Improving Fly Ash Brightness with Carbon and Iron Oxide Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment of Fly Ash
2.2. High Temperature Carbon Removal
2.3. Iron-Containing Oxides Removal by Acid Leaching
2.3.1. Process Method
2.3.2. Box–Behnken Design and Response Surface Methodology
2.4. Characterizations of Samples
2.4.1. Testing of Chemical Composition
2.4.2. Testing of Phase and Crystal
2.4.3. Testing of Powder Brightness
3. Results and Discussion
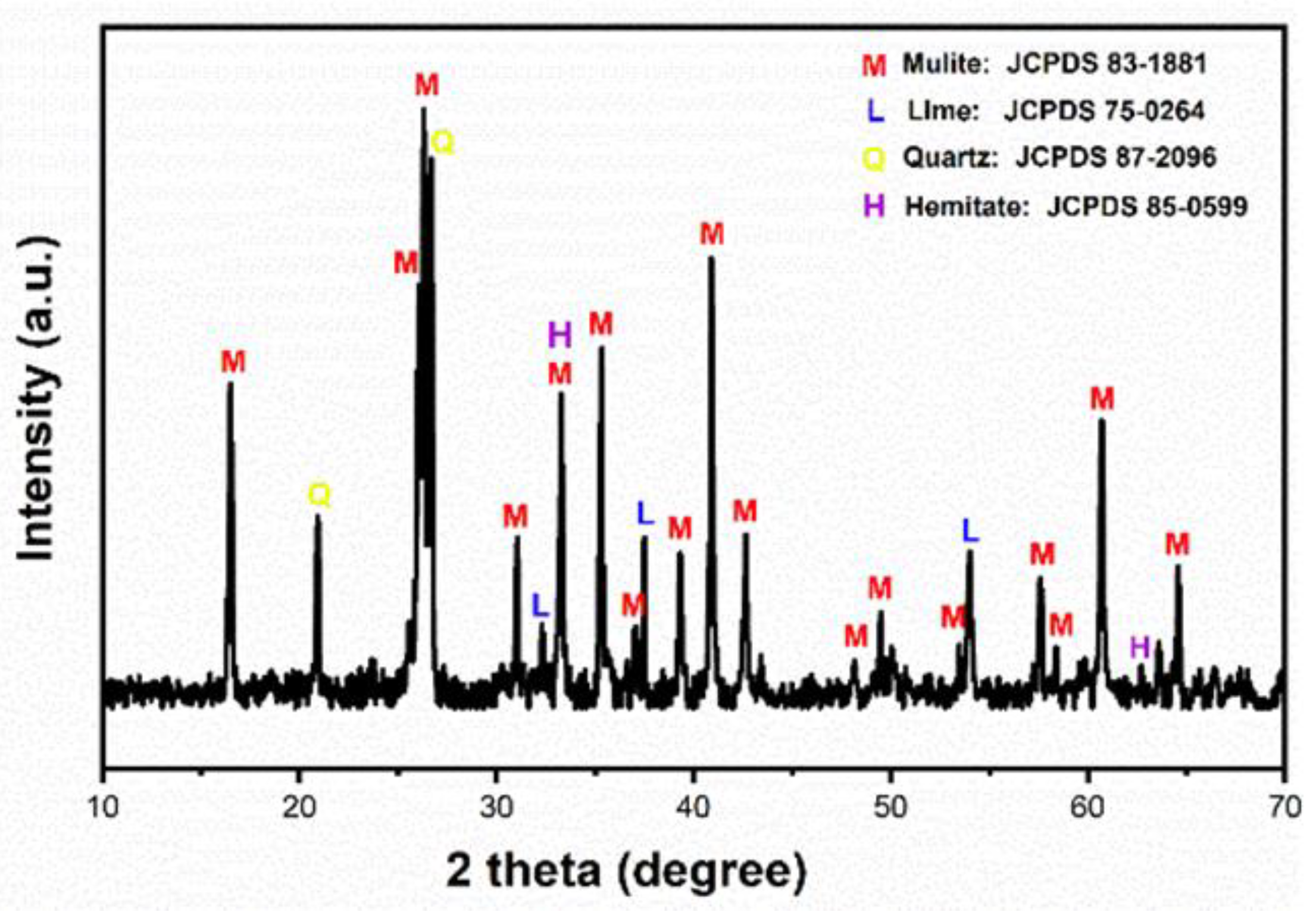
3.1. Analysis of Phase Composition of Original Fly Ash
3.2. Analysis of Chemical Composition of Original Fly Ash
3.3. Screening Analysis of the Original Fly Ash
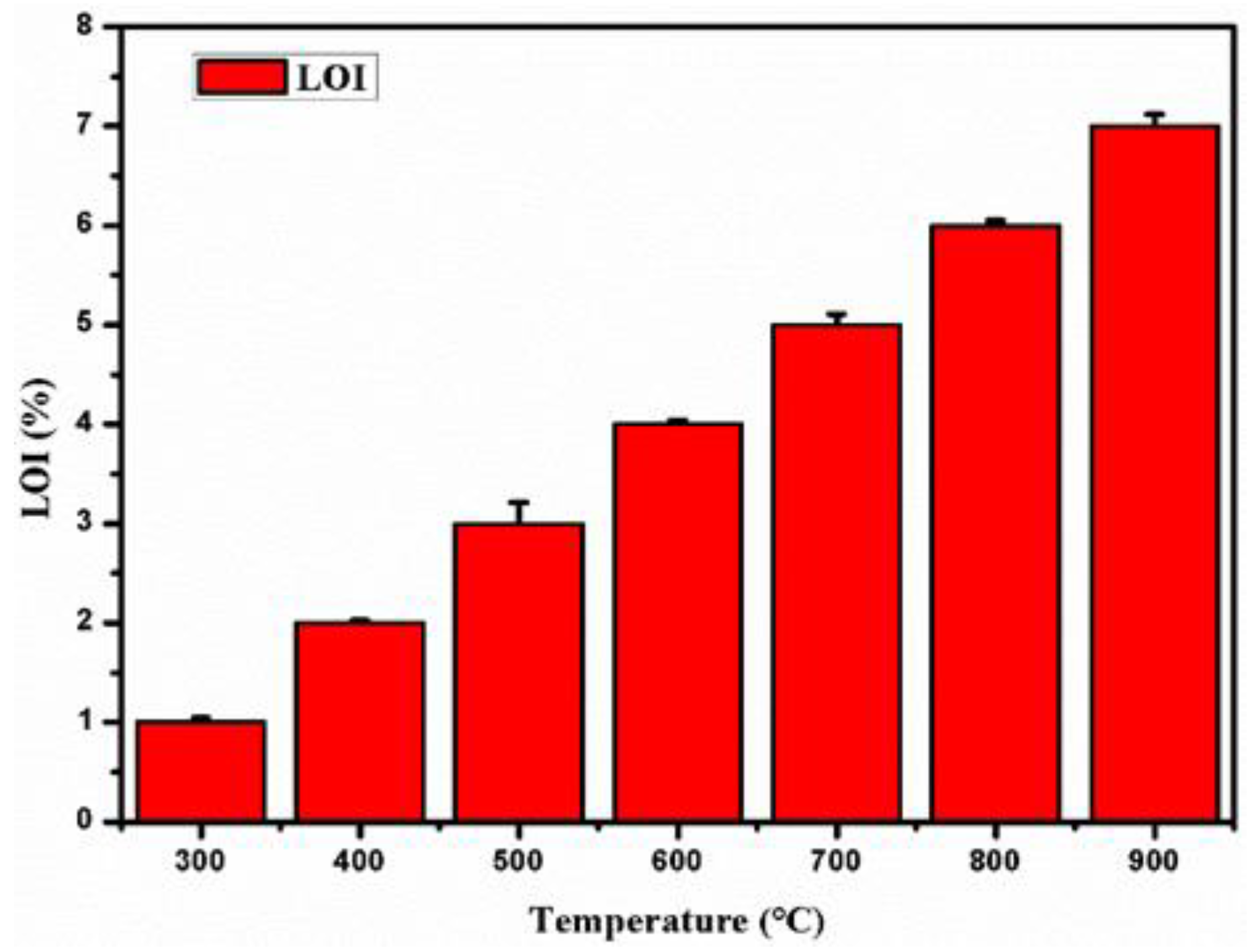
3.4. Analysis of High Temperature Carbon Removal
3.5. Analysis of Iron-Containing Oxides by Acid Treatment
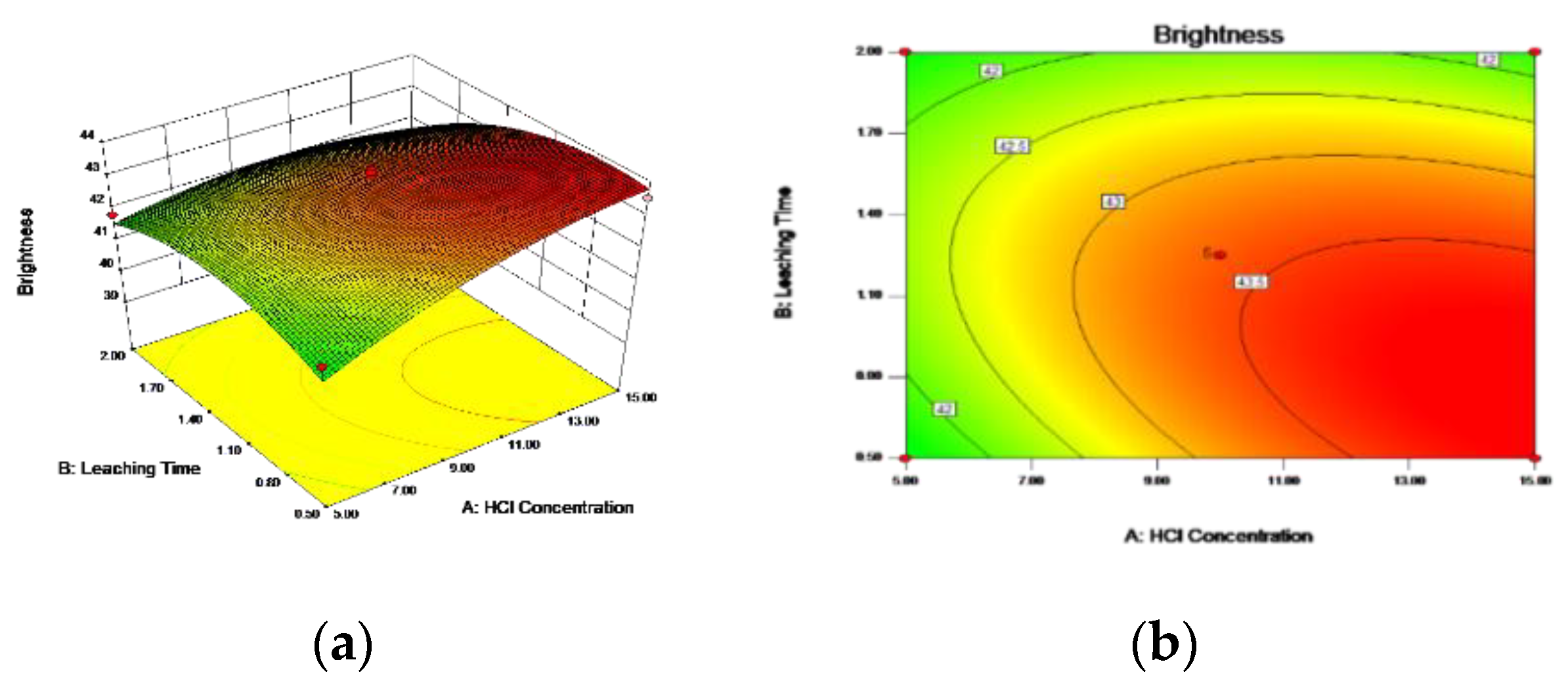
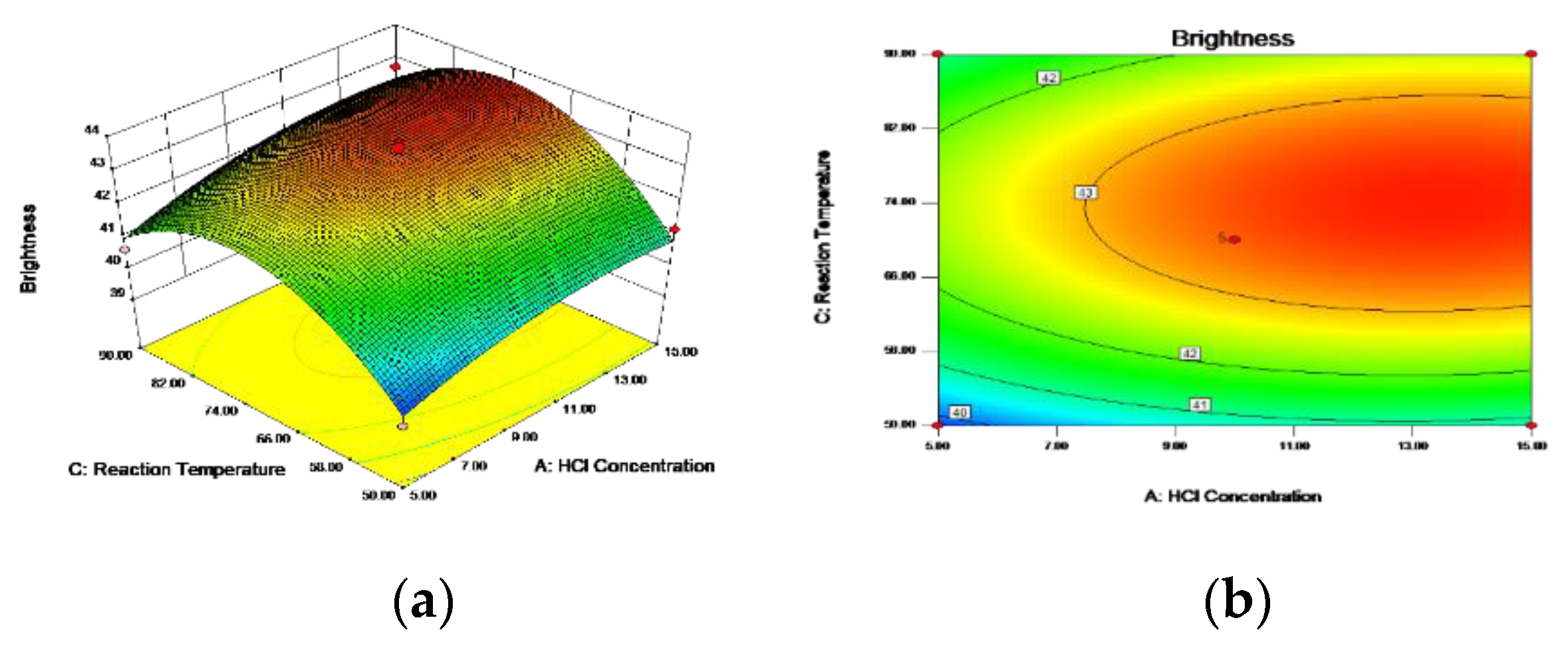
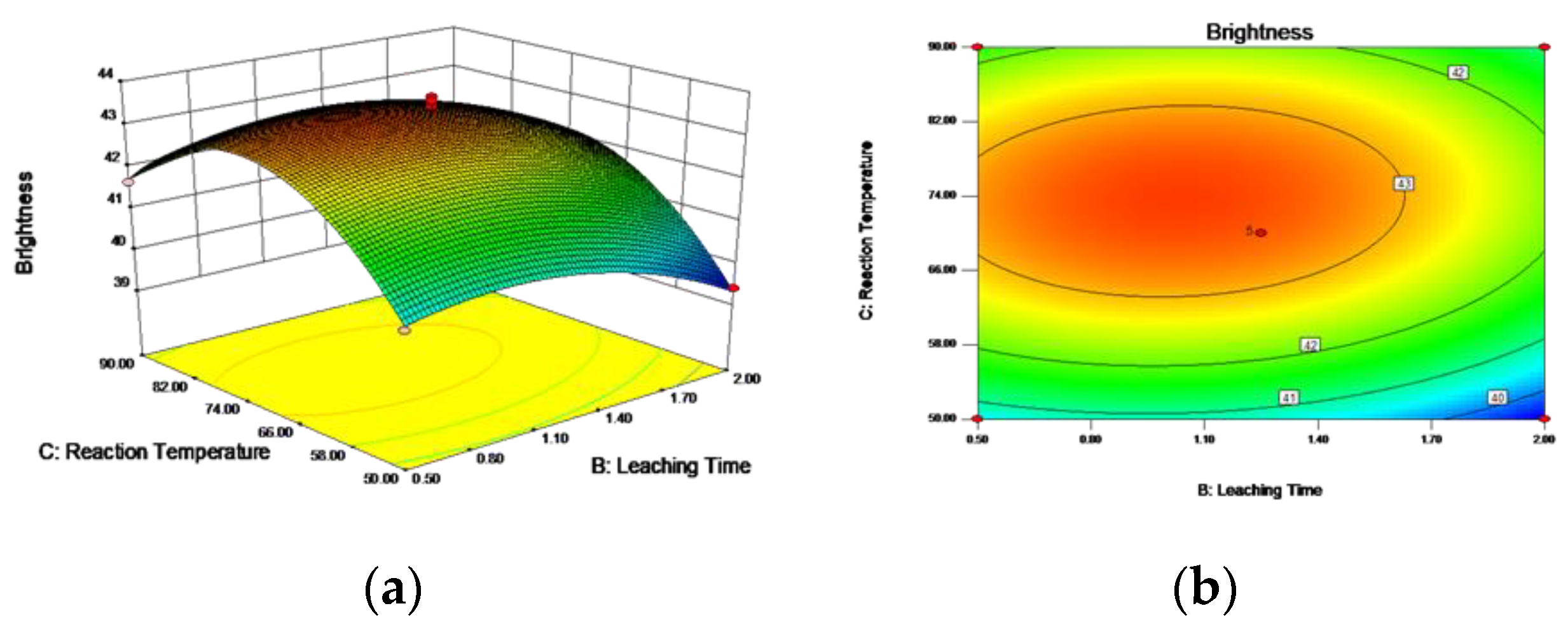
3.5.1. Box–Behnken Design and Response Surface Methodology
3.5.2. Analysis of Variance of Response Surface Regression Model
3.5.3. Optimal Process Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Peng, S.; Luo, L. Optimization of carbon removal technology for fly ash flotation. Coal Sci. Technol. 2001, 8, 21–23. [Google Scholar]
- Golewski, G.L. Studies of natural radioactivity of concrete with siliceous fly ash addition. Cem. Wapno Beton 2015, 20, 106. [Google Scholar]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ramaswamy, A. A review on fly ash characteristics–Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 559. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Kandpal, T.C. Potential of fly ash utilisation in India. Energy 2002, 27, 151–166. [Google Scholar] [CrossRef]
- Wang, S. Application of solid ash based catalysts in heterogeneous catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063. [Google Scholar] [CrossRef]
- Yang, A.L.; Jiang, Y.N.; Zhao, X.M.; Rashid, K.; Zhao, X.; Huang, X.; Zhou, H.; Su, M.; Tang, H.; Li, F. The True Cost Coal: An Investigation into Coal ash in China. Available online: www.greenpeace.org.cn coal-ash2010-rpt/ (accessed on 15 September 2010).
- Wu, Y.; Yi, G.; Liu, Q.; Li, F.; Zhao, L.; Ma, M. Status of Comprehensive Utilization of Fly Ash. Clean Coal Technol. 2013, 19, 100–104. [Google Scholar] [CrossRef]
- Joshaghani, A.; Balapour, M.; Ramezanianpour, A.A. Effect of controlled environmental conditions on mechanical, microstructural and durability properties of cement mortar. Constr. Build. Mater. 2018, 164, 134–149. [Google Scholar] [CrossRef]
- Phoungthong, K.; Shao, L.M.; He, P.J.; Zhang, H. Phytotoxicity and groundwater impacts of leaching from thermal treatment residues in roadways. J. Environ. Sci. 2018, 63, 58–67. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, R.; Fu, T.W. Tenghuan.Experimental Study on Frost Resistance Durability of Low Volume Fly Ash Recycled Concrete. Bull. Chin. Ceram. Soc. 2018, 37, 3522–3527. [Google Scholar]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Yang, L. Treatment of calcium-containing wastewater from alumina extraction by fly ash acid method. Ind. Water Treat. 2018, 38, 45–47. [Google Scholar]
- Zhang, Y.N.; Yu, Y.X. Adsorptive removal of Cr3+, Cu2+, and Ni2+ ions by magnetic Fe3O4@ alkali-treated coal fly ash. Desalin. Water Treat. 2018, 123, 277–287. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, F. Study on Extraction and Purification Technology of Gallium in Fly Ash. Coal Technol. 2018, 37, 336–339. [Google Scholar]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Khatri, C.; Mishra, M.K.; Rani, A. Synthesis and characterization of fly ash supported sulfated zirconia catalyst for benzylation reactions. Fuel Process. Technol. 2010, 91, 1288–1295. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Red mud and fly ash supported Co catalysts for phenol oxidation. Catal. Today 2012, 190, 68–72. [Google Scholar] [CrossRef]
- Sinha, A.S.K. Effects of pulverized coal fly-ash addition as a wet-end filler in papermaking. Tappi J. 2008, 7, 3–7. [Google Scholar]
- Fan, H.; Wang, S.; Liu, J.; Long, J. Improving fly ash whiteness and the influence of modified fly ash on the physical strength of paper. BioResources 2015, 10, 7434–7441. [Google Scholar]
- Metzger, M. Determination of carbon in fly ashes from incineration processes. Fresenius J. Anal. Chem. 1987, 327, 726–727. [Google Scholar] [CrossRef]
- He, X.; Chang, Z.; Zhang, C. Experimental Study on Charcoal Selection for Fly Ash. Fly Ash 1999, 3, 5–8. [Google Scholar]
- Zhang, L.; Yang, F.; Tao, Y. Removal of unburned carbon from fly ash using enhanced gravity separation and the comparison with froth flotation. Fuel 2020, 259, 116282. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Z.; Zhang, Y. Mineral Composition of Fly Ash (Part II). Compr. Util. Fly Ash 2001, 2, 37–41. [Google Scholar]
- Wang, F.; Wu, Z. Fly Ash Utilization Manual; China Electric Power Press: Beijing, China, 1997. [Google Scholar]
- Pan, C. Determination of Carbon Content in Fly Ash. Compr. Util. Fly Ash 1998, 3, 59–60. [Google Scholar]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]


| Factor | Variable Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A HCl concentration/% | 5% | 10% | 15% |
| B Leaching time/h | 0.5 | 1 | 2 |
| C Reaction temperature/°C | 50 | 70 | 90 |
| SiO2 | Al2O3 | CO3 | CaO | Fe2O3 | TiO2 | K2O | Na2O | SO3 | MgO | P2O5 | CeO2 | SrO | ZrO2 | Cl | MnO | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 46.32% | 31.60% | 8.54% | 4.74% | 4.47% | 1.29% | 0.63% | 0.60% | 0.48% | 0.42% | 0.37% | 0.20% | 0.15% | 0.06% | 0.05% | 0.03% | 0.02% |
| 400 Mesh | 500 Mesh | 600 Mesh | 700 Mesh | |
|---|---|---|---|---|
| Mass Percentage/% | 15% | 7% | 50% | 28% |
| Brightness/%ISO | 9.02 | 13.46 | 18.01 | 19.61 |
| Run | Coded Variable Level | Brightness (%ISO) | |||
|---|---|---|---|---|---|
| HCl Concentration (/%) | Time (/h) | Temperature (/°C) | Predicted | Experimental | |
| 1 | 1 | −1 | 0 | 43.79 | 43.52 |
| 2 | 0 | 0 | 0 | 43.37 | 43.55 |
| 3 | 0 | 0 | 0 | 43.37 | 42.33 |
| 4 | 1 | 1 | 0 | 41.69 | 41.26 |
| 5 | 0 | 1 | 1 | 41.01 | 41.11 |
| 6 | 0 | −1 | 1 | 41.73 | 41.65 |
| 7 | −1 | 0 | 1 | 40.97 | 40.62 |
| 8 | 0 | 0 | 0 | 43.37 | 43.55 |
| 9 | 0 | −1 | −1 | 40.59 | 40.50 |
| 10 | 0 | 0 | 0 | 43.37 | 43.76 |
| 11 | −1 | −1 | 0 | 41.45 | 41.90 |
| 12 | 1 | 0 | 1 | 42.39 | 42.73 |
| 13 | 0 | 0 | 0 | 43.37 | 43.67 |
| 14 | −1 | 0 | −1 | 39.73 | 39.40 |
| 15 | 0 | 1 | −1 | 39.31 | 39.40 |
| 16 | −1 | 1 | 0 | 41.55 | 41.82 |
| 17 | 1 | 0 | −1 | 40.79 | 41.15 |
| Variable | Statistical Analysis | ||||
|---|---|---|---|---|---|
| Sum of Square | Df | Mean Square | f-Value | p-Value | |
| Model | 30.86 | 9 | 3.43 | 9.88 | 0.0032a |
| A | 3.03 | 1 | 3.03 | 8.72 | 0.0213 |
| B | 1.98 | 1 | 1.98 | 5.70 | 0.0483 |
| C | 4.00 | 1 | 4.00 | 11.53 | 0.0115 |
| AB | 1.19 | 1 | 1.19 | 3.42 | 0.1068 |
| AC | 0.032 | 1 | 0.032 | 0.093 | 0.7689 |
| BC | 0.078 | 1 | 0.078 | 0.23 | 0.6491 |
| A2 | 0.92 | 1 | 0.92 | 2.66 | 0.1468 |
| B2 | 2.55 | 1 | 2.55 | 7.35 | 0.0301 |
| C2 | 15.66 | 1 | 15.66 | 45.11 | 0.0003 |
| Cor Total | 33.29 | 16 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Fan, H.; Gao, H.-t.; Liu, J.-a.; Mou, H. Improving Fly Ash Brightness with Carbon and Iron Oxide Removal. Recycling 2020, 5, 5. https://doi.org/10.3390/recycling5010005
Song H, Fan H, Gao H-t, Liu J-a, Mou H. Improving Fly Ash Brightness with Carbon and Iron Oxide Removal. Recycling. 2020; 5(1):5. https://doi.org/10.3390/recycling5010005
Chicago/Turabian StyleSong, Helong, Huiming Fan, Hang-tian Gao, Jian-an Liu, and Hongyan Mou. 2020. "Improving Fly Ash Brightness with Carbon and Iron Oxide Removal" Recycling 5, no. 1: 5. https://doi.org/10.3390/recycling5010005
APA StyleSong, H., Fan, H., Gao, H.-t., Liu, J.-a., & Mou, H. (2020). Improving Fly Ash Brightness with Carbon and Iron Oxide Removal. Recycling, 5(1), 5. https://doi.org/10.3390/recycling5010005





