Protective Low-Density Polyethylene Residues from Prepreg for the Development of New Nanocomposites with Montmorillonite: Recycling and Characterization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
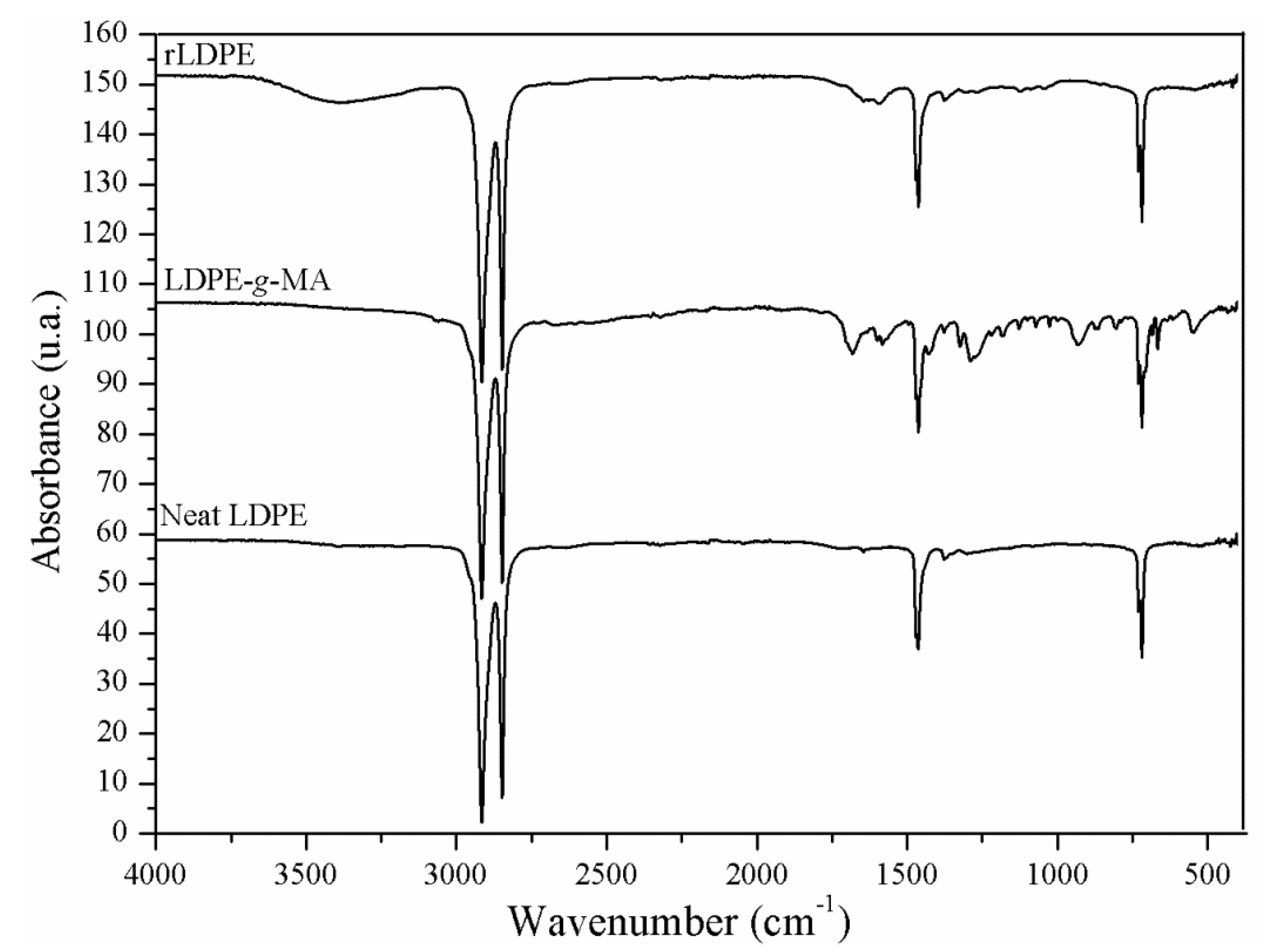
2.2. Preparation of Compatibilizer Agent (LDPE-g-MA)
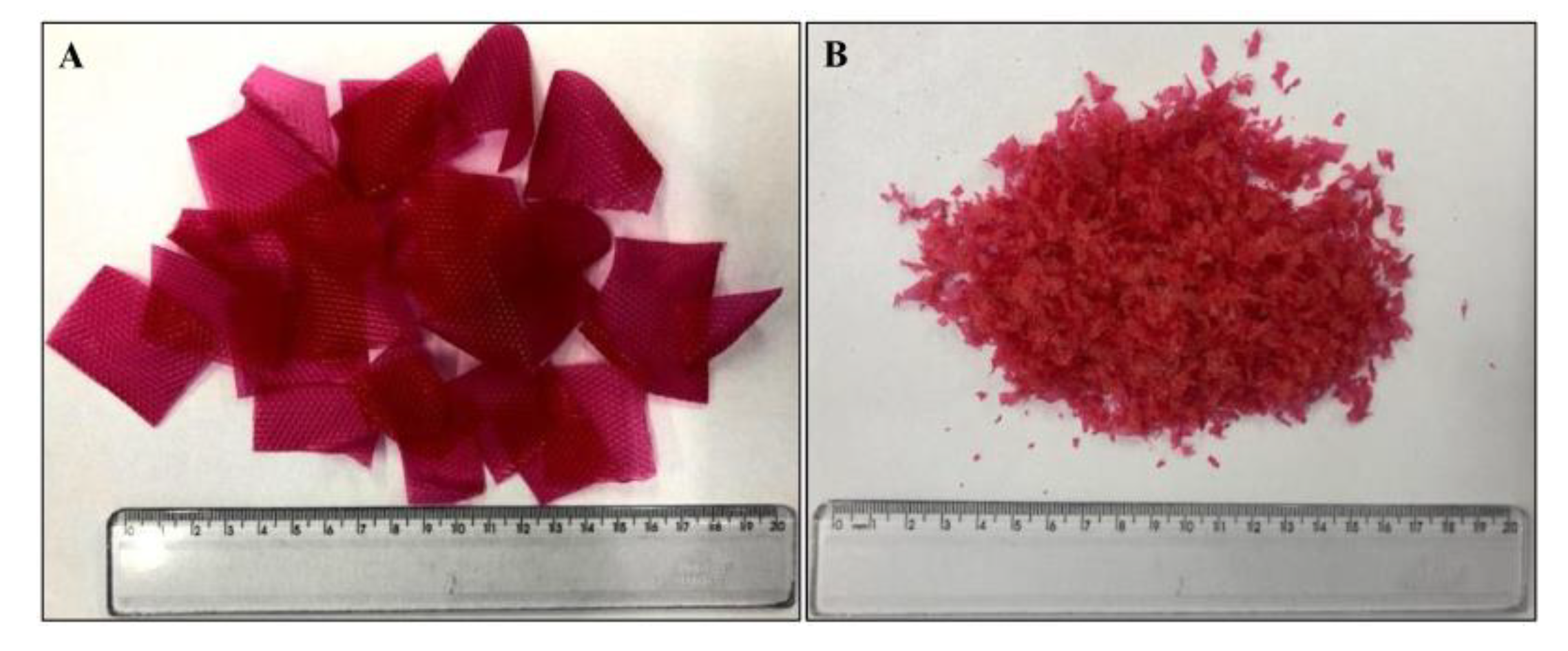
2.3. Preparation of rLDPE by Milling of the LDPE Films Waste
2.4. Sample Processing
3. Composites Obtained: Characterization
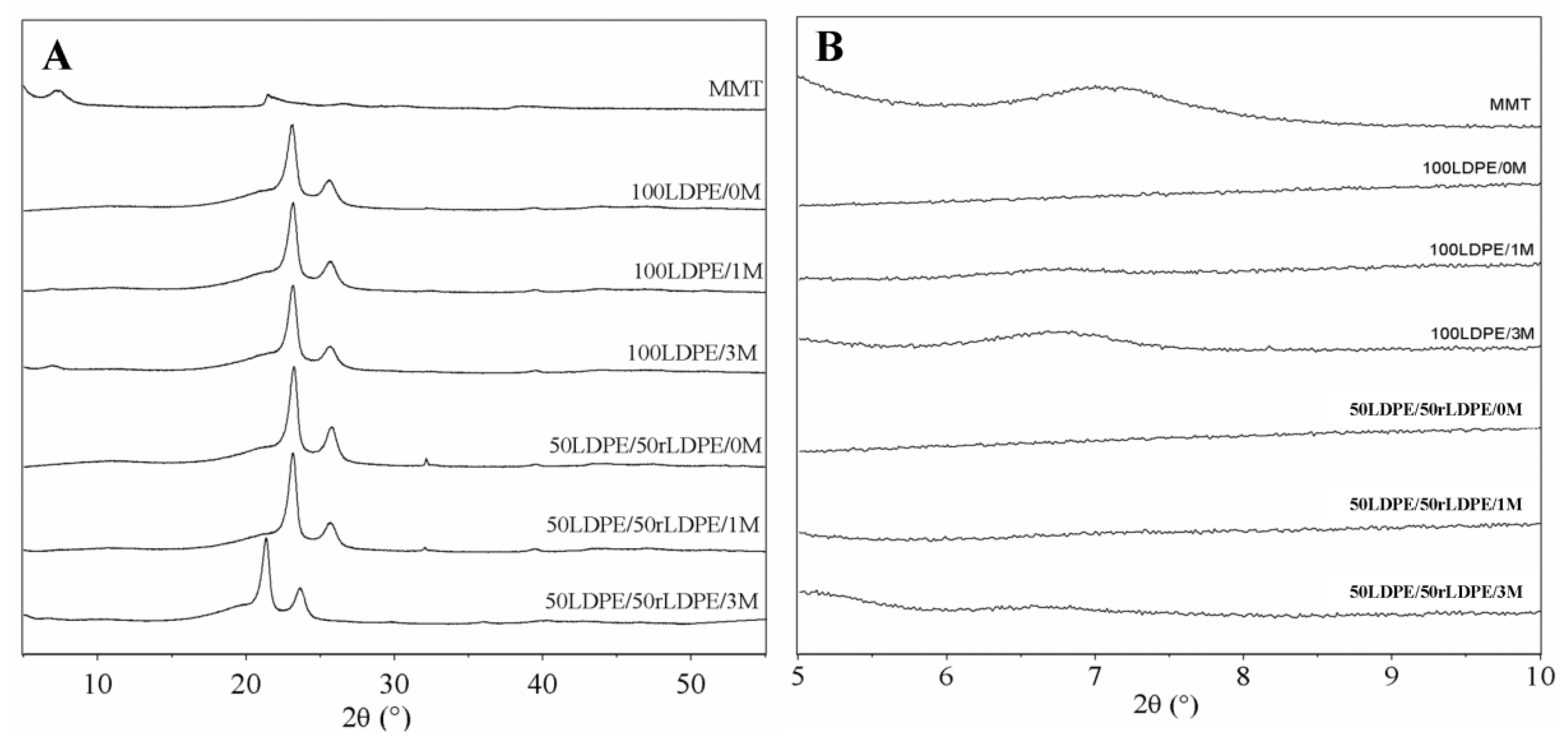
3.1. X-Ray Diffraction (XRD)
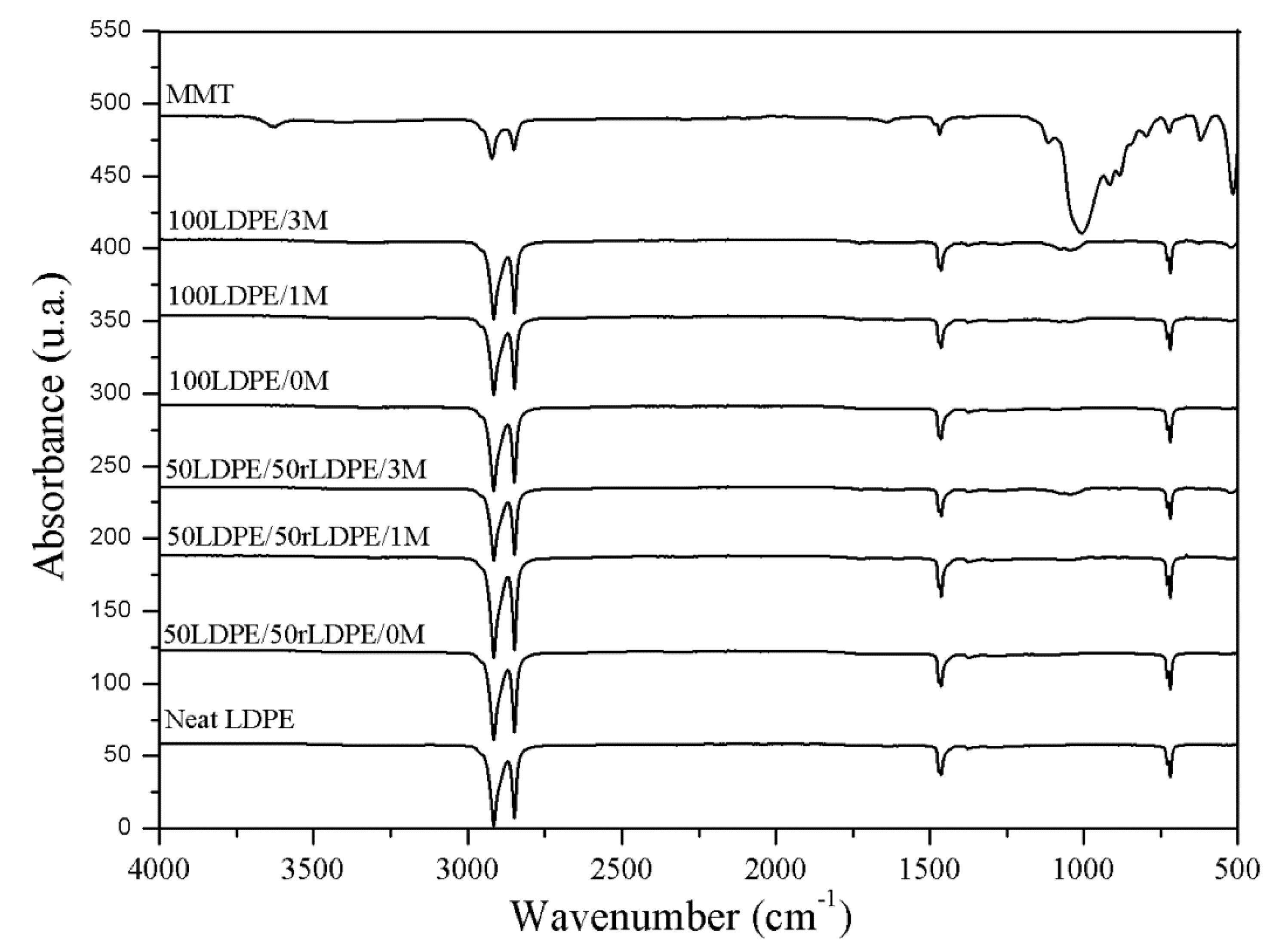
3.2. FT-IR/UATR
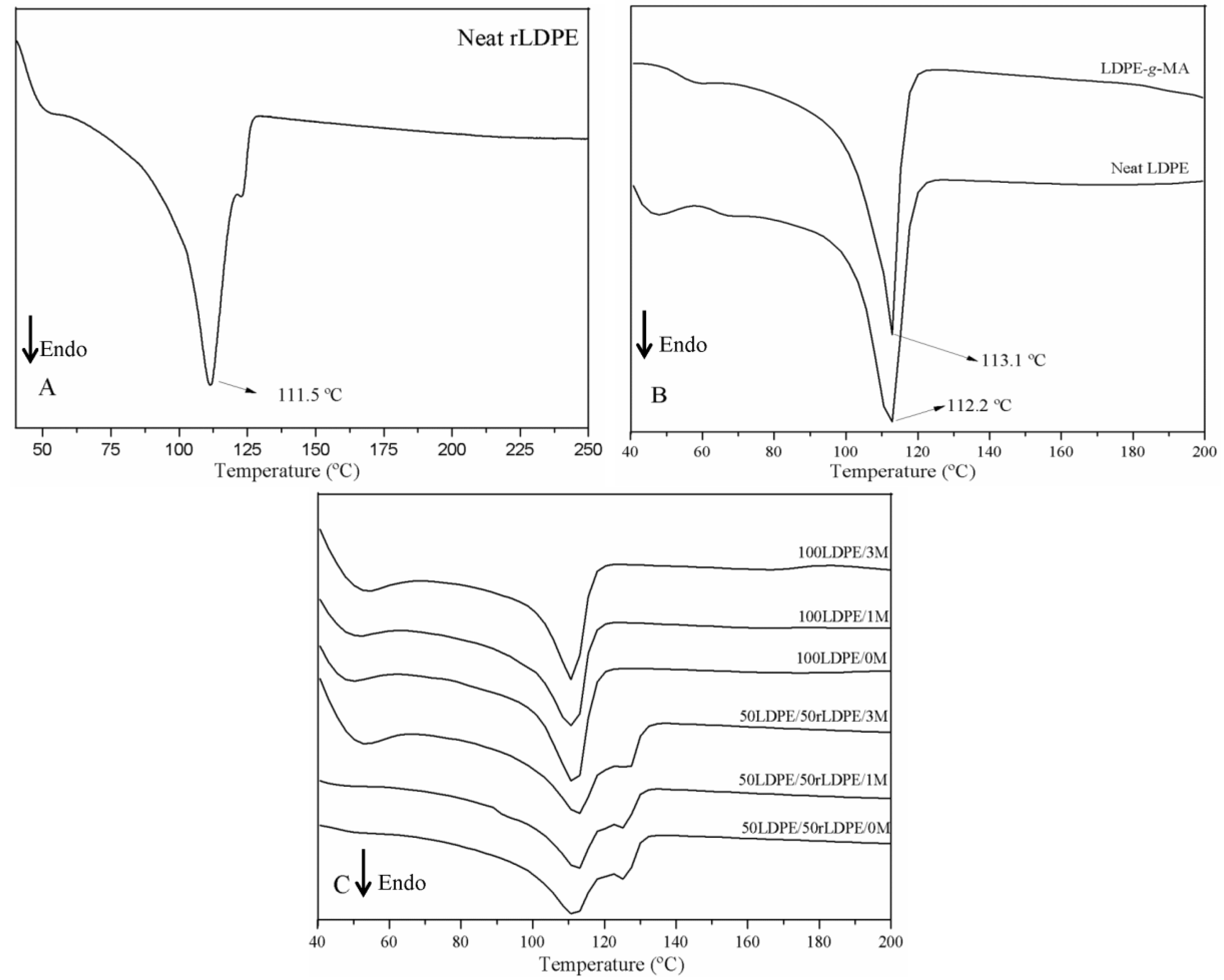
3.3. Differential Scanning Calorimetry (DSC)
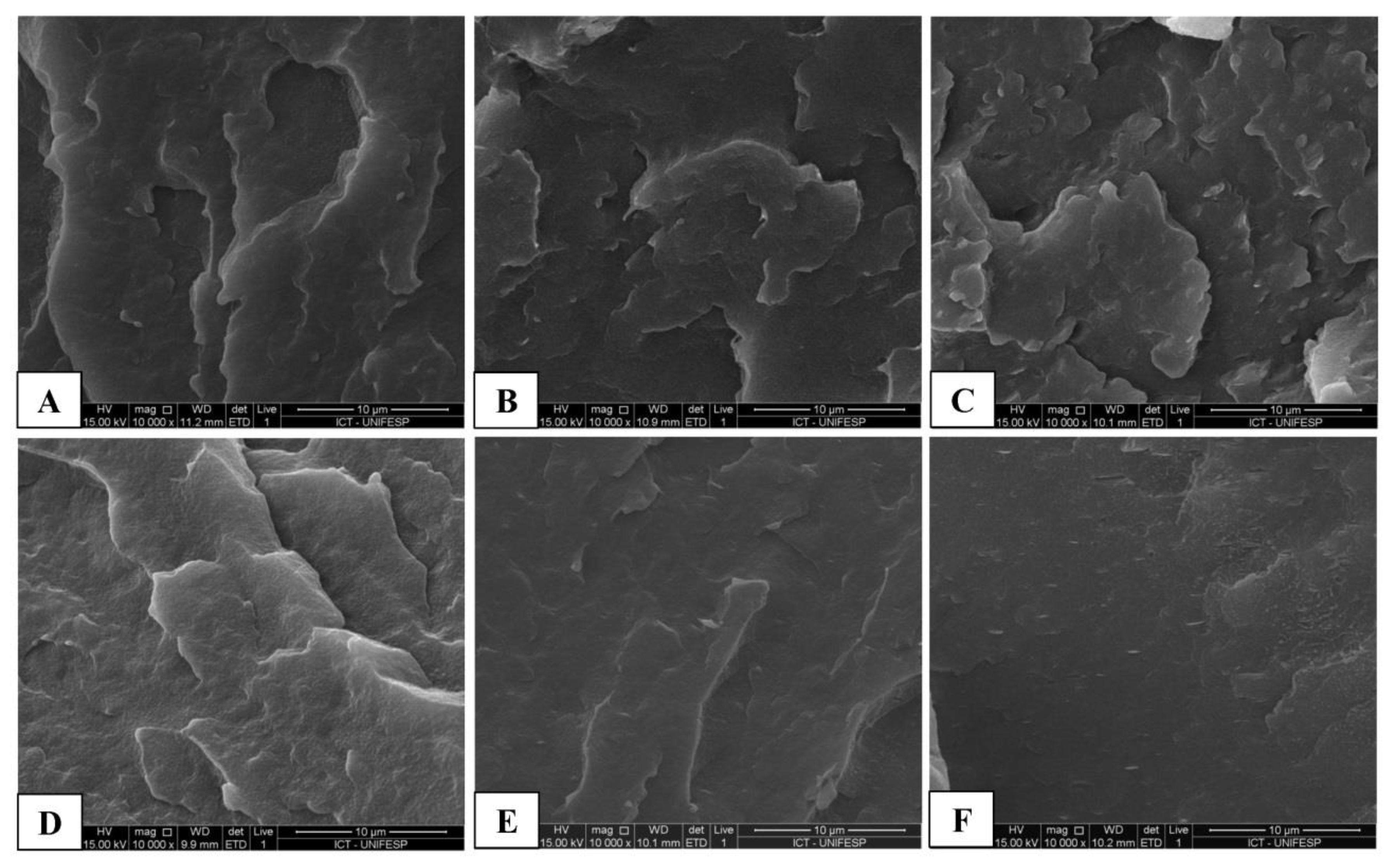
3.4. Scanning Electron Microscopy (SEM)
3.5. Mechanical Properties
4. Results and Discussion
4.1. XRD: MMT and Nanocomposites
4.2. FT-IR/UATR
4.3. Thermal, Morphological and Mechanical Characterization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soto, J.M.; Blázquez, G.; Calero, M.; Quesada, L.; Godoy, V.; Martín-lara, M.Á. A real case study of mechanical recycling as an alternative for managing of polyethylene plastic film presented in mixed municipal solid waste. J. Clean. Prod. 2018, 203, 777–787. [Google Scholar] [CrossRef]
- Jnr, A.K.; Yunana, D.; Kamsouloum, P.; Webster, M.; Wilson, D.C.; Cheeseman, C. Recycling waste plastics in developing countries: Use of low-density polyethylene water sachets to form plastic bonded sand blocks. Waste Manag. 2018, 80, 112–118. [Google Scholar]
- Vaverková, M.D.; Winkler, J.; Adamcová, D.; Radziemska, M.; Uldrijan, D.; Zloch, J. Municipal solid waste land fi ll–Vegetation succession in an area transformed by human impact. Ecol. Eng. 2019, 129, 109–114. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.H. Science of the Total Environment Review of leaching behavior of municipal solid waste incineration. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Xu, Y.; Taiebat, M.; Lastoskie, C.; Miller, S.A. Life cycle assessment of end-of-life treatments for plastic fi lm waste. J. Clean. Prod. 2018, 201, 1052–1060. [Google Scholar] [CrossRef]
- Wagner, T.P.; Science, E.; Maine, S.; States, U. Sustainability and Plastic Waste; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Matar, S.; Hatch, L.F. Synthetic Petroleum-Based Polymers. In Chemistry of Petrochemical Processes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 323–373. [Google Scholar]
- Rahimi, A.; García, J.M. Chemical recycling of wast plastic for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Garofalo, E.; di Maio, L.; Scarfato, P.; di Gregorio, F.; Incarnato, L. Reactive compatibilization and melt compounding with nanosilicates of post-consumer fl exible plastic packagings. Polym. Degrad. Stab. 2018, 152, 52–63. [Google Scholar] [CrossRef]
- Scarfato, P.; Incarnato, L.; di Maio, L.; Dittrich, B. Influence of a novel organo-silylated clay on the morphology, thermal and burning behavior of low density polyethylene composites. Compos. Part B 2016, 98, 444–452. [Google Scholar] [CrossRef]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of nanostructured polymers and advanced polymeric based nanocomposites. Mater. Sci. Eng. R Rep. 2014, 85, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Shugulí, C.; Rodríguez, F.J.; Bruna, J.E.; Galotto, M.J.; Sarantópoulos, C.; Perez, M.A.F.; Padula, M. Cetylpyridinium bromide-modified montmorillonite as filler in low density polyethylene nanocomposite films. Appl. Clay Sci. 2019, 168, 203–210. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A Review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Pessan, L.A.; Passador, F.R.; Coll, A. Structural, Thermal, and Gas Transport Properties of HDPE/LLDPE Blend-Based Nanocomposites Using a Mixture of HDPE-g-MA and LLDPE-g-MA as Compatibilizer. Polym. Eng. Sci. 2016, 56, 765–775. [Google Scholar]
- Passador, F.R.; Ruvolo-Filho, A.C.; Pessan, L.A. Influence of blending protocol on the thermal and mechanical properties of hdpe/lldpe blend-based nanocomposites. AIP Conf. Proc. 2014, 1593, 278–281. [Google Scholar]
- Chang, M.-K. Journal of Industrial and Engineering Chemistry Mechanical properties and thermal stability of low-density polyethylene grafted maleic anhydride/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2015, 27, 96–101. [Google Scholar] [CrossRef]
- Spencer, M.W.; Cui, L.; Yoo, Y.; Paul, D.R. Morphology and properties of nanocomposites based on HDPE/HDPE-g-MA blends. Polymer 2010, 51, 1056–1070. [Google Scholar] [CrossRef]
- Hotta, S.; Paul, D.R. Nanocomposites formed from linear low density polyethylene and organoclays. Polymer 2004, 45, 7639–7654. [Google Scholar] [CrossRef]
- Wilfong, D.L. Crystallization Mechanisms for LLDPE and Its Fractions. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 861–870. [Google Scholar] [CrossRef]
- Høgsaa, B.; Fini, E.H.; Christiansen, J.D.C.; Hung, A.; Mousavi, M.; Jensen, E.A.; Sanporean, C.G. A Novel Bioresidue to Compatibilize Sodium Montmorillonite and Linear Low Density Polyethylene. Ind. Eng. Chem. Res. 2018, 57, 1213–1224. [Google Scholar] [CrossRef]
- Kumanayaka, T.O.; Parthasarathy, R.; Jollands, M. Accelerating effect of montmorillonite on oxidative degradation of polyethylene nanocomposites. Polym. Degrad. Stab. 2010, 95, 672–676. [Google Scholar] [CrossRef]
- da Silva, D.J.; Wiebeck, H. CARS-PLS regression and ATR-FTIR spectroscopy for eco-friendly and fast composition analyses of LDPE/HDPE blends. J. Polym. Res. 2018, 25, 112. [Google Scholar] [CrossRef]
- Sclavons, M.; Franquinet, P.; Carlier, V.; Verfaillie, G.; Fallais, I.; Legras, R.; Thyrion, F.C. Quantification of the maleic anhydride grafted onto polypropylene by chemical and viscosimetric titrations, and FTIR spectroscopy. Polymer 2000, 41, 1989–1999. [Google Scholar] [CrossRef]
- Smith, A.L. Applied Infrared Spectroscopy, Fundamentals, Techniques, and Analytical Problem Solving; Wiley: New York, NY, USA, 1979; Volume 54. [Google Scholar]
- Zhang, J.; Gupta, R.K.; Wilkie, C.A. Controlled silylation of montmorillonite and its polyethylene nanocomposites. Polymer 2006, 47, 4537–4543. [Google Scholar] [CrossRef]
- Xie, L.; Lv, X.Y.; Han, Z.J.; Ci, J.H.; Fang, C.Q.; Ren, P.G. Preparation and Performance of High-Barrier Low Density Polyethylene/Organic Montmorillonite Nanocomposite. Polym. Plast. Technol. Eng. 2012, 51, 1251–1257. [Google Scholar] [CrossRef]
- Baur, E.; Osswald, T.A.; Rudolph, N.; Baur, E.; Osswald, T.A.; Rudolph, N. Plastics Handbook: The Resource for Plastics Engineers, 5th ed.; Carl Hanser Verlag GmbH Co KG: München, Germany, 2019; pp. I–XXI. [Google Scholar]
- David, D.; Moreno, P.; Saron, C. Low-density polyethylene waste/recycled wood composites. Compos. Struct. 2017, 176, 1152–1157. [Google Scholar]
- Lee, D.; Kim, H.; Yoon, K.; Eun, K. Polyethylene/MMT nanocomposites prepared by in situ polymerization using supported catalyst systems. Sci. Technol. Adv. Mater. 2005, 6, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Chrissopoulou, K.; Altintzi, I.; Anastasiadis, S.H.; Giannelis, E.P.; Pitsikalis, M. Controlling the miscibility of polyethylene/layered silicate nanocomposites by altering the polymer/surface interactions. Polymer 2005, 46, 12440–12451. [Google Scholar] [CrossRef]
- Golebiewski, J.; Rozanski, A.; Dzwonkowski, J.; Galeski, A. Low density polyethylene–montmorillonite nanocomposites for film blowing. Eur. Polym. J. 2008, 44, 270–286. [Google Scholar] [CrossRef]
- Passador, F.R. Avaliação de sistemas compatibilizantes nas correlações entre processamento e propriedades térmicas, mecânicas e de transporte em nanocompósitos de blenda HDPE/LLDPE e OMMT. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2012. [Google Scholar]
- Liu, S.; Tu, L. Studies on mechanical properties of dispersing intercalated silane montmorillonite in low density polyethylene matrix. Int. Commun. Heat Mass Transf. 2011, 38, 879–886. [Google Scholar] [CrossRef]
- Opelt, C.V.; Coelho, L.A.F. Reinforcement and toughening mechanisms in polymer nanocomposites—Reinforcement effectiveness and nanoclay nanocomposites. Mater. Chem. Phys. 2016, 169, 179–185. [Google Scholar] [CrossRef]
- Varghai, D.; Maiorana, A.; Meng, Q.; Gross, R.A.; Manas-Zloczower, I. Sustainable, electrically-conductive bioepoxy nanocomposites. Polymer 2016, 107, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Zhong, Q. Stable aqueous foams created with intercalated montmorillonite nanoclay coated by sodium caseinate. J. Food Eng. 2019, 248, 36–45. [Google Scholar] [CrossRef]
| Samples Composition | LDPE (%) | LDPE-g-MA (wt%) | rLDPE (%) | MMT (wt/%) |
|---|---|---|---|---|
| 50LDPE/50rLDPE/0M | 50 | 5 | 50 | 0 |
| 50LDPE/50rLDPE/1M | 50 | 5 | 50 | 1 |
| 50LDPE/50rLDPE/3M | 50 | 5 | 50 | 3 |
| 100LDPE/0M | 100 | 5 | 0 | 0 |
| 100LDPE/1M | 100 | 5 | 0 | 1 |
| 100LDPE/3M | 100 | 5 | 0 | 3 |
| Samples | Tm (°C) | ΔHm (J/g) | XC (%) |
|---|---|---|---|
| Neat rLDPE | 112 | 100.4 | 34.3 |
| Neat LDPE | 112 | 76.86 | 26.2 |
| LDPE-g-MA | 113 | 84.28 | 28.8 |
| 50LDPE/50rLDPE/0M | 112 | 98.38 | 33.6 |
| 50LDPE/50rLDPE/1M | 112 | 96.52 | 34.0 |
| 50LDPE/50rLDPE/3M | 112 | 98.93 | 34.5 |
| 100LDPE/0M | 112 | 84.78 | 28.9 |
| 100LDPE/1M | 111 | 81.94 | 28.2 |
| 100LDPE/3M | 110 | 73.06 | 25.7 |
| Samples | IS (J/m) | UTS (MPa) | E (MPa) | Shore D Hardness |
|---|---|---|---|---|
| 50LDPE/50rLDPE/0M | 40.45 ± 2.81 | 9.5 ± 0.1 | 88.20 ± 14.86 | 52.64 ± 1.09 |
| 50LDPE/50rLDPE/1M | 39.75 ± 0.46 | 9.6 ± 0.3 | 54.93 ± 11.03 | 56.09 ± 0.89 |
| 50LDPE/50rLDPE/3M | 38.17 ± 2.55 | 9.6 ± 0.1 | 47.25 ± 6.01 | 54.84 ± 1.07 |
| 100LDPE/0M | 34.31 ± 3.42 | 8.4 ± 0.1 | 53.70 ± 12.01 | 52.07 ± 0.88 |
| 100LDPE/1M | 36.81 ± 7.30 | 8.7 ± 0.7 | 54.75 ± 10.83 | 52.37 ± 0.91 |
| 100LDPE/3M | 34.59 ± 3.57 | 8.4 ± 0.1 | 51.88 ± 5.05 | 51.51 ± 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Passos Severino, P.; Larissa do Amaral Montanheiro, T.; Ferro, O.; Roberto Passador, F.; Stieven Montagna, L. Protective Low-Density Polyethylene Residues from Prepreg for the Development of New Nanocomposites with Montmorillonite: Recycling and Characterization. Recycling 2019, 4, 45. https://doi.org/10.3390/recycling4040045
Rodrigues Passos Severino P, Larissa do Amaral Montanheiro T, Ferro O, Roberto Passador F, Stieven Montagna L. Protective Low-Density Polyethylene Residues from Prepreg for the Development of New Nanocomposites with Montmorillonite: Recycling and Characterization. Recycling. 2019; 4(4):45. https://doi.org/10.3390/recycling4040045
Chicago/Turabian StyleRodrigues Passos Severino, Pamela, Thaís Larissa do Amaral Montanheiro, Orestes Ferro, Fábio Roberto Passador, and Larissa Stieven Montagna. 2019. "Protective Low-Density Polyethylene Residues from Prepreg for the Development of New Nanocomposites with Montmorillonite: Recycling and Characterization" Recycling 4, no. 4: 45. https://doi.org/10.3390/recycling4040045
APA StyleRodrigues Passos Severino, P., Larissa do Amaral Montanheiro, T., Ferro, O., Roberto Passador, F., & Stieven Montagna, L. (2019). Protective Low-Density Polyethylene Residues from Prepreg for the Development of New Nanocomposites with Montmorillonite: Recycling and Characterization. Recycling, 4(4), 45. https://doi.org/10.3390/recycling4040045






