein content in the extracted products. The standard type I collagen from calfskin was used as a reference sample. Generally, a sample of the triple helical type I fish collagen consists of two α-chains (α1 and α2) and β-chains with different mobilities and intensities [35]. Many studies have reported similar electrophoresis patterns for collagen from different parts (skin, bone, and scale) of bigeye tuna [19] and the skin of channel catfish [3], golden carp [7], and sole fish [8]. According to [23], the expected molar mass (KDa) of three major characteristic bands considered to be the golden standard for the identification of collagen type I peptides are α1 (120–150 KDa), α2 (120–150 KDa), and β (200–250 KDa). From the electrophoretic analysis, our extracted products from the skin and bone of striped catfish contained similar subunits of type I collagen, as shown in Figure 5. The molecular weights of the α1 and α2 for both the skin and bone range between 130 and 150 KDa, and the β band represents the intermolecular crosslinked for all samples at around 250 KDa, which corresponds to the golden standard. However, a blurred band in the β region indicated some impurities or slight denaturation of collagen, which result in the formation of gelatin or collagen protein hydrolysates. In all, the proposed extraction using a single step of CO2-acidified water under the supercritical condition as a green solvent can be successfully used for the co-extraction of collagen/gelatin from the skin and bone of striped catfish. These recovered products (collagen/gelatin) are highly profitable and beneficial compounds that can be applied further for biomaterial engineerings such as cartilage/bone tissue regeneration, bone tissue engineering, or skin regeneration and wound healing [12,23].
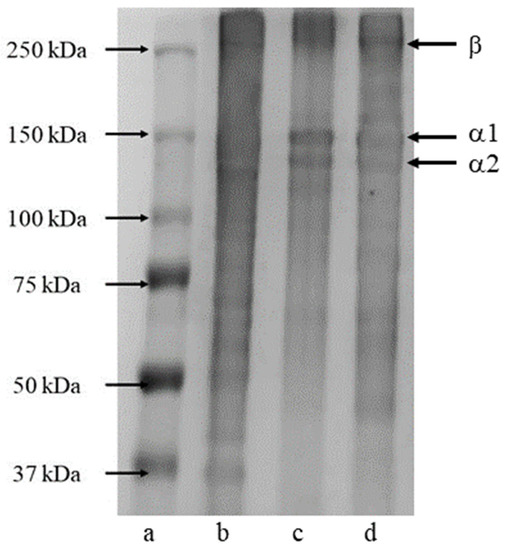
Figure 5.
SDS-PAGE analysis of (a) protein marker, (b) type I calf collagen, (c) the extracted product from the skin, (d) the extracted product from the bone.
2.8. Morphology
After being freeze-dried, the collagen/gelatin extracts were recovered as a soft white sponge. The images of the extracted products obtained under the optimum extraction condition (37 °C, 75 bars, and 24 h) using carbon dioxide-acidified water are presented in Figure 6. The soft white sponges from the skin and bone of striped catfish were observed with the naked eye. However, SEM revealed that dense and homogenous multilayered samples had formed, probably during the lyophilization process. The extracted collagen/gelatin structures were hexagonal at 50× magnification. At 200× magnification, the samples were sponge-like with porous structures. The structures were apparent, with a fibrous meshwork and loose and flaky orientation at further magnification. Our results are consistent with the SEM images of the extracted collagen from the skin of Nile tilapia [36]. The cross-sectional images revealed highly porous and homogenous structures with interconnected pores for both the skin and bone. The pores and the wrinkles on the surface of the products resulted from dehydration during the lyophilization step [32]. This rough surface creates a generous surface area, which results in a high absorption capability. According to [33,37], a well-distributed pore structure is favorable for biomedical applications, serving as matrices for absorption and cell proliferation. From our results, it can be said that the extracted products obtained from carbon dioxide-acidified water have the potential to be further developed as a material for wound dressing, hydrating agents, growth gene expression, and drug delivery [10,38] or grafted on chitosan-based polymer, which can be used for heavy metal removal from drinking water [14].
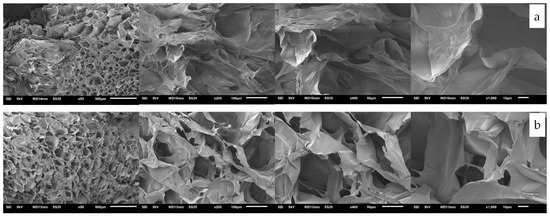
Figure 6.
SEM images of the extracted collagen/gelatin from (a) the skin and (b) the bone.
2.9. Thermal Stability
The thermal stability of the collagen/gelatin products under nitrogen flows was assessed by TGA. This technique measures the stability and degradation of the material, i.e., collagen. The loss of mass relates to hydrogen bond rupture and the loss of intermolecular water, followed by the degradation of protein chains and rupture of the collagen fiber. In this study, the loss of hydrogen bonds and intermolecular water occurred during the lyophilization step (−80 °C freeze-drying). As shown in Figure 7, the mass of collagen/gelatin product is decreased by an increase in temperature. The loss was observed in three phases as follows: denaturation (20–200 °C), collagen combustion (200–500 °C), and residue formation (500–700 °C). The first phase showed denaturation of collagen samples, attributing to 10–16%, at a temperature around 27–32 °C, which is compatible with the values reported earlier: bigeye tuna (31–33 °C) [19], snakehead (34 °C) [6], and southern catfish (34 °C) [35]. The second phase relates to the decomposition of the collagen structure due to the combustion of collagen. The results affirm that our extracted products have compatible thermal stability with ASC obtained from other fish.
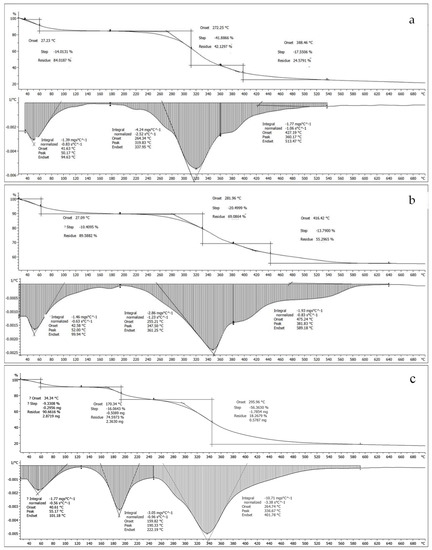
Figure 7.
TGA graphs of extracts from (a) the skin and (b) the bone, and (c) type I calfskin.
3. Materials and Methods
3.1. Materials
Hydrogen peroxide (H2O2), sodium hydroxide (98% NaOH), and sodium chloride (NaCl) were purchased from QRëC, Auckland, New Zealand. Linear alkylbenzene sulfonate sodium salt (LASNa) 13% (w/w) (C18H29NaO3S) was obtained from commercial sources. Standard type I calf collagen was obtained from Sigma–Aldrich, Saint Louis, MO, USA. Carbon dioxide (99.8% CO2) was purchased from Linde, Samut Prakarn, Thailand (HPLC grade). All other reagents were analytical grade.
3.2. Preparation and Pretreatment of Fish Skin and Bone
The fish waste (i.e., skin and bone of striped catfish) was obtained from food vendors of the KMUTT canteen, Bangkok, Thailand. The samples were further cleaned and washed with tap water to remove blood and dirt. After removing the fish meat and fat, the skin and bone were washed again with deionized (DI) water and cut with scissors into small pieces of approximately 10 × 10 mm. The prepared samples were kept in a polyethylene bag and stored at −20 °C.
The samples were pretreated according to the method reported previously [29]. The skin and bone samples were completely lyophilized at −80 °C using a freeze dryer FD8-T-Series, Gold Sim (Gibthai, Bangkok, Thailand). The dried skin and bone were weighed and recorded before mixing in 0.5% (w/v) LASNa with a solid-liquid ratio (S/L) of 1:25 (w/v) for 6 h to remove lipids and minerals. The samples were filtered and washed with cold DI water before mixing in 1% (v/v) H2O2 and 0.05 N NaOH solution with the same S/L for 2 h to remove pigments and noncollagenous substances. The residues were filtered and washed with cold DI water.
3.3. Extraction Method and Extraction Yield
The pretreated samples were transferred into a Teflon reactor vessel, and DI water was added at an S/L ratio of 1:25. Figure 8 shows the experimental workflow of this study. A high-pressure lab-scale autoclave was operated at various pressures (10, 42.5, and 75 bar), temperatures (30, 33.5, and 37 °C), and reaction times (3, 13.5, and 24 h) as shown in Table 6 using the 2k factorial design by Minitab software Version 16. The gas was immediately released after reaching the desired conditions. The obtained solutions were filtered, and the filtrates were centrifuged by a refrigerated laboratory centrifuge MPW-380R (MPW Med. Instruments, Warsaw, Poland) and lyophilized.
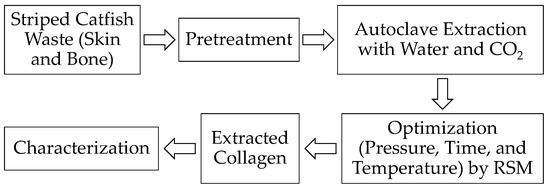
Figure 8.
Schematic representation of experimental flow chart.

Table 6.
Experimental design range and values of independent variables.
The extraction yield was calculated as the percentage between the dry mass of extracted material and the dry weight of striped catfish skin or bone. The calculation is as follows [32,39]:
3.4. Experimental Design and Modeling
RSM is the most common statistical tool for optimal condition prediction and process improvement. The independent variables and their interactions could be studied [28,40,41]. RSM is the most useful method for optimizing independent variables for the desired response. Among all the designs, the 2k factorial design is the most effective for many experiments involving the study of the main effects and interactions of more than two variables. As shown in Table 6, the design of this study consisted of three variables, two replicates, and two center points, which generated 18 random experiments as shown in Table 1.
The data are fitted into a model to predict the response according to the following first-order equation:
where Y is the extraction yield (response), β0 is a constant, ε is an error, βi and βij are regression coefficients, and Xi and Xj are the levels of factors.
3.5. Characterization of the Obtained Product
3.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
The typical functional groups of collagen were confirmed by a Nicolet 6700 FTIR Spectrometer (Thermo Fisher Scientific, Glendale, WI, USA). Approximately 2 mg of dry samples were mixed with approximately 200 mg of potassium bromide (KBr). The FTIR spectral regions of 650–4000 cm−1 at a resolution of 4 cm−1 were selected. The scanning was recorded 32 times for the average of each sample.
3.5.2. Electrophoretic Pattern
Protein patterns were studied by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS–PAGE) according to a previously published method with a slight adjustment [42]. The dry samples were dissolved in 10% SDS before incubation at 85 °C for 1 h. The dissolvable debris was centrifuged from the mixtures at 5000× g for 10 min. Then, 5× sample loading buffer (0.5% bromophenol blue pH 6.8, 0.25 M Tris-HCl, 50% glycerol, 0.5 M dithiothreitol (DTT), and 10% SDS) was added to solubilized samples. The mixtures were incubated again for 2 min in boiling water. The samples were loaded into the wells of a gel consisting of 5% stacking and 7% resolving gel. A constant voltage of 35 mA was applied. After electrophoresis, the resolving gel was cleaned and stained with Coomassie blue R250. Type I calf collagen was utilized as a standard. A protein marker was used to evaluate the molecular weight (MW) of proteins.
3.5.3. Scanning Electron Microscopy (SEM)
The morphology of the products obtained from the skin and bone was evaluated by a JSM-6610LV scanning electron microscope (Jeol Ltd., Tokyo, Japan). The dry samples were stabilized on aluminum stubs with mutual conductive adhesive tape and sputtered with a gold nanolayer. The samples were placed on the sample holder and observed at 1000×, 400×, 200×, and 50× magnifications.
3.5.4. Thermal Gravimetric Analysis (TGA)
The thermal stability of the dry samples was studied using a TGA/DSC 3+ (Mettler Toledo, Nänikon, Switzerland). The instrument was operated at a temperature ranging from 25 to 700 °C and a heating rate of 10 °C/min in a nitrogen (N2) atmosphere. The decomposition temperatures were obtained from the thermogravimetric curves.
3.5.5. UV Absorption Spectrum
The ultraviolet (UV) spectra of the sample were measured using a Lambda 35 UV/Vis Spectrophotometer (Perkin Elmer, Waltham, MA, USA). The samples of 0.1 mg/mL were prepared in 0.05 M acetic acid. The mixtures were centrifuged, and the supernatants were placed into a quartz cell. The UV spectra were scanned in the wavelength range of 190–390 nm with a speed of 2 nm/s and an interval of 1 nm. Type I calf collagen was utilized as a standard.
4. Conclusions
Organic acids are commonly used to extract collagen from fish waste. Our work suggested a process to successfully recover collagen/gelatin from the waste of striped catfish. In this study, a novel technique using a green solvent (water and CO2) can increase yield and/or enhance the quality of the extracted product. The optimum extraction conditions were highlighted. Overall, the findings confirm the effectiveness of the suggested method for improvement of extraction yield compared to the conventional acid/enzymatic method, and the properties of the products indicate the potential for further development in a variety of applications.
Author Contributions
Conceptualization, S.P.T.; methodology, A.L.P. and S.P.; validation, S.P. and S.P.T.; formal analysis, A.L.P., S.P. and S.P.T.; investigation, S.P.; resources, S.P.T.; data curation, S.P.; writing—original draft preparation, S.P.; writing—review and editing, S.P.T; supervision, S.P.T.; project administration, S.P.T.; funding acquisition, S.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Department of Chemistry, Faculty of Science, King Mongkut’s University of Technology, Thonburi. The student was supported by the Petchra Pra Jom Klao Master’s Degree Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are also grateful for the support of Patthra Pason and the PPDTI staff for versatile assistance during the SDS-PAGE analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and Characterization of Acid Soluble Collagen from Fish Waste: Development of Collagen-Chitosan Blend as Food Packaging Film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C. Isolation and Characterization of Collagen Extracted from Channel Catfish (Ictalurus punctatus) Skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and Characterization of Acid-Soluble Collagen from Scales and Skin of Tilapia (Oreochromis niloticus). LWT-Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and Characterization of Fish Scale Collagen from Tilapia (Oreochromis Sp.) by a Novel Extrusion-Hydro-Extraction Process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Cui, N.; Wang, T. Extraction and Characterization of Pepsin-Solubilized Collagen from Snakehead (Channa argus) Skin: Effects of Hydrogen Peroxide Pretreatments and Pepsin Hydrolysis Strategies. Process. Biochem. 2019, 76, 194–202. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Benjakul, S.; Prodpran, T.; Kishimura, H. Extraction and Characterisation of Collagen from the Skin of Golden Carp (Probarbus jullieni), a Processing By-Product. Waste Biomass Valorization 2018, 9, 783–791. [Google Scholar] [CrossRef]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, Optimization and Characterization of Collagen from Sole Fish Skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated Solvent Extraction and Pulsed Electric Fields for Valorization of Rainbow Trout (Oncorhynchus mykiss) and Sole (Dover sole) By-Products: Protein Content, Molecular Weight Distribution and Antioxidant Potential of the Extracts. Mar. Drugs 2021, 19, 207. [Google Scholar] [CrossRef]
- Faralizadeh, S.; Rahimabadi, E.Z.; Bahrami, S.H.; Hasannia, S. Extraction, Characterization and Biocompatibility Evaluation of Collagen from Silver Carp (Hypophthalmichthys molitrix) Skin by-Product. Sustain. Chem. Pharm. 2021, 22, 100454. [Google Scholar] [CrossRef]
- Vidthayanon, C.; Hongan, Z. Pangasianodon hypophthalmus, Striped Catfish. IUCN Red List. Threat. Species 2013, e.T180689A7649971. Available online: https://www.iucnredlist.org/species/180689/7649971 (accessed on 17 February 2023).
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic Cod (Gadus morhua) Skins Extracted Using CO2 Acidified Water with Potential Application in Healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent Advances of Collagen-Based Biomaterials: Multi-Hierarchical Structure, Modification and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, F.; Huang, K.; Wang, Y.; Mei, S.; Zhou, Y.; Jing, T. Environmentally Friendly Chitosan/PEI-Grafted Magnetic Gelatin for the Highly Effective Removal of Heavy Metals from Drinking Water. Sci. Rep. 2017, 7, srep43082. [Google Scholar] [CrossRef]
- Silva, J.C.; Barros, A.A.; Aroso, I.M.; Fassini, D.; Silva, T.H.; Reis, R.L.; Duarte, A.R.C. Extraction of Collagen/Gelatin from the Marine Demosponge Chondrosia Reniformis (Nardo, 1847) Using Water Acidified with Carbon Dioxide—Process Optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; Massuda, L.Á.; Ostwald, B.E.P.; Piccin, J.S.; Dettmer, A. Production and Environmental Applications of Gelatin-Based Composite Adsorbents for Contaminants Removal: A Review. Environ. Chem. Lett. 2021, 19, 2465–2486. [Google Scholar] [CrossRef]
- 정인우; 싸이풀라 론. Chitosan-Gelatin Hydrogels for Heavy Metal Adsorption and Chitosan-Gelatin Hydrogels Manufactured by the Same Method. KR20190027661A, 7 September 2019. [Google Scholar]
- Ahmed, R.; Haq, M.; Chun, B.S. Characterization of Marine Derived Collagen Extracted from the By-Products of Bigeye Tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G. fang Isolation and Characterization of Acid Soluble Collagens and Pepsin Soluble Collagens from the Skin and Bone of Spanish Mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and Carbon Dioxide: Green Solvents for the Extraction of Collagen/Gelatin from Marine Sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and Characterisation of Collagen Extracted from the Skin of Striped Catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- He, G.; Yan, X.; Wang, X.; Wang, Y. Extraction and Structural Characterization of Collagen from Fishbone by High Intensity Pulsed Electric Fields. J. Food Process. Eng. 2019, 42, e13214. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent Advances in Subcritical Water and Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Plant Materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Liao, G.; Liu, L.; E, J.; Zhang, F.; Chen, J.; Deng, Y.; Zhu, H. Effects of Technical Progress on Performance and Application of Supercritical Carbon Dioxide Power Cycle: A Review. Energy Convers. Manag. 2019, 199, 111986. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction Optimization of Pepsin-Soluble Collagen from Eggshell Membrane by Response Surface Methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef]
- Thu Huong, L.T.; Dung, N.H.; Tuan, P.D. Optimization of Conditions for Extraction of Collagen from the Skins of Basa Fish (Pangasius Hypophthalmus) By the Response Surface Method. Vietnam J. Sci. Technol. 2014, 52, 339–347. [Google Scholar]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- de Melo Oliveira, V.; Assis, C.R.D.; Costa, B.D.A.M.; de Arauro Neri, R.C.; Monte, F.T.D.; da Costa Vasconcelos, H.M.S.; França, R.C.P.; Santos, J.F.; de Souza Bezerra, R.; Porto, A.L.F. Physical, Biochemical, Densitometric and Spectroscopic Techniques for Characterization Collagen from Alternative Sources: A Review Based on the Sustainable Valorization of Aquatic by-Products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Hukmi, N.M.M.; Sarbon, N.M. Isolation and Characterization of Acid Soluble Collagen (ASC) and Pepsin Soluble Collagen (PSC) Extracted from Silver Catfish (Pangasius Sp.) Skin. Int. Food Res. J. 2018, 25, 1785–1791. [Google Scholar]
- Zhang, Q.; Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Regenstein, J.M.; Lin, L. Comparison of Collagen and Gelatin Extracted from the Skins of Nile Tilapia (Oreochromis niloticus) and Channel Catfish (Ictalurus Punctatus). Food Biosci. 2016, 13, 41–48. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and Characterization of Collagen from the Outer Skin of Squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Xu, S.; Yang, H.; Shen, L.; Li, G. Purity and Yield of Collagen Extracted from Southern Catfish (Silurus meridionalis Chen) Skin through Improved Pretreatment Methods. Int. J. Food Prop. 2017, 20, S141–S153. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of Acid- and Pepsin-Soluble Collagen Extracted from the Skin of Nile Tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, B. Preparation and Characterization of Malonic Acid Cross-Linked Chitosan and Collagen 3D Scaffolds: An Approach on Non-Covalent Interactions. J. Mater. Sci. Mater. Med. 2012, 23, 1309–1321. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Tylingo, R.; Mania, S. Isolation and Characterization of Acid Soluble Collagen from the Skin of African Catfish (Clarias gariepinus), Salmon (Salmo Salar) and Baltic Cod (Gadus Morhua). J. Biotechnol. Biomater. 2016, 6, 234. [Google Scholar] [CrossRef]
- Woo, J.W.; Yu, S.J.; Cho, S.M.; Lee, Y.B.; Kim, S.B. Extraction Optimization and Properties of Collagen from Yellowfin Tuna (Thunnus albacares) Dorsal Skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. Canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).