Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Separation and Purification
2.2.1. Dissolution Based Polymer Separation and Purification
2.2.2. Dissolution-Based Polymer Separation
2.3. Material Characterization
2.3.1. XRF Analysis
2.3.2. BFR Analysis
2.3.3. FTIR
2.3.4. Melt Flow Rate
2.3.5. Differential Scanning Calorimetry (DSC)
3. Results
3.1. Composition of Mixed Plastic Fractions
3.2. Material Separation Achieved by Dissolution
3.3. Distribution of Elements and Related Materials
3.4. Dissolution Based Separation of BFR from ABS and PS
3.5. Material Properties
4. Discussion
4.1. Identification of Target Polymers
4.2. Characterization and Management of Halogen and BFR Content
4.3. Recycling Concept Derived from Laboratory Scale Trials and Effect of BFR Management
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene copolymer |
| BFR | brominated flame retardants |
| Br | bromine |
| Cl | chlorine |
| CRT | cathode ray tube |
| DF | density fraction |
| FT-IR | Fourier transform infrared |
| g | gramm |
| g/10 min | grams per ten minutes |
| g/mol | Grams per mol |
| g/cm3 | grams per cubic centimeter |
| mm | Millimeter |
| µm | Mikrometer |
| min | minute |
| HIPS | high impact polystyrene |
| ICT | Information and Communication Technology |
| kg | kilograms |
| K/min | Kelvin per minute |
| LCD | liquid-crystal-display |
| MFR | melt flow rate |
| RoHS | restriction of hazardous substances directive |
| SDA | small domestic appliances |
| PA | polyamide |
| PS | polystyrene |
| PC | polycarbonate |
| PP | polypropylene |
| PE | polyethylene |
| PBT | persistant bioaccumulative toxic |
| POM | polyocymethylene |
| POP | persistent organic pollutants |
| PVC | polyvinyl chloride |
| ppm | parts per million |
| WEEE | waste of electrical and electronic equipment |
| XRF | X-ray fluorescence |
| °C | degree celsius |
References
- Wagner, F.; Peeters, J.R.; de Keyzer, J.; Janssens, K.; Duflou, J.R.; Dewulf, W. Towards a more circular economy for WEEE plastics—Part A: Development of innovative recycling strategies. Waste Manag. 2019, 100, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bressanelli, G.; Saccani, N.; Pigosso, D.C.A.; Perona, M. Circular Economy in the WEEE industry: A systematic literature review and a research agenda. Sustain. Prod. Consum. 2020, 23, 174–188. [Google Scholar] [CrossRef]
- Cardamone, G.F.; Ardolino, F.; Arena, U. About the environmental sustainability of the European management of WEEE plastics. Waste Manag. 2021, 126, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Drage, D.S.; Sharkey, M.; Berresheim, H. Brominated flame retardants and perfluoroalkyl substances in landfill leachate from Ireland. Sci. Total Environ. 2019, 695, 133810. [Google Scholar] [CrossRef]
- Leslie, H.A.; Leonards, P.E.G.; Brandsma, S.H.; de Boer, J.; Jonkers, N. Propelling plastics into the circular economy—weeding out the toxics first. Environ. Int. 2016, 94, 230–234. [Google Scholar] [CrossRef]
- Wagner, S.; Schlummer, M. Legacy additives in a circular economy of plastics: Current dilemma, policy analysis, and emerging countermeasures. Resour. Conserv. Recycl. 2020, 158, 104800. [Google Scholar] [CrossRef]
- Schlummer, M.; Gruber, L.; Mäurer, A.; Wolz, G.; van Eldik, R. Characterisation of polymer fractions from waste electrical and electronic equipment (WEEE) and implications for waste management. Chemosphere 2007, 67, 1866–1876. [Google Scholar] [CrossRef]
- Stubbings, W.A.; Abdallah, M.A.-E.; Misiuta, K.; Onwuamaegbu, U.; Holland, J.; Smith, L.; Parkinson, C.; McKinlay, R.; Harrad, S. Assessment of brominated flame retardants in a small mixed waste electronic and electrical equipment (WEEE) plastic recycling stream in the UK. Sci. Total Environ. 2021, 780, 146543. [Google Scholar] [CrossRef]
- Hale, R. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003, 29, 771–779. [Google Scholar] [CrossRef]
- Darnerud, P. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Sindiku, O.; Babayemi, J.O.; Tysklind, M.; Osibanjo, O.; Weber, R.; Watson, A.; Schlummer, M.; Lundstedt, S. Polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) in e-waste plastic in Nigeria. Environ. Sci. Pollut. Res. Int. 2015, 22, 14515–14529. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Schlummer, M. Polymer Green Flame Retardants. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; Volume 25, pp. 869–889. [Google Scholar]
- Aldrian, A.; Ledersteger, A.; Pomberger, R. Monitoring of WEEE plastics in regards to brominated flame retardants using handheld XRF. Waste Manag. 2015, 36, 297–304. [Google Scholar] [CrossRef]
- Jandric, A.; Part, F.; Fink, N.; Huber-Humer, M.; Salhofer, S.; Zafiu, C. Bromierte Flammschutzmittel in Elektroaltgeräten: Untersuchung der Brom-Konzentration nach Kunststofftypen und Gerätekategorien mittels Röntgenfluoreszenzanalyse. Österr Wasser Abfallw 2020, 72, 68–76. [Google Scholar] [CrossRef]
- Schlummer, M.; Mäurer, A.; Leitner, T.; Spruzina, W. Recycling of flame-retarded plastics from waste electric and electronic equipment (WEEE)//Report: Recycling of flame-retarded plastics from waste electric and electronic equipment (WEEE). Waste Manag. Res. 2006, 24, 573–583. [Google Scholar] [CrossRef]
- Maisel, F.; Chancerel, P.; Dimitrova, G.; Emmerich, J.; Nissen, N.F.; Schneider-Ramelow, M. Preparing WEEE plastics for recycling—How optimal particle sizes in pre-processing can improve the separation efficiency of high quality plastics. Resour. Conserv. Recycl. 2020, 154, 104619. [Google Scholar] [CrossRef]
- Araujo-Andrade, C.; Bugnicourt, E.; Philippet, L.; Rodriguez-Turienzo, L.; Nettleton, D.; Hoffmann, L.; Schlummer, M. Review on the photonic techniques suitable for automatic monitoring of the composition of multi-materials wastes in view of their posterior recycling. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2021, 734242X21997908. [Google Scholar]
- Maris, E.; Botané, P.; Wavrer, P.; Froelich, D. Characterizing plastics originating from WEEE: A case study in France. Miner. Eng. 2015, 76, 28–37. [Google Scholar] [CrossRef]
- Dodbiba, G.; Shibayama, A.; Sadaki, J.; Fujita, T. Combination of Triboelectrostatic Separation and Air Tabling for Sorting Plastics from a Multi-Component Plastic Mixture. Mater. Trans. 2003, 44, 2427–2435. [Google Scholar] [CrossRef]
- Martinho, G.; Pires, A.; Saraiva, L.; Ribeiro, R. Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag. 2012, 32, 1213–1217. [Google Scholar] [CrossRef]
- Balart, R.; López, J.; García, D.; Dolores Salvador, M. Recycling of ABS and PC from electrical and electronic waste. Effect of miscibility and previous degradation on final performance of industrial blends. Eur. Polym. J. 2005, 41, 2150–2160. [Google Scholar] [CrossRef]
- Dong Hwang, Y.; Woon Cha, S. The relationship between gas absorption and the glass transition temperature in a batch microcellular foaming process. Polym. Test. 2002, 21, 269–275. [Google Scholar] [CrossRef]
- Arabeche, K.; Delbreilh, L.; Adhikari, R.; Michler, G.H.; Hiltner, A.; Baer, E.; Saiter, J.-M. Study of the cooperativity at the glass transition temperature in PC/PMMA multilayered films: Influence of thickness reduction from macro- to nanoscale. Polymer 2012, 53, 1355–1361. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Moeller, B.C.; Lu, K.; Rager, J.E.; Fry, R.C.; Starr, T.B. Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol. Pathol. 2013, 41, 181–189. [Google Scholar] [CrossRef]
- LaA Guardia, M.J.; Hale, R.C.; Harvey, E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ. Sci. Technol. 2006, 40, 6247–6254. [Google Scholar] [CrossRef]
- Morose, G. An Overview of Alternatives to Tetrabromobisphenol A (TBBPA) and Hexabromocyclododecane (HBCD). Available online: https://webcache.googleusercontent.com/search?q=cache:90hSjjf1xd0J:https://www.chemicalspolicy.org/downloads/AternativestoTBBPAandHBCD.pdf+&cd=2&hl=de&ct=clnk&gl=de&client=firefox-b-e (accessed on 30 March 2021).
- Scientific Opinion on Emerging and Novel Brominated Flame Retardants (BFRs) in Food. EFSA J. 2012, 10, 2908. [CrossRef]
- Schlummer, M.; Maurer, A.; Wagner, S.; Berrang, A.; Fell, T.; Knappich, F. Recycling of flame retarded waste polystyrene foams (EPS and XPS) to PS granules free of hexabromocyclododecane (HBCDD). Adv. Recycl. Waste Manag. 2017, 2. [Google Scholar] [CrossRef]
- Tange, T.; Drohmann, D. Closing the Bromine Cycle for Plastics from Electrical and Electronic Waste Using Staged Gasification. Available online: https://www.researchgate.net/publication/242198374_WASTE_MANAGEMENT_CONCEPT_FOR_WEEE_PLASTICS_CONTAINING_BROMINATED_FLAME_RETARDANTS_INCLUDING_BROMINE_RECYCLING_AND_ENERGY_RECOVERY (accessed on 26 April 2021).
- Hense, P. Development and Optimization of a Thermo-Chemical Process for Recycling Waste Electrical and Electronic Equipment, Amsdottorato. 2019. Available online: http://amsdottorato.unibo.it/id/eprint/7900 (accessed on 14 May 2021).
- Arends, D.; Schlummer, M.; Mäurer, A.; Markowski, J.; Wagenknecht, U. Characterisation and materials flow management for waste electrical and electronic equipment plastics from German dismantling centres. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2015, 33, 775–784. [Google Scholar] [CrossRef]
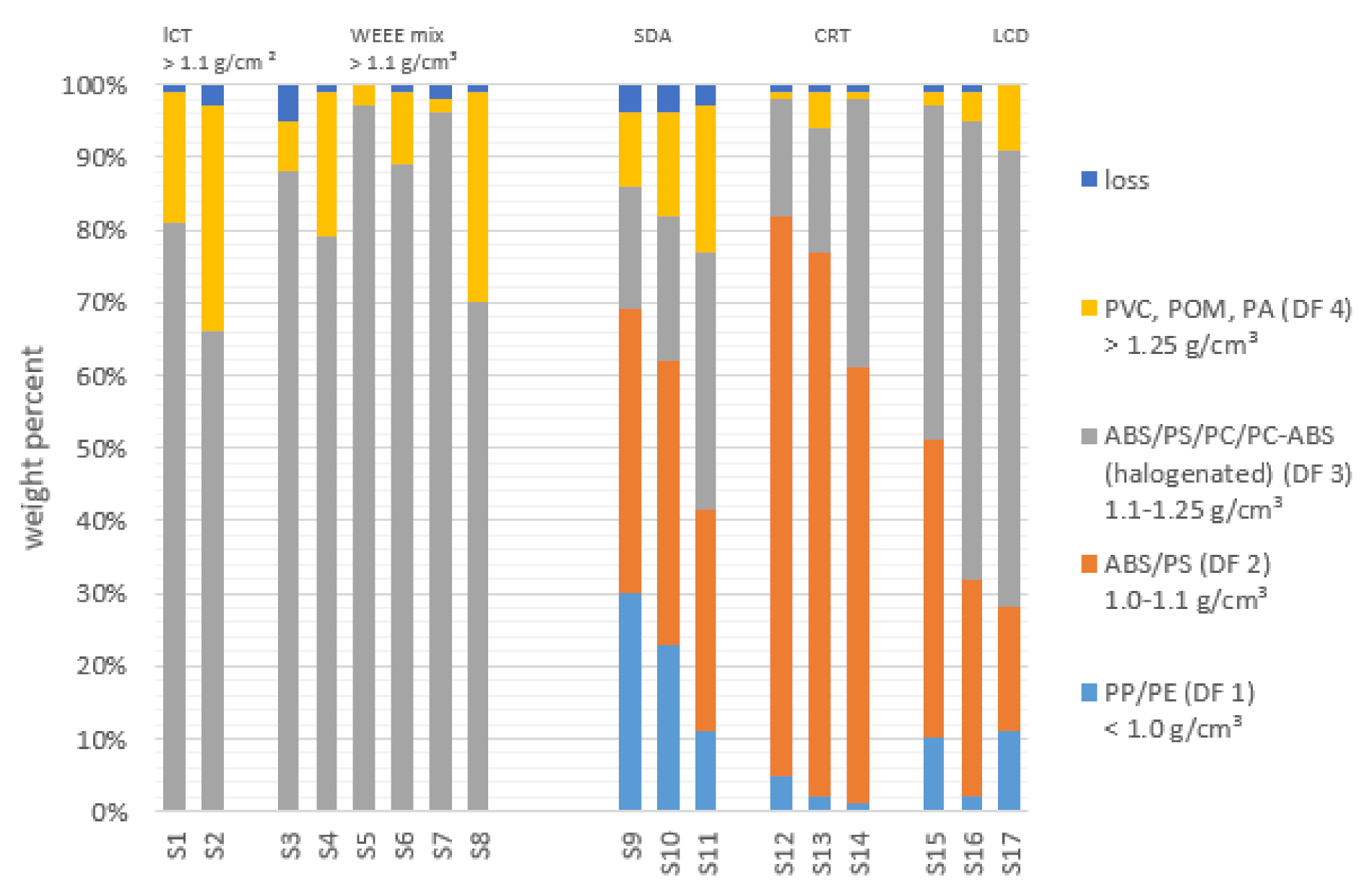
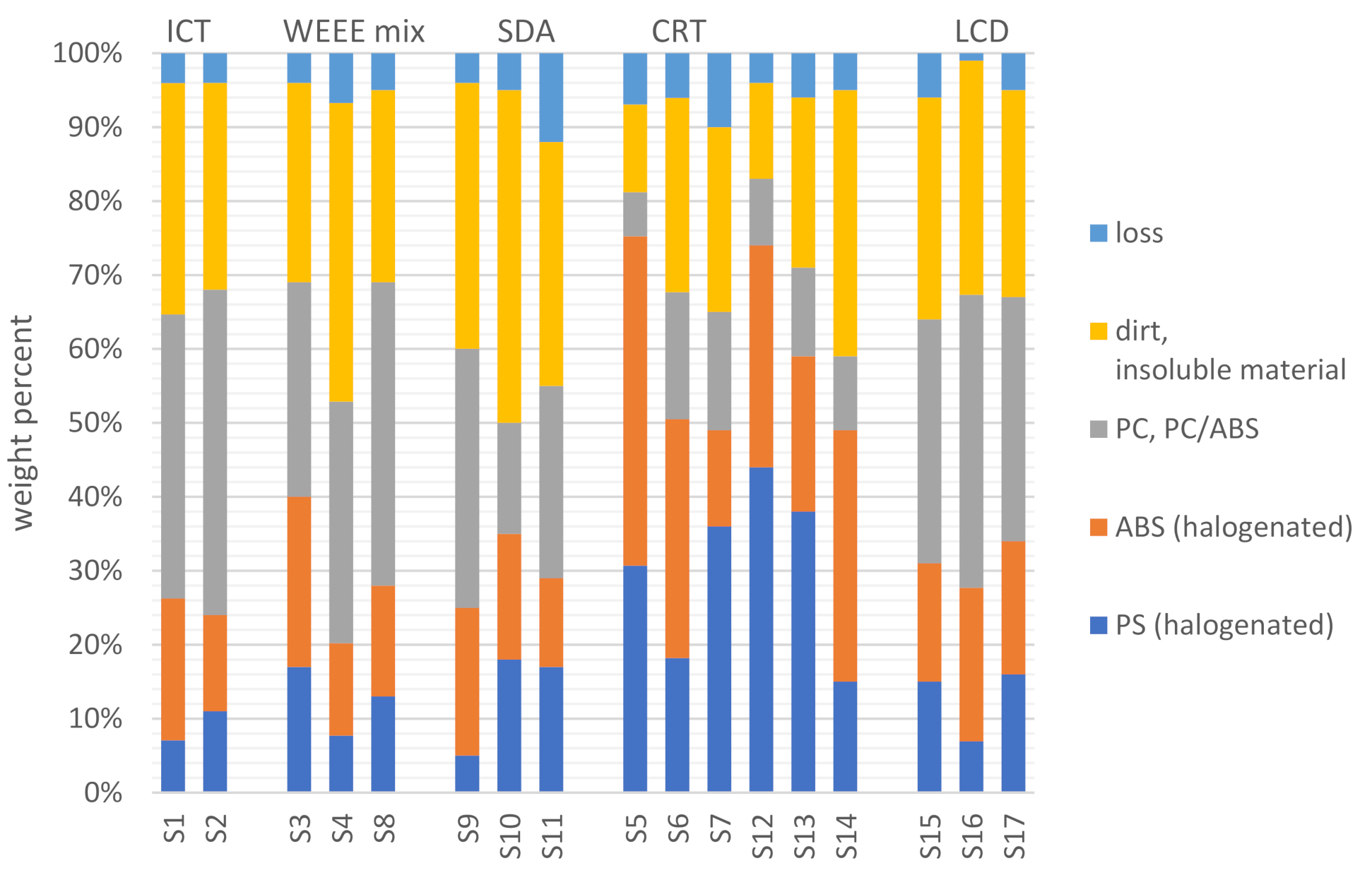
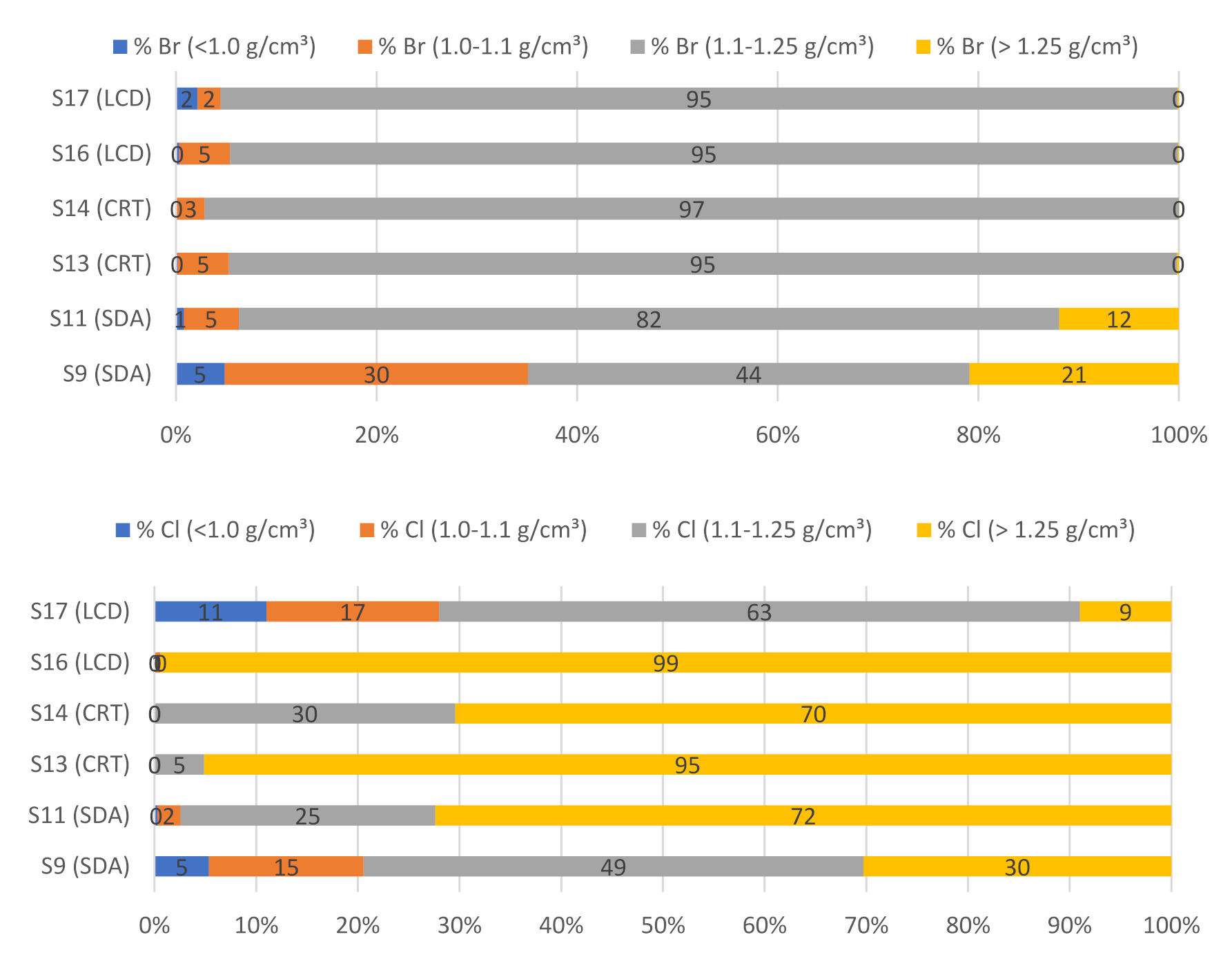
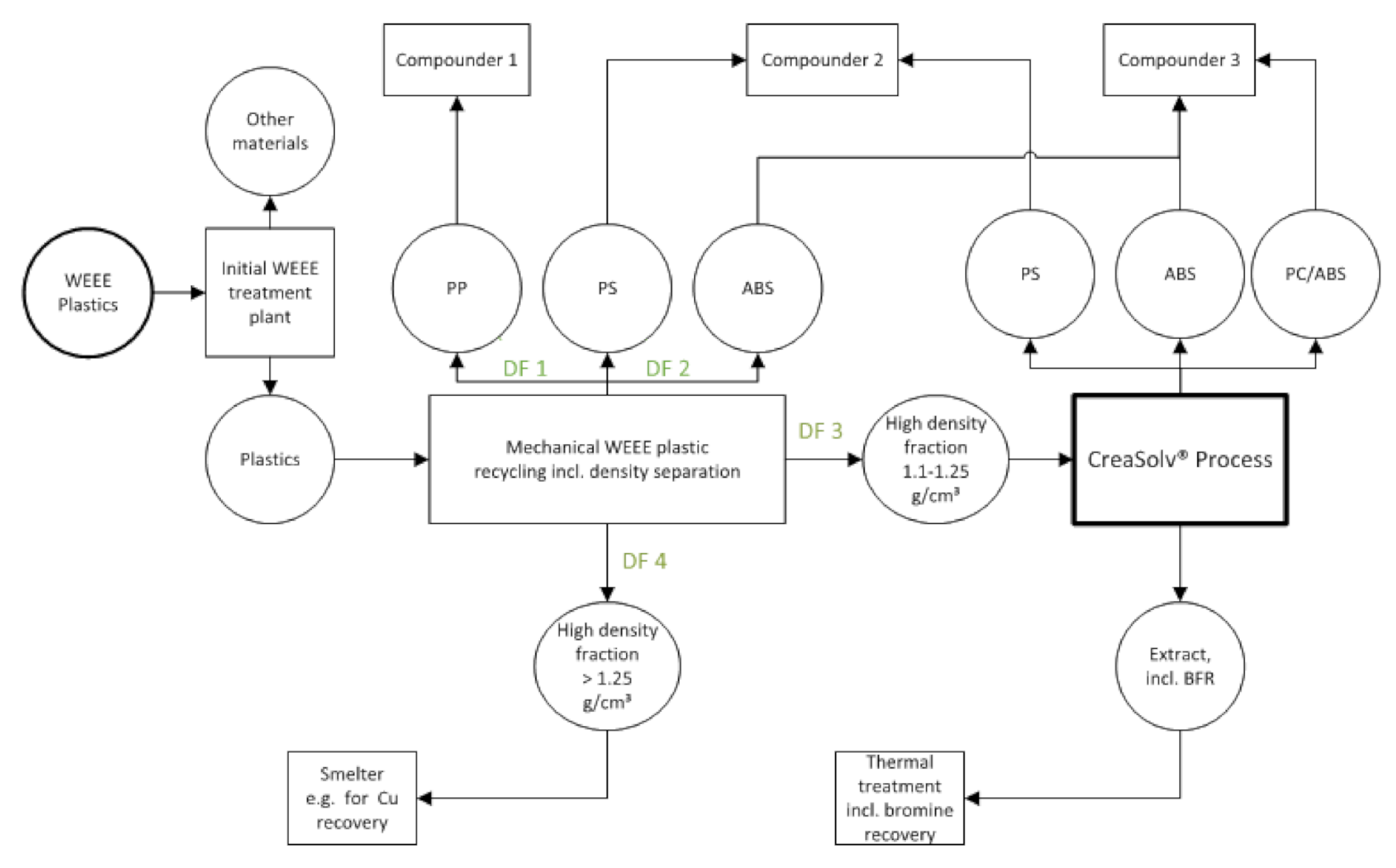
| Sample Name | Source | Description | Sampling Year | Country | Mass [kg] |
|---|---|---|---|---|---|
| Sample 1 | ICT | Plastic mix > 1.1 g/cm3 | 11/2019 | Netherlands | 5 |
| Sample 2 | ICT | Plastic mix > 1.1 g/cm3 | 05/2020 | Netherlands | 20 |
| Sample 3 | WEEE mix (SDA/ICT/Screens) | Plastic mix > 1.1 g/cm3 | 09/2019 | Sweden | 5 |
| Sample 4 | WEEE mix (SDA/ICT) | Plastic mix > 1.1 g/cm3 | 11/2019 | Spain | 5 |
| Sample 5 | WEEE (TV’s) | Plastic mix > 1.1 g/cm3 | 11/2019 | Spain | 5 |
| Sample 6 | CRT | Plastic mix > 1.1 g/cm3 | 09/2019 | Netherlands | 25 |
| Sample 7 | WEEE mix (Monitors) | Plastic mix > 1.1 g/cm3 | 11/2019 | Spain | 5 |
| Sample 8 | SDA | Plastic mix > 1.1 g/cm3 | 09/2019 | Netherlands | 25 |
| Sample 9 | SDA | Plastic mix | 09/2019 | Italy | 5 |
| Sample 10 | SDA | Plastic mix | 09/2019 | Italy | 5 |
| Sample 11 | SDA | Plastic mix | 08/2019 | Italy | 20 |
| Sample 12 | CRT | Plastic mix | 08/2019 | Italy | 20 |
| Sample 13 | CRT | Plastic mix | 04/2020 | Italy | 20 |
| Sample 14 | CRT | Plastic mix | 05/2020 | Italy | 20 |
| Sample 15 | Flat screen (LCD) | Plastic mix | 10/2019 | Italy | 20 |
| Sample 16 | Flat screen (LCD) | Plastic mix | 05/2020 | Italy | 20 |
| Sample 17 | Flat screen (LCD) | Plastic mix | 12/2020 | Italy | 20 |
| Density Fraction (DF) | Density | Polymers |
|---|---|---|
| DF 1 | <1.0 g/cm3 | PE, PP |
| DF 2 | 1.0–1.1 g/cm3 | ABS (non-halogenated) PS (non-halogenated) |
| DF 3 | 1.1–1.25 g/cm3 | ABS (halogenated), PS (halogenated) PC, Soft PVC, PMMA |
| DF 4 | >1.25 g/cm3 | POM, Hard PVC, PA |
| Sample Name | Source | <1.0 g/cm³ | 1.0–1.1 g/cm³ | 1.1–1.25 g/cm³ | >1.25 g/cm³ | ||||
|---|---|---|---|---|---|---|---|---|---|
| [ppm] | Br | Cl | Br | Cl | Br | Cl | Br | Cl | |
| Sample 1 | ICT | - | - | - | - | 10,621 | 10,940 | 6480 | 47,443 |
| Sample 2 | ICT | - | - | - | - | 4335 | 380 | 6069 | 13,410 |
| Sample 3 | WEEE | - | - | - | - | 10,402 | 2875 | 7916 | 22,733 |
| Sample 4 | WEEE | - | - | - | - | 14,296 | 16,190 | 4214 | 28,917 |
| Sample 5 | WEEE | - | - | - | - | 45,856 | 13,653 | 13,760 | 29,363 |
| Sample 6 | CRT | - | - | - | - | 27,618 | 16,100 | 3570 | 16,100 |
| Sample 7 | WEEE | - | - | - | - | 70,193 | 10,210 | 1335 | 266,566 |
| Sample 8 | SDA | - | - | - | - | 9434 | 5442 | 10,498 | 15,800 |
| Sample 9 | SDA | 696 | 2084 | 3346 | 4542 | 11,167 | 33,700 | 9000 | 35,300 |
| Sample 10 | SDA | 2283 | 2655 | 3138 | 4471 | 10,933 | 53,367 | 9667 | 52,033 |
| Sample 11 | SDA | 1027 | 268 | 2598 | 771 | 33,327 | 7303 | 8506 | 36,917 |
| Sample 12 | CRT | 596 | 168 | 2656 | 197 | 58,300 | 10,933 | n.a. | n.a. |
| Sample 13 | CRT | 750 | 72 | 591 | <10 | 48,340 | 2837 | 328 | 188,500 |
| Sample 14 | CRT | 430 | <10 | 358 | <10 | 20,200. | 1800 | 24 | 158,600 |
| Sample 15 | LCD | 2222 | 229 | 5048 | <10 | 16,333 | 1063 | 187 | 283 |
| Sample 16 | LCD | 349 | 69 | 389 | 30 | 3451 | <10 | 56 | 44,510 |
| Sample 17 | LCD | 2546 | <10 | 1757 | <10 | 19,785 | <10 | 196 | <10 |
| Name | Description | Source | Br [ppm] | Cl [ppm] |
|---|---|---|---|---|
| Recycler 1 | ABS | SDA/ICT | 1197 | 1125 |
| PP/PE | SDA/ICT | 380 | 705 | |
| PS | SDA/ICT | 173 | 406 | |
| HIPS | fridge | 76 | 386 | |
| Recycler 2 | ABS | WEEE | 1269 | 641 |
| PP | WEEE | 145 | 605 | |
| HIPS | WEEE | 931 | 570 | |
| Recycler 3 | ABS | WEEE | 141 | 148 |
| PP/PE | WEEE | 942 | 84 | |
| PS | WEEE | 613 | 705 |
| Polymer 1.1–1.25 g/cm³ | Source | Total Bromine a [ppm] | TBBPA [ppm] | BTBPE [ppm] | Hexa-Nona-BDE [ppm] | Deca-BDE [ppm] |
|---|---|---|---|---|---|---|
| Sample 1 | ICT | 10,621 | 4147 | 341 | 288 | 279 |
| Sample 4 | WEEE | 14,296 | 9487 | 1481 | 332 | 1453 |
| Sample 6 | CRT | 27,617 | 13,616 | 6766 | 1231 | 10,686 |
| Sample 9 | SDA | 11,167 | 4172 | 99 | 93 | 784 |
| Sample 11 | SDA | 33,327 | 22,698 | 4538 | 2518 | 2038 |
| Sample 15 | LCD | 16,333 | 6966 | - | 237 | 176 |
| Polymer | Unit | R-PS | R-ABS | R-PC | PS, Virgin | ABS, Virgin | PC, Vigin |
|---|---|---|---|---|---|---|---|
| Melt flow rate * (MFR) | g/10 min | 4.2 + | 19.2 ++ | 12.2 +++ | 9.5 a,+ | 22 b,++ | 6.0 c,++++ |
| Weight average of molecular weight (Mw) ** | g/mol | 163,498 | 106,205 | 44,379 | 194,224 | 105,812 | 37,879 |
| Tg (DSC) | °C | 103.7 | 107.7 | 141.0 | 100 | 120 | 148 |
| GC-ECD | TBBPA [ppm] | <10 | <10 | 47 | - | - | - |
| TBPE [ppm] | <10 | 22 | <10 | - | - | - | |
| DecaBDE [ppm] | 108 | <10 | 16 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strobl, L.; Diefenhardt, T.; Schlummer, M.; Leege, T.; Wagner, S. Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste. Recycling 2021, 6, 33. https://doi.org/10.3390/recycling6020033
Strobl L, Diefenhardt T, Schlummer M, Leege T, Wagner S. Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste. Recycling. 2021; 6(2):33. https://doi.org/10.3390/recycling6020033
Chicago/Turabian StyleStrobl, Laura, Thomas Diefenhardt, Martin Schlummer, Tanja Leege, and Swetlana Wagner. 2021. "Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste" Recycling 6, no. 2: 33. https://doi.org/10.3390/recycling6020033
APA StyleStrobl, L., Diefenhardt, T., Schlummer, M., Leege, T., & Wagner, S. (2021). Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste. Recycling, 6(2), 33. https://doi.org/10.3390/recycling6020033







