New Nitrogen-Containing Recycled Fertilizers: Bioavailability of Nutrients and Harmful Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Granulation
2.2. Analysis Methods
2.3. Leaching Trial
3. Results and Discussion
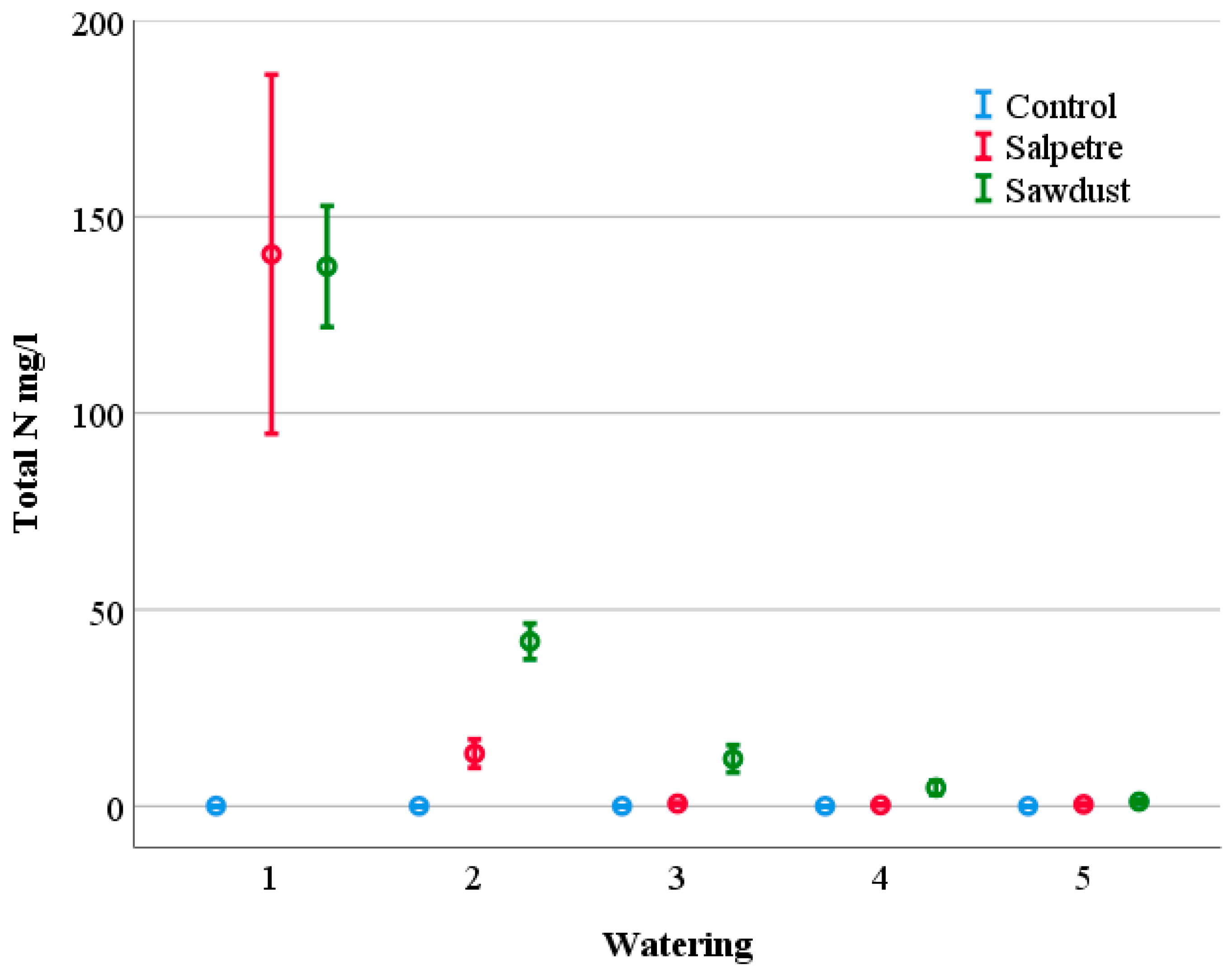
3.1. Contents and Leaching of Nutrients
3.2. Contents and Leaching of Harmful Elements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B
| Source | df | F | Sig. |
|---|---|---|---|
| Intercept | 1/4.6 | 19.88 | 0.008 |
| Treatment | 2/4.6 | 104.31 | 0.000 |
| Watering | 4/17.9 | 22.62 | 0.000 |
| Treatment × Watering | 8/17.9 | 3.98 | 0.007 |
References
- European Commission Circular Economy Strategy. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 19 February 2018).
- European Commission. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Wood, S.; Cowie, A. A Review of Greenhouse Gas Emission Factors for Fertiliser Production; IEA Bioenergy Task 38: Sydney, Australia, 2004. [Google Scholar]
- Aho, M.; Pursula, T.; Saario, M.; Miller, T.; Kumpulainen, A.; Päällysaho, M.; Autio, M.; Hillgren, A.; Descombes, L. Ravinteiden Kierron Taloudellinen Arvo ja Mahdollisuudet SUOMELLE (Econimic Value and Possibilites of Nutrient Recycling for Finland); Sitran selvityksiä; Sitra: Helsinki, Finland, 2015; p. 50. [Google Scholar]
- Ojala, E. Selvitys puu- ja Turvetuhkan Lannoite- Sekä Muusta Hyötykäytöstä (Report on the Utilization of Wood and Peat Based Ash as A Fertilizer and in Other Applications); Motiva: Helsinki, Finland, 2010. [Google Scholar]
- Sanna, M.; Venelampi, O.; Iho, A.; Koikkalainen, K.; Lehtonen, E.; Luostarinen, S.; Rasia, K.; Sarvi, M.; Tampio, E.; Turtola, E.; et al. Kohti Ravinteiden Kierrätyksen Läpimurtoa (Towards the Breakthrough of Nutrent Recycling); Luonnonvara- ja biotalouden tutkimus: Helsinki, Finland, 2017. [Google Scholar]
- Hannam, K.D.; Deschamps, C.; Kwiaton, M.; Venier, L.A.; Hazlett, P.W. Regulations and Guidelines for the Use of Wood Ash as A Soil Amendment in Canadian Forests; Information Report GLC-X-17; Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre: Ottawa, ON, Canada, 2016.
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolinčius, R.; Varnagiryte-Kabasinskiene, I. Wood Ash Recycling—Possibilities and Risks. In Sustainable Use of Forest Biomass for Energy: A Synthesis with Focus on the Baltic and Nordic Region; Röser, D., Asikainen, A., Eds.; Springer Netherlands: Dordrecht, The Nederland, 2008; pp. 79–108. ISBN 978-1-4020-5054-1. [Google Scholar]
- Moilanen, M.; Hytönen, J.; Hökkä, H.; Ahtikoski, A. Fertilization increased growth of Scots pine and financial performance of forest management in a drained peatland in Finland. Silva Fenn. 2015, 49, 1–18. [Google Scholar] [CrossRef]
- Moilanen, M.; Silfverberg, K.; Hökkä, H.; Issakainen, J. Comparing effects of wood ash and commercial PK fertiliser on the nutrient status and stand growth of Scots pine on drained mires. Balt. For. 2004, 10, 2–10. [Google Scholar]
- Pugliese, S.; Jones, T.; Preston, M.D.; Hazlett, P.; Tran, H.; Basiliko, N. Wood ash as a forest soil amendment: The role of boiler and soil type on soil property response. Can. J. Soil Sci. 2014, 94, 621–634. [Google Scholar] [CrossRef]
- Rasmusson, H.; Sarenbo, S.; Claesson, T. Ash Products and Their Economic Profitability. Open Waste Manag. J. 2013, 6, 1–5. [Google Scholar] [CrossRef][Green Version]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Lindberg, R.; Sahlén, K.; Tysklind, M. Occurrence and Distribution of Synthetic Organic Substances in Boreal Coniferous Forest Soils Fertilized with Hygienized Municipal Sewage Sludge. Antibiot. Basel Switz. 2013, 2, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, J.; Kuokkanen, T.; Rautio, P.; Lassi, U. Bioavailability of nutrients and harmful elements in ash fertilizers: Effect of granulation. Biomass Bioenergy 2017, 100, 92–97. [Google Scholar] [CrossRef]
- European Commission. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste; European Commission: Brussels, Belgium, 1999. [Google Scholar]
- European Commission. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge is Used in Agriculture; European Commission: Brussels, Belgium, 1986. [Google Scholar]
- Ministry of the Environment. Valtioneuvoston Asetus Eräiden Jätteiden Hyödyntämisestä Maarakentamisessa (Government Decree on the Recovery of Certain Waste in Earth Construction) 843/2017. Available online: https://www.finlex.fi/fi/laki/alkup/2017/20170843 (accessed on 20 February 2018).
- Ministry of Agriculture and Forestry. Maa—ja Metsätalousministeriön Asetus Lannoitevalmisteista 24/11 (Ministry of Agriculture and Forestry Decree on Fertilizer Products 24/11); Ministry of Agriculture and Forestry: Helsinki, Finland, 2011.
- Kaakinen, J.; Kuokkanen, T.; Kujala, K.; Välimäki, I.; Jokinen, H. The Use of a Five-stage Sequential Leaching Procedure for Risk Assessment of Heavy Metals in Waste Rock Utilized in Railway Ballast. Soil Sediment Contam. Int. J. 2012, 21, 322–334. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Reis, L.S.; de Andrade Gonçalves, É.C.B. Chemical speciation: An instrument for evaluation of mineral bioavailability. Cienc. Rural 2015, 45, 1126–1132. [Google Scholar] [CrossRef]
- Lorentzen, E.M.L.; Kingston, H.M. “Skip” Comparison of Microwave-Assisted and Conventional Leaching Using EPA Method 3050B. Anal. Chem. 1996, 68, 4316–4320. [Google Scholar] [CrossRef]
- Douiri, H.; Louati, S.; Baklouti, S.; Arous, M.; Fakhfakh, Z. Structural, thermal and dielectric properties of phosphoric acid-based geopolymers with different amounts of H3PO4. Mater. Lett. 2014, 116, 9–12. [Google Scholar] [CrossRef]
- International Organization of Standardization. ISO 11466:1995—Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia; International Organization of Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. JEM 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Pöykiö, R.; Kuokkanen, T.; Perämäki, P.; Välimäki, I. Sequential leaching of trace elements in bottom ash from a fluidized bed co-combustion boiler at a pulp and paper mill complex. J. Solid Waste Technol. Manag. 2005, 31, 115–121. [Google Scholar]
- Kuokkanen, T.; Pöykiö, R.; Nurmesniemi, H.; Rämö, J. Sequential leaching of heavy metals and sulfur in bottom ash and fly ash from the co-combustion of wood and peat at a municipal district heating plant. Chem. Speciat. Bioavailab. 2006, 18, 131–142. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Pöykiö, R.; Kuokkanen, T.; Rämö, J. Chemical sequential extraction of heavy metals and sulphur in bottom ash and in fly ash from a pulp and paper mill complex. Waste Manag. Res. 2008, 26, 389–399. [Google Scholar] [CrossRef]
- Pöykiö, R.; Nurmesniemi, H.; Perämäki, P.; Kuokkanen, T.; Välimäki, I. Leachability of metals in fly ash from a pulp and paper mill complex and environmental risk characterisation for eco-efficient utilization of the fly ash as a fertilizer. Chem. Speciat. Bioavailab. 2005, 17, 1–10. [Google Scholar] [CrossRef]
- Finnish Standards Association. SFS-EN 12880 Characterization of Sludges. Determination of Dry Residue and Water Content; Finnish Standards Association: Helsinki, Finland, 2001. [Google Scholar]
- Kosson, D.S.; Van, D.S.; Sanchez, F.; Garrabrants, A.C. An Integrated Framework for Evaluating Leaching in Waste Management and Utilization of Secondary Materials. Environ. Eng. Sci. 2002, 19, 159–204. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
| Sample | Composition (Mass Fraction (%)) |
|---|---|
| FA | 60% fly ash; 40% water |
| FAPA | 61.3% fly ash; 12.3% phosphoric acid; 26.4% ammonium sulphate solution |
| FASD | 19.2% fly ash; 19.2% sawdust; 9.6% lignosulfonate; 3.8% phosphoric acid; 48.1% ammonium sulphate solution |
| SD | 28.6% sawdust; 14.3% lignosulfonate; 57.1% ammonium sulphate solution |
| Step | Fraction | Extractant | Experimental Conditions |
|---|---|---|---|
| F1 | Water-soluble | 40 cm3 H2O at pH 4 (with HNO3) | 16 h at 22 ± 5 °C, constant shaking |
| F2 | Exchangeable and acid soluble | 40 cm3 HOAc 0.11 mol L−1 | 16 h at 22 ± 5 °C, constant shaking |
| F3 | Reducible | 40 cm3 NH2OH∙HCl 0.5 mol L−1 at pH 1.5 (with HNO3) | 16 h at 22 ± 5 °C, constant shaking |
| F4 | Oxidizable | 10 cm3 H2O2 300 g L−1 | 1 h at 22 ± 5 °C, occasional manual shaking, then 1 h 85 ± 2 °C. Reduce the volume to less than 3 cm3 |
| 10 cm3 H2O2 300 g L−1 | 1 h at 85 ± 2 °C | ||
| 50 cm3 NH4OAc 1 mol L−1 at pH 2 (with HNO3) | 16 h at 22 ± 5 °C |
| Granule | Ca (g kg−1) | K (g kg−1) | P (g kg−1) | Mg (g kg−1) | S (g kg−1) | N (mass fraction (%)) |
|---|---|---|---|---|---|---|
| FA | 83.9 | 8.05 | 9.97 | 14.7 | 7.89 | 0.0 |
| FAPA | 76.7 | 5.67 | 67.1 | 13.8 | 38.8 | 2.5 |
| FASD | 37.6 | 2.72 | 24.6 | 5.45 | 75.8 | 7.2 |
| SD | 0.82 | 0.58 | 0.07 | 0.70 | 113 | 7.0 |
| Limit value | ≥60 | K + P ≥ 20 |
| Granule | Ca (g kg−1) | K (g kg−1) | P (g kg−1) | Mg (g kg−1) | S (g kg−1) |
|---|---|---|---|---|---|
| FA | |||||
| Easily bioavailable (F1 + F2) | 36.0 ± 0.9 | 0.87 ± 0.03 | 2.69 ± 0.07 | 0.110 ± 0.001 | 6.49 ± 0.22 |
| Total bioavailability (F1 + F2 + F3 + F4) | 60.5 ± 0.2 | 3.12 ± 0.01 | 3.94 ± 0.01 | 5.24 ± 0.08 | 6.89 ± 0.22 |
| FAPA | |||||
| Easily bioavailable (F1 + F2) | 44.5 ± 0.1 | 1.58 ± 0.05 | 5.81 ± 0.1 | 28.9 ± 0.5 | 36.3 ± 0.7 |
| Total bioavailability (F1 + F2 + F3 + F4) | 59.8 ± 0.2 | 3.40 ± 0.04 | 6.36 ± 0.01 | 44.3 ± 0.3 | 36.4 ± 0.7 |
| FASD | |||||
| Easily bioavailable (F1 + F2) | 29.5 ± 0.1 | 1.19 ± 0.01 | 2.82 ± 0.09 | 11.1 ± 0.1 | 79.7 ± 1.4 |
| Total bioavailability (F1 + F2 + F3 + F4) | 34.8 ± 0.4 | 2.12 ± 0.07 | 3.46 ± 0.2 | 20.5 ± 0.1 | 80.1 ± 1.4 |
| SD | |||||
| Easily bioavailable (F1 + F2) | 0.71 ± 0.01 | 0.55 ± 0.01 | 0.59 ± 0.01 | 0.04 ± 0.01 | 136.6 ± 2.9 |
| Total bioavailability (F1 + F2 + F3 + F4) | 0.72 ± 0.01 | 0.55 ± 0.01 | 0.59 ± 0.01 | 0.04 ± 0.01 | 136.8 ± 2.9 |
| As (mg kg−1) | Cd (mg kg−1) | Cr (mg kg−1) | Cu (mg kg−1) | Ni (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) | |
|---|---|---|---|---|---|---|---|
| FA | 18 | 1.4 | 66 | 76 | 47 | 33 | 270 |
| FAPA | 17 | 1.8 | 52.2 | 64.6 | 44.2 | 22.3 | 217 |
| FASD | 7.2 | 0.67 | 21.2 | 34.9 | 16.5 | 8.7 | 95.4 |
| SD | <1.05 | <0.07 | 1.2 | 0.71 | 46 | <1.03 | 8.42 |
| Limit value Field/forest fertilizers | 25/40 | 2.5/25 | 300/300 | 600/700 | 100/150 | 100/150 | 1500/4500 |
| As (mg kg−1) | Cd (mg kg−1) | Cr (mg kg−1) | Cu (mg kg−1) | Ni (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) | |
|---|---|---|---|---|---|---|---|
| FA | |||||||
| F1 | <1.2 | <0.04 | 0.4 ± 0.1 | <0.1 | <0.1 | <0.4 | <0.1 |
| F2 | <1.2 | 0.41 ± 0.01 | 0.9 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.1 | 0.9 ± 0.1 | 19 ± 1 |
| F3 | 5.2 ± 0.1 | 0.33 ± 0.01 | 1.8 ± 0.1 | 7.3 ± 0.5 | 0.9 ± 0.1 | 9.0 ± 1 | 18 ± 1 |
| F4 | <1.5 | <0.05 | 1.0 | 2.5 | 0.3 | 3.5 | 3.1 |
| FAPA | |||||||
| F1 | 2.0 ± 0.1 | 0.04 ± 0.01 | 0.08 ± 0.01 | 7.1 ± 0.4 | 1.9 ± 0.1 | <0.40 | 0.10 ± 0.01 |
| F2 | 1.2 ± 0.1 | 0.21 ± 0.01 | 0.08 ± 0.01 | 1.3 ± 0.1 | 1.3 ± 0.1 | <0.40 | 29 ± 0.9 |
| F3 | 3.4 ± 0.1 | 0.15 ± 0.01 | 7.5 ± 0.1 | 5.3 ± 0.1 | 0.85 ± 0.01 | 5.5 ± 0.1 | 17 ± 1 |
| F4 | 2.0 ± 0.1 | 0.06 ± 0.01 | 5.2 ± 0.9 | 5.8 ± 0.8 | 1.6 ± 0.5 | 3.3 ± 0.2 | 9.0 ± 2.6 |
| FASD | |||||||
| F1 | 3.1 ± 0.1 | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 1.3 ± 0.1 | <0.39 | <0.1 |
| F2 | 1.2 ± 0.1 | 0.27 ± 0.01 | 0.08 ± 0.01 | 1.2 ± 0.1 | 3.2 ± 0.1 | <0.39 | 11 ± 1 |
| F3 | 7.5 ± 0.1 | 0.74 ± 0.01 | 5.4 ± 0.1 | 12 ± 1 | 0.98 ± 0.01 | 2.6 ± 0.01 | 57 ± 1 |
| F4 | 1.5 ± 0.1 | 0.05 ± 0.01 | 1.8 ± 0.1 | 0.77 ± 0.06 | 0.36 ± 0.06 | 1.8 ± 0.1 | 1.7 ± 0.1 |
| SD | |||||||
| F1 | <1.2 | <0.04 | 0.83 ± 0.03 | 0.37 ± 0.08 | 40 ± 1 | <0.39 | 7.9 ± 0.1 |
| F2 | <1.2 | <0.04 | <0.08 | 0.08 ± 0.01 | 2.4 ± 0.3 | <0.39 | 0.56 ± 0.07 |
| F3 | <1.2 | <0.04 | <0.08 | 0.13 ± 0.02 | 0.71 ± 0.15 | <0.39 | 0.16 ± 0.06 |
| F4 | <1.5 | <0.05 | <0.10 | 0.17 ± 0.01 | <0.15 | <0.49 | 0.16 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesonen, J.; Rautio, P. New Nitrogen-Containing Recycled Fertilizers: Bioavailability of Nutrients and Harmful Elements. Recycling 2019, 4, 17. https://doi.org/10.3390/recycling4020017
Pesonen J, Rautio P. New Nitrogen-Containing Recycled Fertilizers: Bioavailability of Nutrients and Harmful Elements. Recycling. 2019; 4(2):17. https://doi.org/10.3390/recycling4020017
Chicago/Turabian StylePesonen, Janne, and Pasi Rautio. 2019. "New Nitrogen-Containing Recycled Fertilizers: Bioavailability of Nutrients and Harmful Elements" Recycling 4, no. 2: 17. https://doi.org/10.3390/recycling4020017
APA StylePesonen, J., & Rautio, P. (2019). New Nitrogen-Containing Recycled Fertilizers: Bioavailability of Nutrients and Harmful Elements. Recycling, 4(2), 17. https://doi.org/10.3390/recycling4020017






