A Proof-of-Concept Portable Water Purification Device Obtained from PET Bottles and a Magnetite-Carbon Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
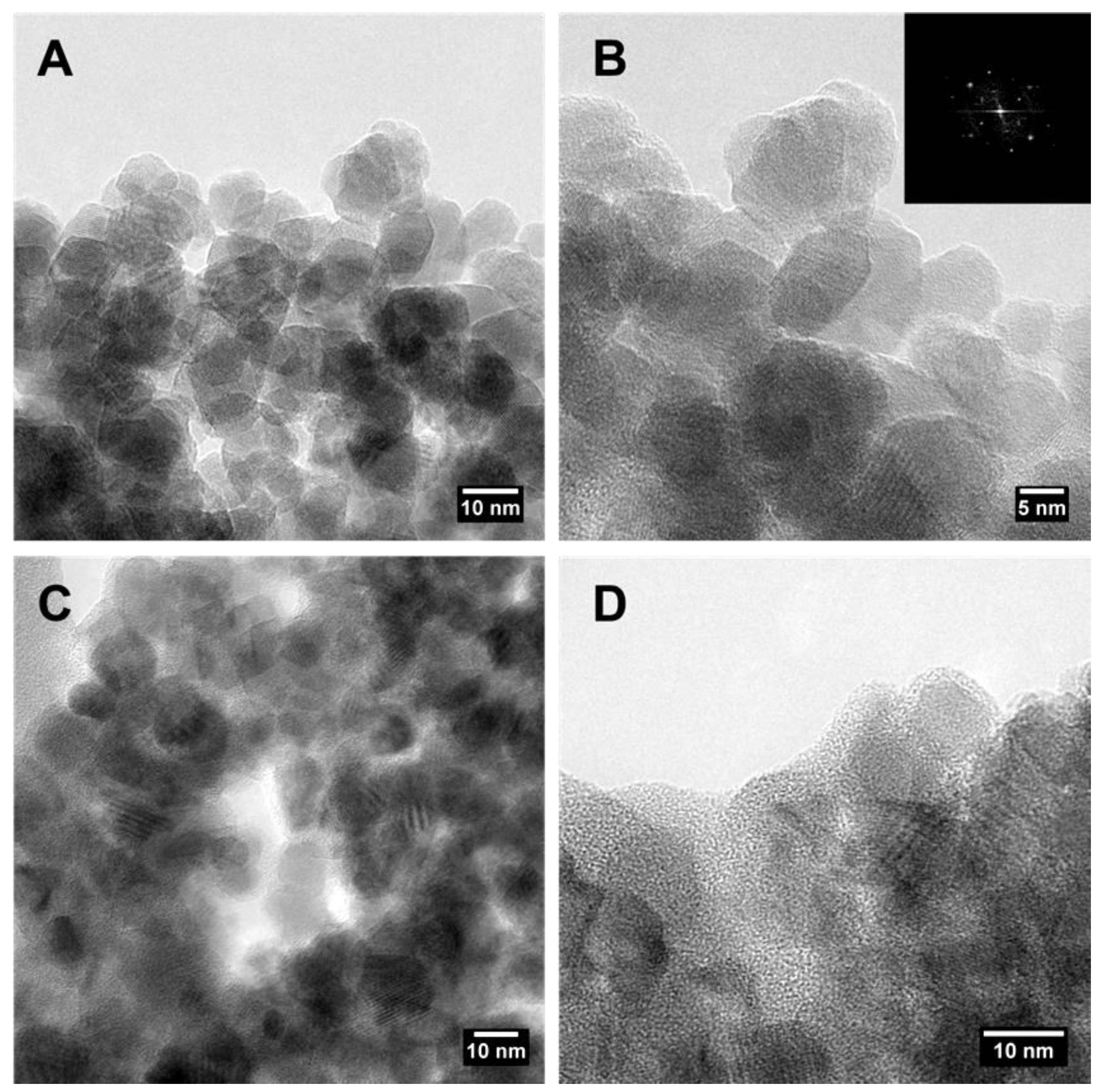
2.1. Synthesis and Characterization of Magnetite Nanoparticles and of the Activated Carbon-Magnetite Nanocomposite
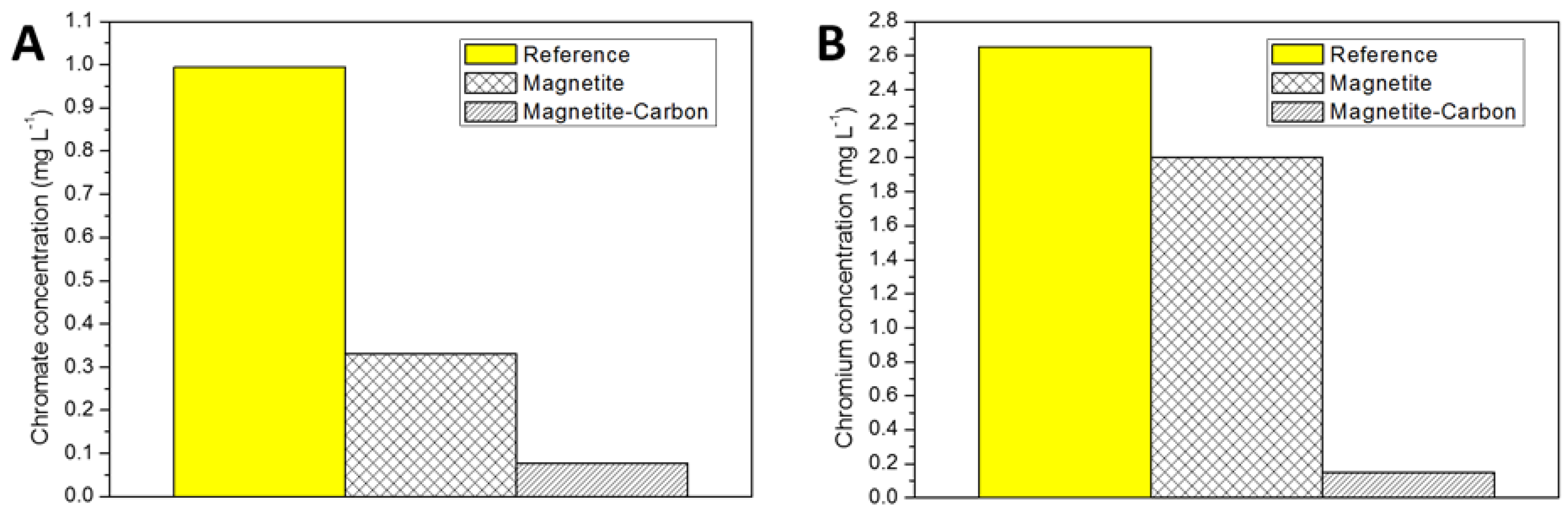
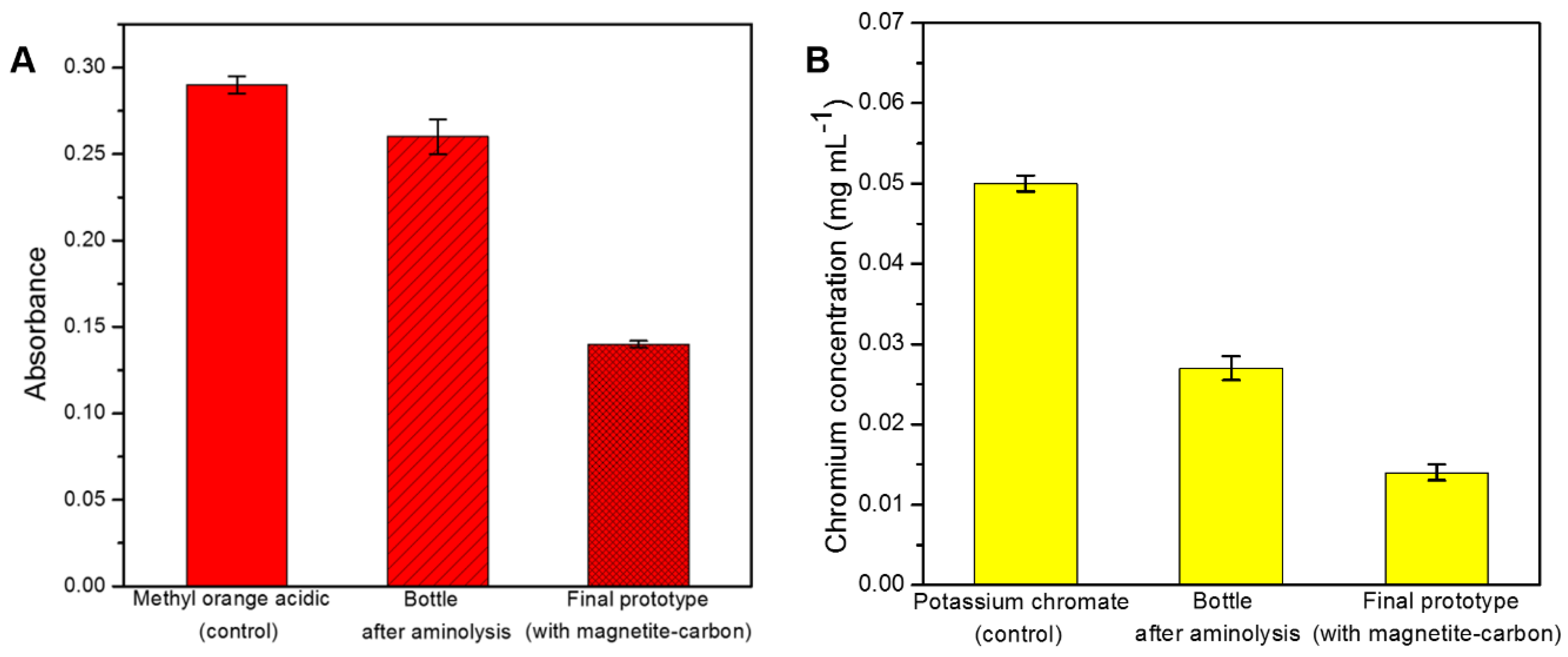
2.2. Removal Efficiency for Hexavalent Chromium
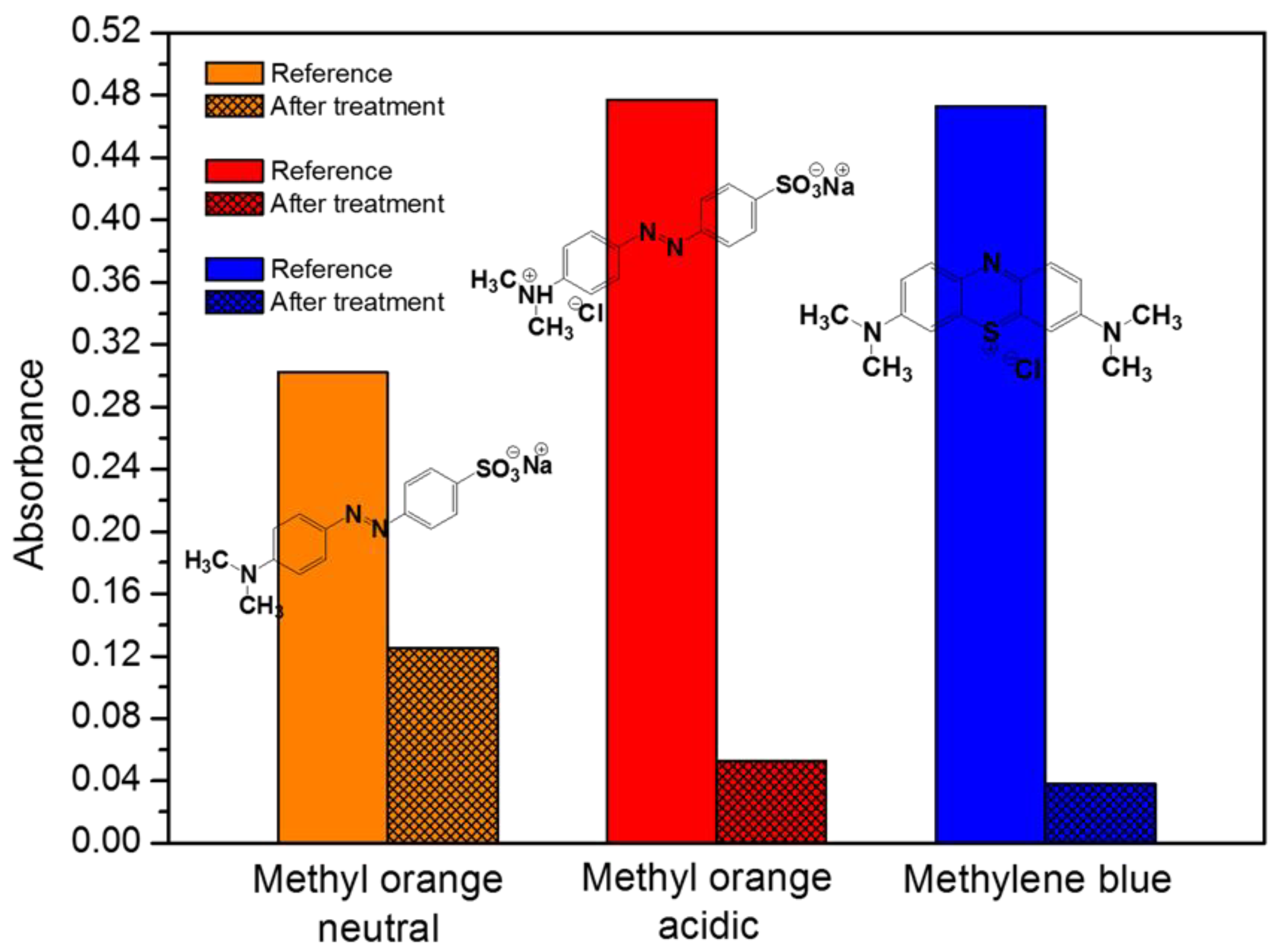
2.3. Removal Efficiency for Industrial Dyes
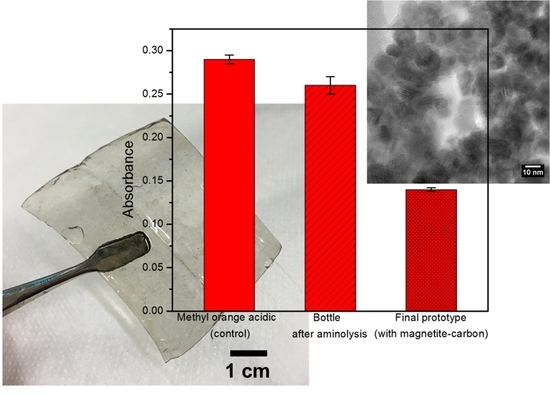
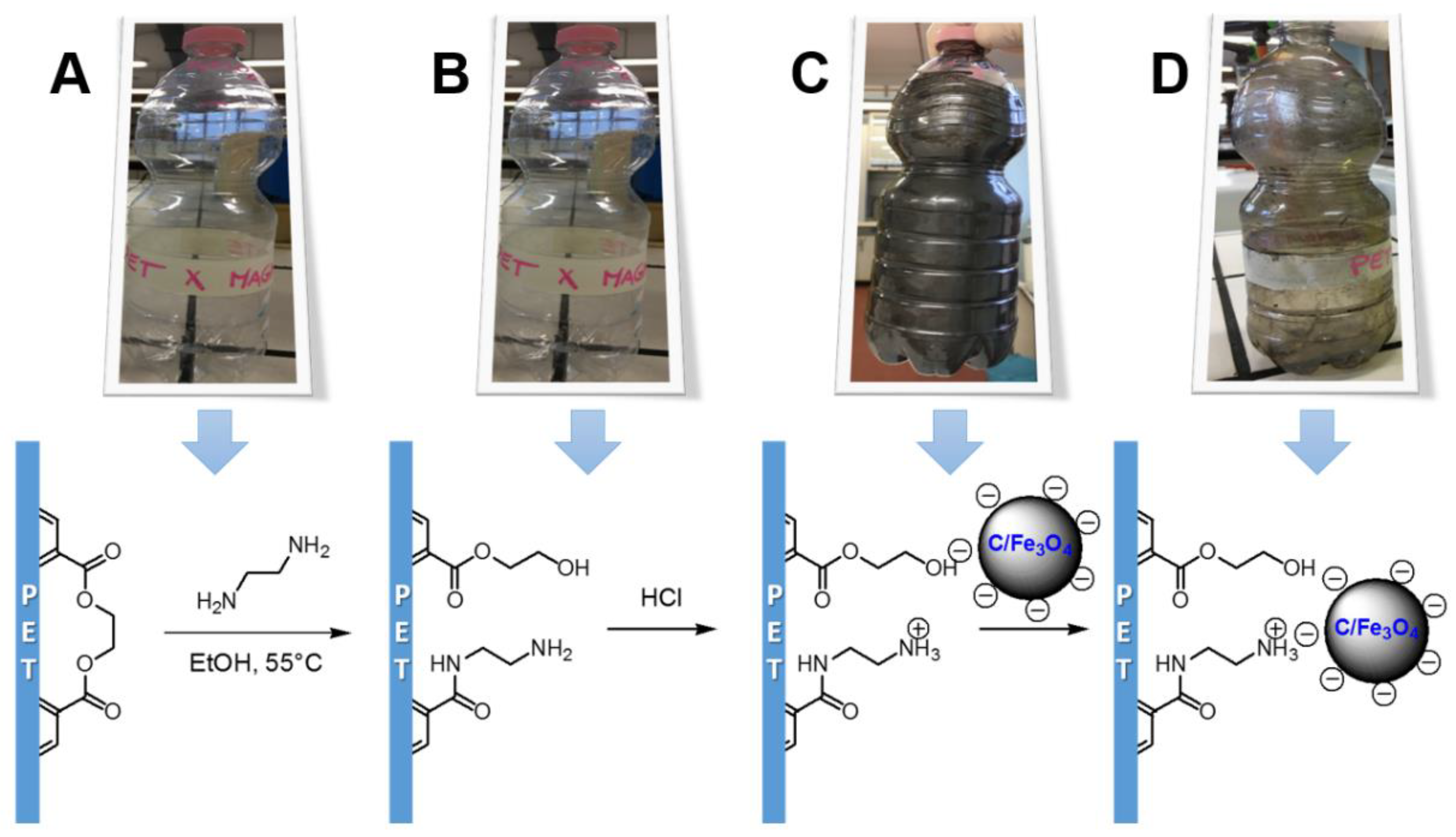
2.4. Development of a Proof-of-Concept Portable Water Purification Device
2.5. Challenges and Opportunities for Real-World Applications
3. Materials and Methods
3.1. Synthesis of Magnetite Nanoparticles and of the Magnetite-Carbon Nanocomposite
3.2. Preparation of the Portable Water Purification Device
3.3. Characterization Techniques
Determination of Adsorption Efficiency for Hexavalent Chromium and Organic Dyes
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Moe, C.L.; Rheingans, R.D. Global challenges in water, sanitation and health. J. Water Health 2006, 4, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Imdad, S.; Yaldram, K.; Butt, N.M.; Pervez, A. Emerging nanotechnology-based methods for water purification: A review. Desalin. Water Treat. 2014, 52, 4089–4101. [Google Scholar] [CrossRef]
- Kwon, J.H.; Wilson, L.D.; Sammynaiken, R. Synthesis and characterization of magnetite and activated carbon binary composites. Synth. Met. 2014, 197, 8–17. [Google Scholar] [CrossRef]
- Linley, S.; Leshuk, T.; Gu, F.X. Magnetically separable water treatment technologies and their role in future advanced water treatment: A patent review. Clean Soil Air Water 2013, 41, 1152–1156. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon N. Y. 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Ma, J.; Sun, S.; Chen, K. Facile and scalable synthesis of magnetite/carbon adsorbents by recycling discarded fruit peels and their potential usage in water treatment. Bioresour. Technol. 2017, 233, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011. [CrossRef]
- Mancini, S.D.; Schwartzman, J.A.S.; Nogueira, A.R.; Kagohara, D.A.; Zanin, M. Additional steps in mechanical recyling of PET. J. Clean. Prod. 2010. [Google Scholar] [CrossRef]
- Nakatani, J.; Fujii, M.; Moriguchi, Y.; Hirao, M. Life-cycle assessment of domestic and transboundary recycling of post-consumer PET bottles. Int. J. Life Cycle Assess. 2010. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, Z.G. The consumption and recycling collection system of PET bottles: A case study of Beijing, China. Waste Manag. 2014. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Meegan, M.E.; Joyce, T.; McGuigan, K.; Barnes, J. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch. Dis. Child. 2001. [Google Scholar] [CrossRef]
- Mäusezahl, D.; Christen, A.; Pacheco, G.D.; Tellez, F.A.; Iriarte, M.; Zapata, M.E.; Cevallos, M.; Hattendorf, J.; Cattaneo, M.D.; Arnold, B.; Smith, T.A.; Colford, J.M. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: A cluster-randomized, controlled trial. PLoS Med. 2009. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Elmore-Meegan, M.; Joyce, T.; McGuigan, K.G.; Barnes, J. Solar disinfection of drinking water and diarrhoea in Maasai children: A controlled field trial. Lancet 1996. [Google Scholar] [CrossRef]
- Caslake, L.F.; Connolly, D.J.; Menon, V.; Duncanson, C.M.; Rojas, R.; Tavakoli, J. Disinfection of Contaminated Water by Using Solar Irradiation. Appl. Environ. Microbiol. 2004. [Google Scholar] [CrossRef]
- Acher, A.; Fischer, E.; Turnheim, R.; Manor, Y. Ecologically friendly wastewater disinfection techniques. Water Res. 1997. [Google Scholar] [CrossRef]
- Ciochetti, D.A.; Metcalf, R.H. Pasteurization of naturally contaminated water with solar energy. Appl. Environ. Microbiol. 1984. [Google Scholar]
- Burch, J.D.; Thomas, K.E. Water disinfection for developing countries and potential for solar thermal pasteurization. Sol. Energy 1998. [Google Scholar] [CrossRef]
- Hillie, T.; Hlophe, M. Nanotechnology and the challenge of clean water. Nat. Nanotechnol. 2007, 2, 663–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Carotenuto, M.; Libralato, G.; Domingos, R.F.; Markus, A.; Dini, L.; Gautam, R.K.; Baldantoni, D.; Rossi, M.; Sharma, S.K.; Chattopadhyaya, M.C.; Giugni, M.; Meric, S. Polymer functionalized nanocomposites for metals removal from water and wastewater: An overview. Water Res. 2016, 92, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Su, C.X.H.; Low, L.W.; Teng, T.T.; Wong, Y.S. Combination and hybridisation of treatments in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2016, 4, 3618–3631. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Xia, Q.; Wu, J.; Liu, J.; Huang, M.; Xie, J. Conductive 3D Sponge for Affordable and Highly-efficient Water Purification. Nanoscale 2018. [Google Scholar] [CrossRef] [PubMed]
- Panzarasa, G.; Osypova, A.; Consolati, G.; Quasso, F.; Soliveri, G.; Ribera, J.; Schwarze, F.W.M.R. Preparation of a sepia melanin and poly(ethylene-alt-maleic anhydride) hybrid material as an adsorbent for water purification. Nanomaterials 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002. [Google Scholar] [CrossRef]
- Mirabello, G.; Lenders, J.J.M.; Sommerdijk, N.A.J.M. Bioinspired synthesis of magnetite nanoparticles. Chem. Soc. Rev. 2016. [CrossRef] [PubMed]
- Schladt, T.D.; Schneider, K.; Schild, H.; Tremel, W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalt. Trans. 2011, 40, 6315. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Danhier, F.; Préat, V. Iron oxide-loaded nanotheranostics: Major obstacles to in vivo studies and clinical translation. J. Control. Release 2015, 198, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Lattuada, M.; Ren, Q.; Zuber, F.; Galli, M.; Bohmer, N.; Matter, M.T.; Wichser, A.; Bertazzo, S.; Pier, G.B.; Herrmann, I.K. Theranostic body fluid cleansing: Rationally designed magnetic particles enable capturing and detection of bacterial pathogens. J. Mater. Chem. B 2016, 4, 7080–7086. [Google Scholar] [CrossRef]
- McCloskey, K.E.; Chalmers, J.J.; Zborowski, M. Magnetic Cell Separation: Characterization of Magnetophoretic Mobility. Anal. Chem. 2003, 75, 6868–6874. [Google Scholar] [CrossRef] [PubMed]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; Liu, Z.F. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.C.; Chin, S.F.; Anderson, M.A. Redox equilibria of iron oxides in aqueous-based magnetite dispersions: Effect of pH and redox potential. J. Colloid Interface Sci. 2007, 311, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Camacho, C.; Villalobos, M.; de la Luz Rivas-Sánchez, M.; Arenas-Alatorre, J.; Alcaraz-Cienfuegos, J.; Gutiérrez-Ruiz, M.E. Characterization and surface reactivity of natural and synthetic magnetites. Chem. Geol. 2013, 347, 233–245. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Kim, J.H.; Yang, H.M.; Lee, J.W.; Kim, J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Sun, Z.X.; Su, F.W.; Forsling, W.; Samskog, P.O. Surface characteristics of magnetite in aqueous suspension. J. Colloid Interface Sci. 1998, 197, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sedman, R.M.; Beaumont, J.; McDonald, T.A.; Reynolds, S.; Krowech, G.; Howd, R. Review of the evidence regarding the carcinogenicity of hexavalent chromium in drinking water. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 155–182. [Google Scholar] [CrossRef] [PubMed]
- Dai, M. Mechanism of Adsorption for Dyes on Activated Carbon. J. Colloid Interface Sci. 1998, 198, 6–10. [Google Scholar] [CrossRef]
- Ravindranath, K.; Mashelkar, R.A. Polyethylene terephtalate-II. Engineering analysis. Chem. Eng. Sci. 1986, 41, 2969–2987. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Costa, L.; Pardoue, S.; Dragoé, D.; Mazerat, S.; Roger, P. Hydrophilic PET surfaces by aminolysis and glycopolymer brushes chemistry. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2689–2697. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of primary amine groups available for subsequent biofunctionalization of polymer surfaces. Bioconjug. Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Furlan, P.Y.; Melcer, M.E. Removal of aromatic pollutant surrogate from water by recyclable magnetite-activated carbon nanocomposite: An experiment for general chemistry. J. Chem. Educ. 2014, 91, 1966–1970. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaita, E.; Evangelisti, C.; Panzarasa, G. A Proof-of-Concept Portable Water Purification Device Obtained from PET Bottles and a Magnetite-Carbon Nanocomposite. Recycling 2018, 3, 31. https://doi.org/10.3390/recycling3030031
Gaita E, Evangelisti C, Panzarasa G. A Proof-of-Concept Portable Water Purification Device Obtained from PET Bottles and a Magnetite-Carbon Nanocomposite. Recycling. 2018; 3(3):31. https://doi.org/10.3390/recycling3030031
Chicago/Turabian StyleGaita, Elisabetta, Claudio Evangelisti, and Guido Panzarasa. 2018. "A Proof-of-Concept Portable Water Purification Device Obtained from PET Bottles and a Magnetite-Carbon Nanocomposite" Recycling 3, no. 3: 31. https://doi.org/10.3390/recycling3030031