Turning Waste into Wealth: Sustainable Amorphous Silica from Moroccan Oil Shale Ash
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Material
2.1.1. Brunauer–Emmett–Teller (BET) and NLDFT Analyses
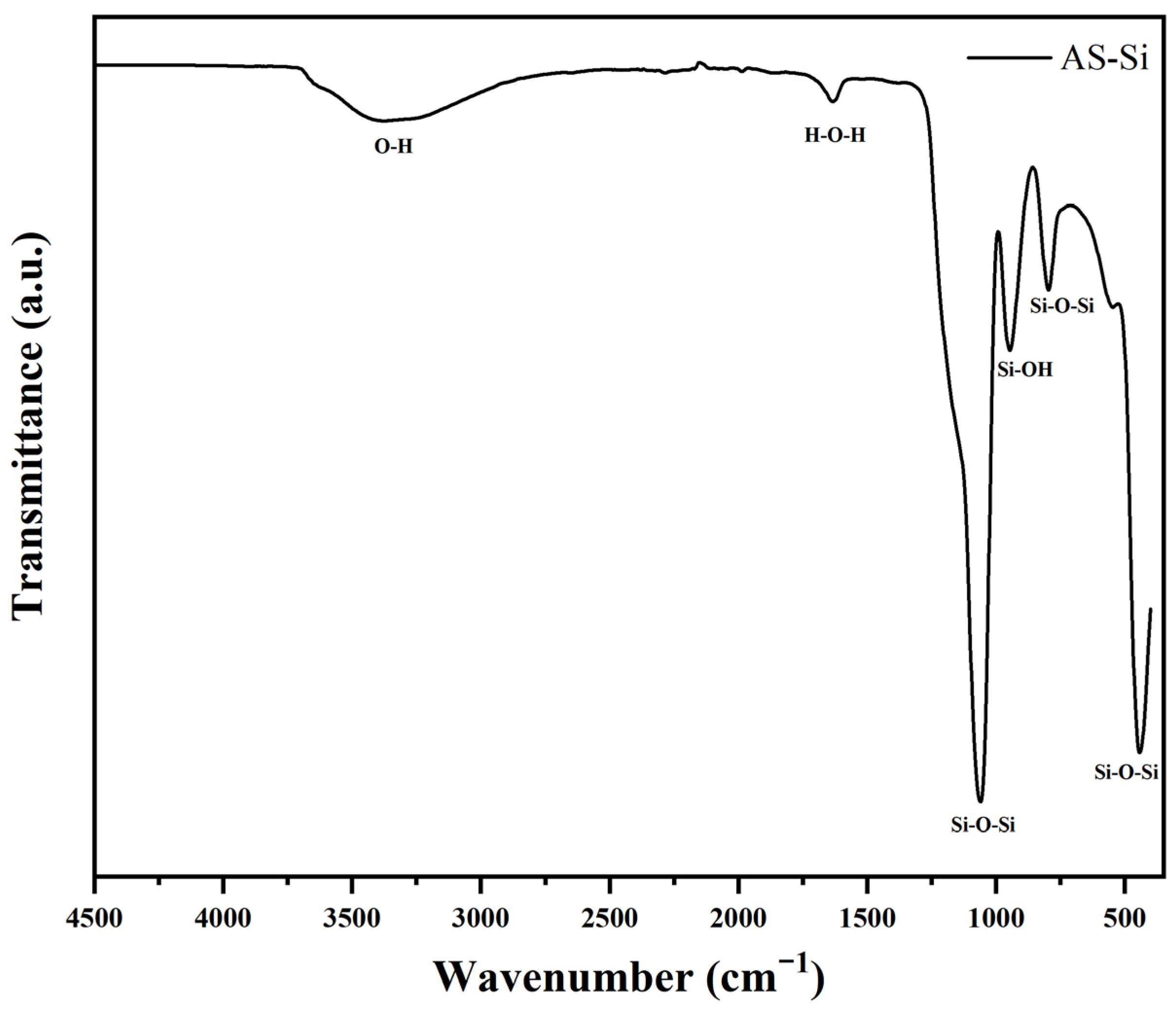
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
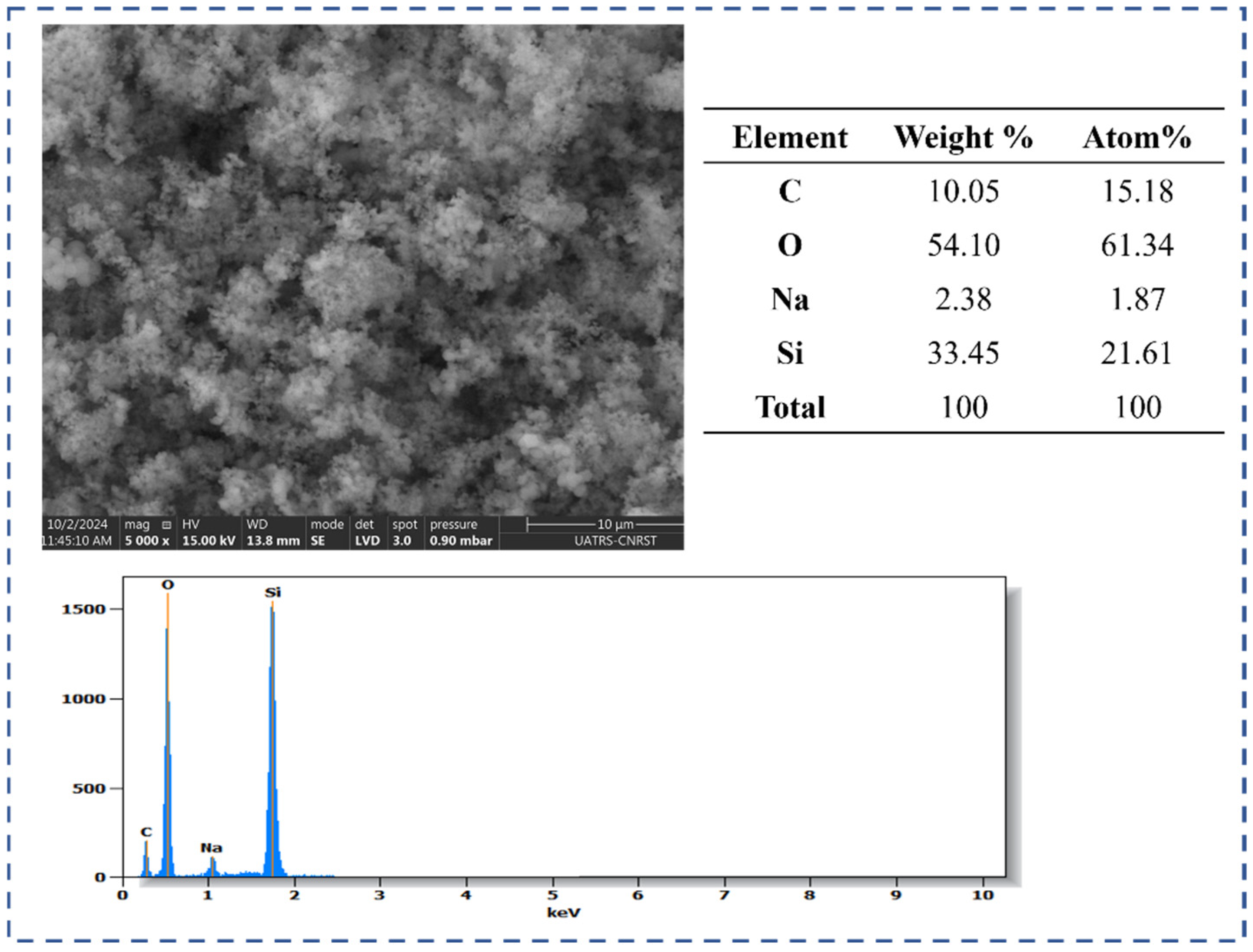
2.1.3. Scanning Electron Microscopy (SEM)
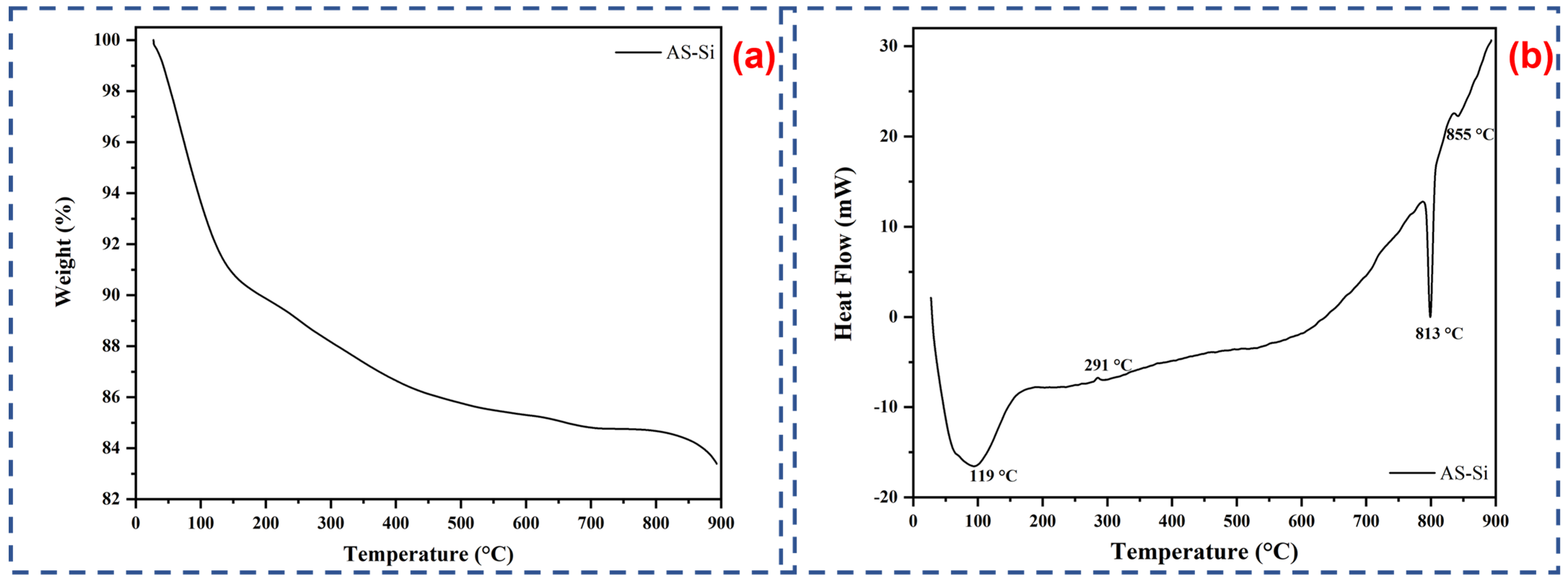
2.1.4. Thermal Analysis (TGA and DSC)
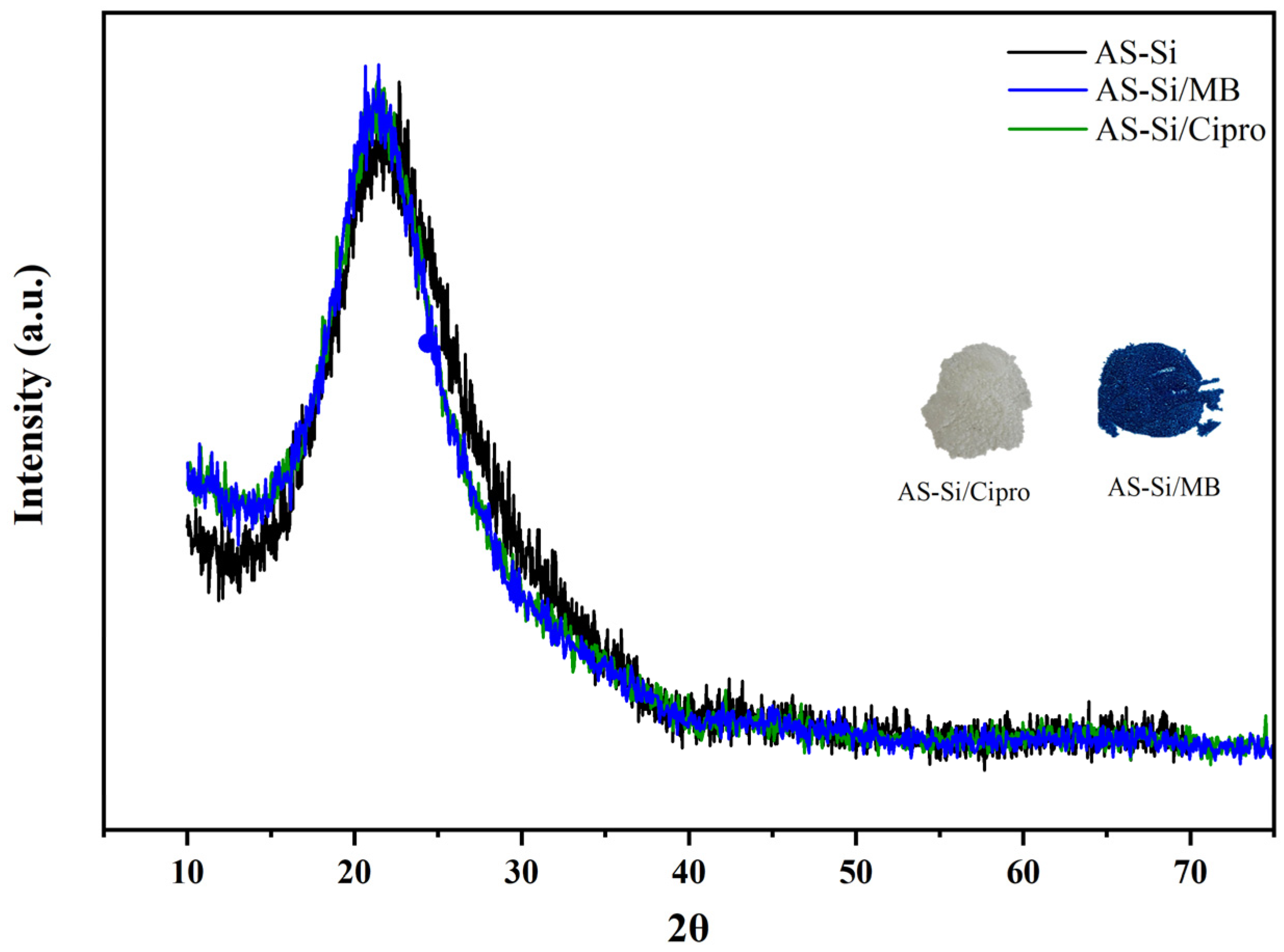
2.1.5. X-Ray Diffraction Analysis (XRD)
2.1.6. Hydrodynamic Diameter, Polydispersity Index (PDI), and Zeta Potential
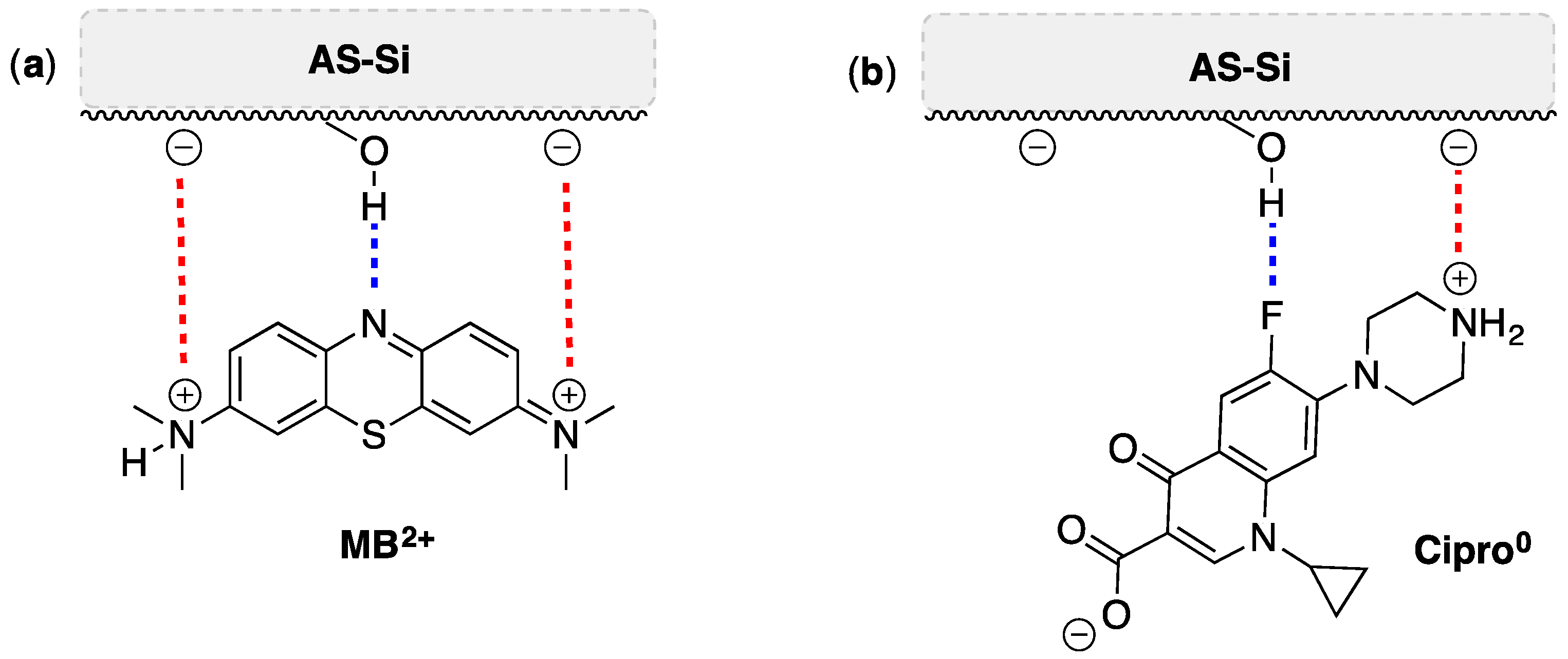
2.2. Tests of Adsorption of Methylene Blue and Ciprofloxacin onto AS-Si
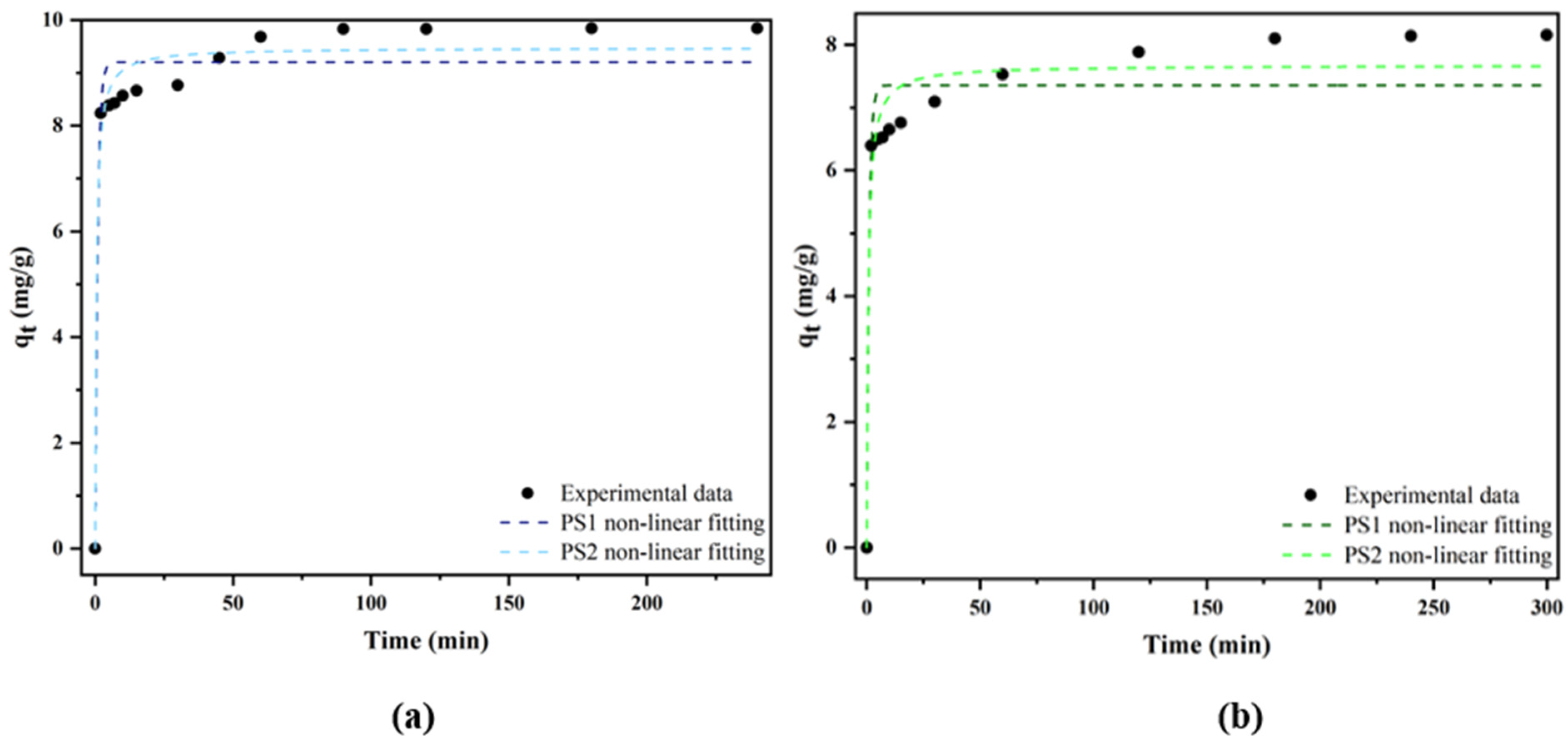
2.2.1. Kinetic Models
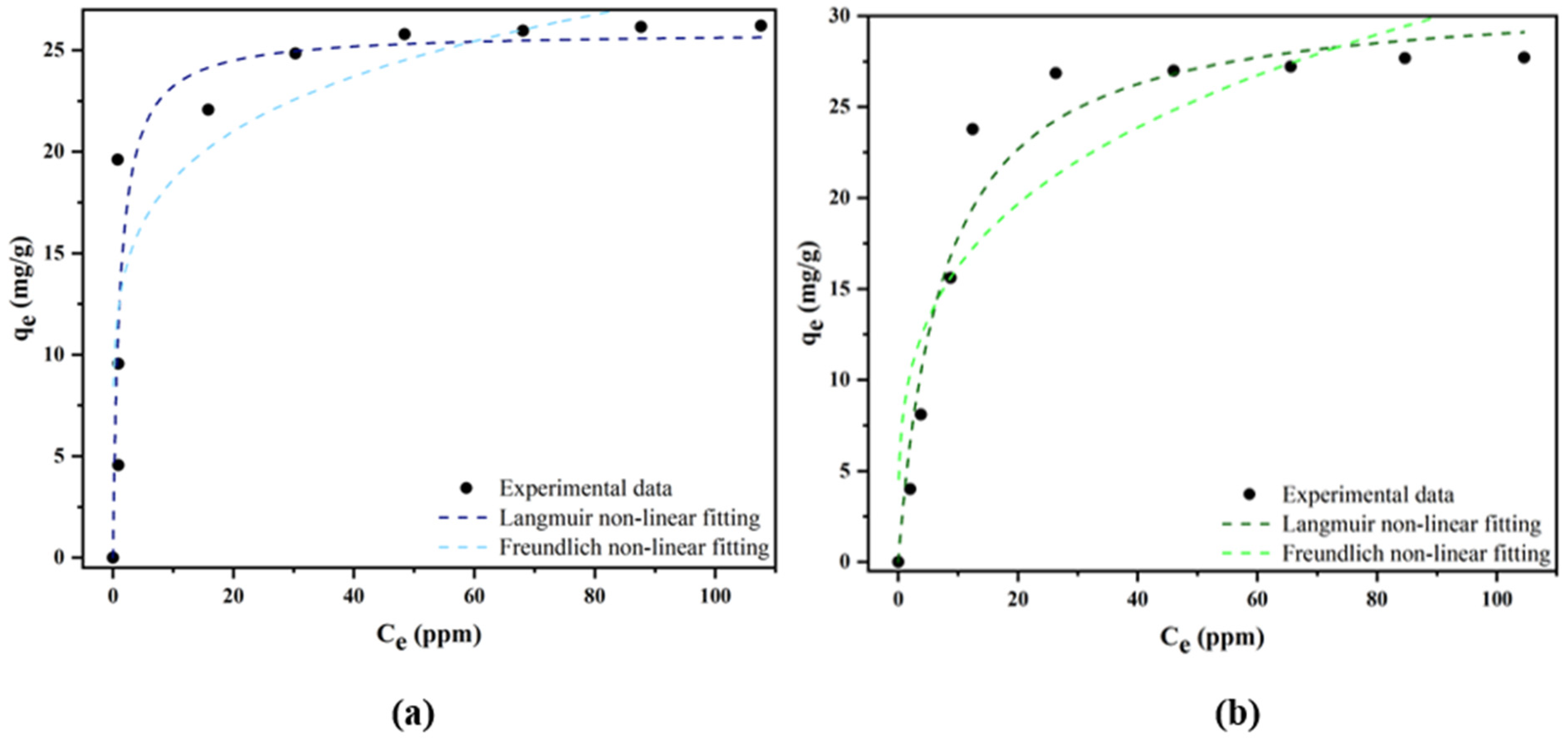
2.2.2. Isotherms
2.3. Comparative Study
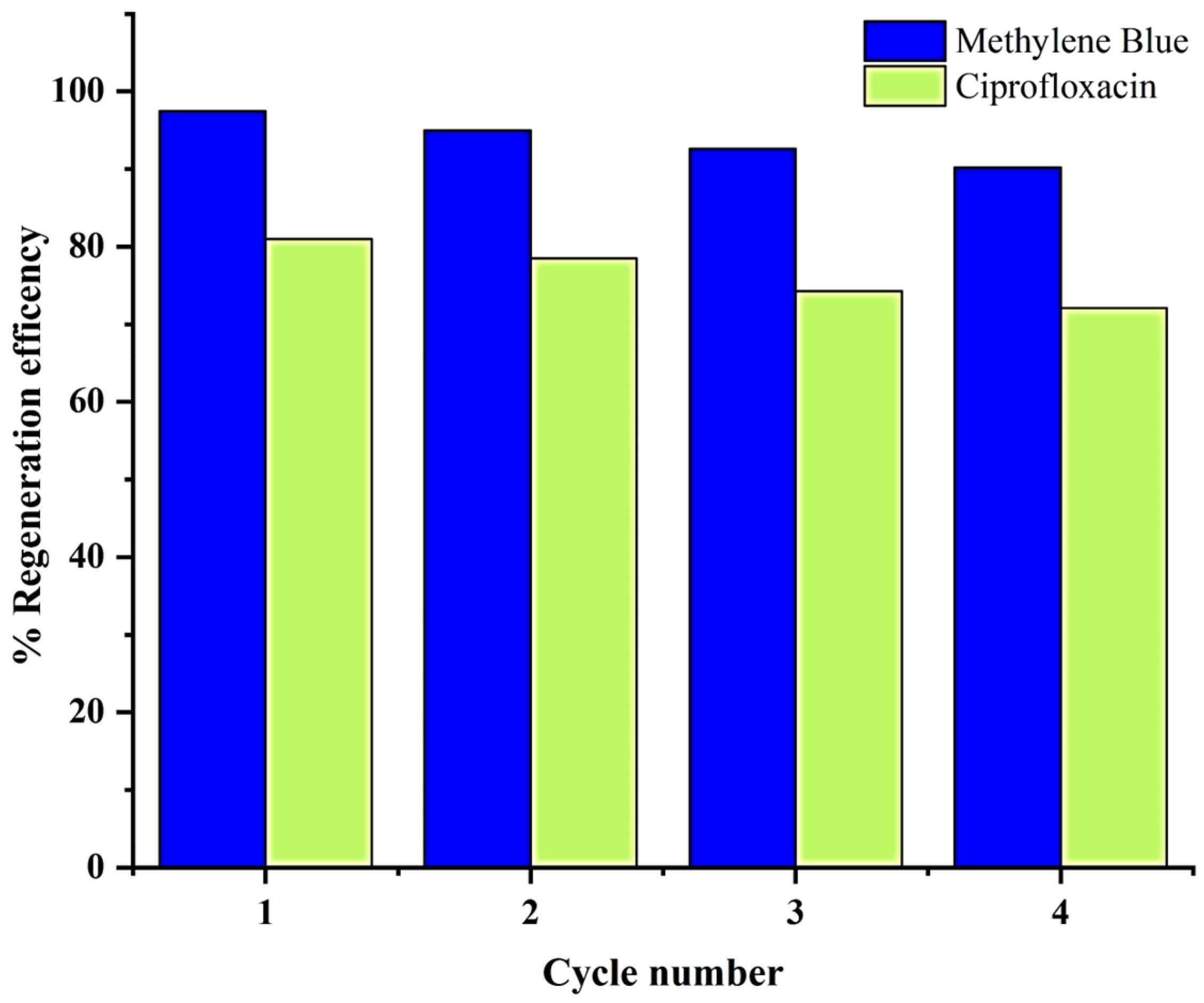
2.4. Regeneration Study
2.5. FTIR and XRD After Adsorption
3. Materials and Methods
3.1. Materials
3.2. Sol–Gel Process
3.3. Adsorption from Solution Experiments
3.4. Models for Isotherm and Kinetic Studies
- -
- Pseudo-first-order kinetic non-linear model [49]:
- -
- Pseudo-second-order kinetic non-linear form [50]:
- -
- Langmuir isotherm non-linear form [51]:
- -
- -Freundlich isotherm non-linear shape [52]:
3.5. Regeneration Process
3.6. Characterization of Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Lu, Y.; Li, W.; Wang, L.; Huang, X.; Yang, D. Effect of Supercritical Water Temperature on Pyrolysis Characteristics of Oil Shales. Energy Fuels 2025, 39, 8482–8494. [Google Scholar] [CrossRef]
- Zhai, Y.; Yang, T.; Liu, B.; Zhu, Y.; Wang, X. Enhancing Oil Shale Pyrolysis through Swelling Pretreatment: Mechanisms and Product Distribution. Fuel 2025, 399, 135637. [Google Scholar] [CrossRef]
- Krime, A.; Saoiabi, S.; Berrahou, S.; Latifi, S.; El Hammari, L.; Saoiabi, A. Adsorption of Organic Pollutants Using Moroccan Oil Shales: Optimization of the Adsorption Process Using a Factorial Design. NanoWorld J. 2023, 9, S396–S400. [Google Scholar] [CrossRef]
- Doumbouya, M.; Kacemi, K.E.; Kitane, S. Production of Portland Cement Using Moroccan Oil Shale and Comparative Study between Conventional Cement Plant and Cement Plant Using Oil Shale. J. Chem. Soc. Pak. 2012, 34, 885–889. [Google Scholar]
- Alsafasfeh, A.; Alawabdeh, M.; Alfuqara, D.; Gougazeh, M.; Amaireh, M.N. Oil Shale Ash as a Substitutional Green Component in Cement Production. Adv. Sci. Technol. Res. J. 2022, 16, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Ma, H.; Yan, Y.; Zhang, Y.; Sun, R.; Wang, D. Enhancement Mechanism of Early Age Strength in Cement Paste Induced by Oil Shale Residue. Constr. Build. Mater. 2025, 465, 140277. [Google Scholar] [CrossRef]
- Bai, S.; Chang, Z.; Ren, Z.; Zhao, Y.; Pang, L. Adsorption of Cd2+ Ions onto Zeolites Synthesized from a Mixture of Coal Fly Ash and Oil Shale Ash in Aqueous Media. RSC Adv. 2025, 15, 11293–11300. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.M.; Miao, L.N.; Ji, G.J.; Zou, H.F.; Gan, S.C. Preparation and Characterization of Silica Aerogels from Oil Shale Ash. Mater. Lett. 2009, 63, 2721–2724. [Google Scholar] [CrossRef]
- Koreeda, H.; Ishijima, M.; Kajihara, K. Cosolvent-Free Sol–Gel Synthesis of Macroporous Silica Gels from Tetramethoxysilane–Tetraethoxysilane Mixtures. J. Solgel Sci. Technol. 2024, 113, 48–55. [Google Scholar] [CrossRef]
- Navik, R.; Wang, E.; Ding, X.; Qiu, K.X.; Li, J. Atmospheric Carbon Dioxide Capture by Adsorption on Amine-Functionalized Silica Composites: A Review. Environ. Chem. Lett. 2024, 22, 1791–1830. [Google Scholar] [CrossRef]
- Kim, J.S.; Woo, S.Y.; Lee, J.G.; Kim, Y.D. Effect of Adsorption Isotherm and Kinetics on the Performance of Silica Gel-Based Adsorption Desalination System with Heat and Mass Recovery. Desalination 2024, 577, 117375. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-Based Nanomaterials and Their Adsorptive Removal Efficiency for Dyes and Antibiotics: A Review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Bano, K.; Singh, P.P.; Kumar, S.; Saeed, S.M.; Aggarwal, S.; Kumar, R.; Kaushal, S. Construction of Honey Bee Hive-like CuO/PbO Heterojunction Photocatalysts with Enhanced Antibiotic and Dye Degradation Activity under Visible Light. Environ. Sci. 2024, 10, 1714–1725. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Tahir, B.; Sanati, S.; Zheng, Y.; Tahir, M.; Gao, J. Iron-Based Metal–Organic Frameworks and Their Derived Materials for Photocatalytic and Photoelectrocatalytic Reactions. Coord. Chem. Rev. 2024, 499, 215538. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Negash, E.A.; Brehane Tesfamariam, B.; Mengesha, G.A.; Shasho, Y.; Mekuriya, Y.S.; Beyene, S.T. High-Purity Amorphous Silica from Industrial Filter Cake Waste: Synthesis and Process Optimization. Mater. Res. Express 2025, 12, 015201. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Chandrasekaran, N.; T, S.K.; Sasthri, G.; Al-Dayan, N. Green Synthesis of Silica Nanoparticles Using Enhalus Acoroides: Characterization and Antioxidant Activity. Biomass Convers. Biorefin. 2025, 1–8. [Google Scholar] [CrossRef]
- Zothansanga, C.; Dawngliana, K.M.S.; Zathang, B.; Rai, S. Investigation of Judd-Ofelt Parameters, Luminescent Properties and Energy Transfer Analysis of Eu3+ Doped Al in Silica Glass Matrix Synthesized via Sol-Gel Technique. Opt. Mater. 2025, 159, 116598. [Google Scholar] [CrossRef]
- Jin, C.; Wang, J.; Wang, Y.; Tang, H.; Lu, T. Fabrication of Hierarchically Porous Silica Nanospheres through Sol-Gel Process and Pseudomorphic Transformation. J. Solgel Sci. Technol. 2014, 70, 53–61. [Google Scholar] [CrossRef]
- Santos, G.M.; Leong, C.A.; Grootes, P.M.; Seiler, M.; Svarva, H.; Nadeau, M.J. Radiocarbon Step-combustion Oxidation Method and Ftir Analysis of Trondheim CaCO3 Precipitates of Atmospheric CO2 samples: Further investigations and insights. Radiocarbon 2024, 66, 1289–1301. [Google Scholar] [CrossRef]
- Leventaki, E.; Baena-Moreno, F.M.; Sardina, G.; Ström, H.; Ghahramani, E.; Naserifar, S.; Ho, P.H.; Kozlowski, A.M.; Bernin, D. In-Line Monitoring of Carbon Dioxide Capture with Sodium Hydroxide in a Customized 3D-Printed Reactor without Forced Mixing. Sustainability 2022, 14, 10795. [Google Scholar] [CrossRef]
- Krime, A.; Eloufir, M.R.; Saoiabi, S.; Tlemcani, M.; Morais, M.; Saoiabi, A. Extracting High Purity Nano-Silica from Oil Shale: Valorising a Neglected Natural Resource. Mater. Res. Bull. 2025, 192, 113561. [Google Scholar] [CrossRef]
- Gorbunova, O.V.; Baklanova, O.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Drozdov, V.A. Poly(Ethylene Glycol) as Structure Directing Agent in Sol–Gel Synthesis of Amorphous Silica. Microporous Mesoporous Mater. 2014, 190, 146–151. [Google Scholar] [CrossRef]
- Hatem, R.S.; Hussain, A.F.; Mihsen, H.H. Green Synthesis, Characterization, and Adsorption of Co(II) and Cu(II) Ions from Aqueous Solution by Mesoporous Silica MCM-41. Chem. Pap. 2024, 78, 6331–6342. [Google Scholar] [CrossRef]
- Sawangprom, A.; Jampreecha, T.; Maensiri, S. Synthesis and Characterization of High-Purity SiO2 Nanoparticles Utilizing Greater Club Rush: Exploring a Promising Natural Source. Int. J. Miner. Metall. Mater. 2025, 32, 1234–1244. [Google Scholar] [CrossRef]
- Guo, S.; Ali, M.A.; Mohamed, M.A.; Han, X.; Liu, X.; Qiu, J. Reduction of Injection Molded Silica Glass Defects and Enhancement of Glass Quality via Water Debinding. Mater. Chem. Front. 2024, 8, 1400–1408. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, J.; Xiang, T.; Cheng, Y.; Zhang, H. Effect of Hydrophobic Fumed Silica on Bending Strength of Sodium Silicate-Bonded Sand Cores. Materials 2024, 17, 5714. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, G.; Kunc, F.; Piras, C.C.; Stranik, O.; Edwards, A.A.; Hall, A.J.; Gubala, V. Stabilizing Silica Nanoparticles in Hydrogels: Impact on Storage and Polydispersity. RSC Adv. 2017, 7, 19924–19933. [Google Scholar] [CrossRef]
- Breznan, D.; Nazemof, N.; Kunc, F.; Hill, M.; Vladisavljevic, D.; Gomes, J.; Johnston, L.J.; Vincent, R.; Kumarathasan, P. Acellular Oxidative Potential Assay for Screening of Amorphous Silica Nanoparticles. Analyst 2020, 145, 4867–4879. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Abid, J.; Waqar-Un-Nisa; Gul, S.; Nouman, M.; Idris, A.M.; Ullah, H. Nickel-Modified Orange Peel Biochar for the Efficient Adsorptive Removal of Eriochrome Black T from Aqueous Solution. Water 2025, 17, 1484. [Google Scholar] [CrossRef]
- Vinu, A.A.; Murugesan, V.; Tangermann, O.; Hartmann, M. Adsorption of Cytochrome c on Mesoporous Molecular Sieves: Influence of pH, Pore Diameter, and Aluminum Incorporation. Chem. Mater. 2004, 16, 3056–3065. [Google Scholar] [CrossRef]
- Hemdan, S.S. The Shift in the Behavior of Methylene Blue Toward the Sensitivity of Medium: Solvatochromism, Solvent Parameters, Regression Analysis and Investigation of Cosolvent on the Acidity Constants. J. Fluoresc. 2023, 33, 2489–2502. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of Ciprofloxacin from Water: A Comprehensive Review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Lafi, W.K.; Al-Anber, Z.; Al-Shannag, M.; Harahsheh, A. Adsorption of Methylene Blue by Acid and Heat Treated Diatomaceous Silica. Desalination 2007, 217, 212–224. [Google Scholar] [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive Adsorption of Metal Ions by Lignocellulosic Materials: A Review of Applications, Mechanisms and Influencing Factors. Separations 2025, 12, 70. [Google Scholar] [CrossRef]
- Daizy, M.; Ali, M.R.; Bacchu, M.S.; Aly, M.A.S.; Khan, M.Z.H. ZnO Hollow Spheres Arrayed Molecularly-Printed-Polymer Based Selective Electrochemical Sensor for Methyl-Parathion Pesticide Detection. Environ. Technol. Innov. 2021, 24, 101847. [Google Scholar] [CrossRef]
- Yuan, N.; Cai, H.; Liu, T.; Huang, Q.; Zhang, X. Adsorptive Removal of Methylene Blue from Aqueous Solution Using Coal Fly Ash-Derived Mesoporous Silica Material. Adsorpt. Sci. Technol. 2019, 37, 333–348. [Google Scholar] [CrossRef]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the Mechanism Involved during the Sorption of Methylene Blue onto Fly Ash. J. Colloid. Interface Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.G.; Sharma, A. Kinetics and Thermodynamics of Methylene Blue Adsorption on Neem (Azadirachta indica) Leaf Powder. Dye. Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Hongo, T.; Moriura, M.; Hatada, Y.; Abiko, H. Simultaneous Methylene Blue Adsorption and PH Neutralization of Contaminated Water by Rice Husk Ash. ACS Omega 2021, 6, 21604–21612. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, W.; Li, X.; Wang, H.; Lan, Y.; Zhang, S.; Wang, Y.; Liu, H. Effect of Environmental Factors on Adsorption of Ciprofloxacin from Wastewater by Microwave Alkali Modified Fly Ash. Sci. Rep. 2024, 14, 19831. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Ali, G.; Akbar, A.R.; Khan, K.U.; Al-Khattaf, F.S.; Saleem, A. Physicochemical Synthesis of Zeolite Modified Nano-Flakes to Eliminate Emerging Pollutants in Aquatic Environment. J. Water Process Eng. 2025, 69, 106832. [Google Scholar] [CrossRef]
- Moreira, C.A.; Scanferla, C.E.; Oliveira, A.G.; Duarte, V.A.; Arroyo, P.A.; de Lara Andrade, J.; Bittencourt, P.R.S.; Garcia, J.C.; de Oliveira, D.M.F. Bio-Adsorbents Based on Mesoporous Silica Produced from Rice Husks with Tunable Architecture and Surface Area for Remediation of Industrial Effluents. J. Porous Mater. 2024, 32, 27–46. [Google Scholar] [CrossRef]
- Lu, D.; Xu, S.; Qiu, W.; Sun, Y.; Liu, X.; Yang, J.; Ma, J. Adsorption and Desorption Behaviors of Antibiotic Ciprofloxacin on Functionalized Spherical MCM-41 for Water Treatment. J. Clean. Prod. 2020, 264, 121644. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Li, L.; Xu, K.; Iqbal, J.; Tian, Z.; Xing, X.; Li, H.; Hou, Y.; Wang, J.; et al. Preparation of Mg-Doped Coffee Waste for Efficient Removal of Methylene Blue and Lead from Water. Heliyon 2025, 11, e43091. [Google Scholar] [CrossRef]
- Mujiyanti, D.R.; Santoso, U.T.; Saptarini, M.D.; Emi, N.H. Synthesis and Characterization Nanosilica from Rice Husk Ash Using Sol-Gel Method with Addition of PEG-6000 and PVA. JKPK (J. Kim. Dan Pendidik. Kim.) 2021, 6, 252–263. [Google Scholar] [CrossRef]
- Jabłońska, B.; Jabłoński, P.; Gęga, J. Kinetics and Thermodynamics of Pb(II), Zn(II), and Cd(II) Adsorption from Aqueous Solutions onto Activated Biochar Obtained from Tobacco Waste. Materials 2025, 18, 2324. [Google Scholar] [CrossRef] [PubMed]
- Haoufazane, C.; Zaaboul, F.; El Monfalouti, H.; Sebbar, N.K.; Hefnawy, M.; El Hourch, A.; Kartah, B.E. A Sustainable Solution for the Adsorption of C.I. Direct Black 80, an Azoic Textile Dye with Plant Stems: Zygophyllum Gaetulum in an Aqueous Solution. Molecules 2024, 29, 4806. [Google Scholar] [CrossRef] [PubMed]
- Zaaboul, F.; Kaichouh, G.; Haoufazane, C.; Abuelizz, H.A.; Karrouchi, K.; Zarrouk, A.; El Hourch, A. Adsorption of Reactive Blue Day 49 from Aqueous Solution on Commercial Activated Carbon and Polyaniline Electrochemically Deposited on Carbon Felt: Kinetic Modeling and Equilibrium Isotherm Analysis. Int. J. Electrochem. Sci. 2024, 19, 100713. [Google Scholar] [CrossRef]
- Benazouz, K.; Bouchelkia, N.; Moussa, H.; Boutheldja, R.; Zamouche, M.; Amrane, A.; Parvathiraja, C.; Al-Lohedan, H.A.; Bollinger, J.C.; Mouni, L. Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water 2025, 17, 851. [Google Scholar] [CrossRef]
- Karleuša, R.; Marinić, J.; Tomić Linšak, D.; Dubrović, I.; Antunović, D.; Broznić, D. The Hidden Legacy of Dimethoate: Clay Binding Effects on Decreasing Long-Term Retention and Reducing Environmental Stability in Croatian Soils. Toxics 2025, 13, 219. [Google Scholar] [CrossRef] [PubMed]



| AS-Si | |||
|---|---|---|---|
| MB | Cipro | ||
| Pseudo-first-order model | qe(Cal) (mg g−1) | 9.2031 ± 0.1821 | 7.3544 ± 0.2122 |
| K1 (min−1) | 1.082 ± 0.2943 | 0.9508 ± 0.3127 | |
| R2 | 0.9473 | 0.9103 | |
| SSE | 3.9355 | 4.3558 | |
| RMSE | 1.9838 | 2.0970 | |
| Pseudo-second-order model | qe(Cal) (mg g−1) | 9.4750 ± 0.1615 | 7.6750 ± 0.1879 |
| K2 (g mg−1 min−1) | 0.2246 ± 0.694 | 0.1925 ± 0.0640 | |
| R2 | 0.9714 | 0.9530 | |
| SSE | 2.1322 | 2.2822 | |
| RMSE | 1.4602 | 1.5106 | |
| AS-Si | |||
|---|---|---|---|
| MB | Cipro | ||
| Langmuir | qm (mg g−1) | 25.9118 ± 1.8458 | 31.1804 ± 1.6416 |
| KL (L mg−1) | 0.28654 ± 0.3569 | 0.1333 ± 0.0300 | |
| R2 | 0.8278 | 0.9506 | |
| SSE | 137.3734 | 42.3328 | |
| RMSE | 11.7206 | 6.5063 | |
| Freundlich | KF ((mg g−1) ((L mg−1)1/n)) | 12.4672 ± 2.3616 | 8.5223 ± 2.3527 |
| 1/n | 0.1742 ± 0.0490 | 0.2792 ± 0.704 | |
| R2 | 0.6916 | 0.7474 | |
| SSE | 139.9143 | 152.4881 | |
| RMSE | 11.8285 | 12.3486 | |
| Adsorbent Material | Process | Pollutant | Initial Concentration (ppm) | Adsorbent Dose | Contact Time (min) | Adsorbed Quantity (mg/g) | Reference |
|---|---|---|---|---|---|---|---|
| Fly ash | Pretreated with hot distilled water and dried at 105 °C | MB | 100 | 600 mg | 120 | 5.7169 | [39] |
| Neem leaves | Pretreatment including: washing with distilled water, dried in an oven at 333–343 K, crushing | MB | 40 | 200 mg | 60 | 8.76 | [40] |
| Raw RHA | No pretreatment | MB | 10 | 500 mg | 180 | 3.01 | [41] |
| Treated RHA | Mechanochemical treatment | MB | 10 | 500 mg | 180 | 5.02 | [41] |
| AS-Si | Sol-gel | MB | 20 | 200 mg | 90 | 9.82 | This work |
| Modified fly ash | Modification trough microwave power | Cipro | 100 | 100 mg | 120 | 11.76 | [42] |
| Zeolite-modified nano-flakes | Modification trough different steps: alkaline treatment with NaOH, acid and re-precipitation using HCL, Filtration, neutralization with distilled water and dried at 280 °C using an electro-thermostatic blast oven (YLD-2000) | Cipro | 20 | 100 mg | 120 | 7.67 | [43] |
| AS-Si | Sol–gel | Cipro | 20 | 200 mg | 180 | 8.10 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krime, A.; Saoiabi, S.; Tlemcani, M.; Saoiabi, A.; Carreiro, E.P.; Carrott, M.R. Turning Waste into Wealth: Sustainable Amorphous Silica from Moroccan Oil Shale Ash. Recycling 2025, 10, 143. https://doi.org/10.3390/recycling10040143
Krime A, Saoiabi S, Tlemcani M, Saoiabi A, Carreiro EP, Carrott MR. Turning Waste into Wealth: Sustainable Amorphous Silica from Moroccan Oil Shale Ash. Recycling. 2025; 10(4):143. https://doi.org/10.3390/recycling10040143
Chicago/Turabian StyleKrime, Anas, Sanaâ Saoiabi, Mouhaydine Tlemcani, Ahmed Saoiabi, Elisabete P. Carreiro, and Manuela Ribeiro Carrott. 2025. "Turning Waste into Wealth: Sustainable Amorphous Silica from Moroccan Oil Shale Ash" Recycling 10, no. 4: 143. https://doi.org/10.3390/recycling10040143
APA StyleKrime, A., Saoiabi, S., Tlemcani, M., Saoiabi, A., Carreiro, E. P., & Carrott, M. R. (2025). Turning Waste into Wealth: Sustainable Amorphous Silica from Moroccan Oil Shale Ash. Recycling, 10(4), 143. https://doi.org/10.3390/recycling10040143









