Abstract
Following the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident caused by the 2011 Tōhoku earthquake and tsunami, radioactive caesium (r-Cs) was detected in the ash generated by municipal solid waste (MSW) incineration facilities in Fukushima Prefecture. This has led to concerns of r-Cs leaching and subsequent environmental contamination during recycling or landfill disposal. Therefore, it is crucial that the relevant authorities have a thorough understanding of r-Cs leaching behavior to establish suitable prevention methods. In this study, we collected r-Cs-contaminated fly and bottom ash (FA and BA) samples from five MSW incineration facilities in Fukushima Prefecture and conducted tests to clarify their basic physical properties and r-Cs leaching properties. We also examined the possibility of preventing r-Cs leaching by adding 5 wt% acid clay to the FA. FA had greater chloride content and r-Cs leaching rate than BA and was found to absorb moisture and deliquesce when stored under high-humidity conditions. However, the addition of acid clay effectively prevented r-Cs leaching upon contact with moisture. From the results, we propose some specific recommendations to counter the leaching of r-Cs from FA at MSW incineration facilities, which will limit r-Cs leaching during recycling or landfill processes.
1. Introduction
The 2011 Fukushima Daiichi Nuclear Power Plant (FDNPP) accident, caused by the 2011 Tōhoku earthquake and tsunami, resulted in the environmental release of radioactive caesium (r-Cs) to residential areas [1,2]. The estimated amount of r-Cs released into the environment was approximately 10% of that released by the Chernobyl accident [3].
However, the release of r-Cs into residential environments is of significant concern because of the risk of r-Cs entering sewage treatment processes along with rainwater and then concentrating in sewage sludge [4].
Moreover, r-Cs can attach to vegetation, soil and other substances, and be unintentionally collected in municipal solid waste (MSW) incineration facilities along with waste generated from daily outdoor activities. This waste is subsequently incinerated, which greatly reduces its volume [5].
However, r-Cs is not removed by incineration, and instead becomes concentrated in the MSW incineration ash. r-Cs has been detected in the incineration ash of MSW incineration facilities in Fukushima Prefecture following the FDNPP accident.
Standards set by the Japanese government allow MSW incineration ash with r-Cs concentrations below 8000 Bq/kg to be recycled or sent to landfills [6]. However, r-Cs in fly ash (FA) may be prone to leaching upon contact with moisture [7,8]; therefore, there are significant concerns that r-Cs will leach out of the ash during recycling or landfill disposal processes and result in environmental contamination of the surrounding area.
To prevent leaching during these processes and to ensure the appropriate handling of r-Cs-contaminated MSW incineration ash, the relevant authorities need to understand its basic physical and leaching properties upon contact with moisture.
In this study, field investigations were conducted at five MSW incineration facilities generating r-Cs-contaminated MSW incineration ash. Ash samples were collected and analyzed to determine the basic physical properties of the ash and the r-Cs leaching properties upon contact with moisture.
Moreover, as acid clay is expected to be effective at capturing r-Cs [9], we examined the effect of adding acid clay to r-Cs-contaminated FA on r-Cs leaching. Finally, we considered whether the addition of acid clay interferes with the inhibitory effect of chelates on heavy-metal leaching.
2. Experimental
2.1. Samples
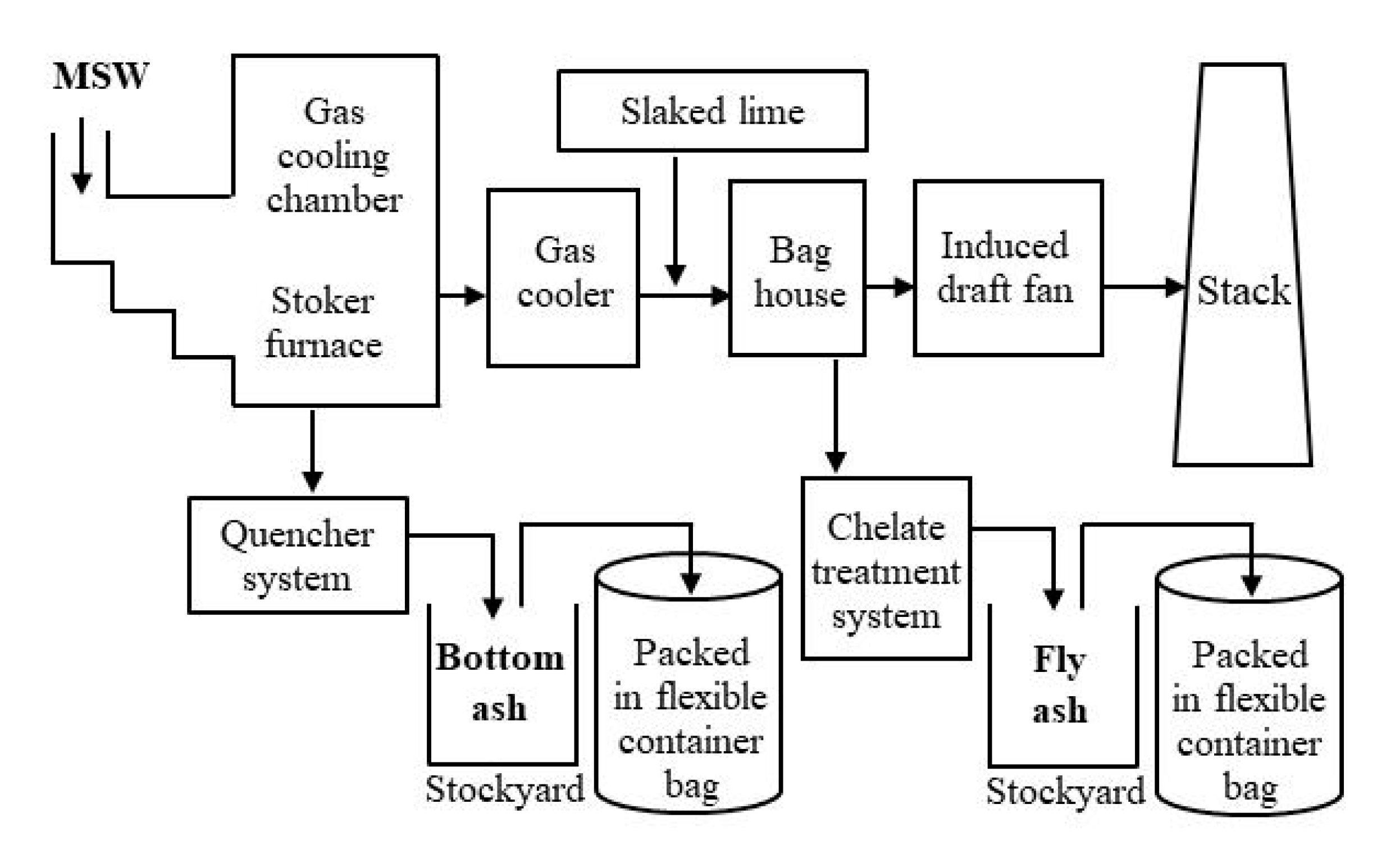
Incineration ash samples were collected from five municipal solid waste (MSW) incineration facilities in Fukushima Prefecture in August 2018. The furnaces that were sampled were all stoker-type furnaces. Figure 1 shows an example of the process flow in a stoker-type MSW incineration facility. Samples of bottom ash (BA) and chelate-treated fly ash (FA) were taken from each furnace. BA is the residue left at the bottom of the furnace when MSW is incinerated, which is sprayed with cooling water for extinguishing fire. FA consists of fine particles contained in the flue gas produced during incineration, which are collected in baghouse filters; chelates are then added to inhibit heavy-metal leaching. Particles with a size of ≤2 mm were selected using a stainless-steel sieve.
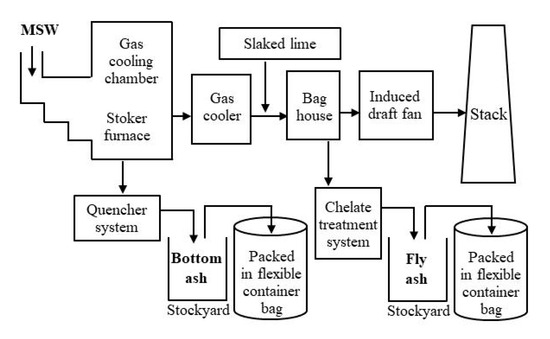
Figure 1.
Example of the process flow in a municipal solid waste (MSW) incineration facility.
2.2. Elemental Analysis
To investigate the elemental composition of the ash samples, energy dispersive X-ray fluorescence (ED-XRF) spectroscopy (JSX-3220; JEOL Ltd., Tokyo, Japan) was conducted in a vacuum using the bulk fundamental parameter (FP) method. The voltage was set to 30.0 keV, the current was set automatically (between minimum and maximum values), the measurement time was set to 600 s, and the measured elements were Na, Mg, Al, Si, P, S, Cl, K, Ca, Fe, Cu, Zn, and Pb. The samples were prepared using the pellet method, wherein each sample was compacted in an aluminum ring with an inner diameter of 3.1 cm and a height of 0.5 cm using a machine press.
2.3. Analysis of Water Absorption and Deliquescence
To investigate the extent to which the sample weight changed, and whether deliquescence occurred in the presence of moisture, such as when samples are temporarily stored outdoors during the recycling or landfill disposal processes, water absorption and deliquescence tests were conducted as follows. Approximately 10 g of ash was placed in a glass Petri dish and stored for one month in a thermo-hygrostat (PR-2KPH; ESPEC, Tokyo, Japan) maintained at a constant temperature of 25 ± 5 °C and a humidity of 75 ± 5%. The samples were then removed from the thermo-hygrostat, weighed, and visually checked for the occurrence of dissolution.
2.4. Measurement of r-Cs Concentration and Leaching Properties
The r-Cs concentration of the ash samples was measured using a Ge semiconductor detector (GC3018 CC-HI-U; CANBERRA, CT, USA). Each sample was compacted in a 100 mL U-8 polypropylene container to a height of 5 cm and then placed in the Ge semiconductor detector. Measurements were performed for 1 h to determine the r-Cs concentration. The measured detection limit for 134Cs and 137Cs was 5 Bq/kg. The decay correction date used to calculate the concentrations of 134Cs and 137Cs was taken as the date on which the sample was collected.
JIS K 0058-1 leaching tests [10] were performed to investigate the leaching properties of r-Cs when the ash samples came into contact with moisture, which may occur during recycling or landfill disposal processes. Approximately 300 g of ash was placed into a 5 L polyethylene bottle. Next, 3 L of ultrapure water was added and the mixture was stirred for 6 h at 200 rpm using an agitator. The mixture was then passed through a filtration unit using a membrane filter with a pore size of 0.45 μm to obtain a filtrate. Subsequently, a 2 L acrylic container was filled to a height of 12 cm with the filtrate and placed in the Ge semiconductor detector. Measurements were performed for 6 h to determine the r-Cs concentration. The measured detection limit for 134Cs and 137Cs was 0.1 Bq/L. The pH and electrical conductivity (EC) of the filtrate were also measured (pH: DS-52; Horiba, Kyoto, Japan; EC: F-52; Horiba).
2.5. Analysis of the Inhibitory Effect of Acid Clay on r-Cs Leaching
To examine the potential inhibitory effect of acid clay on r-Cs leaching when the samples come into contact with moisture, 5 wt% acid clay (Mizuka Catcher DX; Mizusawa Industrial Chemicals, Ltd., Tokyo, Japan; specific surface area: 100 m2/g; average particle size: 25 μm) was added to the samples. Measurements were then performed using the Ge semiconductor detector and by leaching tests under the same conditions as those described in Section 2.4. Equation (1) was used to determine the amount of ultrapure water that should be added to obtain a moisture content of 30 wt% in the samples after adding 5 wt% acid clay.
where WSample, WAcid clay, and WUltrapure water represent the weights (kg) of the sample, acid clay, and ultrapure water, respectively, while MSample, MAcid clay, and MUltrapure water represent their respective moisture contents (kg).
To confirm the long-term inhibitory effect of acid clay on r-Cs leaching, in addition to the normal leaching test (6 h), leaching tests lasting 7, 14, and 30 days were also conducted.
2.6. Analysis of the Influence of Acid Clay on the Sorption Capacity
To elucidate whether the addition of acid clay interferes with the inhibitory effect of chelating agents on heavy-metal leaching, 6 h and 30 days leaching tests were conducted on FA with and without 5 wt% acid clay, and the concentrations of heavy metals in the filtrate were then measured. The heavy-metal concentrations were measured using an ion chromatography system (ICS-2000; Waltham, MA, USA) with an inductively coupled plasma (ICP) emission spectrometer (iCAP 6300; Thermo Fisher Scientific, Waltham, MA, USA) and an anion-exchange column (Dionex IonPac AS18; Thermo Fisher Scientific, Waltham, MA, USA). For ICP emission spectrometry, quantification was performed using a four-point calibration curve method with element standard solutions containing element concentrations of 0.5, 5, 10, and 50 mg/L. The standard solutions were prepared by diluting ICP Multi standard IV (Merch Group, Darmstadt, Germany) with 0.1 mol/L nitric acid. For the sample solution, a 10-fold dilution with 0.1 mol/L nitric acid was employed.
For ion chromatography, quantification was performed using a three-point calibration curve with standard ion concentration solutions (Anion Mixture Standard Solution; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) diluted 5, 10, and 50-fold with ultrapure water. For the sample solution, 250 and 5000-fold dilutions with ultrapure water were employed. In addition, a potassium hydroxide aqueous solution generated from an eluent generator cartridge for anion analysis (EGC III-KOH; Thermo Fisher Scientific, Waltham, MA, USA) was used as the eluent.
3. Results and Discussion
3.1. Elemental Composition of Ash Samples
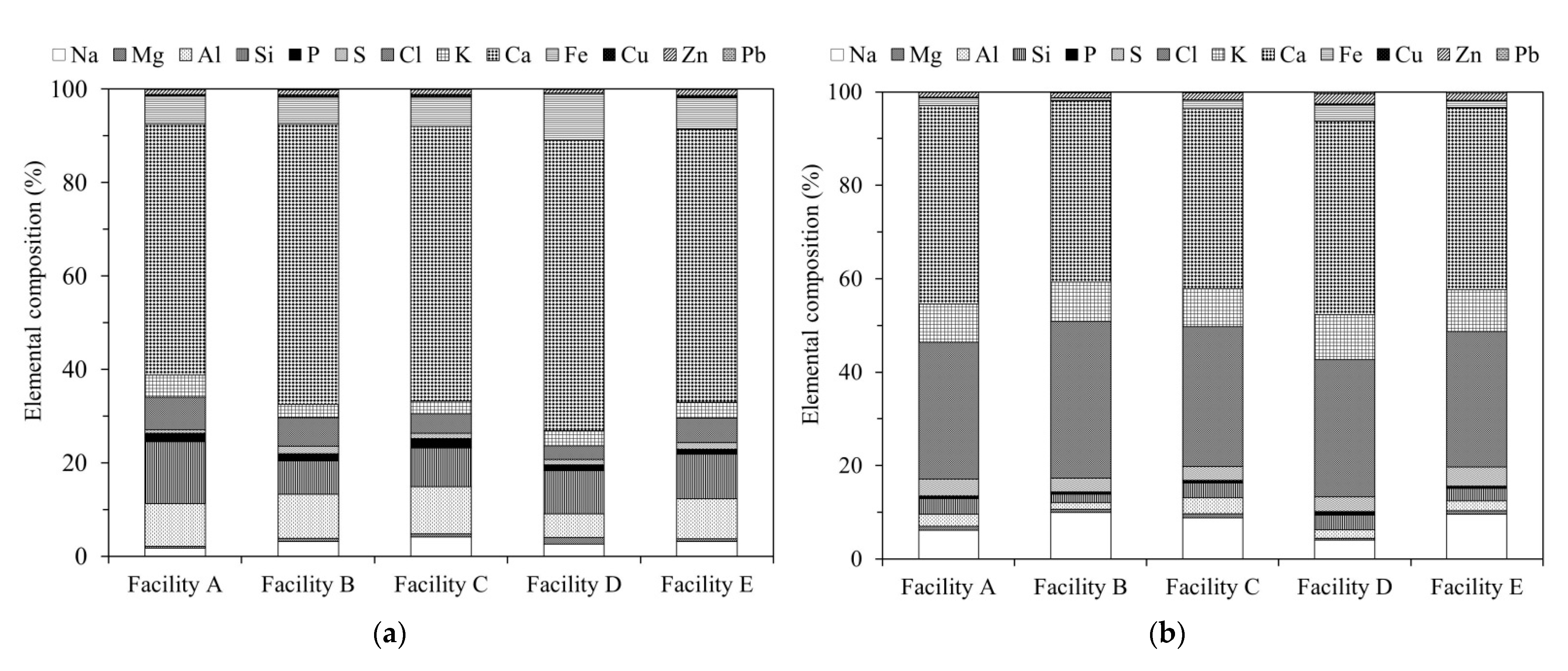
Figure 2a,b show the results of the ED-XRF analysis. The bottom ash (BA) samples contained high concentrations of Ca, Si, and Al, indicating that the main components were CaO, SiO2, and Al2O3 [11].
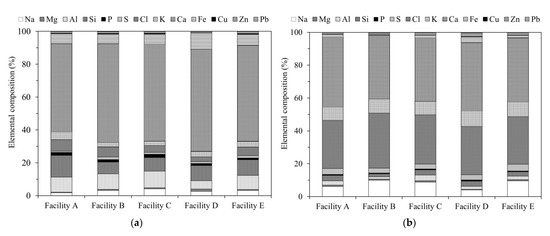
Figure 2.
Energy dispersive X-ray fluorescence spectroscopy (ED-XRF) results showing the elemental composition of incineration ash samples from different facilities: (a) bottom ash (BA); (b) fly ash (FA).
Conversely, the fly ash (FA) samples contained large amounts of Ca, Cl, and Na. The Ca in the FA, which is considered to exist as Ca(OH)2, CaCl2, CaO, and CaSO4 [12,13], is derived from the slaked lime that is blown into the furnace as a measure to neutralize hydrogen chloride gas (Figure 1).
The Na in the FA is likely to have been generated by the incineration of household food waste, and exists as NaCl [8,12,13,14]. Thus, the Cl content is higher in FA than in BA, suggesting the presence of large amounts of water-soluble chlorides.
Cs is known to be a volatile element [15]. In addition, the Cs present in BA and FA exists in different forms [11,16,17,18]. This is because most r-Cs enters municipal solid waste (MSW) incineration facilities via vegetation and soil and is volatilized into a gaseous state during incineration, following which it migrates into the flue gas [19].
The r-Cs cools near the baghouse filter, which is controlled at a temperature of approximately 200 °C. The r-Cs then solidifies and concentrates in the FA. Therefore, Cs may exist in FA in the form of highly water-soluble CsCl [8,17,18], CsOH, and Cs2SO4 [20,21].
3.2. Moisture Absorption and Deliquescence of Ash Samples
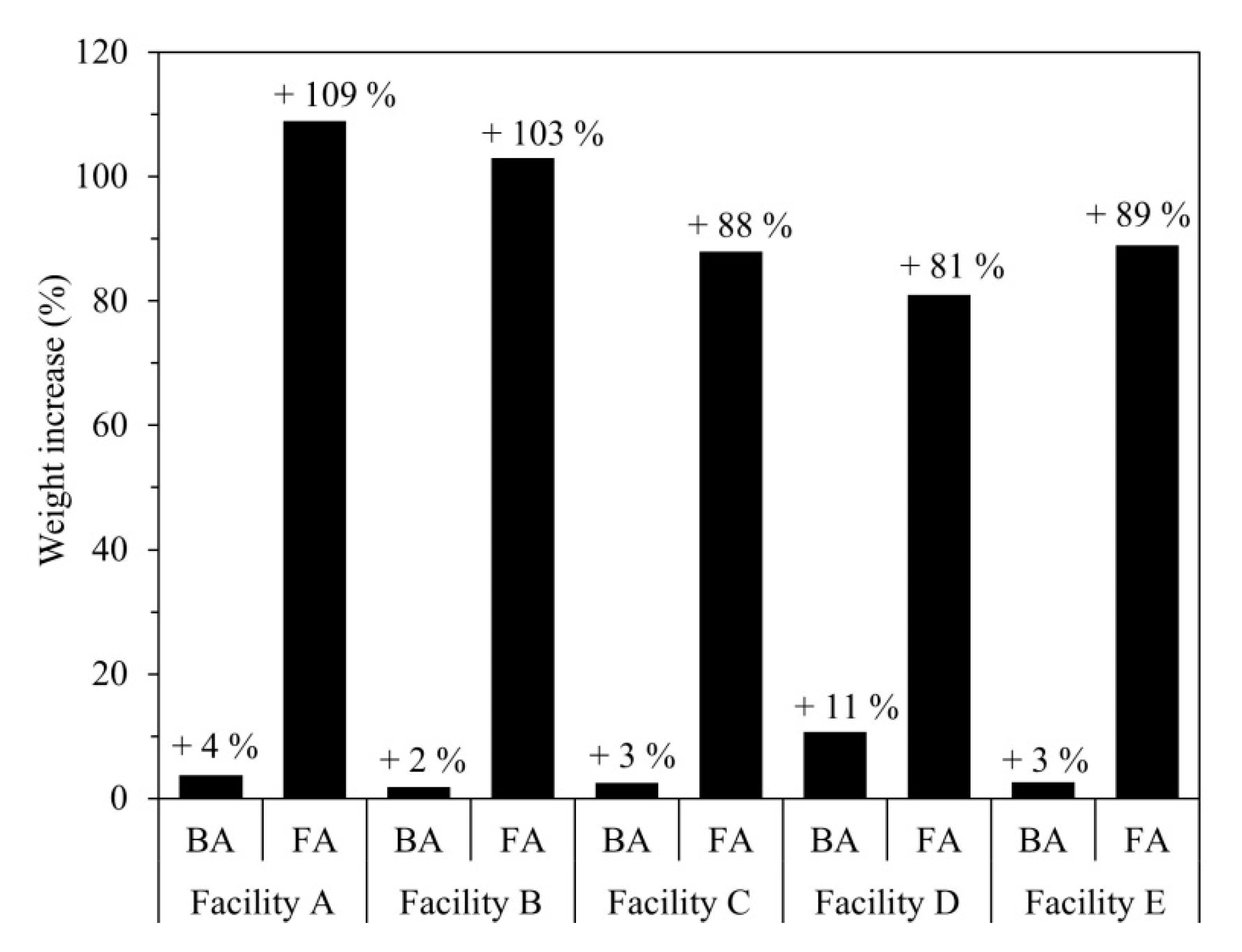
Figure 3 shows the change in the weight of samples retained for 30 days at a temperature of 25 ± 5 °C and humidity of 75 ± 5%. The weight increase was calculated relative to the initial sample weight as follows:
where WBefore and WAfter represent the sample weights (g) before and after being placed in the thermo-hygrostat chamber, respectively. Although the BA and FA samples were maintained under the same conditions, the latter showed significantly higher moisture absorbency and a greater weight increase.
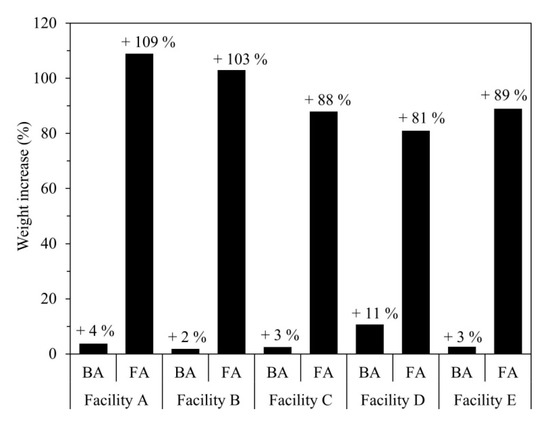
Figure 3.
Weight increase of BA and FA after exposure to moisture for 30 days (temperature: 25 ± 5 °C; humidity: 75 ± 5%).
The FA from Facility A exhibited the greatest weight gain of 109% (2.09 times the original sample weight). Furthermore, all five FA samples showed deliquescence (Figure 4). A comparison of these results with the ED-XRF results indicates that components such as CaCl2, NaCl, and KCl influence the moisture absorption and deliquescence of incineration ash [22,23].

Figure 4.
Photographs showing the deliquescence of FA after exposure to moisture.
In Fukushima Prefecture, r-Cs-contaminated MSW incineration ash tends to be packed into flexible container bags during the recycling or landfill process and temporarily stored in the MSW incineration facility. In addition, MSW incineration ash that cannot be recycled or disposed of in landfills because of excess r-Cs concentration tends to be packed into flexible container bags and stored long-term in concrete boxes until its r-Cs concentration decreases (Figure 5).

Figure 5.
Example of temporary (left) and long-term (right) ash storage conditions in an MSW incineration facility.
The flexible container bags block the flow of air and water to a certain extent; however, considering the results of this test, if MSW incineration ash is stored long-term in a humid location the liquid produced as a result of moisture absorption and deliquescence could leak from the flexible container bag and contaminate the storage area.
In addition, the increase in weight due to water absorbance could affect the durability of the flexible container bags and place a burden on operators when removing the containers. Therefore, in the case of long-term storage, it is advisable to regularly monitor the humidity and temperature around the storage area and check the flexible container bags for deterioration.
3.3. r-Cs Concentrations and Leaching Properties of Contaminated MSW Incineration Ash
Table 1 shows the r-CS concentrations and leaching test results of the ash samples. The r-Cs concentrations are expressed as the sum of the 134Cs and 137Cs concentrations. The leaching rate of r-Cs was calculated using Equation (3):
where CSample and CFiltrate represent the r-Cs concentrations (Bq/kg) of the sample and the filtrate used in the leaching test, respectively, while WSample and WFiltrate represent their respective weights (kg). ρ represents the density of the solvent used in the leaching test (ρ = 1.0 kg/L). If the r-Cs concentration of the filtrate was below the detection limit (below 0.1 Bq/L), it was calculated as 0 Bq/L, and shown as ‘ND’ in Table 1. The water content of the ash samples, which was determined using an air oven drying method, is also shown in Table 1.

Table 1.
Radioactive caesium (r-Cs) concentration and leaching test results of BA and FA samples from different facilities.
Comparison of the r-Cs concentrations in Table 1 demonstrates that there is substantial variation among incineration facilities. The reason for this wide variation may be that the type of waste differs between facilities owing to different industrial structures and regional characteristics, such as whether they are located in an urban or rural area.
For example, the amount of vegetation, leaf litter, and attached soil generated by agricultural work can differ depending on the incineration facility. This agrees with previous findings that the type of waste supplied to the incinerator influences the properties of the incineration ash [24,25].
Despite the differences among facilities, the FA samples had higher r-Cs concentrations than the BA samples at all facilities. Moreover, the r-Cs concentrations and leaching rates of the filtrates were higher for FA than BA.
Our results for MSW incineration ash disagree with previous findings that the r-Cs leaching rate from the incineration ash of decontamination waste and sewage sludge is typically not high [8,26]. The fact that r-Cs is present in different forms in the BA and FA could explain the difference in the r-Cs leaching rates between BA and FA [11,16,17,18].
The pH values and EC of the filtrates are also shown in Table 1. All filtrates were strongly alkaline; however, there was no correlation between the pH and r-Cs leaching rate. In contrast, there was a certain correlation between the EC and r-Cs leaching rate, with the FA samples having higher EC values than the BA samples.
Considering the difficulty in preventing r-Cs leaching during recycling or landfill disposal using only chelating agents, additional measures are required to inhibit r-Cs leaching from FA, particularly for MSW incineration ash.
3.4. Inhibitory Effect of Acid Clay on r-Cs Leaching
Table 2 shows a summary of the r-Cs concentrations of samples prepared by adding 5 wt% acid clay to each FA sample and adjusting the water content to 30% using ultrapure water. Table 3 shows a summary of the r-Cs concentrations of the filtrates obtained after leaching tests on each prepared sample.

Table 2.
r-Cs concentration results of FA samples with 5 wt% acid clay addition.

Table 3.
Leaching test results of FA with 5 wt% acid clay addition.
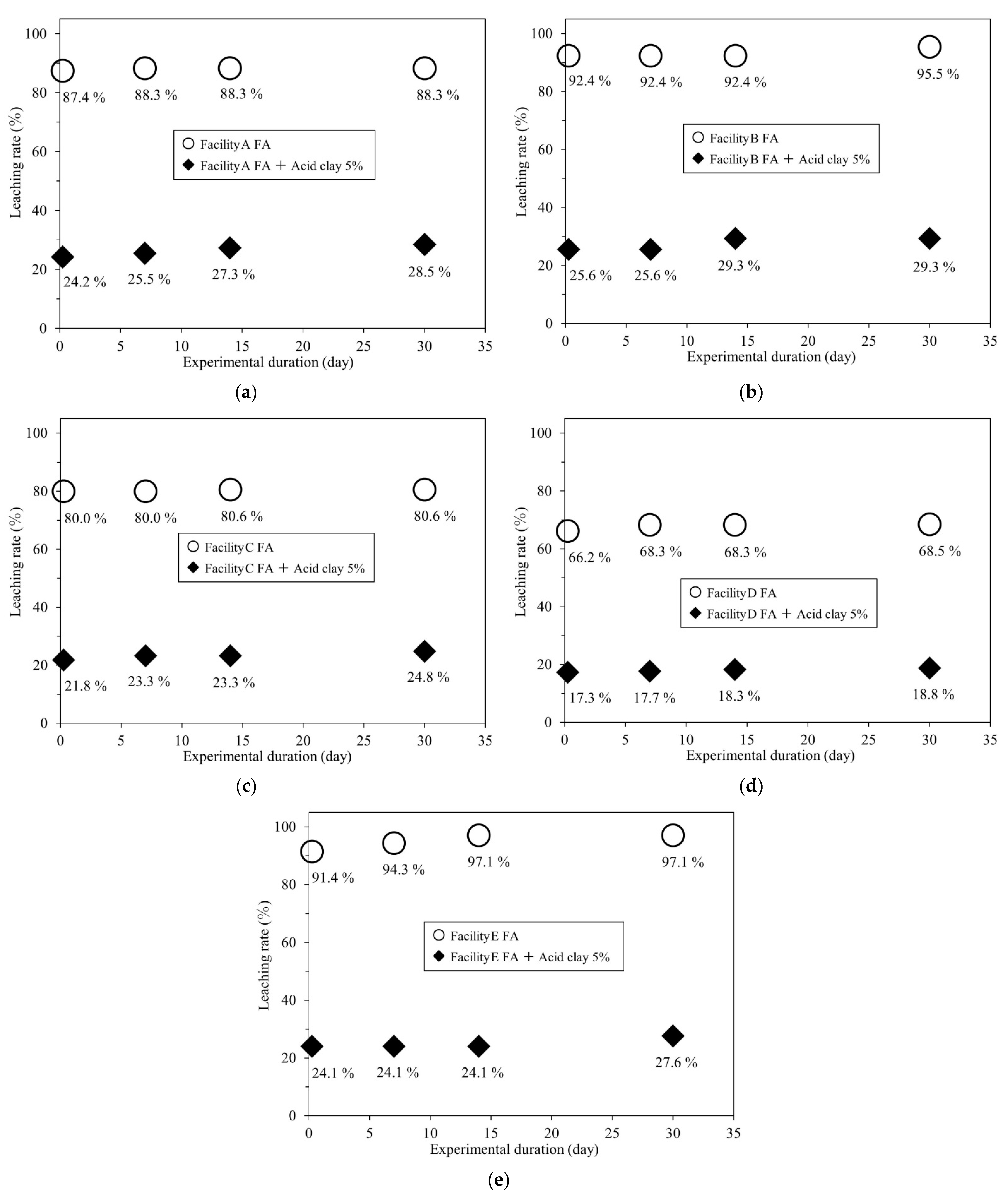
For comparison, leaching tests were conducted under the same conditions on FA without adding acid clay (Table 4). Figure 6a–e summarize the r-Cs leaching rates of the samples as a function of the number of days of leaching.

Table 4.
Leaching test results of FA with no acid clay addition.
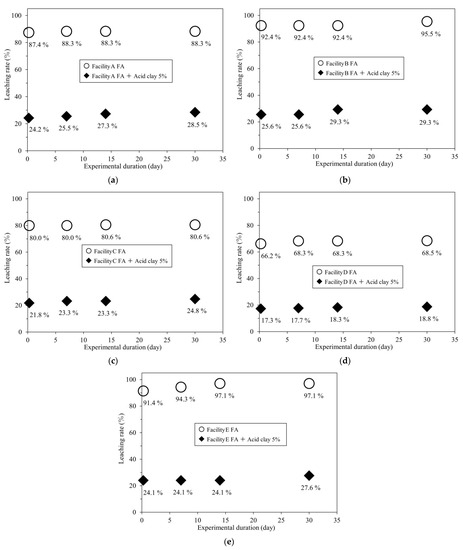
Figure 6.
r-Cs leaching rates of FA from different facilities with and without 5 wt% acid clay: (a) Facility A; (b) Facility B; (c) Facility C; (d) Facility D; (e) Facility E.
Previous researchers have exploited the high r-Cs leaching rate from FA to develop a method for r-Cs removal by washing [27]. However, this method requires the construction of washing facilities. Further, even if the r-Cs can be removed, other problems may arise, such as the safe management of contaminated water generated by the washing process. In addition, other methods of removing or inhibiting r-Cs leaching using chemicals are being considered [28,29].
However, chemical treatment may lead to restrictions on where the ash can be recycled, and other problems related to landfill disposal. Therefore, with the aim of inhibiting r-Cs leaching from FA, preferably using materials that exist in nature, this study examined the effect of added acid clay, which can be mined stably in areas of Japan on the Sea of Japan.
Some clay minerals such as zeolites are considered capable of capturing r-Cs [30,31]. However, few studies have analysed the inhibition of r-Cs leaching from r-Cs-contaminated MSW incineration ash by adding clay minerals that are thought to be effective for capturing r-Cs. Therefore, this study is the first to examine the extent to which r-Cs leaching can be inhibited by adding acid clay to FA.
According to the results in Table 3 and Table 4, adding 5 wt% of acid clay to FA reduced the r-Cs leaching rate from >80% to ≤30% in all FA samples, even after a maximum testing duration of 30 days.
This indicates that acid clay has a long-term inhibiting effect on r-Cs leaching, which will limit r-Cs leaching when FA is recycled or disposed of in landfills. Furthermore, Table 1 shows that the r-Cs concentration of the filtrate differed depending on the r-Cs concentration of the FA; however, the r-Cs leaching rate was often higher than 80%. This can be used to approximate the r-Cs concentration of contaminated water produced by leaching.
Therefore, the amount of acid clay to be added could also be determined by estimating the r-Cs concentration of contaminated water produced by leaching from the r-Cs concentration of FA. For example, we estimated that the concentration of Cs in contaminated water generated by leaching was reduced to 10 Bq/L or less by adding acid clay. However, the excessive addition of acid clay could hinder recycling or lead to pressure on landfill sites. Therefore, we propose a maximum limit of 20 wt% acid clay.
3.5. Interference of Acid Clay with the Sorption Capacity
Table 5 and Table 6 show the concentration of heavy metals contained in the filtrate after 6 h and 30 days leaching tests of FA, respectively, with and without the addition of 5 wt% acid clay. For comparison, leaching tests were also performed with only acid clay to confirm the heavy-metal concentration in the filtrate obtained from acid clay. If the heavy-metal concentration of the filtrate was below the limit of quantification (shown in the bottom row of Table 5 and Table 6), it was noted as ‘–’.

Table 5.
Leaching test results indicating the effect of acid clay on the sorption capacity of FA (6 h duration).

Table 6.
Leaching test results indicating the effect of acid clay on the sorption capacity of FA (30 days duration).
No significant change was observed in the sorption capacity with the addition of 5 wt% acid clay. Moreover, no unusual heavy-metal concentrations were observed in the leaching test results performed on only acid clay. Therefore, it is inferred that the addition of acid clay did not interfere with the sorption capacity, and that the inhibitory effect of chelating agents on heavy-metal leaching was maintained.
The Cl concentration tended to increase with increasing leaching test duration in all samples. Previous research suggests that Cs leaching is caused by an increase in the concentrations of K, Na, or Cl in the surrounding area (Cs leaching by ion exchange) [32,33,34]. Therefore, the gradual increase in the r-Cs leaching rate with the test duration, as shown in Figure 6, may be because the r-Cs captured by the acid clay was then leached out by ion exchange.
4. Safety Measures of r-Cs-Contaminated MSW Incineration Ash during Recycling or Landfill Disposal Processes
The results of this study show that the r-Cs present in municipal solid waste (MSW) incineration ash, particularly fly ash (FA), will gradually leach out due to moisture absorption and contact with rainwater during the recycling or landfill disposal processes.
In addition, because of the strong alkalinity and high Cl concentration of the filtrate, the possibility of corrosion and damage to concrete containers must be considered during the long-term storage of FA until its r-Cs concentration decreases.
Therefore, we recommend adding 5–20 wt% acid clay to r-Cs-contaminated FA to inhibit the leaching of r-Cs without interfering with the effect of chelating agents. Some clay minerals may also be able to simultaneously inhibit heavy-metal leaching [35].
For example, acid clay is considered to be effective at capturing Pb [36,37]. The clay minerals should be added at certain points in the MSW incineration and ash storage processes (Figure 1).
The following recommendations are proposed as specific countermeasures to the leaching of r-Cs from FA at MSW incineration facilities.
- Clay minerals can be premixed with slaked lime, which is blown into the furnace to neutralize hydrogen chloride gas.
- A clay mineral tank and additional equipment can be installed such that clay minerals are added at the same time as the chelates during chelate treatment.
- Clay minerals can be added to MSW incineration ash storage containers (and when repacking waste into new containers during container inspection) to improve safety during long-term storage. This will facilitate the recycling and landfill disposal of MSW incineration ash.
5. Conclusions
- Based on the experimental results, the following conclusions were drawn.
- Bottom ash (BA) contains high concentrations of elements such as Ca, Si, and Al, and the main components are CaO, SiO2, and Al2O3. Conversely, fly ash (FA) contains large amounts of Ca, Cl, and Na. The Cl content of FA is higher than that of BA, suggesting the presence of large amounts of water-soluble chlorides.
- Under the same temperature and humidity conditions, FA shows higher moisture absorbency and a greater weight increase than BA. Furthermore, all five types of FA show deliquescence.
- When municipal solid waste (MSW) incineration ash is stored long-term in a humid location, the liquid produced as a result of moisture absorption and deliquescence could leak from the flexible container bag and contaminate the storage area. Therefore, in the case of long-term storage, it is advisable to regularly monitor the humidity and temperature around the storage area and check the flexible container bag for deterioration.
- A comparison of r-Cs concentrations in different incineration ash samples demonstrated that there is substantial variation among incineration facilities. However, FA had higher r-Cs concentrations than BA at all facilities. Moreover, both, the r-Cs concentration and leaching rate of the filtrate were higher for FA than BA.
- Considering the difficulty in preventing r-Cs leaching during recycling or landfill disposal using only chelating agents, additional measures are required to inhibit r-Cs leaching from FA, particularly for MSW incineration ash.
- Adding 5 wt% of acid clay reduced the r-Cs leaching rate from >80% to ≤30% in all FA samples. This indicates that adding acid clay can achieve the long-term inhibition of r-Cs leaching when recycling or disposing of FA in landfills. However, excess acid clay addition could hinder recycling or lead to pressure on landfill sites. Therefore, we propose a maximum limit of 20 wt% acid clay.
- No significant change was observed in the sorption capacity with the addition of 5 wt% acid clay. Moreover, no unusual concentrations of heavy metals were observed in the leaching test results performed on only acid clay. Therefore, it is inferred that the addition of acid clay does not interfere with the effect of chelating agents, and that the inhibitory effect on heavy-metal leaching was maintained. Consequently, we recommend that acid clay should be added at certain points in the MSW incineration and ash storage processes, in order to inhibit the leaching of r-Cs from FA without interfering with the effect of chelating agents.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prime Minister of Japan and His Cabinet. Report of Japanese Government to the IAEA Ministerial Conference on Nuclear Safety: The Accident at TEPCO’s Fukushima Nuclear Power Stations. Available online: http://www.kantei.go.jp/foreign/kan/topics/201106/iaea_houkokusho_e.html (accessed on 7 July 2021).
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation, UNSCEAR 2013 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 2014; Volume I. [Google Scholar]
- Chino, M.; Nakayama, H.; Nagai, H.; Terada, H.; Katata, G.; Yamazawa, H. Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. J. Nucl. Sci. Technol. 2011, 48, 1129–1134. [Google Scholar] [CrossRef]
- Kamei-Ishikawa, N.; Ito, A.; Tagami, K.; Umita, T. Fate of radiocesium in sewage treatment process released by the nuclear accident at Fukushima. Chemosphere 2013, 93, 689–694. [Google Scholar] [CrossRef]
- Ministry of the Environment of Japan. Solid Waste Management and Recycling Technology of Japan: Toward a Sustainable Society. Available online: http://www.env.go.jp/recycle/circul/venous_industry/en/brochure.pdf (accessed on 7 July 2021).
- Ministry of the Environment of Japan. Environmental Remediation in Affected Areas in Japan. Available online: http://josen.env.go.jp/en/pdf/environmental_remediation_1905.pdf (accessed on 7 July 2021).
- Oshita, K.; Aoki, H.; Fukutani, S.; Shiota, K.; Fujimori, T.; Takaoka, M. Behavior of cesium in municipal solid waste incineration. J. Environ. Radioact. 2015, 143, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, H.; Fujiwara, H.; Yui, K. Behavior of radioactive cesium during thermal treatment of radioactively contaminated wastes in the aftermath of the Fukushima Daiichi Nuclear Power Plant accident. Glob. Environ. Res. 2016, 20, 91–100. [Google Scholar]
- Wu, J.; Li, B.; Liao, J.; Feng, Y.; Zhang, D.; Zhao, J.; Wen, W.; Yang, Y.; Liu, N. Behavior and analysis of cesium adsorption on montmorillonite mineral. J. Environ. Radioact. 2009, 100, 914–920. [Google Scholar] [CrossRef]
- Japanese Industrial Standards Committee. JIS K 0058-1, Test Methods for Chemicals in Slags. Part 1: Leaching Test Method; Japanese Standards Association: Tokyo, Japan, 2005. [Google Scholar]
- Saffarzadeh, A.; Shimaoka, T.; Kakuta, Y.; Kawano, T. Cesium distribution and phases in proxy experiments on the incineration of radioactively contaminated waste from the Fukushima area. J. Environ. Radioact. 2014, 136, 76–84. [Google Scholar] [CrossRef]
- Bogush, A.; Stegemann, J.A.; Wood, I.; Roy, A. Element composition and mineralogical characterisation of air pollution control residue from UK energy-from-waste facilities. Waste Manag. 2015, 36, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Koval, L.; Škrobánková, H.; Matýsek, D.; Winter, F.; Purgar, A. Possibilities of municipal solid waste incinerator fly ash utilisation. Waste Manag. Res. 2015, 33, 740–747. [Google Scholar] [CrossRef]
- Zhu, F.; Takaoka, M.; Shiota, K.; Oshita, K.; Kitajima, Y. Chloride chemical form in various types of fly ash. Environ. Sci. Technol. 2008, 42, 3932–3937. [Google Scholar] [CrossRef]
- MacKay, K.M.; MacKay, R.A.; Henderson, W. Introduction to Modern Inorganic Chemistry, 6th ed.; Nelson Thornes: Cheltenham, UK, 2002. [Google Scholar]
- Yoon, I.-H.; Choi, W.-K.; Lee, S.-C.; Min, B.-Y.; Yang, H.-C.; Lee, K.-W. Volatility and leachability of heavy metals and radionuclides in thermally treated HEPA filter media generated from nuclear facilities. J. Hazard. Mater. 2012, 219–220, 240–246. [Google Scholar] [CrossRef]
- Shiota, K.; Takaoka, M.; Fujimori, T.; Oshita, K.; Terada, Y. Cesium speciation in dust from municipal solid waste and sewage sludge incineration by synchrotron radiation micro-X-ray analysis. Anal. Chem. 2015, 87, 11249–11254. [Google Scholar] [CrossRef]
- Yui, K.; Kuramochi, H.; Osako, M. Understanding the behavior of radioactive cesium during the incineration of contaminated municipal solid waste and sewage sludge by thermodynamic equilibrium calculation. ACS Omega 2018, 3, 15086–15099. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Kuramochi, H.; Maeseto, T.; Nomura, K.; Takeuchi, Y.; Kawamoto, K.; Yamasaki, S.; Kokubun, K.; Osako, M. Influence of the type of furnace on behavior of radioactive cesium in municipal solid waste thermal treatment. Waste Manag. 2018, 81, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Eun, H.-C.; Lee, D.-G.; Oh, W.-Z.; Lee, K.-W. Behavior of radioactive elements during thermal treatment of nuclear graphite waste thermodynamic model analysis. J. Nucl. Sci. Technol. 2005, 42, 869–876. [Google Scholar] [CrossRef]
- Yoo, J.-I.; Shinagawa, T.; Wood, J.; Linak, W.P.; Santoianni, D.A.; King, C.J.; Seo, Y.-C.; Wendt, J.O.L. High-temperature sorption of cesium and strontium on dispersed kaolinite powders. Environ. Sci. Technol. 2005, 39, 5087–5094. [Google Scholar] [CrossRef]
- Lipasek, R.A.; Ortiz, J.C.; Taylor, L.S.; Mauer, L.J. Effects of anticaking agents and storage conditions on the moisture sorption, caking, and flowability of deliquescent ingredients. Food Res. Int. 2012, 45, 369–380. [Google Scholar] [CrossRef]
- Bhowmik, M.; Haldar, S.; Dharmalingam, K.; Muthukumar, P.; Anandalakshmi, R. Evaluation of thermo-kinetic and absorption characteristics of pure desiccants and desiccant mixtures. Mater. Today Proc. 2020, 26, 1967–1971. [Google Scholar] [CrossRef]
- Wu, H.; Glarborg, P.; Frandsen, F.J.; Dam-Johansen, K.; Jensen, P.A.; Sander, B. Trace elements in co-combustion of solid recovered fuel and coal. Fuel Process. Technol. 2013, 105, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Vainio, E.; Yrjas, P.; Zevenhoven, M.; Brink, A.; Laurén, T.; Hupa, M.; Kajolinna, T.; Vesala, H. The fate of chlorine, sulfur, and potassium during co-combustion of bark, sludge, and solid recovered fuel in an industrial scale BFB boiler. Fuel Process. Technol. 2013, 105, 59–68. [Google Scholar] [CrossRef]
- Tsushima, I.; Ogoshi, M.; Harada, I. Leachate tests with sewage sludge contaminated by radioactive cesium. J. Environ. Sci. Health Part A 2013, 48, 1717–1722. [Google Scholar] [CrossRef]
- Namiki, Y.; Ueyama, T.; Yoshida, T.; Watanabe, R.; Koido, S.; Namiki, T. Hybrid micro-particles as a magnetically-guidable decontaminant for cesium-eluted ash slurry. Sci. Rep. 2015, 4, srep06294. [Google Scholar] [CrossRef] [Green Version]
- Awual, R.; Yaita, T.; Taguchi, T.; Shiwaku, H.; Suzuki, S.; Okamoto, Y. Selective cesium removal from radioactive liquid waste by crown ether immobilized new class conjugate adsorbent. J. Hazard. Mater. 2014, 278, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Mallampati, S.R.; Mitoma, Y.; Okuda, T.; Sakita, S.; Simion, C. Preferential removal and immobilization of stable and radioactive cesium in contaminated fly ash with nanometallic Ca/CaO methanol suspension. J. Hazard. Mater. 2014, 279, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Harjula, R.; Lehto, J. Effect of sodium and potassium ions on cesium absorption from nuclear power plant waste solutions on synthetic zeolites. Nucl. Chem. Waste Manag. 1986, 6, 133–137. [Google Scholar] [CrossRef]
- Faghihian, H.; Marageh, M.G.; Kazemian, H. The use of clinoptilolite and its sodium form for removal of radioactive cesium, and strontium from nuclear wastewater and Pb2+, Ni2+, Cd2+, Ba2+ from municipal wastewater. Appl. Radiat. Isot. 1999, 50, 655–660. [Google Scholar] [CrossRef]
- Gast, R.G. Alkali metal cation exchange on chambers montmorillonite. Soil Sci. Soc. Am. J. 1972, 36, 14–19. [Google Scholar] [CrossRef]
- Delvaux, B.; Kruyts, N.; Cremers, A. Rhizospheric mobilization of radiocesium in soils. Environ. Sci. Technol. 2000, 34, 1489–1493. [Google Scholar] [CrossRef]
- Mon, J.; Deng, Y.; Flury, M.; Harsh, J.B. Cesium incorporation and diffusion in cancrinite, sodalite, zeolite, and allophane. Micropor. Mesopor. Mat. 2005, 86, 277–286. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: Influence of acid activation of the clays. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 191–200. [Google Scholar] [CrossRef]
- Eloussaief, M.; Benzina, M. Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions. J. Hazard. Mater. 2010, 178, 753–757. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).