Abstract
The volume of discarded solid wastes, especially plastic, which accumulates in large quantities in different environments, has substantially increased. Population growth and the consumption pattern of societies associated with unsustainable production routes have caused the pollution level to increase. Therefore, the development of materials that help mitigate the impacts of plastics is fundamental. However, bioplastics can result in a misunderstanding about their properties and environmental impacts, as well as incorrect management of their final disposition, from misidentifications and classifications. This chapter addresses the aspects and factors surrounding the biodegradation of bioplastics from natural (plant biomass (starch, lignin, cellulose, hemicellulose, and starch) and bacterial polyester polymers. Therefore, the biodegradation of bioplastics is a factor that must be studied, because due to the increase in the production of different bioplastics, they may present differences in the decomposition rates.
1. Introduction
Consumption demands for industrialized materials such as plastics in their various applications have increased over the past years. This consumption is generating residues, which require alternatives for their proper disposal and recycling. Disposal, recycling, and plastic substitution are potential research areas towards urgent and necessary solutions. Most commercial plastics come from the petrochemical industry, which uses natural gas and fossil hydrocarbons as feedstock. Such synthetic plastics are biodegradable and degradable only for a long period. Therefore, they are considered neither biodegradable nor renewable [1]. Synthetic polymers, such as polypropylene (PP), polyethylene (PE), polytetrafluoroethylene (PTFE), nylon, polyester (PS), and epoxy are examples of plastic components of high resistivity, chemical and biological inertness, resistance, flexibility, and other interesting properties [2,3,4,5].
At the beginning of the large-scale production of synthetic plastic materials, their properties seemed adequate for good quality development. However, such materials are non-biodegradable, thus generating large accumulations of residues in different landscapes. Thus, they have been a cause of growing concerns due to environmental problems. New materials based on biological sources have been developed towards solving or reducing the above-mentioned problems. However, in addition to be renewable and biodegradable, bioplastics must have vapors barrier properties and mechanical properties that meet the different applications of this material, and the attention has now evolved towards the possible ecotoxic effects of bioplastics and active properties for a cover of food.
The names of biodegradable and/or bioplastic products given by companies and reported in the literature, when drawn up wrongly, can lead to misunderstandings by the general public due to incorrect classifications of the polymeric materials [6,7,8,9]. A bioplastic can be biodegradable or not. However, a biodegradable material does not necessarily come from a biological source. Towards the avoidance of errors, the following definitions, reported in this article, must be clarified:
- Plastics are polymeric matrices comprised of organic polymers of high molecular weight and other substances, such as fillers, colors, and additives [6]. In general, the synthetic route is predominant in the synthesis of the material.
- Bioplastic refers to materials that are biodegradable, bio-based, or both. Although the term bioplastic is generally used to distinguish polymers derived from fossil resources, it is worth mentioning that bioplastics may come from petroleum [6]. The prefix “bio” of bioplastic does not necessarily mean this material is environmentally friendly [6].
- Biomass is a source of natural organic carbon that may originate from animals or vegetables raised/cultivated by humans or that spontaneously emerge in terrestrial and marine environments [10].
Different biotic and abiotic factors contribute to the different degradation processes [11]. Thermal, mechanical, and chemical degradation, as well as photodegradation, are examples of abiotic degradation. A degradation process is related to the fragmentation of material into small elements or molecules, or just physical and chemical changes in a polymer. Due to high temperatures, polymers can be thermally degraded. The chemical bonds in their chains are broken by a thermo-degradation effect [12].
Mechanical degradation is an abiotic degradation mechanism that occurs through shear forces (due to aging, turbulence in water and air, snow pressure, and other factors), tension and/or compression. Under environmental conditions, it acts synergistically with different abiotic factors [13].
Abiotic chemical degradation occurs by the degradative effect of chemicals substances, and represent one of the most important mechanisms of abiotic degradation, since the polymer matrix is affected by atmospheric or agrochemical pollutants, such as oxygen (i.e., O2 or O3), which produce free radicals through oxidation, attacking covalent bonds [13]. Abiotic chemical degradation differs from biotic chemical degradation, mainly regarding the origin of the chemical with a degrading effect.
Photodegradation is the process of degradation of polymers by the action of light, resulting in the oxidation of the material. UV rays interact with chromophores groups of polymers (carbonyl, hydroxyls, and aldehydes), which are degraded by chain fission, photoionization, crosslinking, and oxidation reaction [11,13,14,15].
Microbial biodegradation is a degradation process of polymers and other materials through the action of microorganisms [11] resulting in CO2 and/or methane, water, cell biomass, and energy. However, in the natural environment and even in the process of controlled biodegradation, abiotic effects help or even occur synergistically with biodegradation. This consideration of synergism is important for the elaboration of biodegradation procedures.
With environmental concern, this review evaluated the biodegradation process (considering the synergistic action of biotic and abiotic agents) of bioplastics elaborated with polysaccharides from plant biomass and microbial polyesters. Moreover, addressing the definitions, biodegradation mechanism, and factors that affect the biodegradative process of bioplastics. The scope of this review does not address the biodegradation of bioplastics produced from polymers of animal origin (natural polymer), and bioplastics derived from petroleum (PBAT, PBS, PVA, PCL, and PGA [16]. However, the definitions presented in this review do not exclude these types of bioplastics.
2. Problems Related to Plastics
The current geological era, the so-called Anthropocene, is exposed to the influence of human actions in different environments. Indicators from such anthropic actions are biodiversity reduction, deforestation, climate, and other environmental changes [17]. However, materials produced by human society, like plastics, are also indicators of the Anthropocene. Plastics directly (i.e., environmental impacts from the plastic production chain) affect different environments (e.g., terrestrial and marine).
An environment in which plastic waste currently generates several problems, is the oceans, due to the large accumulation of these materials. The plastic that reaches the oceans mostly is generated in coastal population regions, where the disposal and management of this waste is destined for uncontrolled landfills [18]. Due to urban runoff and inland waterways, such plastics reach oceans and are transported via tide and winds. It is estimated that between 4.8 and 12.7 million metric tons of plastics produced in the continent (distribution varies according to the analyzed location) reached the marine environment in 2010 [18].
In recent years, environmental concerns (e.g., harmful effects of plastics on the environment, since they are not biodegradable [19], or slowly degraded) have been intensified. Large accumulations of floating plastics in the oceans have been reported- approximately 1.8 trillion pieces of plastic have been quantified in the Great Pacific Garbage Patch (GPGP) [20]. The ingestion of plastic fragments by the marine fauna is a major concern due to their small size [18,21,22], the so-called microplastics, which are smaller than 5 mm [21]. Besides, since plastic fragments are present on the surface and floor of oceans, as well as in several maritime regions (coastal areas) and the Arctic sea ice, strategies, as a reduction in inputs [18] and the elaboration/utilization of biodegradable materials, would be adequate measures to reduce the impacts of plastics.
Even with the area of studies on the impacts of plastics on fauna and for the various organisms still under development, some studies point to the occurrence of toxicological effects of this synthetic waste [23,24]. A plastic intake and entanglement can lead to the lower life quality of organisms, loss of mobility, external and internal injuries, blockage of digestion, and other harms [25]. Goldstein and Goodwin [22] identified the presence of microplastics (mainly PE, PS, and PP) in the digestive tract of 33.5% of Gooseneck crustaceans (Lepas spp.).
The development of innovative technologies represents a means for both sustainable development and the growth of emerging countries, such as Brazil, whose sustainable energy has been highlighted by innovation technologies. Thus, even with bioplastic not representing a material for total replacement of non-biodegradable plastic, researches and production of bioplastics are a technological alternative for the development of a more sustainable and balanced society. Therefore, aligning with the current trend (socio-political and environmental), in which concerns with the environment is growing.
3. Biodegradation Process
The microbial biodegradation of materials occurs by the action of microorganisms, such as fungi and bacteria [26], and is classified as physical, chemical, and enzymatic according to modifications in the materials. Biodegradation is a natural process of vital importance for nutrients and energy recycling [27]. Microorganisms use organic material as a source of nutrition for their metabolism; except for the substances used in metabolic incorporation, the rest is oxidized by cellular respiration, thus leading to the formation of simple and small submetabolites, released in the environment [28,29].
Biodegradation due to physical degradation occurs from the adhesion of microorganism species to the surface of organic materials through the secretion of a gum [30] produced by microorganisms. This gum represents a complex matrix made of natural polymers (e.g., polysaccharides and proteins). Such a thick complex, together with microorganisms, infiltrates the material and changes its volume, size, pores distribution, moisture content, and thermal transfers. A few microorganisms (e.g., filamentous fungi) lead to cracks in the materials due to mycelial growth, i.e., both their durability and resistance properties are reduced [13,31]. Microorganism biofilms are a matrix that protects microorganisms from different environmental conditions and results in a major change in materials [13,32].
Biodegradation by chemical degradation refers to the production of chemical substances by living organisms, which facilitate and increase the speed of the process. Emulsifying substances produced by microorganisms help the exchange between hydrophobic and hydrophilic phases, which are important interactions for the penetration of microorganisms in the polymeric material [33]. Such a lime formation (polymers secreted by microorganisms mixed with different microbial species) improves the material deterioration. It represents a point of accumulation of polluting and chemical substances (abiotic chemical degradation), thus benefitting microbial proliferation [34].
Examples of chemical substances released into the environment by microorganisms, which play an important role in chemical biodegradation, are nitrous acid, nitric acid, and sulfuric acid. All of these compounds are produced by chemolithotrophic bacteria, such as Nitrosomonas spp., Nitrobacter spp., and Thiobacillus spp., respectively [29,33,35]. Apart from the action of chemical substances generated by those organisms, chemoorganotrophic microorganisms generate organic acids with potential for chemical degradation (e.g., oxalic, citric, gluconic, glutaric, glyoxalic, oxaloacetic, and fumaric acids).
The action mechanisms of such acids (organic or inorganic) are diverse and include an increase in surface erosion when adhering to the material surface [36]. The use of those acids as nutrients benefits the growth of filamentous fungi and bacteria [13]. Another action mechanism of biotic chemical degradation is the oxidation of organic material. Certain fungi and bacteria have specific proteins in their membrane that capture iron-chelating compounds (siderophores) [37]. With this mechanism microorganisms capture cations from a matrix.
Biodegradation by enzymatic degradation occurs due to the depolymerization of polymeric chains of a matrix through the action of hydrolase enzymes that catalyze the reactions of chemical bonds breakage adding a water molecule. These bonds are ether, peptide-like, and ester, present in biodegradable bioplastics. The main enzymes are amylases and cellulases, which cleave starch and cellulose polymers, respectively. However, other enzymes (breakage of ester bonds), such as esterases and lipases, can degrade co-polyesters.
A mechanism that explains the action of hydrolases (e.g., depolymerase) in polyesters hydrolysis (synthetic and natural) through biodegradation is related to three amino acids, namely serine, histidine, and aspartate. A hydrogen bond is formed when a component reacts with the histidine ring, thus guiding interaction between histidine and serine, and forming an alcohol group of high nucleophilic character (-O). Histidine plays a deprotonating role for serine, i.e., as a base. The alkoxide group includes an ester bond and generates an acyl-enzyme and an alcohol group. Finally, a free enzyme and a terminal carboxyl group are generated by the action of the water molecule under an acyl-enzyme. This entire enzymatic degradation process is termed catalytic triad [30,38,39], and the products generated are metabolized or not by microorganisms that have depolymerizing enzymes. Therefore, a consortium of microorganisms is important for complete biodegradation [13]. Figure 1 depicts the mechanism of action of depolymerizes and the catalytic triad.
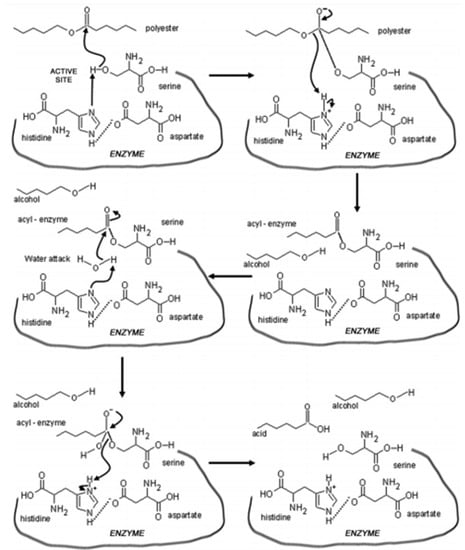
Figure 1.
Enzymatic hydrolysis of polymers and catalytic site of depolymerase enzymes [13].
Apart from the biodegradation of cellulose, starch, and polyesters, hemicellulose is another polymer that can be degraded by microbial enzymes. A catalytic action of hemicellulases (enzymatic pool) on different types of hemicellulose polysaccharides produces monomeric sugars, acetic acids [40]. For example, enzymes that degrade xylan (hemicellulose from grasses) are endo-1,4-β-xylanase (cleavage results in oligosaccharides), xylan 1,4-β-xylosidase (cleavage of oligosaccharides generate by xylan, which forms xylose monomers), and accessory enzymes, such as xylan-esterases, ferulic and p-coumaric-esterases, α-l-arabinofuranosidases, and α-4-O-methyl glucuronidase [41]. Both enzymes act synergistically so that xylans and hemicellulose mannans of some types of plant cell walls are depolymerized [42]. Nevertheless, some polymers are not biodegraded by common enzymatic hydrolysis, i.e., polymers can be oxidized by enzymes such as laccase, dioxygenase, peroxides, monooxygenase, and oxidases [43]. Thus, such enzymes are not hydrolases and influence the cleavage process of polymers differently from hydrolases (oxygen insertion, hydroxylation, oxidation, and free radical formation lead to polymer cleavage) [43]. Figure 2 depicts the enzymatic biodegradation process.
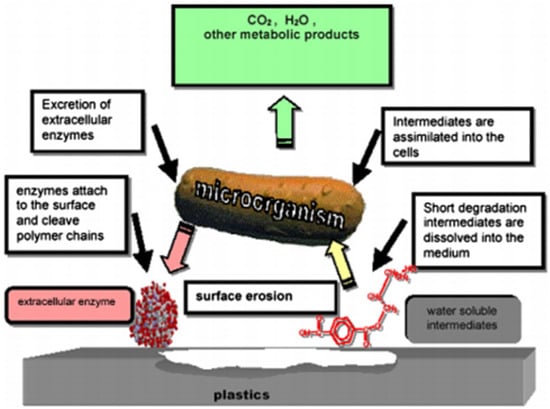
Figure 2.
Enzymatic biodegradation process [44].
The result of biodegradation, for example of bioplastic from natural polymers (e.g., polysaccharides) is the generation of small molecules from a polymer. Microorganisms cannot employ large substances insoluble in water for obtaining organic or inorganic nutrients for their metabolism. They produce enzymes and chemicals used in extracellular environments and, therefore, depolymerize the materials. After hydrolysis and/or oxidative action of microorganism enzymes on different polymers, which results in monomers, metabolism oxidation occurs. In this system, organic compounds lead to a loss of electrons and the consequent production of ATP molecule (adenosine triphosphate). This is the last biodegradation stage, in which organic matter is mineralized. The microorganisms use smaller and simple organic molecules, such as oligomers and monomers, for their metabolic activities. However, byproducts are generated from microbial metabolism (e.g., carbon dioxide—aerobic degradation), water, biomass, methane, and hydrogen sulfide (anaerobic degradation) [45,46]. Figure 3 displays the biotic and abiotic degradation of plastic.
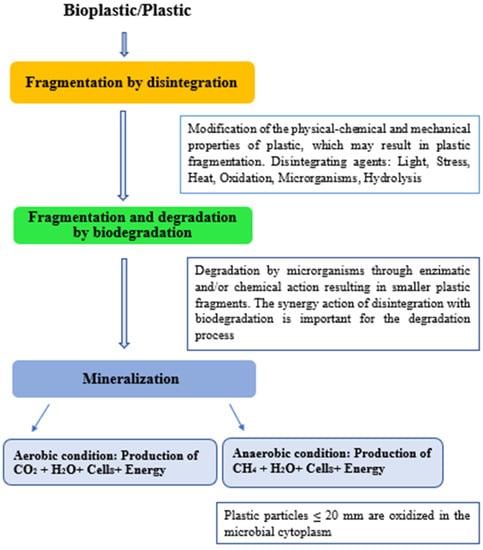
Figure 3.
Disintegration, biodegradation, and mineralization process of plastic polymeric materials. Adapted [47].
3.1. Factors That Influence Biodegradation
The microbial population available is a key factor for biodegradation in an environment (soil, air, and water), and several properties (e.g., the chemical constitution of materials) affects the efficiency of the biodegradation process. Chemical composition influences the biodegradation of plastics through different patterns of crystallinity, hydrophilic and hydrophobic character, conformational flexibility, polymer accessibility, surface area, molecular weight, melting temperature, hydrolyzable and oxidizable bonds in polymer chains, morphology, and stereoconfiguration [27,48,49].
Crystallinity influences biodegradability because it affects the accessibility of the enzyme to the material polymer. More organized regions of polymers (crystalline) tend to hinder enzymatic hydrolysis since catalytic proteins diffuse with greater difficulty. On the other hand, water molecules diffuse more easily between amorphous (less organized) regions, and enzymes can easily access the material polymers in such regions [28].
The polarity of bioplastics directly influences biodegradation, since materials developed with hydrophobic polymers are less susceptible to enzymatic attack. Degrading microorganisms depend on a hydrophilic surface to adhere to and catalyze the depolymerization reaction by means of hydrolytic enzymes. However, this enzymatic accessibility to the material is reduced on hydrophobic polymeric surfaces. This impediment occurs not only because the microorganisms and enzymes are more hydrophilic, but also due to the aqueous medium (usual water), in which the enzyme is contained, to have their contact with the material (bioplastic) reduced. For example, glycolic polyacids (PGA) are more easily biodegraded than poly (lactic acids) (PLA), since PLA is more hydrophobic) [50].
Blends in a polymeric bioplastic matrix are common when it is desired to obtain materials with certain characteristics, and also interfere with biodegradation (increase or reduce biodegradation), since the different components of biocomposites can influence the accessibility of the enzyme to the polymeric material in different ways.
The molecular weight of polymers affects the biodegradability of plastics, since the heavier the molecular weight, the greater the difficulty for microorganisms to break it down and assimilate. Therefore, the lower the molecular weight of the polymer, the easier the biodegradation, since the need for extracorporeal digestion is reduced. Aliphatic polyester is one of the few biodegradable polymers of high molecular weight [13]. However, it is worth mentioning that in addition to the molecular weight, the types of bonds in the polymeric chain (considering that bioplastic, like plastic, is formed by a polymeric matrix), and different chemical groups in polymers influence the biodegradation process.
Although the term “bio” degradation is directly correlated with the fragmentation of a polymer by the action of microorganisms, these microorganisms do not act in isolation on the polymeric material, since abiotic agents influence the fragmentation efficiency. The abiotic degradation of organic matter such as thermal, mechanical, chemical, and by the action of light are examples of degradative processes. These processes work synergistically with biodegradation, reducing the material to dimensions that allow microbial assimilation [13,51].
3.2. Assessment and Biodegradation Quantification
Biodegradation can be measured through metabolic products, physical and chemical properties of plastics/bioplastic, acidification of the medium, and other ways. CO2 is a product of biodegradation, more specifically, of the oxidation of organic matter, and can be used for direct or indirect measurement of material biodegradation over a period of time. Its content released in a degradation process is quantified by the respirometry technique, which can use a closed CO2 production and a capture system. International methods, such as ASTM D5338-15 [52] and ISO 14855-2: 2018 [53] are applied for the quantification of the CO2 produced in a microbial degradation process.
The measurement of consumed oxygen (ISO 17556: 2003) [54] is another method of quantifying biodegradation by respirometry. Respirometry involves techniques that measure parameters indicative of cellular respiration. The higher the consumption of oxygen and the release of CO2 by microorganisms, the better the biodegradation indicator. For details and examples of other standard methods of respirometry analysis (ASTM, EN, and ISO), see specialized literature [55].
Methane molecules can also be used for measurements of materials biodegradation. However, unlike the above-mentioned respirometry techniques, CH4, CO2, and other gases quantification is generally conducted under anaerobic conditions. Analysis methods such as ASTM D5511-02 [56], is used for this purpose.
Apart from microbial proliferation in plastic/bioplastics materials, analyses of color change, surface roughness, cracks, and holes are also alternatives for checking the deterioration of materials [13,36]. Analysis parameters can be used especially for materials of difficult biodegradation and low CO2 release. However, the results of such analyses (e.g., microbial growth in the polymeric matrix) are not recommended for the conclusion of biodegradation or abiotic degradation directly [13]. Additional techniques, such as electron microscopy, photon microscopy, microscopy of polarization, and atomic force microscopy reinforce the results [13,57,58].
The physical properties of plastics/bioplastics (e.g., tensile strength, elongation at break, modulus of elasticity, crystallinity, cold crystallization temperature, and glass transition temperature) can be measured as biodegradation indicators. The weight loss of a sample determined by the burial method can be used in plastic/bioplastic biodegradation analyses, although it may result from the solubility and volatility of certain substances [13]. The analysis of weight loss of bioplastics by burying in soil, or composting systems, may result in conclusion errors, since in addition to the mass of the soil or compost account for the variation in the bioplastic mass, in bioplastics washing processes (a step which precedes weighing procedures), can cause fragmentation and loss of material derived from bioplastic. Thus, even though the method of analyzing mass loss is frequently reported in the literature, as is usual in determining the biodegradation of bioplastics, this technique ends up being difficult to perform [59]. Recent articles evaluating the biodegradation of bioplastics by burying in soil and compost has used image evaluation as a tool for analysis, that is, the reduction of the area of bioplastics, detected by image registration (from the insertion of the bioplastic in a mold/grid with known dimensions) [60,61].
The indication of biodegradation through products generated by microorganisms is another way of measuring the process. For example, the biodegradation of polymeric cellulose materials can be measured according to the release of glucose [62], or the quantification of 1,4-butanediol as an indicator of the biodegradation of PBA and PBS polymers [63].
The increase in microbial biomass (weight or number of cells) is indicative of a biodegradation process since a single source of carbon (plastic or bioplastic material) in a closed environment can point out the occurrence of biodegradation and/or surface changes and molecular rearrangements. However, conclusive statements about the amount of mineralized material cannot be directly made.
The evaluation and quantification of bioplastic and/or plastic biodegradation by the above-mentioned methods can be conducted in an aqueous medium and soil. However, each condition of analysis imposes different requirements, which leads to different responses from different methods.
3.3. Biodegradation of Bio-Based Polymers Bioplastics
In this topic, biodegradation of bioplastics developed with polysaccharides from plant biomass/lignocellulose and microbial polyesters was followed as the scope of this review. It was exemplified the biodegradation of a category of bioplastics, those developed with natural polymers (vegetable and microbial). Therefore, this review does not intend to address issues related to the development of bioplastics of vegetable and microbial origin, advantages and disadvantages in addition to the viability of this material (related to the economic aspects and properties of bioplastics). To obtain this information, it is recommended reading of the specialized literature [6,64,65,66].
3.3.1. Biodegradation of Plant-Based Polymers Bioplastics
The mass loss of bioplastics from rice straw showed complete degradation after 105 days [67]. Rice straw bioplastics were composed mainly of cellulose and trifluoroacetic acid. On the first day of contact with the soil, the bioplastic showed an increase in mass, due to the phenomenon of water absorption by the material. According to the authors, its mechanical properties are similar to those of polystyrene (bioplastic in the dry state).
The mass loss of bioplastics consisting of acetylated starch and acetylated sugarcane fibers (lignin, hemicellulose, and cellulose) resulted in 24.2 to 39.3% degradation after 5 weeks [68]. The acetyl group may have created stable biodegradable sites; however, an increasing effect on the crystallinity of the bioplastic with the addition of cellulose may have contributed to the low biodegradation rate due to the restriction effect of the microbial enzyme’s activity. In addition to the crystallinity and chemical structure of cellulose, microbial diversity, carbon availability and the period of biodegradation considered can influence its depolymerization.
Bioplastics (glycerol, acetylated starch, and acetylated nanocellulose composition) subjected to biodegradation in a petri dish with Trametes versicolor were completely degraded in 60 days, and after 40 days with starch and non-acetylated reinforcement. The starch bioplastics were completely biodegraded after 30 days, and the addition of cellulose to the formulation of bio-based plastics resulted in a longer biodegradation time [69]. Water and moisture absorption is important in the biodegradation process of bioplastics [70]. The starch-based bioplastics investigated in this study were composed of different concentrations of oxidation starch (20, 40, and 60%). Oxidation decreases biodegradation due to reduced swelling and water absorption from the soil by bioplastic.
Hemicellulose is another natural plant-derived polymer of potential application for the development of bioplastics. However, in addition to the elaboration that biomaterial, the study of the biodegradation of these carbohydrates in bioplastics have not received attention, as the area of use of hemicellulose for bioplastic focus on physicochemical properties and modifications of this macromolecule. The bioplastic based on xylan (of the hemicellulose type of grasses) and blended with gelatine was completely biodegraded after 15 days of conditioning (determined by the burial procedure) [71]. This bioplastic was considered 100% biodegradable since the sample could not be recovered for weighing. A bioplastic made with 50% xylan (from beechwood) and PVA (polyvinyl alcohol) was 56% biodegraded after 30 days by burial in soil [72]. PVA reduced the biodegradation of the bioplastic produced by the PVA/xylan mixture. The sample with 25% xylan was 42.2% biodegraded after 30 days of burial in soil.
Xylan was grafted with poly-(ε-caprolactone) (PCL), and biodegradation was evaluated by BOD (biological oxygen demand). The biodegradation (aerobic and activated sludge) kinetics of bioplastics with high concentrations of PCL was delayed in comparison to materials made with pure hemicellulose or with lower graft concentrations [66]. Despite changes in the kinetics, the biodegradation property of the bioplastic was not altered and ranged between 95.3 and 99.7%.
Recalcitrant substances also influence the biodegradation of natural polymers. Lignin is a constituent of lignocellulosic fibers, shows the highest degree of recalcitrance in the plant cell wall [73,74]. This polymeric complex of phenylpropane units hinders the biodegradation of the material or products that contain it, such as bioplastics, and reduces the contact surface of lignocellulosic fibers with degrading enzymes [74]. Lignin requires different enzymes to degrade due to the different units that comprise its polymeric complex [75]. In anaerobic environments lignin may persist biodegradation for a longer time, with this process is primarily more efficient in aerobic environments [76], due to the catalytic action involved in oxygen.
Starch and lignin (lignosulfonate) bioplastics were completely biodegraded after 4-month burial [77]. Biodegradation was measured through the analysis of CO2 and morphological characteristics. The samples with lignin analyzed after 5 weeks of biodegradation tests were fragmented, however, small residues of the bioplastic were identified. After the 2-month burial, the samples with lignin showed a significant biodegradation effect, with small fragments of the material still observed. After 4 months of testing, residues of bioplastic fragments were no longer detected. A bioplastic made from the addition of lignin (1.2% w/t) to the bio-PTT matrix (Bio-poly (trimethylene terephthalate)) increased its weight loss through biodegradation in soil [78]. In 140-day burial, bio-PTT/lignin bioplastic showed more than 50% mass weight loss.
A higher CO2 emission was reported from films with lignin in comparison to the bioplastic composed only of starch, due to the greater amount of carbon atoms in its formulation [77]. However, such CO2 may have originated from the metabolism of soil organic compounds, i.e., the bioplastic may have stimulated the microbial degradation of stable organic compounds in the soil through the priming effect. A strategy for the biodegradation of bioplastics composed of lignin, due to the recalcitrance of this phenolic complex, is the application of UV radiation prior to chemical, microbiological and/or enzymatic treatments. Lignin is susceptible to photodegradation due to the UV effect [79]. After photodegradation, other treatment combinations can be applied for the degradation or biodegradation of lignocellulosic fibers, such as enzymatic or oxidative treatments. One of the advantages of using lignin in the development of thermoplastic formulations is its processing at high temperatures [80]. However, studies using lignin in the bioplastic formulation, have not received much attention.
3.3.2. Biodegradation/Enzymatic Degradation of Plant-Based Polymers Bioplastics in Relation to Derivatization
The assessment of biodegradation, disintegration, and enzymatic degradation of bioplastics made with natural polymers (such as proteins, starch, cellulose, and hemicellulose) is not recurrent in the literature. Biodegradation has received lower attention when compared to the objective of most studies, which is to evaluate the physicochemical and mechanical properties of the materials. However, this limitation in the studies is even greater when compared to the biodegradation of bioplastics made with modified polymers.
The comparison between bioplastics developed from unmodified and modified hemicellulose presents few studies intending to analyze the enzymatic degradation [81], and biodegradation. This low number of studies with hemicellulose could be related to the difficulties in obtaining a plastic polymer matrix from this heterogeneous vegetable polysaccharide. However, in addition to the analysis of the physicochemical properties of modified bioplastics, the effects of chemical, physical, and biological (and enzymatic) modifications of polymers on biodegradation must be considered. The enzymes involved in the enzymatic degradation of unmodified and modified polysaccharides may be different. Moreover, a more complex enzymatic pool will be required for modified polysaccharides.
As pending groups are attached to the polysaccharides chain, new enzymes will be required for further hydrolysis. According to a recent review article, physical modifications of polysaccharides hardly result in a change in the biodegradation process [82]. However, chemical changes result in different degradation mechanisms. Considering a chemical similarity, the enzymatic degradation of cellulose acetate can be catalyzed by acetyl esterases, an enzyme common for xylan deacetylation. The modification or functionalization of polysaccharides may result in a reduction in biodegradation since modified bioplastics (acetylated cellulose, acetylated xylan, acetylated starch, starch propionate, starch butyrate, starch valerate, and starch hexanoate) showed a reduction in anaerobic biodegradation [83]. For example, the degree substitution (DS) > 1.5, 1.5, 1.2 for starch, cellulose, and modified xylan (acetylated) respectively, represented the minimum modification necessary to delay the biodegradation of bioplastics.
The chemical modifications of the polysaccharides that make up bioplastics, such as acetylation, increase the degree of hydrophobicity of the polymers and the plastic matrix. This has the advantage of reducing the solubilization of the polymers in polar solutions. However, resulted in a decrease the enzymatic degradation. It was observed a reduction in two mannases of Cellvibrio japonicus (CjMan5A and CjMan26A), with reduced catalytic activities on galactoglucomannan substrates (hemicellulose) due to the decrease of the solubility of the polymers [81]. Other studies in the literature showed the influence of chemical modification of hemicellulose in relation to solubility, thermal resistance, crystallinity [84], and biodegradation rate [85]. Therefore, the diffusion of water by the composite and biodegradation is a parameter affected by chemical derivatization.
Modified xylans with an increase in the DS reduced enzymatic degradation by xylanolitic enzyme [86]. However, a rapid biodegradation rate (80%) on the first day of the evaluation was achieved for (hydroxypropyl)xylan. Substitutions above 1.5 reduced enzymatic degradability by 10%. However, the modification of cellulose with hydroxypropyl led to a reduction in biodegradation (20% in 18 days). Regarding the DS and the enzymatic activity, the article justifies the limitation of the recognition of the xylanolitic enzyme to the substrate due to chemical modification. In addition to the sterile impediment, when it changes the polysaccharide polarity through modification, it may be another explanation for the degradability reduction [87].
Modifications of polysaccharides may result in a less hydrophobic bioplastic, favoring the process of biological and abiotic degradation. Xylan carboxymethylation for bioplastic production showed an increase in water absorption at high relative humidity, demonstrating, therefore, the hydrophilic character of the carboxymethyl groups [88]. Carboxymethylation is a procedure for the production of hemicellulose-based bioplastics with increases in hydrophilic characteristics [89]. This procedure results in the development of environmentally favorable materials considering biodegradation.
A modification of hemicellulose by subtraction of chemical constituents may result in a different biodegradation process. An enzymatic modification of arabinoxylan resulted in an increase in the bioplastic crystallinity as the arabinose content was reduced [90,91]. In both of these studies, the effects of enzymatic modification of hemicellulose in relation to biodegradation were not evaluated. However, the increase in crystallinity may be a retarding factor in the bioplastic biodegradation due to the degree of organization of the molecules limiting enzymatic action, probably reducing the water absorption effect and reducing microbial growth.
The different modifications in natural bio-based polymers (for example, polysaccharides) may result in a difficulty in biodegradation or enzymatic degradation. The rate of degradation of these materials can reduce in a given period. However, the material can still be metabolized or degraded using enzymes. For example, acetylated xylan is the form found in natural lignocellulosic materials, therefore, although acetyl groups result in a delay in biodegradation, these polysaccharides are biodegradable by microbial enzymes, such as xylanases and esterases, whereas the acetylated xylan form is predominant in the environment.
3.3.3. Biodegradation of Microbe-Based Polymers Bioplastics
Under the nutritional abundance of carbon and nitrogen, some bacteria can synthesize energy reserve polymer (inclusions). Polymers like polyhydroxyalkanoate (PHA) (intracellular granules), can be produced via microbial fermentation of biomass (animal or vegetable). Regarding applications, these natural polymers are an important alternative for the manufacture of bioplastic materials since they are biodegradable and biocompatible, and used in the medical field [92]. With the 41% increase in world production of PHAs between 2010–2017, this polyester has become a polymer of significant interest in the development of bioplastics. The properties of this microbial polyester can contribute to a reduction of environmental impacts due to the closed carbon cycle generated by biodegradation [93].
There is a growing interest in the development of materials formulated with PHAs, the study of the biodegradability of these materials. However, factors that influence the degradation of composites and bioplastics are necessary. Some of the marine microorganisms that are known to degrade PHAs [94] are Aestuariibacter halophilus S23; Alcanivorax sp. 24; Alcanivorax dieselolei B-5; Pseudoalteromonas haloplanktis; Alteromonas sp. MH53; Bacillus sp.; Bacillus sp. strain NRRL B-14911; Bacillus sp. MH10; Comamonas testosteroni YM1004; Enterobacter sp.; Aliiglaciecola lipolytica; Gracilibacillus sp.; Marinobacter sp. NK-1; Nocardiopsis aegyptia; Pseudoalteromonas sp. NRRL B-30083; Pseudoalteromonas gelatinilytica NH153; Pseudoalteromonas shioyasakiensis S35; Pseudomonas stutzeri YM1006; Psychrobacillus sp. PL87; Rheinheimera sp. PL100; Shewanella sp. JKCM-AJ-6,1α; Streptomyces sp. SNG9. Terrestrial microbial representatives degraders PHAs [95] are Alcaligenes faecalis; Pseudomonas lemoignei; Acientobacter sp.; Acientobacter schindleri; Bacillus sp.; Pseudomonas sp.; Stenotrophomonas maltophilia; Variovorax paradoxus; Stenotrophomonas rhizophilia; Penicillium sp.; Purpureocillium lilacinum; Verticillium lateritium; Burkholderia sp.; Nocardiopsis sp.; Streptomyces sp.; Bacillus cereus; Burkholderia sp.; Cupriavidus sp.; Gongronella butleri; Penicillium oxalicum.
As in polysaccharide-based bioplastics, crystallinity in polyester bioplastics from microbial synthesis plays an important role in the biodegradation process. In bioplastics with higher proportions of amorphous regions, depolymerization occurs more quickly through abiotic or biotic action. For example, higher biodegradation was obtained with hydroxybutyrate (PHB), hydroxybutyrate-co-hydroxyvalerate (PHBV-40), PHBV-20, and P (3HB, 4HB) (10% mol of 4HB) and PHBV-3 [96]. According to the quantification of CO2 in a composting vessel, PHBV-40 and P (3HB, 4HB) (10% mol 4HB) showed the highest degrees of biodegradation, due to a reduction in crystallinity with the addition of higher percentages of HV (valerate hydroxide—indicated by the numbering in front of the acronym) and 4HB. Biodegradation was 90.5%, 89.3%, 80.2%, 90.3% and 79.7% in 110 days of analysis for bioplastics formulated by PHBV-40, PHBV-20, PHBV-3, P (3HB, 4HB) and PHB, respectively.
The advantage of using PHBV in comparison to PHB is the ease of processing and good toughness. Certain PHBV disadvantages such as low thermal stability and a high degree of crystallinity must be overcome [96]. Improvements, mediated by chemical changes, must be performed together with the preservation of the material’s biodegradation property, which, depending on the HV percentage, maybe rigidity or flexibility, similarly to commercial synthetic plastics (polyethylene, polypropylene, and polyvinylchloride), and assurance of biodegradation of the formulated bioplastic [96].
A commercial Ecoflex bioplastic (commercial product of BASF) was compared to PHB and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) in activated sludge for 18 days. Bioplastics composed of PHBHHx showed a higher degree of biodegradability than Ecoflex and PHB, with weight losses of 40, 20, and 5%, respectively [97]. The low crystallinity and morphology of the surface of the bioplastic proved a determining factor in the biodegradation process, observed mainly in bioplastics with 12% HHx (hydroxyhexanoate), which displayed a rough and porous surface before and after undergoing activated exposure to sludge and lipases (Figure 4).
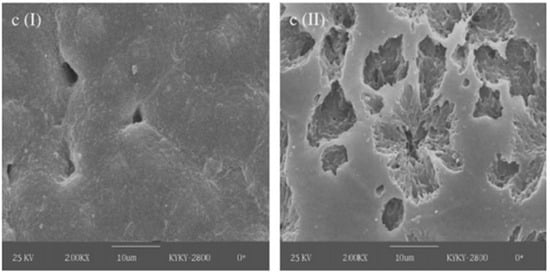
Figure 4.
Surface morphology of bioplastic made with PHB (12% HHx) before (left) and after degradation (right) [97].
Besides surface morphology and crystallinity of the bioplastics, other factors, such as mixing components, depth of burial (due to environmental and/or microbial differences), and time of exposure to the soil also determine the biodegradability degree. In the study performed by Weng et al. [98], evaluating through appearance and fragmentation, the following results were achieved for the biodegradation of polymeric blends (poly (3-hydroxybutyrate-co-4-hydroxybutyrate and poly (lactic acid)—(P (3HB, 4HB)/PLA)): In the first month of testing, blends composed of 100% P (3HB, 4HB) and those with 25% PLA showed loss of integrity (appearance), whereas in the second month, both bioplastics had been almost completely biodegraded. This behavior was similar for the different depths of burial used (20 and 40 cm); however, at 20 cm and 2 months of testing, a greater difficulty was observed in the collection of fragments of blends with 75% of P (3HB, 4HB). For both depths of burial, the higher the concentration of PLA in the blends, the longer the biodegradation time. However, the biodegradation behavior was the opposite for higher concentrations of P (3HB, 4HB).
Polymer blends with 100% and 75% P (3HB, 4HB) were degraded more easily at 20 cm depth, although the presence of PLA in the bioplastics represented a delay in biodegradability at both depths tested. At 40 cm, PLA suffered greater disintegration, due to the anaerobic conditions, providing better conditions for degradation of the PLA, as reported by the authors [98].
In addition to temperature, bioplastic composition, crystallinity, degree of hydrophilicity and environmental conditions in relation to oxygen concentration, another factor that must be taken into account is the abundance of microbial biomass and the efficiency of fungi and bacteria biodegradation in different environments. The biodegradation of microbial polyesters by fungi was reported as dominant in soil [95]. However, the biodegradation of polymers in aquatic (marine) medium was faster with the use of bacteria [99,100].
PHA bioplastics as well as other bioplastics have limitations in their applications and achievements due to the high cost, low mechanical resistance, and impairment of biodegradation in functionalization processes and mixtures with other polymers [93]. In addition to the low ductility property, one of the main disadvantages of using PHAs in the production of bioplastics is the formation of brittle bioplastics, properties that can be improved by mixing biodegradable polymers from oil. However, the sustainability of bioplastic manufacturing is affected since the use of oil in the extraction and refinement stage generates the carbon dioxide production [101]. Another impact that should be considered in the production of PHA bioplastics with synthetic polymers such as the use of PCL is the reduction in the rate of biodegradation [102]. However, the development of polyester bioplastics with bio-based fibers origin (blends development), such as lignocellulosic fibers, can assist in overcoming the low ductility property of bioplastics [93]. This blend reduces costs, ensuring a biodegradable and renewable product.
The application of polyhydroxyalkanoate as a bioplastic has major limitations (e.g., its production costs for replacing conventional plastics [103]). A potential alternative for the optimization of PHA production technologies is the use of organic residues, such as lignocellulosics [104]. As an example, hemicellulose [105,106,107], cellulose [108] and a mixture of hemicellulose and cellulose hydrolyzate [109] have been used for the production of microbial polyesters, such as PHA and P3HB (PHB). However, the use of lignocellulosic fibers poses limitations mainly related to the production yield and generation of inhibitory substances for PHA-producing microorganisms [104]. Table 1 shows some properties and biodegradation times of different biodegradable bioplastics produced from biopolymers.
Poly(lactic acid) (PLA) is another polyester that may be partially derived from the microbial fermentation of biopolymers. The lactic acid produced by the bacteria is polymerized by a chemical route, thus forming PLA, which offers several advantages, such as rigidity and miscibility with other biodegradable plastics. However, in several application areas (e.g., manufacture of 3D printers), its fibers have been used by Brazilian companies due to the PLA lower heat loss in comparison to oil-derived plastics [110]. Nevertheless, bioplastics from bacterial polyesters should be considered, since PHAs like PBH present several advantages in comparison to PLA [111]. Table 1 shows some properties and biodegradation times of different biodegradable bioplastics produced from biopolymers.

Table 1.
Properties and biodegradation time of different biodegradable bioplastics produced from biopolymers.
Table 1.
Properties and biodegradation time of different biodegradable bioplastics produced from biopolymers.
| Bioplastic Type | Polymer Type | CONB | BPR | PAB | TS (MPa) | E (%) | Reference |
|---|---|---|---|---|---|---|---|
| PHB nanofiber | Polyester | Soil, 30 °C and 80% humidity | 100% in 21 days | Weight loss | … | … | [112] |
| PHB/Starch | Polyester | Sludge, 35 °C, anaerobic | 93.8% in 190 days | Biogas quantification | … | … | [113] |
| PHB | Polyester | Compost, 55 °C and 70% humidity | Approx. 80% in 28 days | CO2 quantification | 1015 | … | [114] |
| PHA | Polyester | Seawater | 100% in 1.5 to 3.5 years | Literature review | … | … | [115] |
| PHA | Polyester | Soil, 12–15 cm depth and 35% humidity | 30% in 60 days | Weight loss | 16.2 | 600.3 | [116] |
| PHA/Rice husk | Polyester/Fibers | Soil, of 12–15 cm depth and 35% humidity | >90% in 60 days | Weight loss | 7.5 | <400 | [116] |
| PHA | Polyester | Soil, 20 °C and 60% humidity | 48.5% in 280 days | CO2 quantification | … | … | [117] |
| PHBV/Starch | Polyester/Polysaccharide | Liquid medium | 100% in 31 days | CO2 quantification | 21.01 | 10.85 | [118] |
| PHBV/NPK | Polyester/Fertilizer | Soil, 25–30 °C and 65% humidity | 68.66% in 112 days | Weight loss | … | … | [119] |
| PHBV/Starch | Polyester/ Polysaccharide | Soil, 25 °C and 20% humidity | >60% in 150 days | Weight loss | ~7 | ~3.2 | [120] |
| Starch | Polysaccharide | Soil, 3.5 cm depth and 65% humidity | 30% in 5 days | Weight loss | 1.88 | 0.45 | [70] |
| Starch | Polysaccharide | Marine water with sediment | Approx. 69% in 236 days | BOD | 4.7 * | 211 | [121] |
| Starch | Polysaccharide | Microorganisms in a plate, 25 °C and 75% humidity | 100% in 30 days | Weight loss | 8.6 | 52 | [69] |
| Starch/Cellulose | Polysaccharide/Modified | Microorganisms in plate, 25 °C and 75% humidity | 100% in 60 days | Weight loss | 14.7 | 50 | [69] |
| Cellulose | Polysaccharide | Soil | 100% in 105 days | Weight loss | 45 | 6.1 | [68] |
| Cellulose/Starch | Polysaccharide | Soil/ humus, 25 °C and 75% humidity | 24.2 to 39.3% in 35 days | Weight loss | 5.6 to 35.0 | 13.1 to 21.7 | [69] |
| Hemicellulose/ Gelatine | Polysaccharide/Partially hydrolyzed protein | Soil/manure | 100% in less than 15 days | Weight loss | … | … | [71] |
| PLA/Starch | Poly(lactic acid)/ Polysaccharide | Compost and 58 °C | 79.7% in 90 days | Weight loss | … | … | [122] |
CONB = Biodegradation conditions; BPR = Biodegradation period and rate; PAB = Biodegradation analysis procedure; TS = Tensile strength; E = elongation; … = not reported; * = Newton (N).
Bioplastics are renewable and/or biodegradable and display good mechanical properties such as tensile strength similar to certain synthetic plastics in common use (Table 1). Polypropylene and polystyrene, fossil-based synthetic plastics, show TS between 25–40 MPa and 30–55 MPa, respectively [68], whereas it ranges between 55–124 and 9–17 for CellophaneTM and low-density polyethylene (LDPE), respectively [123,124]. This similarity of mechanical properties of bioplastics and plastics was also reported by Hansen et al. [125]. Therefore, even if at present and in the future, the total replacement of non-biodegradable plastic from petroleum, is something unlikely, for some applications, such as bioplastics for use in agriculture (mulch) and packaging (short lifetime), this technology can represent one of the alternatives (along with other actions and technologies) to mitigate the environmental impacts related to plastics.
3.4. Effect of the Bio-Based Polymer Addition on the Biodegradation Rate of PHAs Bioplastics
The addition of bio-based polymers in polyester microbial biocomposites is vast in the literature [93,126,127]. The bio-based polymers application, mainly lignocellulosic fibers, is due to the improvement in the biodegradation rate of the formulated bioplastic [93]. This improvement in the biodegradation of PHAs biocomposite is related to the increase in hydrophilicity and water absorption by bioplastics. A mixture of 30% Sisal fibers (wt) and PHBV resulted in increased water absorption of 14% compared to pure PHBV (0.8%) [128]. The use of Kenaf fibers (main cellulose) in the blend with poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HHx)], resulted in a greater loss of mass in biodegradation in soil due to greater water absorption and microbial binding sites in the microbial polyester from binding with Kenaf fiber [129]. This study suggested that the accelerated deterioration of the blend (reduction of mechanical properties), after 6 burial weeks (soil) was due to the weakening of the adhesion between the fiber/[P(3HB-co-3HHx )], with the access of water to the internal hydrophobic regions of the polymer.
The use of hemicellulose with PHAs is also an alternative to increase the blends biodegradation rate in relation to pure microbial polyesters [93]. Bioplastics from PHBV/Peach Palm Particles (lignocellulosic fiber with considerable hemicellulose content) were biodegraded faster than pure PHBV in soil [130]. The authors reported cracks, corrosion, and discoloration after 2 months of biodegradation. The poor adhesion between the fiber/PHBV interface, which resulted in greater water absorption and accessibility of soil microorganisms, was suggested as contributed to the deterioration.
Starch, another polysaccharide from vegetable biomass, can also be used in the production of bioplastics with reduced biodegradation time. The mixture of starch and PHBV (50/50% wt) was fully biodegraded in the soil after 33 days, i.e., there was a 50% reduction in biodegradation time compared to pure PHBV [131]. The addition of starch reduced the crystallinity of the blend, facilitating the absorption of water by the matrix and increased the enzymatic activity on the surface and in the inner region of the blend. There was an increase in the biodegradation of PHA/starch blends as the starch content increased in the formulation, with biodegradation of PHA/30% starch (wt) corresponding to 44% in 6 months of burial in soil [132].
Chemical modifications of polysaccharides, such as cellulose acetylation (cellulose acetate) can result in partial or total inhibition of blends biodegradation, due to reduced solubilization and hydrophobicity of the fibers. The acetyl and butyryl group in cellulose reduced the rate of biodegradation of the PHB blend/modified cellulose due to the impediment of the substituents and reduced the blends/water interactions [133]. However, in the same study, the mechanical properties were improved by increasing the concentration of cellulose acetate butyrate. Related to cellulose, the degree of substitution above 2.5 results in the inhibition of biodegradation [93]. However, some chemical modifications can positively influence the biodegradation of microbial and bio-based polymer blends. This improvement in biodegradation is due to the increase in the contact area surface of the fibers, resulting from the surface treatments of the fibers, such as, an increase in the fiber rugosities with the application of NaOH, which removes the hemicellulose and lignin fibers [93].
Lignin is the most recalcitrant constituent of lignocellulosic fibers due to the complexity of the composition of this phenolic macromolecule [82,134]. The lignin enzymatic catalytic degradation needs different enzymes [75,82], or even the synergy of an enzyme complex. The inclusion of lignin in the blends of PHAs and PLA results in steric impediment of the enzyme and reduction of the degree of hydrophilicity, which is shown in the literature as a factor in reducing the biodegradation of polyester blends [93,132]. For example, the biodegradation in soil of the PHA/lignin blend was 4% after 24 weeks, which was lower than the rate of biodegradation of the PHA/10% starch, PHA/cellulose (11.1 and 100% respectively) [132]. An alternative for obtaining bioplastics from microbial polyesters, with guaranteed polymer biodegradability, is the use of enzymes and microorganisms capable of catalyzing the breakdown of lignin. The main enzymes involved in lignin oxidoreduction are laccases (Lac), lignin peroxidase (LiP), and manganese peroxidase (MnP) [82], and the recently discovered enzymes dye-decolorizing peroxidases and unspecific peroxygenase [134,135].
The application of specific microorganisms that degrade lignin and/or PHAs can be an alternative to improve the biodegradation of the blends of these polymers. In nature, these enzymes act synergistically, and some microorganisms can produce the three enzymes, while others only produce a few of the necessary enzymes [82]. LiP has a key role in the degradation of lignin, due to the distinct characteristics of the active site of the enzyme. However, the catalytic action of LiP is mediated by H2O2, which is generated by Lac [135]. The effectiveness of the microorganisms is essential for the biodegradation of PHA/lignin blends, as some microorganisms such as Phanerochaete chrysosporium are considered excellent for the degradation of lignin [134,136]. However, Brown-rot fungi due to the degradation mechanisms of lignin not being oxidative, presents a reduced degradation process of lignin [82]. Another example is the case of the bacteria Streptomyces viridosporus, which can result in a reduced degradation process of lignin since this bacterium acts in the non-phenolic regions of lignin [82,134].
4. Conclusions
Advances in the development of materials and technologies with fewer environmental impacts are highly expected, mainly due to the progress in the area of biopolymers over the past two decades. However, biopolymers application and use in the various sectors of society is limited, i.e., the annual production of bioplastics compared to plastics is still low. In this way, the use of plant biomass and microbial polyesters can help the development of bioplastic feasible, due to the availability of resources, biocompatibility, biodegradability and generally does not result in ecotoxicity. However, the physicochemical and biodegradation properties must be considered for the study of the optimization of bioplastic from natural polymers. Several actions must be taken so that bioplastic can become a reality on a large scale. The state of São Paulo (Brazil) has established a law that prohibits the supply of disposable plastic products to commercial establishments, which may increase the production scale of some bioplastics, thus reducing costs. The approval of a law by the Chinese government that prohibits the import of international plastic wastes for recycling can also encourage the production of bioplastics. An increase in the production and distribution of bioplastics is not sufficient for the development of a more conscious and sustainable society, i.e., care must be taken for the identification of a bioplastic and/or biodegradable material towards no final consumers’ mistakes and no unsuitable actions or disposal habits.
Funding
The authors acknowledge the São Paulo Research Foundation (FAPESP) for the financial support of this research project (process number 2019/16853-9; 2019/12997-6; and 2017/22401-8).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, A.A.; Afrin, S.; Karim, Z. Green Composites: Versatile Material for Future. Green Energy Technol. 2017, 29–44. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic Compatibilization of Cellulose Nanocrystals with Quaternary Ammonium Salt and Their Melt Extrusion with Polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef]
- Mariano, M.; Pilate, F.; De Oliveira, F.B.; Khelifa, F.; Dubois, P.; Raquez, J.M.; Dufresne, A. Preparation of Cellulose Nanocrystal-Reinforced Poly(lactic acid) Nanocomposites through Noncovalent Modification with PLLA-Based Surfactants. ACS Omega 2017, 2, 2678–2688. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Nechyporchuk, O.; El Kissi, N.; Dufresne, A. Melt extrusion of polystyrene reinforced with cellulose nanocrystals modified using poly[(styrene)-co-(2-ethylhexyl acrylate)] latex particles. Eur. Polym. J. 2017, 91, 297–306. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Afrin, S.; Malladi, R.P.; Elkoun, S.; Robert, M.; Ansari, M.A.; Svedberg, A.; Karim, Z. Biocomposites: Present trends and challenges for the future. Green Compos. Automot. Appl. 2019, 197–215. [Google Scholar] [CrossRef]
- Lackner, M. Bioplastics-biobased plastics as renewable and/or biodegradable alternatives to petroplasticsle. Kirk-Othmer Encycl. Chem. Technol. 2015, 6, 1–41. [Google Scholar] [CrossRef]
- Nazareth, M.; Marques, M.R.C.; Leite, M.C.A.; Castro, Í.B. Commercial plastics claiming biodegradable status: Is this also accurate for marine environments? J. Hazard. Mater. 2019, 366, 714–722. [Google Scholar] [CrossRef]
- Harding, K.G.; Gounden, T.; Pretorius, S. “Biodegradable” Plastics: A Myth of Marketing? Procedia Manuf. 2017, 7, 106–110. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chemie Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Consumi, M.; Donati, A.; Leone, G.; Magnani, A.; Tamasi, G.; Rossi, C. Biomass: An Overview, Bioenergy Systems for the Future: Prospects for Biofuels and Biohydrogen; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–42. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Physiochemical properties and degradation. Microplastic Pollut. 2017, 57–100. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Fairbrother, A.; Hsueh, H.-C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.-P. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Niaounakis, M. Properties. In Biopolymers: Applications and Trends; Elsevier Science: Amsterdam, The Netherlands, 2015; pp. 91–138. [Google Scholar]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Crutzen, P.J. The anthropocene. Earth Syst. Sci. Anthr. 2006, 13–18. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Ji, Q.; Zhang, Y.-G.; Liu, D.; Grossnickle, D.M.; Luo, Z.-X. Plastic waste inputs from land into the ocean. Science. 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Goldstein, M.; Goodwin, D. Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the North Pacific Subtropical Gyre. PeerJ. 2013, 1, 184. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Pathak, V.M.; Navneet. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Palmisano, A.C.; Pettigrew, C.A. Biodegradability of Plastics. Bioscience 1992, 42, 680–685. [Google Scholar] [CrossRef]
- De Paoli, M.A. Degradação e Estabilização de Polímeros. Artliber 2008, 1, 286. [Google Scholar]
- Crispim, C.A.; Gaylarde, C.C. Cyanobacteria and biodeterioration of cultural heritage: A review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Capitelli, F.; Principi, P.; Sorlini, C. Biodeterioration of modern materials in contemporary collections: Can biotechnology help? Trends Biotechnol. 2006, 24, 350–354. [Google Scholar] [CrossRef]
- Bonhomme, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Flemming, H.C. Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym. Degrad. Stab. 1998, 59, 309–315. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeter. Biodegr. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Zanardini, E.; Abbruscato, P.; Ghedini, N.; Realini, M.; Sorlini, C. Influence of atmospheric pollutants on the biodeterioration of stone. Int. Biodeterior. Biodegrad. 2000, 45, 35–42. [Google Scholar] [CrossRef]
- Rubio, C.; Ott, C.; Amiel, C.; Dupont-Moral, I.; Travert, J.; Mariey, L. Sulfato/thiosulfato reducing bacteria characterization by FT-IR spectroscopy: A new approach to biocorrosion control. J. Microbiol. Methods 2006, 64, 287–296. [Google Scholar] [CrossRef]
- Lugauskas, A.; Levinskaite, L.; Peĉiulyte, D. Micromycetes as deterioration agents of polymeric materials. Int. Biodeterior. Biodegrad. 2003, 52, 233–242. [Google Scholar] [CrossRef]
- Pelmont, J. Biodégradations et métabolismes. Les bactéries pour les technologies de l’environnement. EDP. Sciences 2005, 1, 798. [Google Scholar]
- Abou Zeid, D.-M. Anaerobic Biodegradation of Natural and Synthetic Polyesters. Ph.D. Dissertation, Technischen Universität Carolo-Wilhelmina zu Braunschweig, Braunschweig, Germany, 2001. [Google Scholar]
- Belal, E.S. Investigations on Biodegradation of Polyesters by Isolated Mesophilic Microbes. Ph.D. Dissertation, Technischen Universität Carolo-Wilhelmina zu Braunschweig, Braunschweig, Germany, 2003. [Google Scholar]
- Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre. 2019, 18, 100–184. [Google Scholar] [CrossRef]
- Terrone, C.C.; Nascimento, J.M.F.; Terrasan, C.R.F.; Brienzo, M.; Carmona, E.C. Salt-tolerant α-arabinofuranosidase from a new specie Aspergillus hortai CRM1919: Production in acid conditions, purification, characterization and application on xylan hydrolysis. Biocatal. Agric. Biotechnol. 2020, 23, 101460. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Costa, C.Z.; Albuquerque, M.C.C.; Brum, M.; Castro, A. Microbial and enzymatic degradation of polymers: A review. Quim. Nova. 2015, 38, 259–267. [Google Scholar] [CrossRef]
- Mueller, R.J. Biological degradation of synthetic polyesters-Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Premraj, R.; Doble, M. Biodegradation of Polymers. Indian J. Biotechnol. 2005, 4, 186–193. [Google Scholar]
- Kumar, S.; Maiti, P. Controlled biodegradation of polymers using nanoparticles and its application. RSC Adv. 2016, 6, 67449–67480. [Google Scholar] [CrossRef]
- Krzan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and certification in the area of environmentally degradable plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Faria, A.U.; De Campos, A.; Gonçalves, S.P.C.; Martins-Franchetti, S.M. Biodegradation of additive PHBV/PP-co-PE films buried in soil. Polimeros 2016, 26, 161–167. [Google Scholar] [CrossRef]
- Priyanka, N.; Archana, T. Biodegradability of Polythene and Plastic by the Help of Microorganism: A Way for Brighter Future. J. Environ. Anal. Toxicol 2011, 1, 111. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- ASTM D5338-15. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, Incorporating Thermophilic Temperatures; ASTM Int.: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ISO 14855-2. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 2: Gravimetric Measurement of Carbon Dioxide Evolved in a Laboratory-Scale Test; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ISO 17556:2003. Determination of the Ultimate Aerobic Biodegradability in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbono Dioxide Evolved; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Pavel, S.; Khatun, A.; Haque, M.M. Nexus Among Bio-plastic, Circularity, Circular Value Chain & Circular Economy. SSRN Electron. J 2020, 47. [Google Scholar] [CrossRef]
- ASTM D5511-02. Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials under High-Solids Anaerobic-digestion Conditions; ASTM Int.: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Zhao, J.H.; Wang, X.Q.; Zeng, J.; Yang, G.; Shi, F.H.; Yan, Q. Biodegradation of poly(butylene succinate-co-butylene adipate) by Aspergillus versicolor. Polym. Degrad. Stab. 2005, 90, 173–179. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Nishimura, Y. Photodegradation of biodegradable polyesters: A comprehensive study on poly(l-lactide) and poly(ε-caprolactone). Polym. Degrad. Stab. 2006, 91, 1128–1137. [Google Scholar] [CrossRef]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polymers. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, M.P.; Villanova, J.; Cesar, G.; Gavara, R.; Hernandez-Munoz, P. Compostable properties of antimicrobial bioplastics based on cinnamaldehyde cross-linked gliadins. Chem. Eng. J. 2015, 262, 447. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Aburto, J.; Alric, I.; Thiebaud, S.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Synthesis, characterization, and biodegradability of fatty-acid esters of amylose and starch. J. Appl. Polym. Sci. 1999, 74, 1440–1451. [Google Scholar] [CrossRef]
- Lindström, A.; Albertsson, A.C.; Hakkarainen, M. Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym. Degrad. Stab. 2004, 83, 487–493. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernández-de-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Ochi, S. Development of high strength biodegradable composites using Manila hemp fiber and starch-based biodegradable resin. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1879–1883. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Camacho-Hernández, I.L.; Martínez-Bustos, F.; Islas-Rubio, A.R.; Carrillo-Cañedo, K.I.; Calderón-Castro, A.; Jacobo-Valenzuela, N.; Carrillo-López, A.; Delgado-Nieblas, C.I.; Aguilar-Palazuelos, E. Mechanical, physical and microstructural properties of acetylated starch-based biocomposites reinforced with acetylated sugarcane fiber. Carbohydr. Polym. 2019, 219, 378–386. [Google Scholar] [CrossRef]
- Babaee, M.; Jonoobi, M.; Hamzeh, Y.; Ashori, A. Biodegradability and mechanical properties of reinforced starch nanocomposites using cellulose nanofibers. Carbohydr. Polym. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Olaleye, F.K.; Olusegun, S.J.; Oluwasina Olayinka, O.; Mohallem, N.D.S. Influence of oxidized starch on physicomechanical, thermal properties, and atomic force micrographs of cassava starch bioplastic film. Int. J. Biol. Macromol. 2019, 135, 282–293. [Google Scholar] [CrossRef]
- Lucena, C.A.A.; Da Costa, S.C.; Eleamen, G.R.D.A.; Mendonça, E.A.D.M.; Oliveira, E.E. Desenvolvimento de biofilmes à base de xilana e xilana/gelatina para produção de embalagens biodegradáveis. Polimeros 2017, 27, 35–41. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Tyhoda, L.; Brienzo, M. Sugarcane biomass influenced by lignin. Biofuels Bioprod. Bioref. 2020, 14, 469–480. [Google Scholar] [CrossRef]
- Melati, R.B.; Shimizu, F.L.; Oliveira, G.; Pagnocca, F.C.; de Souza, W.; Sant’Anna, C.; Brienzo, M. Key Factors Affecting the Recalcitrance and Conversion Process of Biomass. Bioenergy Res. 2019, 12. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994; p. 2. [Google Scholar]
- Campagner, M.R.; Da Silva Moris, V.A.; Pitombo, L.M.; Do Carmo, J.B.; De Paiva, J.M.F. Polymeric films based on starch and lignosulfonates: Preparation, properties and evaluation of biodegradation. Polimeros 2014, 24, 740–751. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Influence of addition of vapor grown carbon fibers on mechanical, thermal and biodegradation properties of lignin nanoparticle filled bio-poly(trimethylene terephthalate) hybrid nanocomposites. RSC Adv. 2015, 5, 56028–56036. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Review: Raw Natural Fiber-Based Polymer Composites. Int. J. Polym. Anal. Charact. 2014, 19, 256–271. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaushik, N.; Biswas, S. Derivatives and Applications of Lignin–An Insight. Sci. Tech. J. 2014, 1, 30–36. [Google Scholar]
- Arnling Bååth, J.; Martínez-Abad, A.; Berglund, J.; Larsbrink, J.; Vilaplana, F.; Olsson, L. Mannanase hydrolysis of spruce galactoglucomannan focusing on the influence of acetylation on enzymatic mannan degradation. Biotechnol. Biofuels. 2018, 11, 1. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Rivard, C.; Moens, L.; Roberts, K.; Brigham, J.; Kelley, S. Starch esters as biodegradable plastics: Effects of ester group chain length and degree of substitution on anaerobic biodegradation. Enzyme Microb. Technol. 1995, 17, 848–852. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Kokkou, S.; Panayiotou, C. Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I. Surface chemical modification and characterization of waste flour. Compos. Part A Appl. Sci. Manuf. 2005, 36, 965–974. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Panayiotou, C. Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part II. Development of biodegradable composites using treated and compatibilized waste flour. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1231–1238. [Google Scholar] [CrossRef]
- Glasser, W.G.; Ravindran, G.; Jain, R.K.; Samaranayake, G.; Todd, J. Comparative Enzyme Biodegradability of Xylan, Cellulose, and Starch Derivatives†. Biotechnol. Prog. 1995, 11, 552–557. [Google Scholar] [CrossRef]
- Mitchell, D.; Grohmann, K.; Himmel, M.; Dale, B.; Schroeder, H. Effect of the degree of acetylation on the enzymatic digestion of acetylated xylans. J. Wood Chem. Technol. 1990, 10, 111–121. [Google Scholar] [CrossRef]
- Alekhina, M.; Mikkonen, K.S.; Alén, R.; Tenkanen, M.; Sixta, H. Carboxymethylation of alkali extracted xylan for preparation of bio-based packaging films. Carbohydr. Polym. 2014, 100, 89–96. [Google Scholar] [CrossRef]
- Geng, W.; Venditti, R.A.; Pawlak, J.J.; Pal, L.; Ford, E. Carboxymethylation of hemicellulose isolated from poplar (Populus grandidentata) and its potential in water-soluble oxygen barrier films. Cellulose 2020, 27, 3359–3377. [Google Scholar] [CrossRef]
- Heikkinen, S.L.; Mikkonen, K.S.; Pirkkalainen, K.; Serimaa, R.; Joly, C.; Tenkanen, M. Specific enzymatic tailoring of wheat arabinoxylan reveals the role of substitution on xylan film properties. Carbohydr. Polym. 2013, 92, 733–740. [Google Scholar] [CrossRef]
- Höije, A.; Stememalm, E.; Heikkinen, S.; Tenkanen, M.; Gatenholm, P. Material properties of films from enzymatically tailored arabinoxylans. Biomacromolecules 2008, 9, 2042–2047. [Google Scholar] [CrossRef]
- Kjeldsen, A.; Price, M.; Lilley, C.; Guzniczak, E.; Archer, I. A Review of Standards for Biodegradable Plastics. Ind. Biotechnol. Innov. Cent. IBioIC 2018, 33. [Google Scholar]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanaoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Kasuya, K. Biodegradability of poly(3-hydroxyalkanoate) and poly(ε-caprolactone) via biological carbon cycles in marine environments. Polym. J. 2020, 53, 47–66. [Google Scholar] [CrossRef]
- Volovaa, T.G.; Boyandina, A.N.; Prudnikovab, S.V. Biodegradation of polyhydroxyalkanoates in natural soils. J. Sib. Fed. Univ. Biol. 2015, 2, 152–167. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Wang, Y.W.; Mo, W.; Yao, H.; Wu, Q.; Chen, J.; Chen, G.Q. Biodegradation studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Degrad. Stab. 2004, 85, 815–821. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, L.; Zhang, M.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Morohoshi, T.; Ogata, K.; Okura, T.; Sato, S. Molecular characterization of the bacterial community in biofilms for degradation of poly (3-Hydroxybutyrate-co-3-Hydroxyhexanoate) films in seawater. Microbes Environ. 2018, 33. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Bioplastics missing link in the era of microplastics. Sci. Total Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energ. Sources Part A 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef]
- Bertrand, J.I.; Ramsay, B.A.; Ramsay, J.A.; Chavarie, C. Biosynthesis of poly-βhydroxyalkanoates from pentoses by Pseudomonas pseudoflava. Appl. Environ. Microbiol. 1990, 6, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.G.; Rocha, R.C.S.; Zanotto, S.P.; Gomez, J.G.C.; Silva, L.F. Screening of bacteria to produce polyhydroxyalkanoates from xylose. World J. Microbiol. Biotechnol. 2009, 25, 1751–1756. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, D.; Han, S.O. Effect of natural fiber surface treatments on the interfacial and mechanical properties of henequen/polypropylene biocomposites. Macromol. Res. 2008, 16, 411–417. [Google Scholar] [CrossRef]
- Nduko, J.M.; Suzuki, W.; Matsumoto, K.; Kobayashi, H.; Ooi, T.; Fukuoka, A.; Taguchi, S. Polyhydroxyalkanoates production from cellulose hydrolysate in Escherichia coli LS5218 with superior resistance to 5-hydroxymethylfurfural. J. Biosci. Bioeng. 2012, 113, 70–72. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.D.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef]
- Jones, F. A Promessa dos Bioplásticos. FAPESP. Available online: https://revistapesquisa.fapesp.br/a-promessa-dos-bioplasticos/ (accessed on 20 June 2020).
- Chen, G. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. Springerplus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Wing, M.T.; Stevens, B.E.; Theegala, C.S.; Negulescu, I.I.; Rusch, K.A. Anaerobic biodegradation of polyhydroxybutyrate in municipal sewage sludge. J. Environ. Eng. 2010, 136, 709–718. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S. Preparation and Characterization of Polyhydroxyalkanoate Bioplastic-Based Green Renewable Composites from Rice Husk. J. Polym. Environ. 2014, 22, 384–392. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Coelho, N.S.; Almeida, Y.M.B.; Vinhas, G.M. A biodegradabilidade da blenda de poli(β-Hidroxibutirato-co-Valerato)/amido anfótero na presença de microrganismos. Polímeros 2008, 18, 270–276. [Google Scholar] [CrossRef][Green Version]
- Harmaen, A.S.; Khalina, A.; Ali, H.M.; Azowa, I.N. Thermal, Morphological, and Biodegradability Properties of Bioplastic Fertilizer Composites Made of Oil Palm Biomass, Fertilizer, and Poly(hydroxybutyrate-co-valerate). Int. J. Polym. Sci. 2016, 1–8. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Andrade, C.T. Properties of melt-processed poly (hydroxybutyrate-co-hydroxyvalerate)/starch 1:1 blend nanocomposites. Polimeros 2013, 23, 366–372. [Google Scholar] [CrossRef]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Innocenti, F.D. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Sarasa, J.; Gracia, J.M.; Javierre, C. Study of the biodisintegration of a bioplastic material waste. Bioresour. Technol. 2009, 100, 3764–3768. [Google Scholar] [CrossRef]
- Allen, W.R. Structural applications for flexible packaging: Innovations in pouch forms and uses. Polym. Plast. Technol. Eng. 1986, 25, 295–320. [Google Scholar] [CrossRef]
- Briston, J.H. Plastic Films, 3rd ed.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Hansen, N.M.L.; Blomfeldt, T.O.J.; Hedenqvist, M.S.; Plackett, D.V. Properties of plasticized composite films prepared from nanofibrillated cellulose and birch wood xylan. Cellulose 2012, 19, 2015–2031. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Cabedo, L.; Gamez-Perez, J. Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications. Int. J. Mol. Sci. 2018, 19, 2102. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.M.; Meira, H.M.; Silva, I.D.L.; Galdino, C.J.S.; Almeida, F.C.G.; Amorim, J.D.P.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a bacterial cellulose/poly(3-hydroxybutyrate) blend activated with clove essential oil for food packaging. Polym. Polym. Compos. 2020. [Google Scholar] [CrossRef]
- Dangtungee, R.; Tengsuthiwat, J.; Boonyasopon, P.; Siengchin, S. Sisal natural fiber/clay-reinforced poly(hydroxybutyrate-co-hydroxyvalerate) hybrid composites. J. Thermoplast. Compos. Mater. 2014, 28, 6–879. [Google Scholar] [CrossRef]
- Joyyi, L.; Ahmad Thirmizir, M.Z.; Salim, M.S.; Han, L.; Murugan, P.; Kasuya Ki Maurer, F.H.J.; Zainal Arifin, M.I.; Sudesh, K. Composite properties and biodegradation of biologically recovered poly(3HB-co-3HHx) reinforced with short kenaf fibers. Polym. Degrad. Stab. 2017, 137, 100–108. [Google Scholar] [CrossRef]
- Batista, K.C.; Silva, D.A.K.; Coelho, L.A.F.; Pezzin, S.H.; Pezzin, A.P.T. Soil Biodegradation of PHBV/Peach Palm Particles Biocomposites. J. Polym. Environ. 2010, 18, 346–354. [Google Scholar] [CrossRef]
- Rosa, D.S.; Rodrigues, T.C.; Graças Fassina Guedes, C.; Calil, M.R. Effect of thermal aging on the biodegradation of PCL, PHB-V, and their blends with starch in soil compost. J. Appl. Polym. Sci. 2003, 89, 3539–3546. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Schrader, J.A.; McCabe, K.G.; Srinivasan, G.; Grewell, D.; Graves, W.R. Performance and biodegradation in soil of novel horticulture containers made from bioplastics and biocomposites. Hort. Technol. 2015, 25, 119–131. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, G.; Ma, S.; Cai, Z.; Zhang, L. Crystallization behavior, mechanical properties, and environmental biodegradability of poly(?-hydroxybutyrate)/cellulose acetate butyrate blends. J. Appl. Polym. Sci. 2003, 89, 2116–2122. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Santacruz-Juarez, E.; Buendia-Corona, R.E.; Ramírez, R.E.; Sanchez, S. Fungal enzymes for the degradation of polyethylene: Molecular docking simulation and biodegradation pathway proposal. J. Hazard. Mater. 2021, 411, 125118. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sust. Energ. Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).