1. Introduction
Most products, which new technologies depend on, are reliant on particular raw elements supply. Silicon, for instance, is the base for the production of fiber optics; rare earth elements are used for the production of smartphones, and new batteries for electric vehicles need cobalt, graphite, and lithium. Countries accounting for the largest share of global production of battery materials are Chile, DRC, and China [
1]. In a few words, the European Community, and in general, all of the world’s economy, depends on such raw materials classified as ‘critical’. Thus, critical raw materials (CRMs) are those materials that are extremely important for the new world economy but suffer, at the same, time of a high supply risk [
2]. The supply risk is not directly related to the ore’s scarcity in Earth’s crust but rather to their uneven geographical distribution. For example, 95% of rare earth elements used in the European Union (EU) come from China. Furthermore, some of those exporting countries may suffer from poor governance or internal conflicts; thus, they cannot guarantee the required material supply.
Fortunately, there are mitigating actions that can be implemented in order to overcome the problems related to the EU CRMs dependency. The first one is the substitution. Some CRMs can be substituted with other ones that are not critical. However, that is not always possible because of the unique properties of CRMs used in particular applications. The second possibility is improving the component performance. That means practically using less material and thus less CRMs. The last possibility is recycling. A great effort is spent in CRMs recycling. For example, in their recent work [
3], Mayyas et al. emphasized the importance of lithium ion battery recycling to overcome the increasing demand for electric vehicles and reduce the criticality issues of cobalt, lithium, and graphite, which batteries are made of. Afterward, Jo and Myung published a paper [
4] in which they described efficient direct physical and intermediate recycling processes to minimize the environmental burden of lithium-ion batteries.
Redmaker et al. [
5] focused on the rare earth elements (REE) recycling as a potential strategy to reduce their criticality (mainly the EU dependence on China). They estimated the annual waste flows of neodymium and dysprosium from permanent magnets during the 2011–2030 period and found that compared to the growing global REE demand, waste flows from permanent magnets remained small in the first period. Therefore, in the next decade, REE recycling will be not able to contribute to the global supply security. However, in the long term, REE recycling will meet a substantial part of the total demand for these metals.
Technologies were also developed aimed at recovering critical raw materials from waste waters resulting from materials production. These include cementation, precipitation, reduction, ion exchange, solvent extraction, electrochemical methods, and adsorption [
6]. The most promising of them is the adsorption technology because of its low cost and high availability. Conventional technologies in metal recovery from various streams of electronic waste have also been used with particular reference to printed circuits boards, hard disc drives, and displays. These are physical, pyrometallurgical, and (bio)hydrometallurgical metal recovery technologies [
7]. Despite their utility, such conventional recycling strategies can be improved in terms of energy requirement and usage of chemicals. As an alternative, Auerbach et al. [
8] proposed the bioleaching where leaching of waste material is performed by microorganisms. In their work, they studied the recycling potential of end-of-life magnets by means of bioleaching with various bacteria. A review about biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment can be found in the work of Işıldar et al. [
9], as well.
A recent work by Bartl et al. [
10] deals with the substitution possibility and supply chain of CRMs such as cobalt, niobium, tungsten, yttrium, and REE. For each element, the most relevant data concerning mining, abundance, recycling rates, and possible substitutes are updated.
A model was developed by Hache et al. [
11] that is able to assess the future raw material market and could be extended to critical raw materials for more efficient regional and sectorial screening. Peck et al. [
12] highlighted how all the criticality definitions present in literature are made by experts outside the field of product design, and the resulting definitions make it difficult for product designers, and the wider product development team, to engage in activity to address the critical materials challenge. With the aim to fill this gap, a strategy for the design of components in a CRMs perspective was therefore proposed in [
13,
14]. In those works, an alloy criticality parameter was defined that allowed defining a material index (in the frame of Ashby’s approach) for the materials selection containing information about materials criticality.
Any eco-design strategy starts with an analysis of the product life cycle (Life Cycle Assessment (LCA)). Ore and feedstock are first mined and processed to yield a material. These are manufactured into a product that is used and, at the end of its life, discarded or recycled. Energy and materials are consumed in each phase, generating waste heat and solid, liquid, and gaseous emissions. Each phase of the product life cycle is quantified by an eco-attribute that, for the sake of simplicity, could be the energy consumption or the corresponding CO2 emission.
Most of the energy consumed in all the phases of the lifecycle of the product is derived from fossil fuels. In this scenario, sustainable recycling [
15] plays a fundamental role in facing the CRMs drawbacks, whatever the strategy used to reduce the product’s environmental impact. It should include product R&D, collection and sorting of end-of-life materials and goods, scrapes recycling, and recycling technologies [
16]. This contribution is focused on CRMs recycling applied to product R&D.
After a short review of CRMs recycling, a systematic strategy is proposed that allows selecting materials when CRMs recycling is the main objective to be maximized. The method is finally illustrated with an example.
2. CRMs End-of-Life Recycling Input Rate
In 2017 the European Commission (EC) identified 27 critical raw materials: Antimony, Beryllium, Borates, Cobalt, Coking Coal, Fluorspar, Gallium, Germanium, Indium, Magnesium, Natural Graphite, Niobium, Phosphate Rock, Silicon Metal, Tungsten, Platinum Group Metals, Light Rare Earths and Heavy Rare Earths, Baryte, Bismuth, Hafnium, Helium, Natural Rubber, Phosphorus, Scandium, Tantalum, and Vanadium. According to its criticality assessment criteria (EC 2011, 2014), the recyclability of CRMs is quantified by the end-of-life recycling input rate (EOL-RIR). The end-of-life recycling input rate (EOL-RIR
i) of the CRM ‘ì’ is ‘the input of secondary material to the European Union (EU) from old scrap to the total input of material (primary and secondary)’. EOL-RIR (%) refers only to functional recycling that is that portion of end-of-life recycling in which the metal in a discarded product is separated and sorted to obtain recyclates that are returned to raw material production processes that generate a metal or metal alloy. Often it is not the specific alloy that is remelted to make the same alloy, but any alloys within a certain class of alloys that are remelted to make one or more specific alloys. For example, a mixture of austenitic stainless steel alloys might be remelted, and the resulting composition adjusted by the addition of reagents or virgin metal to make a specific stainless steel grade. Recyclates obtained by functional recycling are used for the same functions and applications as when obtained from primary sources. Non-functional recycling is that portion of end-of-life recycling in which the metal is collected as old metal scrap and incorporated in an associated large magnitude material stream as a “tramp” or impurity elements. This prevents dissipation into the environment but represents the loss of its function, as it is generally impossible to recover it from the large magnitude stream. It is noted that non-functional recycling will lead to an open metal life cycle. Examples are small amounts of copper in iron recyclates that are incorporated into recycled carbon steel. The EOL-RIR always refers to functional recycling, and includes recycling as a pure metal (e.g., copper) and as an alloy (e.g., brass).
Table 1 summarizes the last updated EOL-RIR value of different CRMs [
17,
18]
The end-of-life (EOL) recycling rate for antimony is estimated to be 28% [
17]. Secondary antimony is mainly recovered from lead-acid batteries and is an important part of the supply chain in some countries such as the United States of America, Japan, Canada, and the EU. On the other hand, beryllium is not recuperated from end-finished products because of the small size of components and the tiny fraction of beryllium contained in appliances. Furthermore, it is mainly used in dissipative applications such as pigments and pharmaceuticals. However, it can be recovered from new scrap generated during the manufacture of beryllium products and from old scrap or from solder alloys found in electronic devices thanks to their low melting points. For this reason, the end-of-life (EOL) recycling input rate for bismuth is estimated at less than 1%.
Cobalt is mainly used in the EU for the production of battery chemicals (42%), superalloys, hard facing, high-speed steel, and other alloys (23%) and hard materials (10%). However, in the last communication from the EC to the European Parliament on the 2017 list of CRMs for the EU, the end-of-life recycling input rate of cobalt was estimated to be 0% since no functional recycling was carried out. The same value of the EOL-RIR (0%) is attributed to gallium because of the high difficulty and cost to recover it in items where it is highly dispersed. As a matter of fact, it is used to produce a deposition layer, a few microns thick, on top of a much thicker substrate in integrated circuits or light-emitting diodes (LEDs).
Around 30% of global germanium production is supplied by recycling, most deriving from scrap produced during the fiber-optic and infrared optics manufacture. That is also encouraged by the high price of refined germanium. However, once the final products are sold into the market, there is very little recycling at end-of-life of “old scrap”. Thus, the functional recycling rate has been estimated at about 12%, and the end-of-life recycling input rate is assessed at 2% only [
17].
It is likely that little to no post-use recycling of hafnium is being carried out currently, given its contamination in the nuclear industry and the low percentage content in superalloys. That is the reason why its end-of-life recycling input rate value is estimated to be 1%.
World secondary refined indium production resulted almost exclusively from the recycling of manufacturing waste (new scrap) rather than recovery from end-of-life. Very little old scrap (1%) is recycled worldwide because of minor indium concentrations in final products, a lack of appropriate technology, or low economic incentives compared to recycling costs. The EOL-RIR of indium is therefore 0%.
Iridium overall recycling accounts for a small proportion of the metal produced globally each year and the EOL-RIR is estimated to be 14%. Only 1% of lanthanum worldwide is recycled, while at the EU level, the magnesium recycling capacity is about 75,000 t/y (mostly for new scrap). Natural Graphite is an important raw material in a wide range of applications such as metal castings, lubricants, fire retardant in plastics, fuel cell, pebble bed nuclear reactors, and so on. A significant amount of material containing natural graphite is lost during use (lubricants, friction materials, and to some extent, refractories) and, therefore, cannot be recycled. Efforts toward recycling post-consumer products containing natural graphite are dampened by oversupply and low prices. There is some recycling of used refractory material so that the EOL-RIR is assessed equal to 3%.
The EOL-RIR of niobium is very low, 0.3%, because its amount physically recovered from scrap (i.e., functional recycling) is negligible.
The high value of palladium makes it generally attractive for recycling, and sophisticated technology has been developed that permits highly effective recovery of palladium from a variety of waste streams, notably autocatalysts and waste electrical and electronic equipment (WEEE). This makes its EOL-RIR (14%) relatively high compared to previous elements and close to that of praseodymium (10%). The EU is 98% reliant on platinum imports. Platinum supplies are derived from both primary sources (mines) and secondary sources (refineries). In the last (2017) report of CRMs filled by the European Commission (EC), the EOL-RIR of palladium is estimated at about 14% [
15].
Similar to palladium, the high value of rhodium makes it generally attractive for recycling. This is the reason why the EOL-RIR of rhodium is relatively high, 24%, compared to the majority of the other CRMs.
Recycling also makes an important and growing contribution to global ruthenium supply. However, its end-of-life recycling rate varies considerably by country and by application. Closed loop recycling can achieve very high levels of ruthenium recovery, chiefly from catalysts, but also from manufacturing waste and by-products, such as chemicals, solutions, and other chemical scraps. The ruthenium EOL-RIR is assessed as equal to 11%.
The EOL-RIR of scandium is 0%. This is because, due to its limited uses, no recycling circuit is known for scandium in end-of-life products nor at the stage of ‘new scrap’. Unfortunately, the same EOL-RIR value is estimated for silicon metal, as well. As a matter of fact, most chemical applications are dispersive, thus not allowing for any recovery. There is research on recycling of silicon wafers; however, it has not yet materialized in marketable solutions. Finally, there is no functional recycling of silicon metal in aluminum alloys.
Tantalus has a low EOL-RIR, less than 1%. Recycling refers above all to the upstream supply chain, rather than the end-users. The main source of this recycled material is from the electronics industry.
The good news is that tungsten and vanadium have a significant high value of the EOL-RIR, approximately equal to 42% and 44%, respectively. For example, recycling of tungsten in high speed steel is high (a typical melt contains 60% to 70% scrap), including internally generated scrap. Vanadium also finds a good supply source in alloys recycling.
Finally, yttrium, in which the EOL-RIR is estimated at around 31%, is mainly recycled from fluorescent lamps. Minor amounts came from oxygen sensors contained in end-of-life vehicles.
In the next paragraph, a design strategy is described that allows the designer to select a metallic material having the lowest amount of CRMs characterized by the lowest value of the EOL-RIR values.
3. The Method
The proposed method is based on Ashby’s material selection approach. An objective equation is first defined that needs to be minimized. By taking into account recycling as a mitigating action to reduce the CRMs related issues, such an objective equation (P) takes the following form Equation (1):
where m is the mass of the component to be produced, and (1 − χ
a) is an index quantifying the criticality in terms of EOL-RIR per the unit of mass of the alloy itself:
In Equation (2), n is the number of elements in the alloy chemical composition, and wt%i is the amount of element ‘i’ contained in the alloy and measured in weight percent. Further, the EOL-RIR of non-critical elements is assumed as equal to 100%.
Since χ
a quantifies the total EOL-RIR per unit of mass of the alloy, the objective equation P measures the
criticality associated with the critical raw materials EOL-RIR
per unit of function (to be minimized). The minimization of P is aimed at limiting the number of CRMs with the lowest EOL-RIR values required to produce a specified component. In
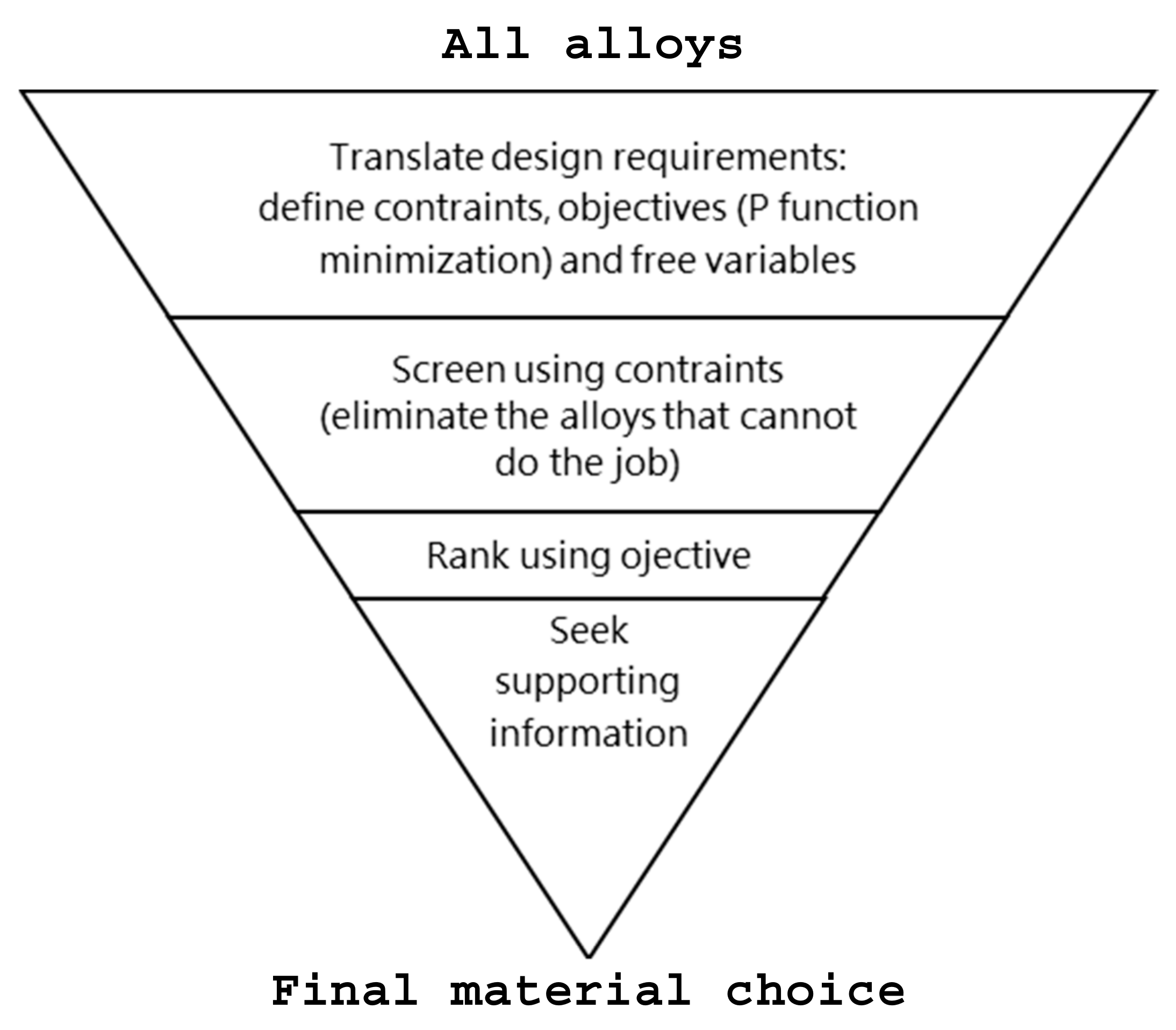
Figure 1, Ashby’s strategy for material selection applied in this work is summarized. First, the design requirements must be translated in terms of not-negotiable conditions that must be met to assure the working of the component (e.g., the component must be strong enough, must be able to be cast, or it must meet some fixed dimensions). The designer also has an objective that, in this case, is the amount reduction of CRMs contained in the alloy having the lowest values of EOL-RIR per unit of function. Fortunately, there are some parameters that can be adjusted in order to optimize the objective, such as the shape and area of the cross section and, above all, the alloy. The second step of the materials selection procedure is
screening (
Figure 1). Candidates that cannot do the job at all because one or more of their attributes lies outside the limits set by the constraints are eliminated. ‘Survived’ alloys are then classified based on an optimization criterion that is found in the material index. Lastly is a functon of materials properties only, and it measures how well a candidate that has passed the screening step can do the job. For example, if the alloy for a cylindrical tie-rod of specified length L, carrying a tensile force F without failure and minimizing the objective equation P has to be selected, Equation (1) must be written as:
where,
is the material density, and A is the area of the cross section. Now, the constraint equation is:
where σ
y the yield stress of the alloy. Using Equation (4) to eliminate the free variable, A, in Equation (3) gives
It is noted that the first bracket contains the specified load F. The second bracket contains the specified geometry, L. The last bracket contains the material properties. Note that (1 − χ
a) is also a material property referring to the recyclability characteristics of the CRMs contained in the alloy itself. The tie that will carry F safely and minimize the supply risks linked to CRMs is that made of the alloy with the smallest value of the following material index:
Obviously, the material index M may change according to the component function and constraint equations.
According to
Figure 1, the final step consists of seeking supporting information about the best alloys obtained in the previous step in order to provide confirmations and seek similar applications, such as case-studies.
In the following paragraph, a case-study describing the material selection for flywheels is illustrated as an example.
4. Case-study: Alloys for Flywheels
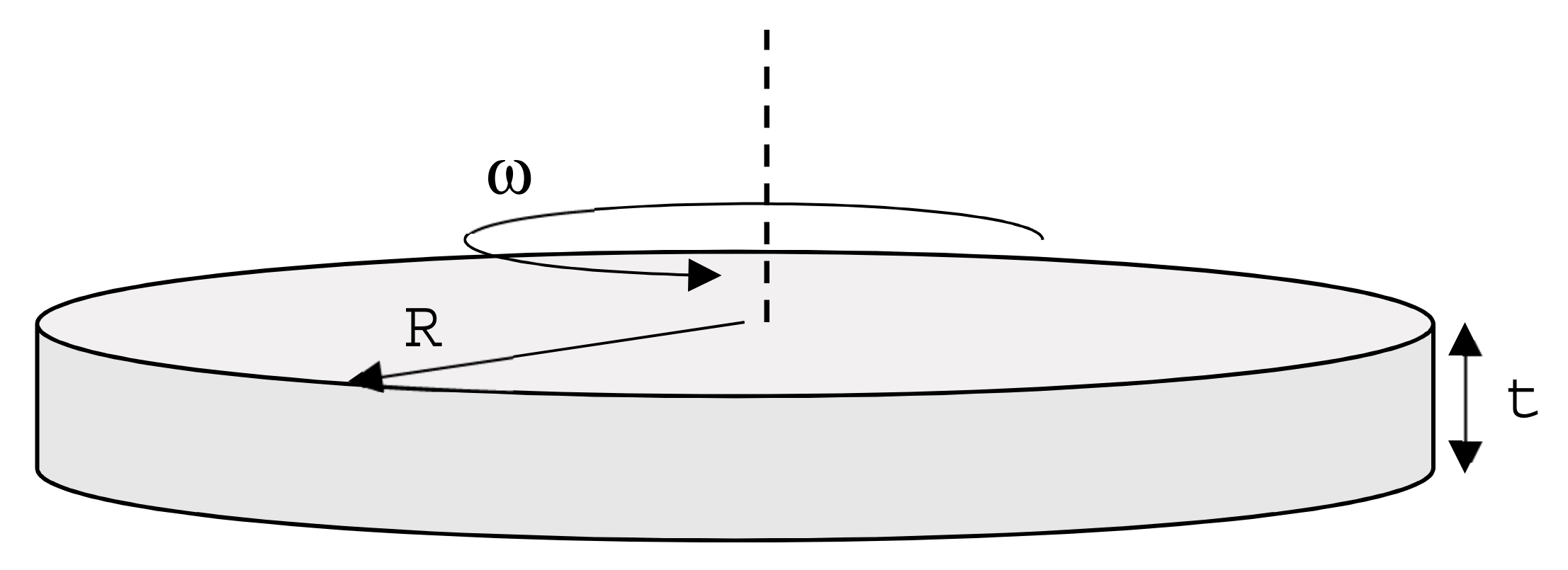
Flywheels store energy. In old steam engines, they are made of cast iron. Cars have them as well, to smooth power-transmission. Recently, flywheels have been proposed for power storage and generative braking systems for vehicles; what is the best choice of alloy for a flywheel in a critical raw materials recycling perspective? An efficient flywheel stores as much energy per unit weight as possible. The limit is set by failure caused by centrifugal loading. It can be schematized by a solid disk of radius R (free variable) and thickness t (constraint), rotating with angular velocity ω (
Figure 2). The energy stored in the flywheel is:
where J = (π/2)ρR
4t is the polar moment of inertia of the disk, and ρ the density of the alloy of which is made, giving
In a CRMs perspective aimed at reducing the amount of those materials with the lowest EOL-RIR values, the quantity to be maximized is the kinetic energy per unit of function P, which is given by the following equation:
As the flywheel is spun up, the energy stored in it increases, but so does the centrifugal stress. The maximum principal stress in a spinning disk of uniform thickness is
where ν is the Poisson’s ratio. This stress must not exceed the failure stress σ
f (whit an appropriated factor of safety, here omitted). Thus, the angular velocity, ω, and the disk radius are limited by:
Substituting Equation (12) in Equation (10) gives
The best materials are those with the high value of the material index
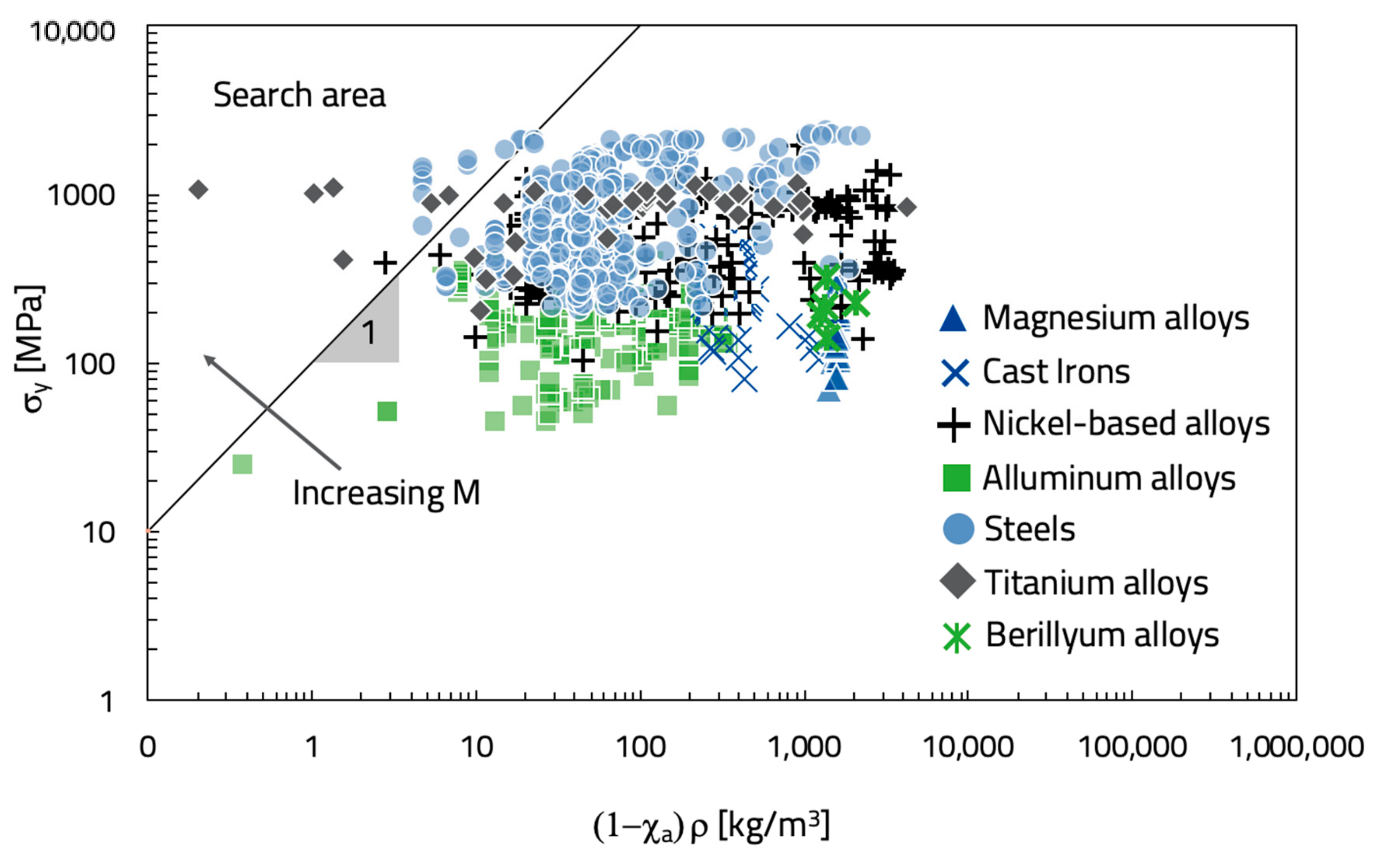
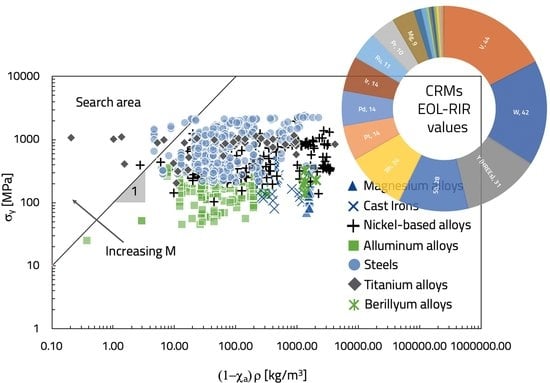
In a σ versus (1 − χ
a)ρ log–log diagram, Equation (14) is represented by a straight line of slope 1, while each material is represented as a point (
Figure 3). All the materials intercepted by the straight line are characterized by the same material index (M) value. Thus, all the materials above that straight line have a higher value of M. By moving the straight line towards the upper left corner, the materials with the highest value of M are selected.
Figure 3 shows that materials with the highest material index value are titanium alloys (e.g., Ti-6Al-2Sn-4Zr-6Mo), some kinds of steel (i.e., UNS S13800), and a nickel-based alloy (i.e., Ni-45Ti Nitinol, UNS N01555). On the other hand, the worst alloys belong to the magnesium alloys family (e.g., ASTM EZ33A). The ASTM EZ33A is used in aerospace, electronics, automotive, sports goods, nuclear, and tools. However, because of its low yield stress (about 80 MPa) and high criticality index, (1 − χ
a) = 0.873, it is not suitable for the flywheel production in a CRMs recycling perspective. Mg, Ce, and La contained in that alloy (
Table 2) are all CRMs with a very low EOL-RIR (
Table 1); moreover, Mg is also the matrix element.
On the contrary, the titanium alloy, Ti-6Al-2Sn-4Zr-6Mo, does not contain CRMs (
Table 2), and its yield strength is very high (1085 MPa). It is used in gas turbine applications or deep sour wells. However, what is most important is that it is made by elements characterized by a high EOL-RIR. Similar considerations can be made for the steels and nickel-based alloys that maximize the material index (
Figure 3). Finally, the obtained solutions should be deeply evaluated on the basis of supporting information required to achieve the final choice.
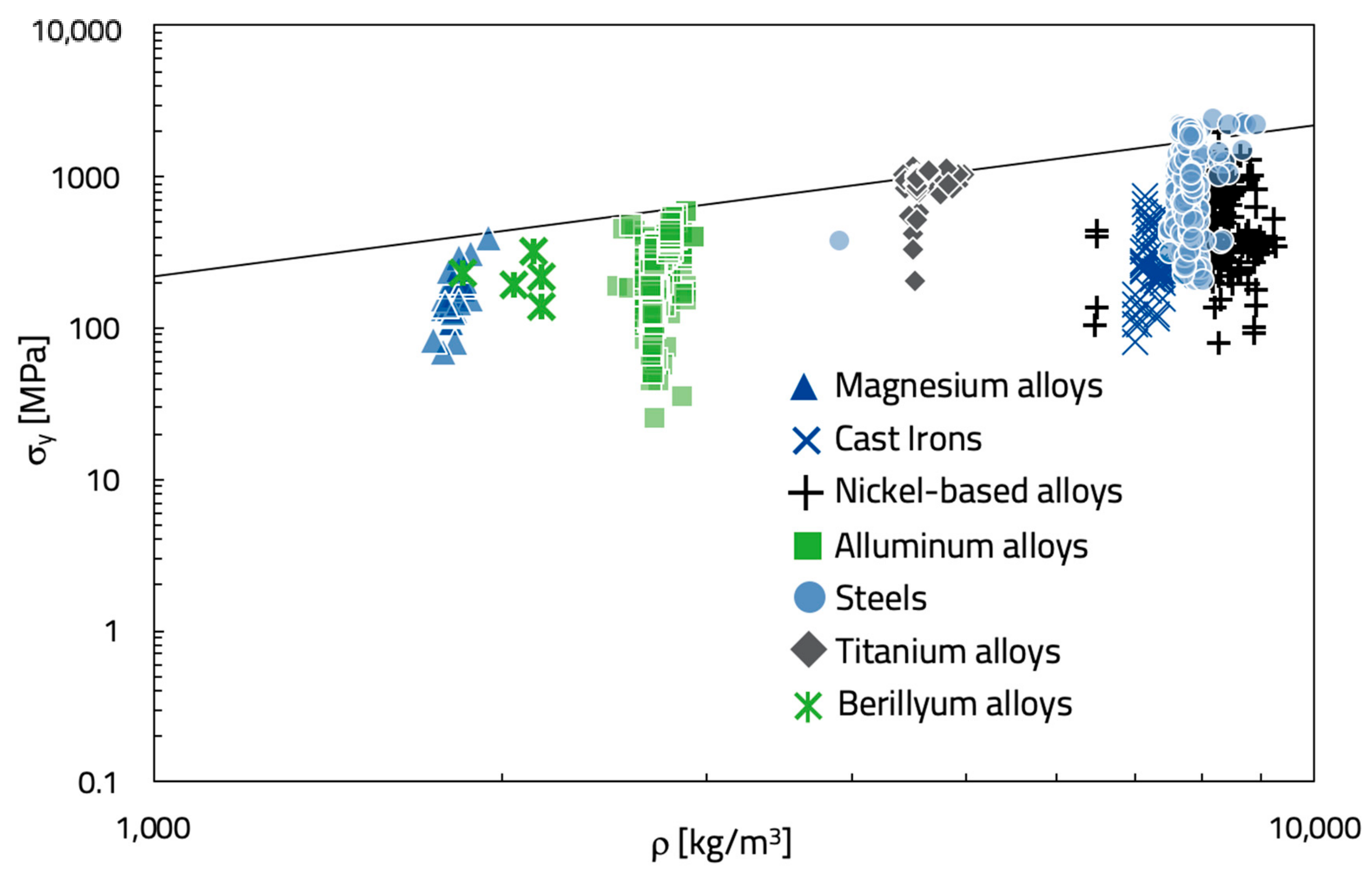
It is noted that if the energy per unit of mass (U/m) needs to be optimized, as in standard design, the materials index is the specific yield stress, σ
y/ρ. The Ashby’s map required to select the best alloys is shown in
Figure 4, where the yield strength is plotted against the density. The log scales allow the material index (M = σ
y/ρ) to be plotted as a set of parallel straight lines of slope 1 and different values of M:
Each line intercepts all materials characterized by the same value of M. All the materials that lie on a line of constant M perform equally well; those above the line are better, and those below, worse. The subset of materials with particularly good values of the index is identified by picking a line that isolates a search area containing a reasonably small number of candidates, as shown schematically in
Figure 4 as a diagonal selection line. In this case, some magnesium alloys (e.g., EA55RS, T4) and aluminum alloys (e.g., EN AW-7055) appear suitable for flywheels design. Because of its near to zero value of (1 − χ
a), the previous titanium alloy remains one of the best solutions, as well. Finally, it is observed that cast iron, which was the original material used to produce flywheels, neither optimize the criticality nor the energy per unit of mass. Probably, IDIs (isothermal ductile irons), that are not included in the present database, could succeed because of their poor chemical composition and high mechanical properties induced by heat treatment [
18].
The final decision requires a deeper analysis of the constraint conditions, verification of geometric details (e.g., the free variable R, must not exceed a limit value), and search for similar case studies in the literature. Despite this, the proposed method suggests, in a systematic way, plausible alternative solutions inside the metallic materials universe.