Rheological, Mechanical and Morphological Characterization of Monopolymer Blends Made by Virgin and Photo-Oxidized Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
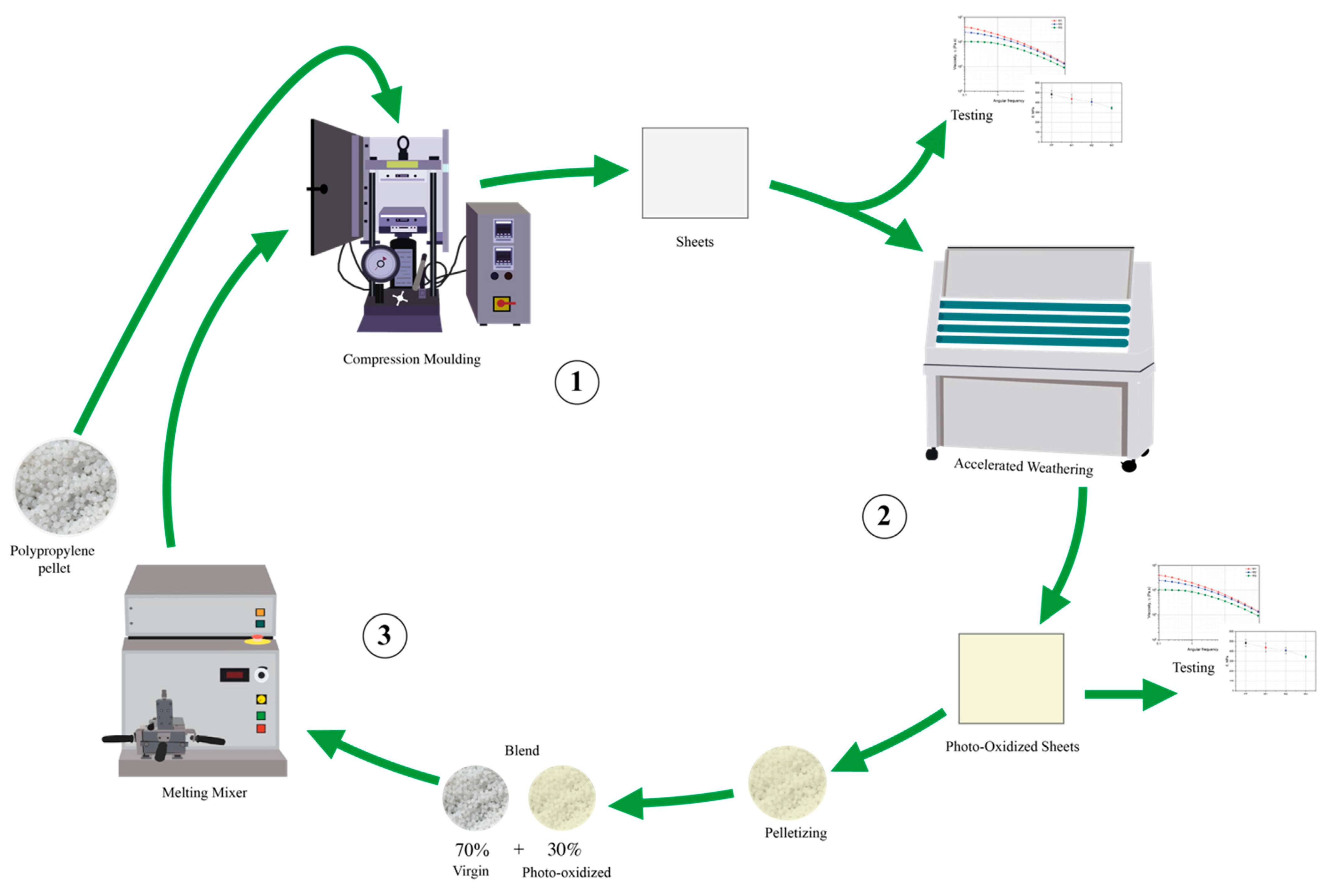
2.1. Materials and Samples Preparation
2.2. Characterization
3. Results and Discussion
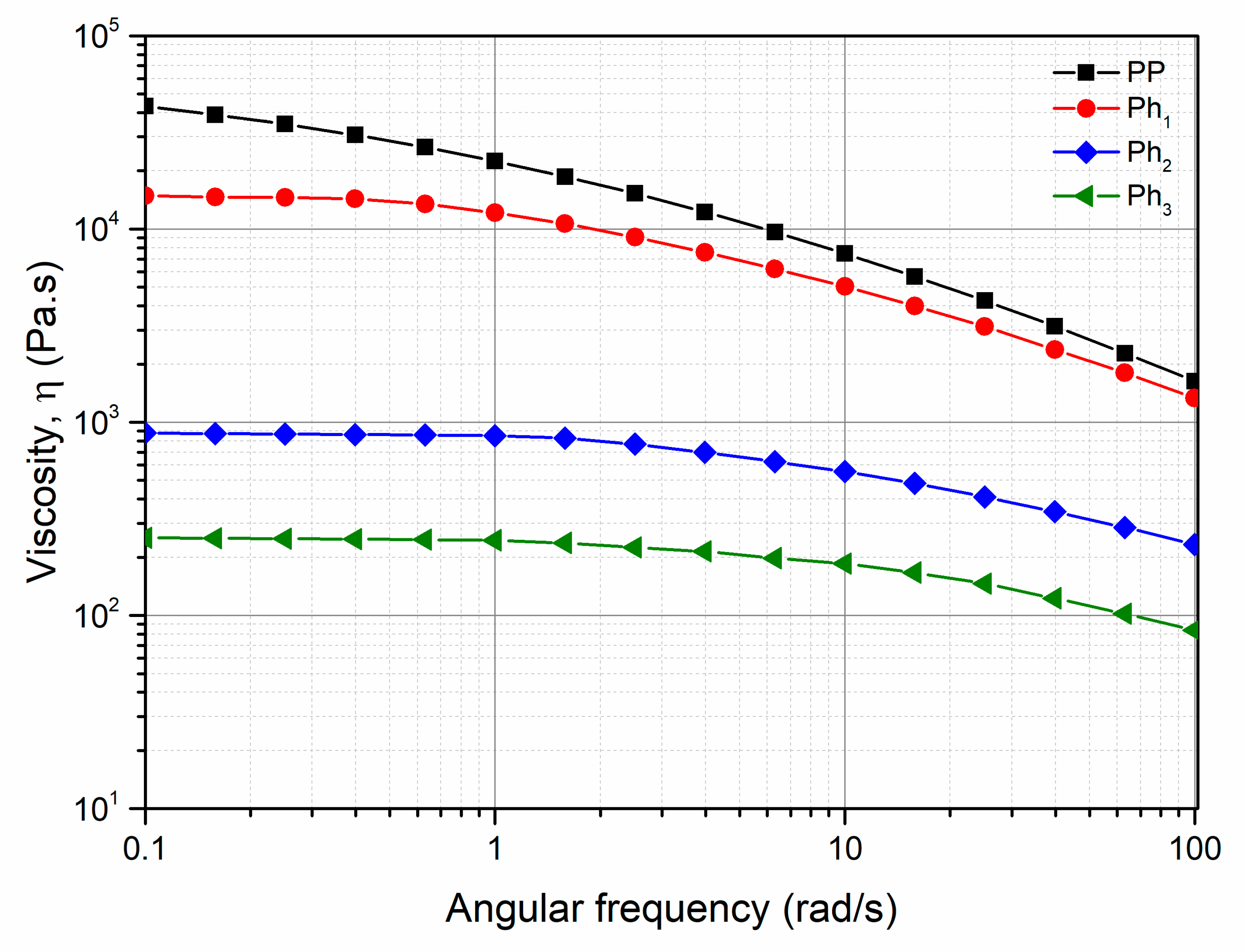
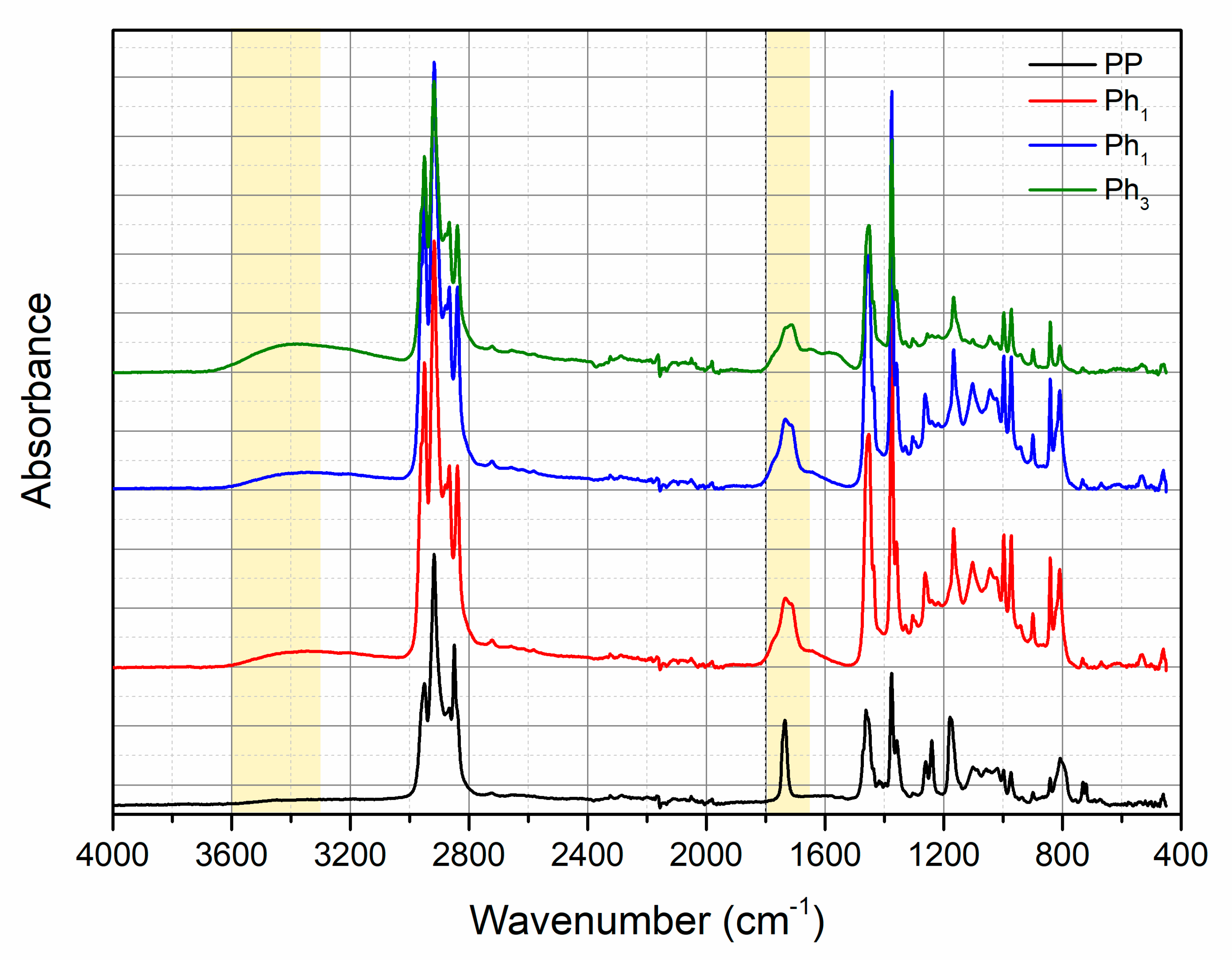
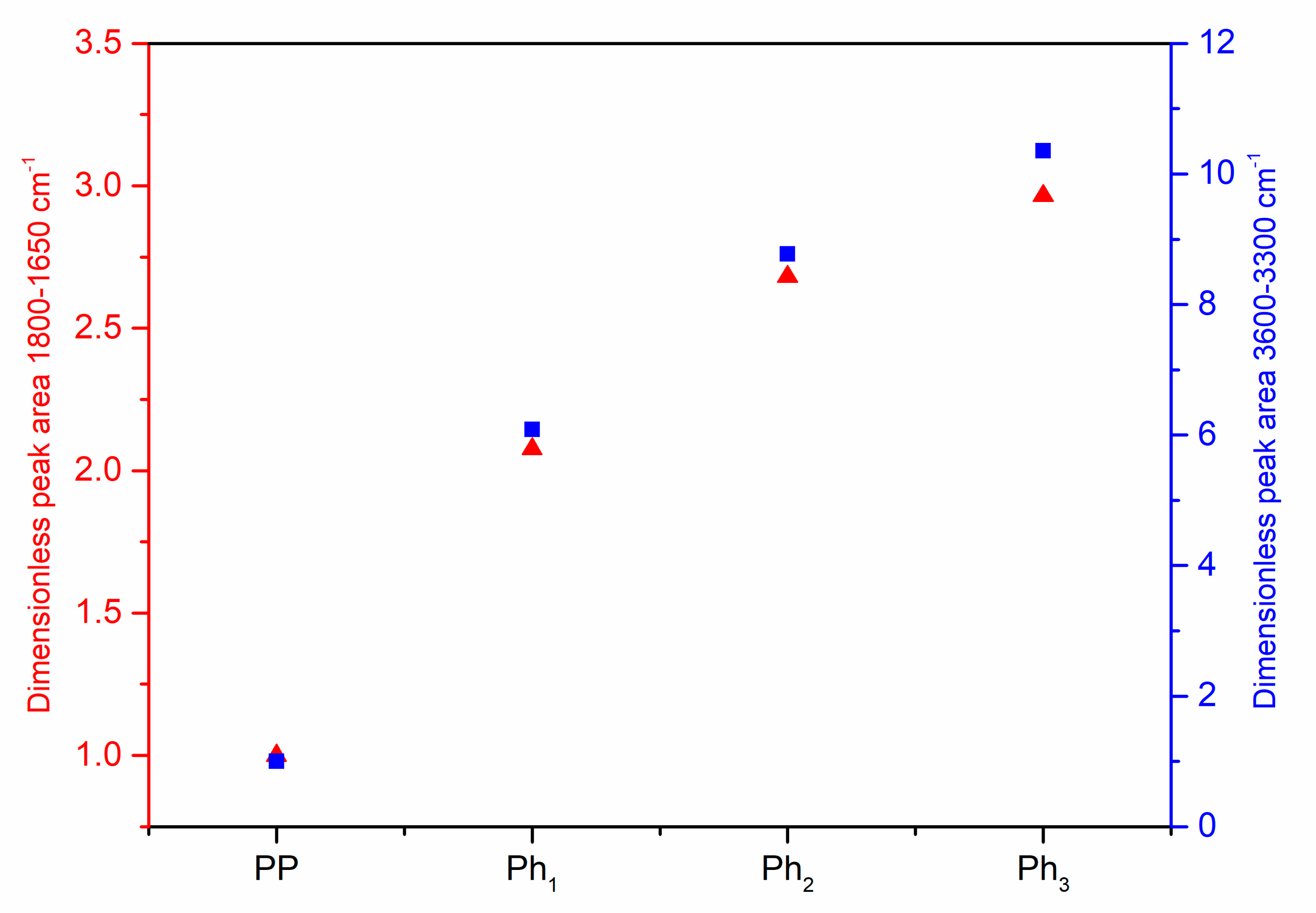
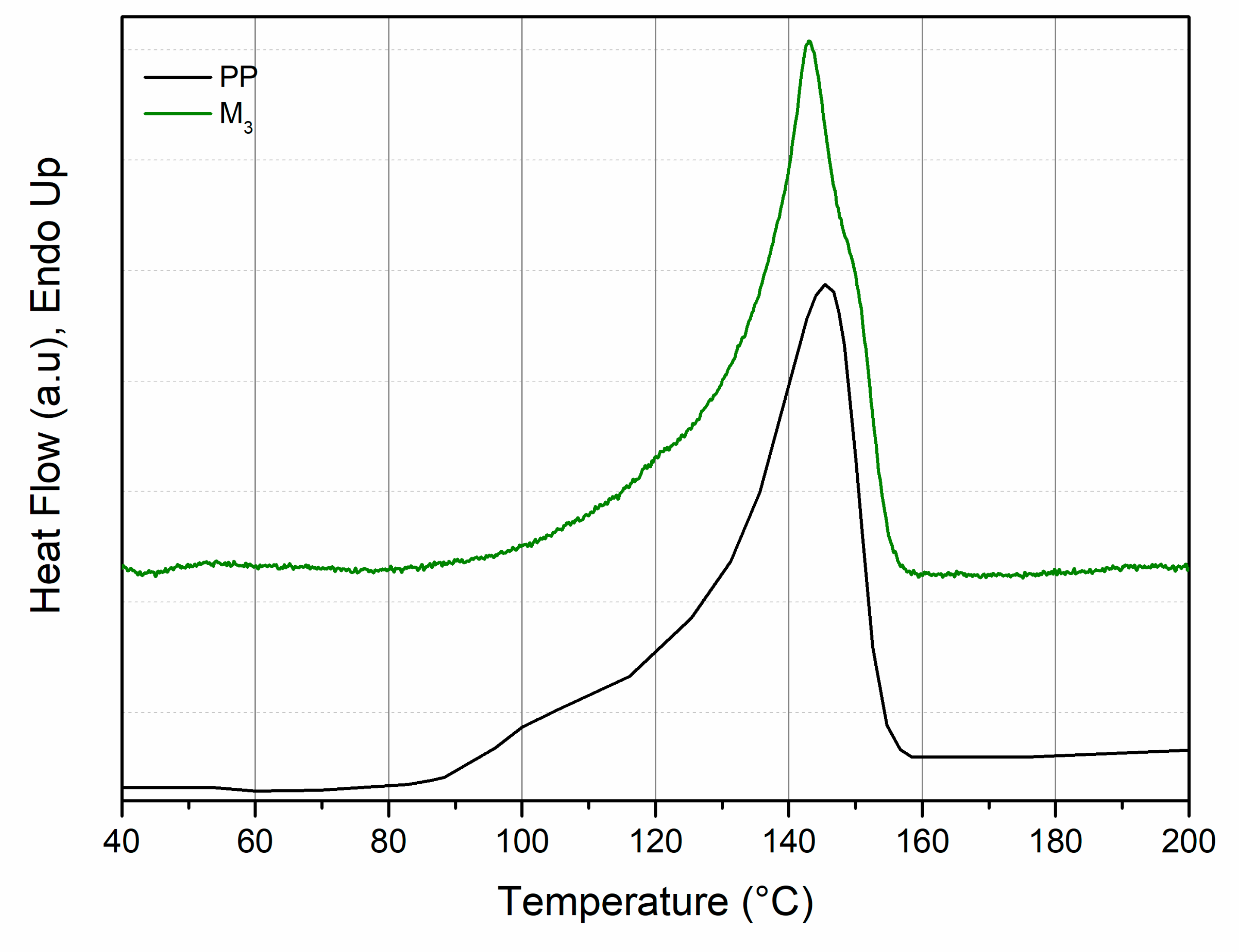
3.1. Characterization of Photo-Oxidized PP
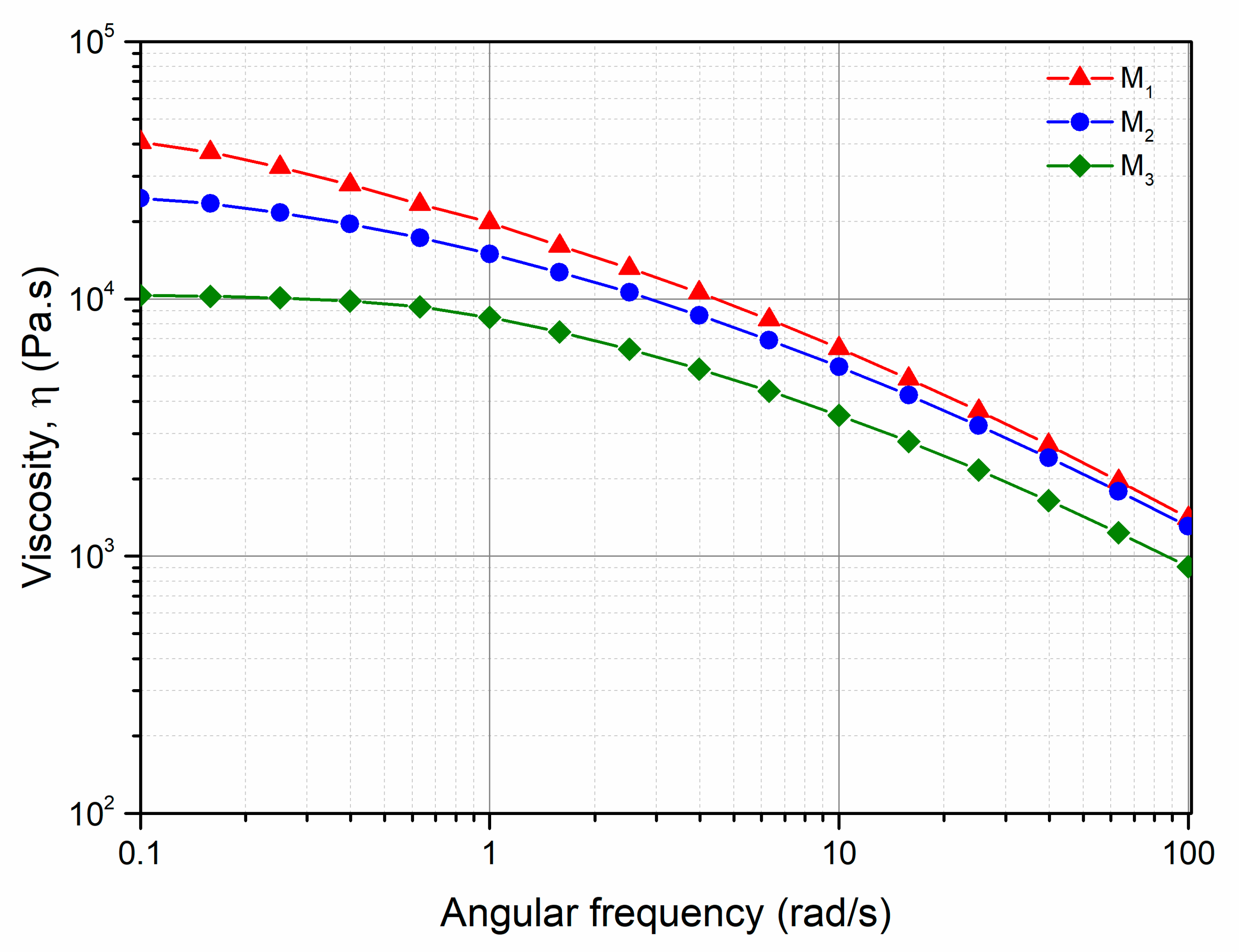
3.2. Characterization of the PP/Photo-Oxidized Blends
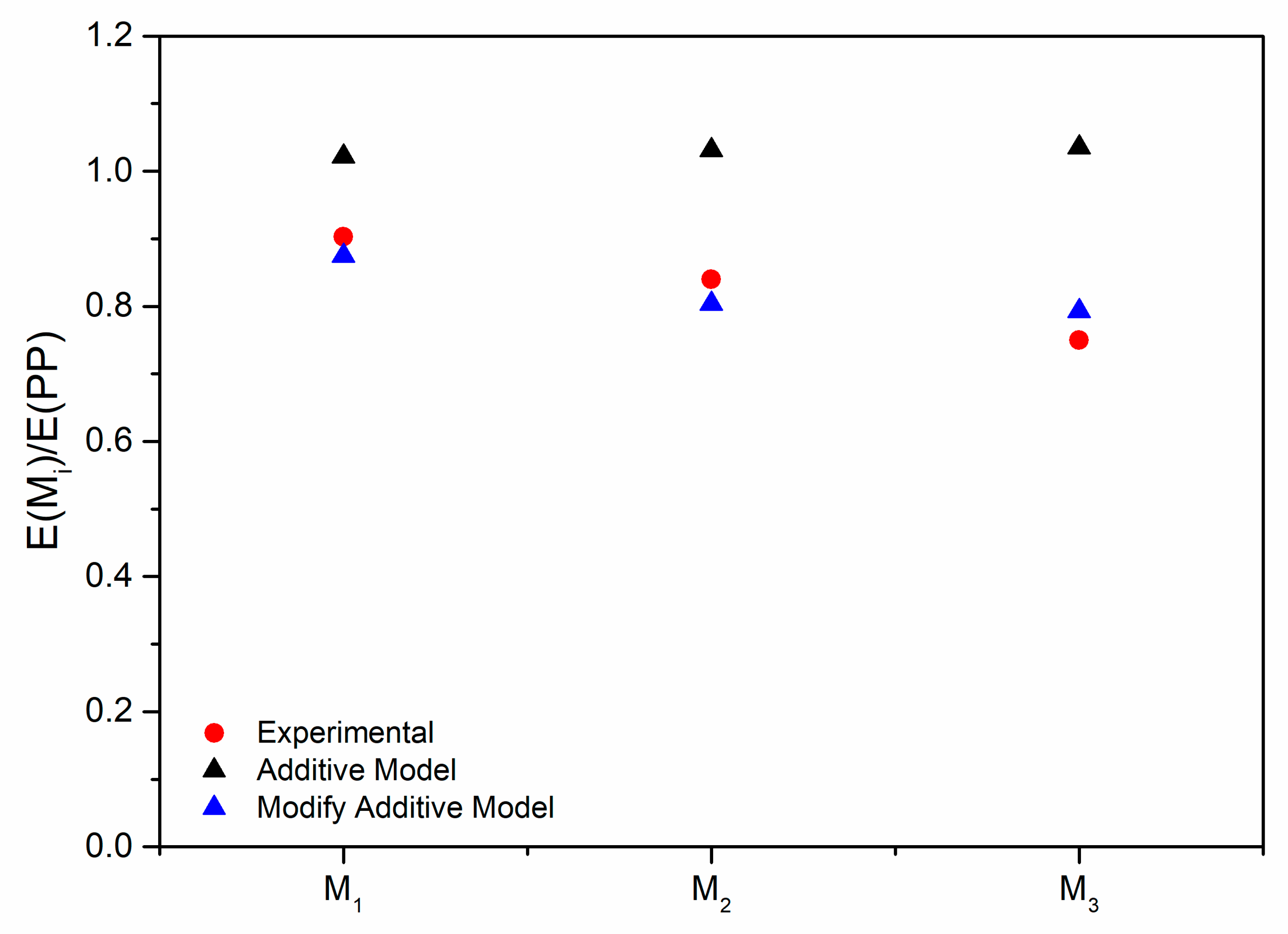
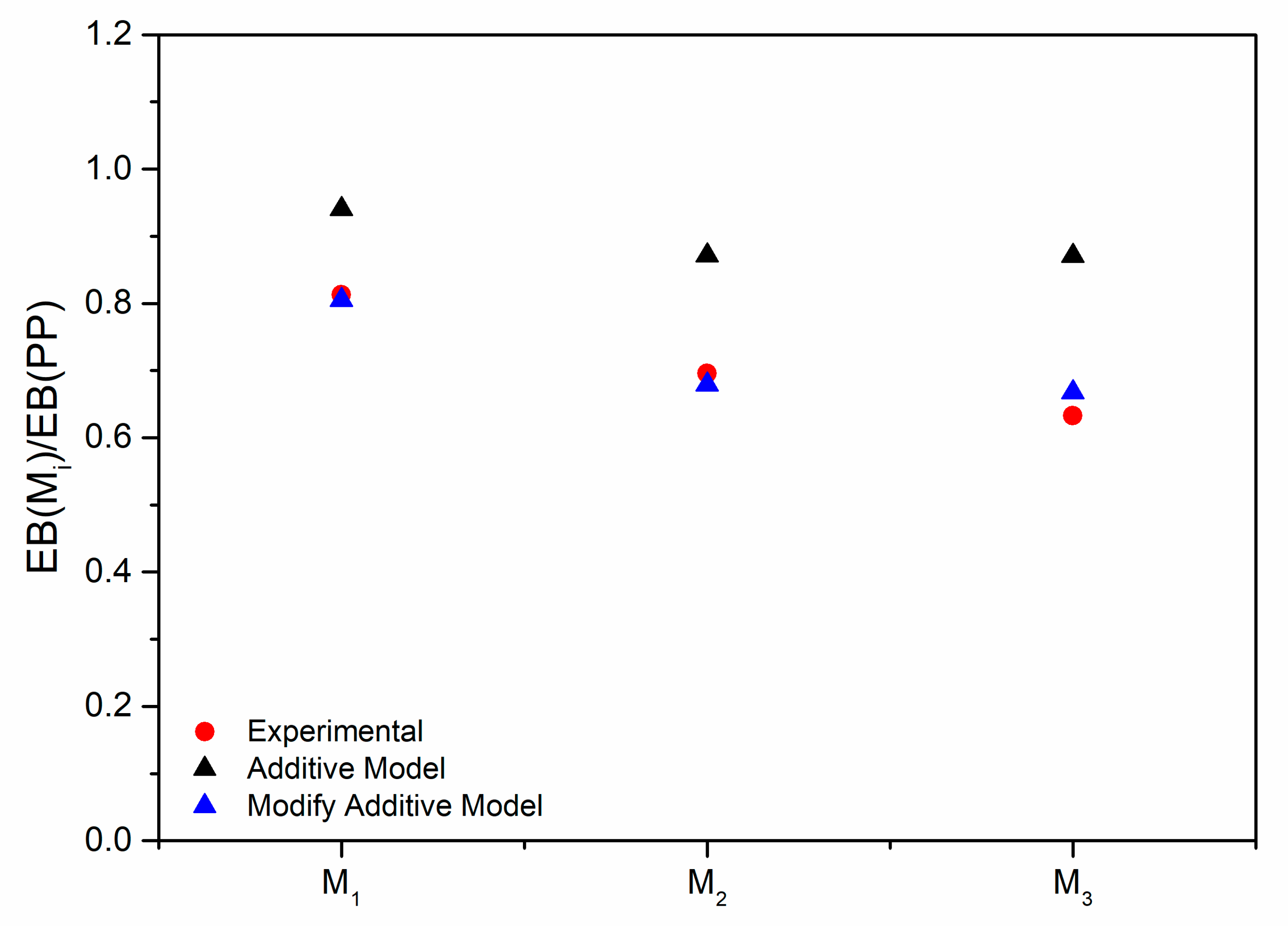
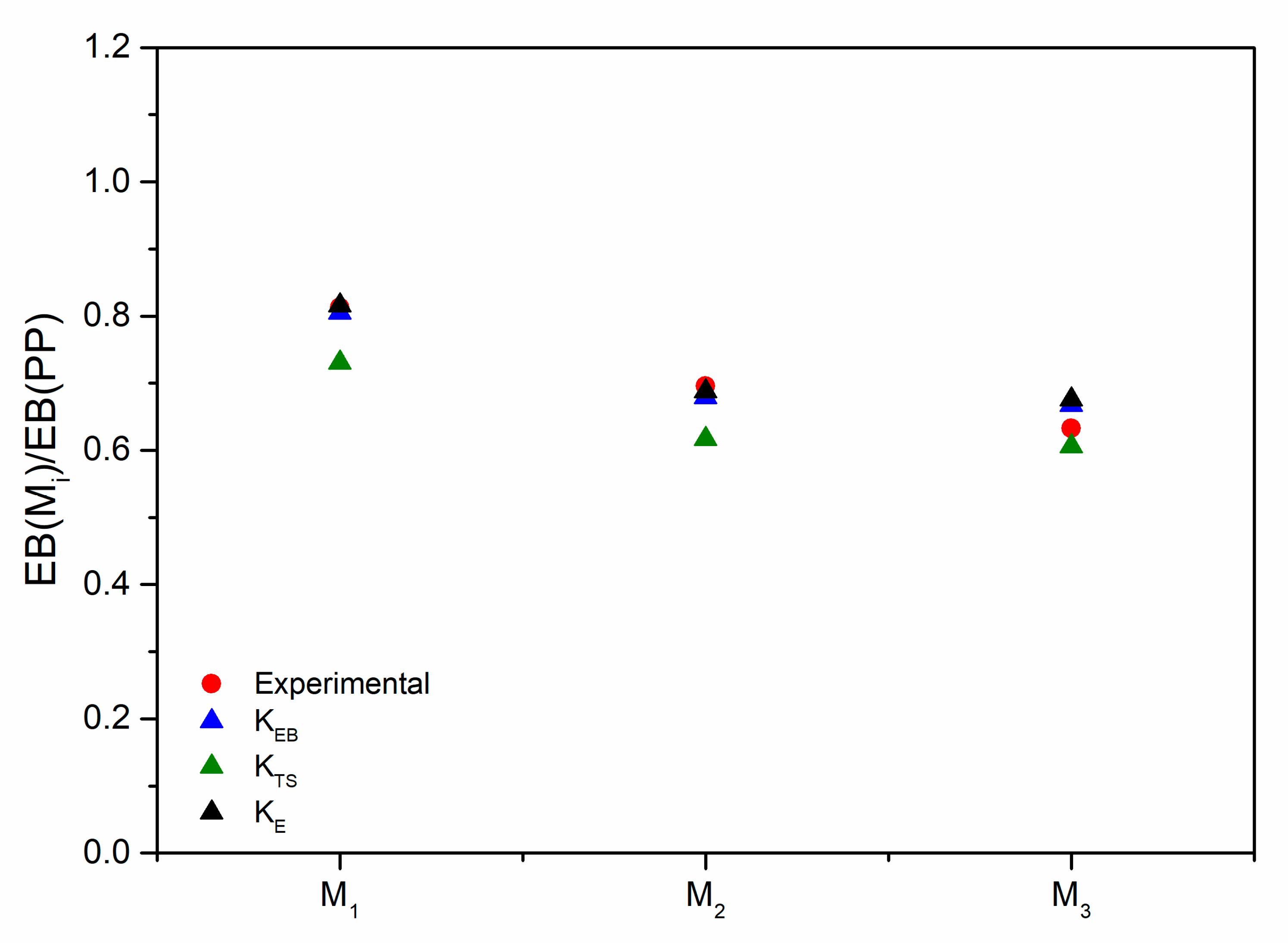
3.3. Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, W.G.; La Mantia, F.P. Recycling of Post-consumer Polyethylene Greenhouse Films: Monopolymer Blends of recycled and virgin Polyethylene. Polym. Netw. Blends 1995, 5, 173. [Google Scholar]
- La Mantia, F.P.; Dintcheva, N.T. Photooxidation and Stabilization of Photooxidized Polyethylene and of its Monopolymer Blends. J. Appl. Polym. Sci. 2003, 91, 2244–2255. [Google Scholar] [CrossRef]
- Marrone, M.; La Mantia, F.P. Monopolymers Blends of Virgin and Recycled Polypropylene. Polym. Recycl. 1996, 2, 9–17. [Google Scholar]
- Valenza, A.; La Mantia, F.P. Recycle of Polymer Waste: Part. 2 Stress degraded polypropylene. Polym. Deg. Stab. 1998, 20, 63. [Google Scholar] [CrossRef]
- Ma, W.G.; La Mantia, F.P. Processing and Mechanical Properties of Recycled PVC and of Homopolymer Blends with Virgin PVC. J. Appl. Polym. Sci. 1996, 59, 5. [Google Scholar]
- La Mantia, F.P.; Scaffaro, R. Reprocessing of Polyethyleneterephtalate and Characterization of Monopolymer Blends of Virgin and Recycled Polyethyleneterephtalate. Polym. Recycl. 1998, 3, 209. [Google Scholar]
- Scaffaro, R.; La Mantia, F.P. Characterization of Monopolymer Blend of Virgin and Recycled Polyamide 6. Polym. Eng. Sci. 2002, 42, 2412–2417. [Google Scholar] [CrossRef]
- Chen, S.-C.; Liao, W.-H.; Hsieh, M.-W.; Chien, R.-D.; Lin, S.-H. Influence of Recycled ABS Added to Virgin Polymers on the Physical, Mechanical Properties and Molding Characteristics. Polym. Technol. Eng. 2011, 50, 306–311. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical Properties of Virgin-Recycled ABS Blends: Effect of Post-consumer Content and of Reprocessing Cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of Reprocessing on Shrinkage and Mechanical Properties of ABS and Investigating the Proper Blend of Virgin and Recycled ABS in Injection Molding. J. Mater. Process. Technol. 2014, 214, 2359–2365. [Google Scholar] [CrossRef]
- Hamarat, I.; Kuram, E.; Ozcelik, B. Investigation the Mechanical, Rheological, and Morphological Properties of Acrylonitrile Butadiene Styrene Blends with Different Recycling Number Content. Proc. Inst. Mech. Eng. Part E J. Process. Mech. Eng. 2018, 232, 449–458. [Google Scholar] [CrossRef]
- Alzerreca, M.; Paris, M.; Boyron, O.; Orditz, D.; Louarn, G.; Correc, O. Mechanical properties and molecular structures of virgin and recycled HDPE polymers used in gravity sewer systems. Polym. Test. 2015, 46, 1–8. [Google Scholar] [CrossRef]
- Cestari, S.P.; Martin, P.J.; Hanna, P.R.; Kearns, M.P.; Mendes, L.S.; Millar, B. Use of virgin/recycled polyethylene blends in rotational moulding. J. Polym. Eng. 2021. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, K.S.; Hahm, W.G.; Kim, S.H. Properties of Recycled and Virgin Poly(ethylene terephthalate)Blend Fibers. J. Appl. Polym. Sci. 2012, 128, 1250–1256. [Google Scholar] [CrossRef]
- Tapia-Picazo, J.C.; Luna-Bárcenas, J.G.; García-Chávez, A.; Gonzalez-Nuñez, R.; Bonilla-Petriciolet, A.; Alvarez-Castillo, A. Polyester fiber production using virgin and recycled PET. Fibers Polym. 2014, 15, 547–552. [Google Scholar] [CrossRef]
- Elamri, A.; Abid, K.; Harzallah, O.; Lallam, A. Characterization of Recycled/Virgin PET Polymers and their Composites. Am. J. Nano Res. Appl. 2015, 3, 11–16. [Google Scholar]
- La Mantia, F.P.; Mistretta, M.C.; Titone, V. An Additive Model to Predict the Rheological and Mechanical Properties of Polypropylene Blends Made by Virgin and Reprocessed Components. Recycling 2021, 6, 2. [Google Scholar] [CrossRef]
- Morlat-Therias, S.; Mailhot, B.; Gonzalez, D.; Gardette, J.L. Photooxidation of Polypropylene/Montmorillonite Nanocomposites. 1. Influence of Nanoclay and Compatibilizing Agent. Chem. Mater. 2004, 16, 377–383. [Google Scholar] [CrossRef]
- Gardette, J.L.; Morlat-Therias, S.; Mailhot, B.; Gonzalez, D. Photooxidation of Polypropylene/Montmorillonite Nanocomposites. 2. Interactions with Antioxidants. Chem. Mater. 2005, 17, 1072–1078. [Google Scholar]
- Morreale, M.; Dintcheva, N.T.; La Mantia, F.P. Accelerated weathering of PP based nanocomposites: Effect of the presence of maleic anydryde grafted polypropylene. eXPRESS Polym. Lett. 2013, 7, 703–715. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Gardette, J.L. Improvement of the Mechanical Properties of Photo-oxidized Film after Recycling. Polym. Deg. Stab. 2002, 75, 1–7. [Google Scholar] [CrossRef]





| Polymer | Density, g/cm3 | MFI, g/10 min (2.16 kg at 230 °C) | Melting Point, °C |
|---|---|---|---|
| Hostalen PP H5416 | 0.897 | 0.3 | 139 |
| E, MPa | TS, MPa | EB, % | |
|---|---|---|---|
| PP | 485 25 | 30.2 2.7 | 706 30 |
| Ph1 | 522 22 | 20.4 1.6 | 568 24 |
| Ph2 | 568 18 | 13.7 0.9 | 30 2.8 |
| Ph3 | 641 19 | 8.4 1.1 | 5.7 1.1 |
| Tm, °C | Xc, % | ||
|---|---|---|---|
| PP | 145.4 | 65.6 1.8 | 31.7 |
| Ph1 | 143.4 | 68.3 2.4 | 33.0 |
| Ph2 | 141.3 | 72.2 2.6 | 34.8 |
| Ph3 | 138.6 | 85.1 3.7 | 41.1 |
| M1 | M2 | M3 | |
|---|---|---|---|
| Newtonian Viscosity, η0 (Pas) | 40,570 | 24,651 | 10,329 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantia, F.P.L.; Mistretta, M.C.; Titone, V. Rheological, Mechanical and Morphological Characterization of Monopolymer Blends Made by Virgin and Photo-Oxidized Polypropylene. Recycling 2021, 6, 51. https://doi.org/10.3390/recycling6030051
Mantia FPL, Mistretta MC, Titone V. Rheological, Mechanical and Morphological Characterization of Monopolymer Blends Made by Virgin and Photo-Oxidized Polypropylene. Recycling. 2021; 6(3):51. https://doi.org/10.3390/recycling6030051
Chicago/Turabian StyleMantia, Francesco Paolo La, Maria Chiara Mistretta, and Vincenzo Titone. 2021. "Rheological, Mechanical and Morphological Characterization of Monopolymer Blends Made by Virgin and Photo-Oxidized Polypropylene" Recycling 6, no. 3: 51. https://doi.org/10.3390/recycling6030051
APA StyleMantia, F. P. L., Mistretta, M. C., & Titone, V. (2021). Rheological, Mechanical and Morphological Characterization of Monopolymer Blends Made by Virgin and Photo-Oxidized Polypropylene. Recycling, 6(3), 51. https://doi.org/10.3390/recycling6030051








