Abstract
Recyclable aluminum-containing industrial solid waste can be used as supplementary cementitious materials (SCMs) to replace cement (30–50%), thereby reducing CO2 emissions during cement production and improving the mechanical properties and durability of concrete. Therefore, the use of SCMs in building materials presents significant potential. Due to the presence of the aluminum phase in the SCMs, the hydration products of cements blended with SCMs are changed. Compared to the primary hydration product of conventional cement, calcium silicate hydrate (CSH), the main hydration product of cement blended with SCMs is calcium aluminosilicate hydrate (CASH), which exhibits a more complex molecular structure. Understanding the role of Al in C-A-S-H at the atomic scale facilitates mechanistic insights and promotes the sustainable utilization of SCMs in eco-friendly construction. Molecular dynamics enables the rapid and accurate structural analysis and property prediction of materials. Therefore, this paper presents a systematic review of molecular dynamics simulations of CASH and discusses the role of Al in the molecular structure, dynamic, and mechanical behavior of CASH. It also analyzes the interfacial properties of CASH composites, the immobilization and transport of ions in CASH, and the temperature effect on the structure and properties of CASH. Finally, the challenges and perspectives for molecular dynamics simulation of CASH are presented.
1. Introduction
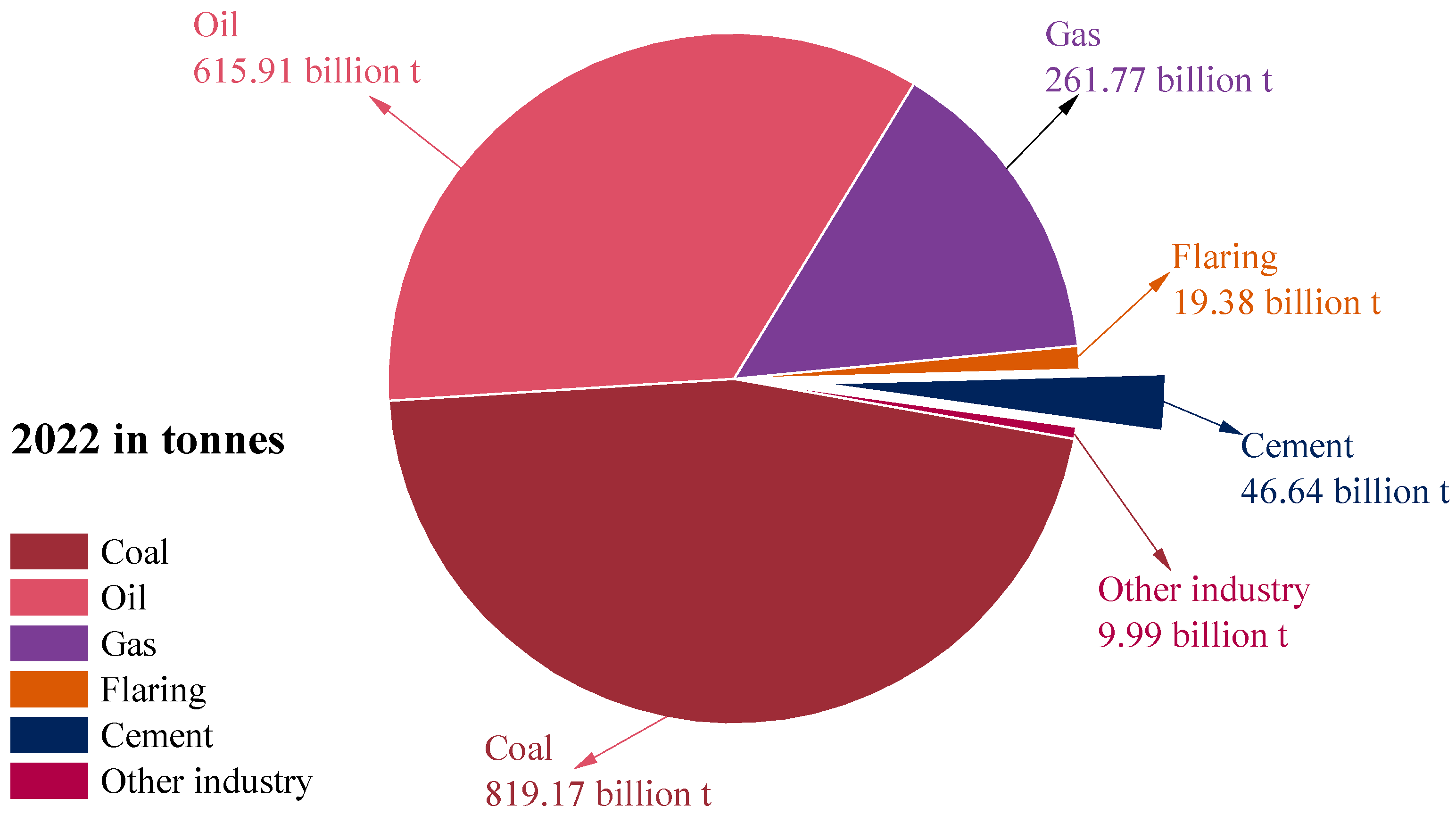
In 2022, CO2 emissions in cement production reached approximately 46.64 billion tons, accounting for 2.64% of global CO2 emissions, as shown in Figure 1. To achieve the greenhouse gas emission reduction goals of the Paris Agreement, sustainable alternatives to cement need to be urgently found. The partial replacement of SCMs with cement provides a favorable pathway for carbon emission reduction in the cement production process. Previous research suggested that the addition of SCMs, such as aluminum-rich industrial solid waste fly ash, granulated blast furnace slag, silica fume, etc., can reduce CO2 emissions in the cement production process by 30–40% [1]. Furthermore, the reaction between the silicoaluminate in SCMs and the alkaline substances in cement can produce the hydrated cementitious product—CASH [2]. Fundamentally, CASH differs from CSH in terms of chemical composition and atomic structure. CSH is primarily composed of Ca2+, silicate chains, and water, but CASH incorporates Al atoms into the silicate chains to replace Si. This Al substitution alters the polymerization of the silicate network, typically resulting in higher degrees of cross-linking and modified interlayer spacing and nanostructure. Chemically, the presence of Al introduces tetrahedral coordination sites with different charge characteristics than Si, affecting the ability of the gel to bind and stabilize ions and ultimately affecting its macroscopic properties. Therefore, the incorporation of SCMs in cement also improves the mechanical properties [3] and long-term durability [4] of concrete. For example, Roman concrete with CASH gel submerged in the ocean for over 2000 years can still maintain structural integrity [5].
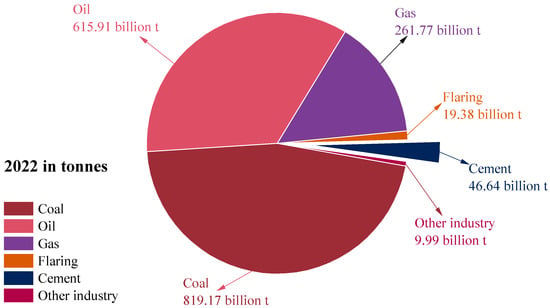
Figure 1.
Share of global CO2 emissions by industry in 2022 (data from [6]).
To promote the research and utilization of SCMs in cement, it is crucial to extensively explore the mechanism of action of SCMs. Extensive experimental studies on the macroscopic properties of cement blended with SCMs (cement-SCMs) and the characterization of their hydration products have been carried out [3,7,8,9,10]. Despite the fact that CASH has been recognized as a main hydration product of cement-SCMs [11,12,13,14], its formation mechanism and complex molecular structure, especially the role of Al in it, need to be further elucidated. The incorporation of Al into the gel phase is significant, as it influences the evolution of the gel phase’s nanostructure, density, and pore structure, thereby enhancing the gel phase’s mechanical strength and permeability resistance. Additionally, it enhances the ability to fix harmful ions, which is crucial for durability in corrosive environments. This leads to difficulties in thoroughly explaining the mechanisms of CASH gel in different applications, such as the interface effects in composites, the immobilization of heavy metal ions, and ion transport in corrosive environments.
With the continuous development of material characterization techniques, it has played a great role in promoting the research of material science. Nuclear magnetic resonance (NMR) techniques can characterize the atomic structure, position, and chemical bonding of cementitious materials but cannot observe the dynamic reaction process. In situ characterization techniques, although they can monitor the reaction process, are unable to observe the reaction details at the atomic level. Importantly, the equipment or characterization to use these techniques is expensive. Molecular dynamics (MD) is a powerful tool in materials science for studying the structure-property relationships of materials at the atomic scale [15]. The advantage of MD in understanding complex systems lies in its ability to complement and extend traditional laboratory methods. MD can provide atomic-level details (e.g., changes in chemical bonds and coordination numbers), observe dynamic reaction processes (e.g., ion diffusion, structural evolution under high temperatures or tensile loads), and predict macroscopic properties (e.g., mechanical properties from simulated atomic trajectories). At the same time, MD is cost-effective and time-saving compared to setting up complex in situ experiments or acquiring extensive synchrotron/NMR time. However, MD simulations of hydration products in cement systems still face challenges. These challenges stem from the inherent amorphous and heterogeneous nature of the materials, making it difficult to define accurate initial structures. Specifically, key processes such as hydration and ion transport involve slow dynamics, with timescales ranging from seconds to years, far exceeding the typical nanosecond to microsecond range of classical MD simulations. Additionally, achieving a realistic composition model that reflects the complex chemical environment of real cement paste imposes extremely high computational demands.
Despite some challenges that remain, MD has provided researchers with a wide range of ideas for characterizing CSH at the atomic level. To date, MD has been employed to study the molecular structure [16], dynamic properties [17], polymerization processes [18], ion immobilization and transport [19], and interface interactions [20,21]. Previous studies have summarized and reviewed relevant MD studies, focusing on modeling and simulation [22], the relationship between structure and properties (the relationship between molecular structural characteristics and their dynamic behavior and mechanical properties) [23], precursor hydration polymerization processes [24], drying shrinkage [25], applications in composite materials [26], and the role of chemical admixtures [27]. These studies have provided critical guidance for the development of MD simulations of CSH. However, there is a lack of comprehensive reviews and critiques on MD studies of CASH. CASH gel is extremely important for enhancing the mechanical properties and durability of concrete. Ma et al. [28] briefly reviewed current computational methods and applications of cementitious materials (CSH, CASH, and NASH) in MD simulations. However, the role of Al in the CASH has not been elucidated systematically at the atomic level. Furthermore, given the many complex factors involved in practical applications, the current understanding of the impact of Al on the properties of cement-based materials is still not comprehensive.
Therefore, this review aims to comprehensively elucidate the MD simulation of CASH systems, exploring the role of Al. Firstly, the impact of Al on the molecular structure, dynamics, and mechanical properties of CASH is discussed. Subsequently, the action mechanisms of CASH gel in real applications are analyzed, including the interfacial characteristics of the composites, the advantages in immobilizing radioactive ions and heavy metals, the transport mechanism of SO42− and Cl− ions in gel pores, and the temperature effect on the structure-properties. Finally, current challenges and potential future research directions are presented.
2. Prediction of Molecular Structure, Dynamics, and Mechanical Properties
With the rapid development of MD, the CASH atomic structure is important to understand because of its influence on the overall cement/concrete. MD can reveal the effect of Al on the molecular structure, dynamics, and mechanical properties of CASH and give accurate predictions.
2.1. Analysis of Molecular Structure
Molecular structure analysis provides the basis for understanding CASH gel at the atomic level. Revealing the interactions and arrangements between CASH atoms through MD helps to explain the macroscopic properties of the material.
2.1.1. Introduction to Molecular Configuration
- (1)
- Substitution method
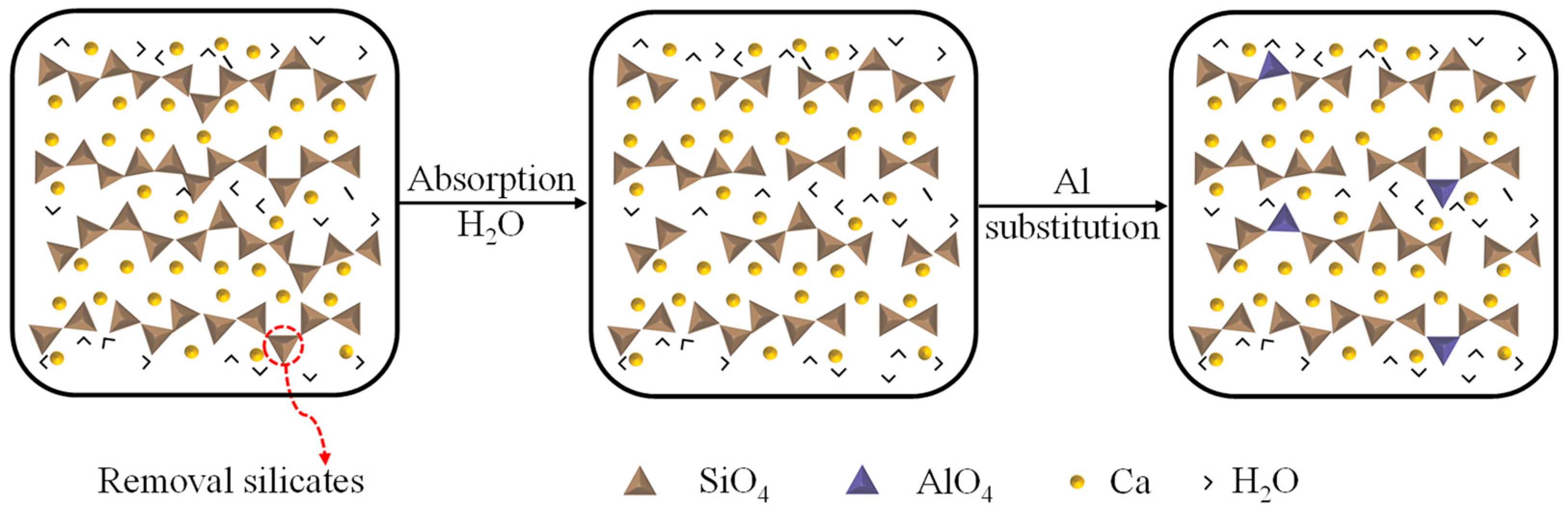
The initial CASH model is usually obtained by modifying the CSH model. CSH is a layered molecular structure, with silicate chains interconnected with Ca ions to form a Ca−Si layer (herein Ca is intralayer calcium), and the Ca ions are also present between the Ca−Si layers to balance the charge (herein Ca is interlayer calcium). Meanwhile, water molecules are present within and between the Ca−Si layers. Based on the CSH model, Si atoms are substituted by Al atoms [29]. As shown in the Figure 2, first of all, CSH is initially configured with tobermorite (Ca/Si = 1) [30]. The Ca/Si ratio is then increased to 1.65 by removing the bridging silicate tetrahedra [31]. Finally, H2O adsorption into the CaO layer of the defective dry tobermorite is simulated [32,33]. The modified model was geometrically optimized by choosing the appropriate force field and ensembles, and the resulting molecular configuration of CASH resembles CSH, which has a layered molecular structure. This C-S-H model accurately describes experimental data such as radial distribution functions and infrared spectra of Ca, CaO, and SiO [34]. Therefore, it has been widely used as a database for studying the chemical structure and corresponding defective and mechanical properties in hydrated cementitious phase [35,36,37,38,39,40,41,42]. However, this process of removing Si atoms generates non-naturally formed vacancy defects, and the actual defect distribution in CASH may be more complex, leading to deviations between the model and the local structure of the real material.
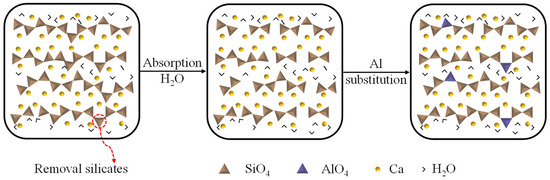
Figure 2.
Molecular modeling of CASH.
- (2)
- Polymerization method
The polymerization method is capable of directly describing the formation of chains during polymerization. Zhao et al. [43] modeled C-A-S-H through the simulation of the monomers polymerization of Si(OH)4, Ca(OH)2, and Al(OH)3. The polymerization process was also studied for Si(OH)4 and Al(OH)3 in the presence of Na(OH)·5H2O as well as Ca(OH)2·4H2O, where the excess positive charge generated by replacing Si4+ by Al3+ is counteracted by OH− [44,45]. This C-A-S-H modeling approach provides direct visualization of the polymerization process of the precursor and the structural evolution at the nanoscopic level, which is difficult to observe in conventional experiments. The polymerization method simulates the polymerization process starting from monomers. However, in actual cement pore solutions, the monomer concentration is quite low [46,47]. The model’s high monomer concentration setting (to shorten simulation time) may distort the polymerization path.
2.1.2. Atomic Bonding
The coordination number is an important parameter that measures the number of nearest neighboring atoms around a specific atom in a molecule, reflecting the potential number of chemical bonds that the atom may form. In the molecular structure of CASH, the silicon atoms are stably present in tetrahedral coordination within the silicate chain [48,49,50], indicating that silicon atoms can form stable bonds. Aluminum atoms exhibit variable coordination numbers, typically 4, 5, and 6 coordination [44,51,52]. The 4-/5-/6-coordinated aluminosilicate structures observed in the simulation are consistent with the 27Al NMR analysis results of CASH gel from other experimental studies [45]. The variability in the coordination of aluminum atoms can make the Al-O-Si bonds prone to hydrolysis compared to Si-O-Si bonds, leading to the instability of Al-O-Si bonds. Calcium atoms primarily exist in a 6-coordinated octahedral structure. However, increasing the aluminum content also increases the coordination number of interlayer calcium [53]. Due to the stable tetrahedral structure of silicate and aluminate in the CASH, constant 4-coordinated silicon atoms make Si-O bonds more stable than Al-O and Ca-O bonds.
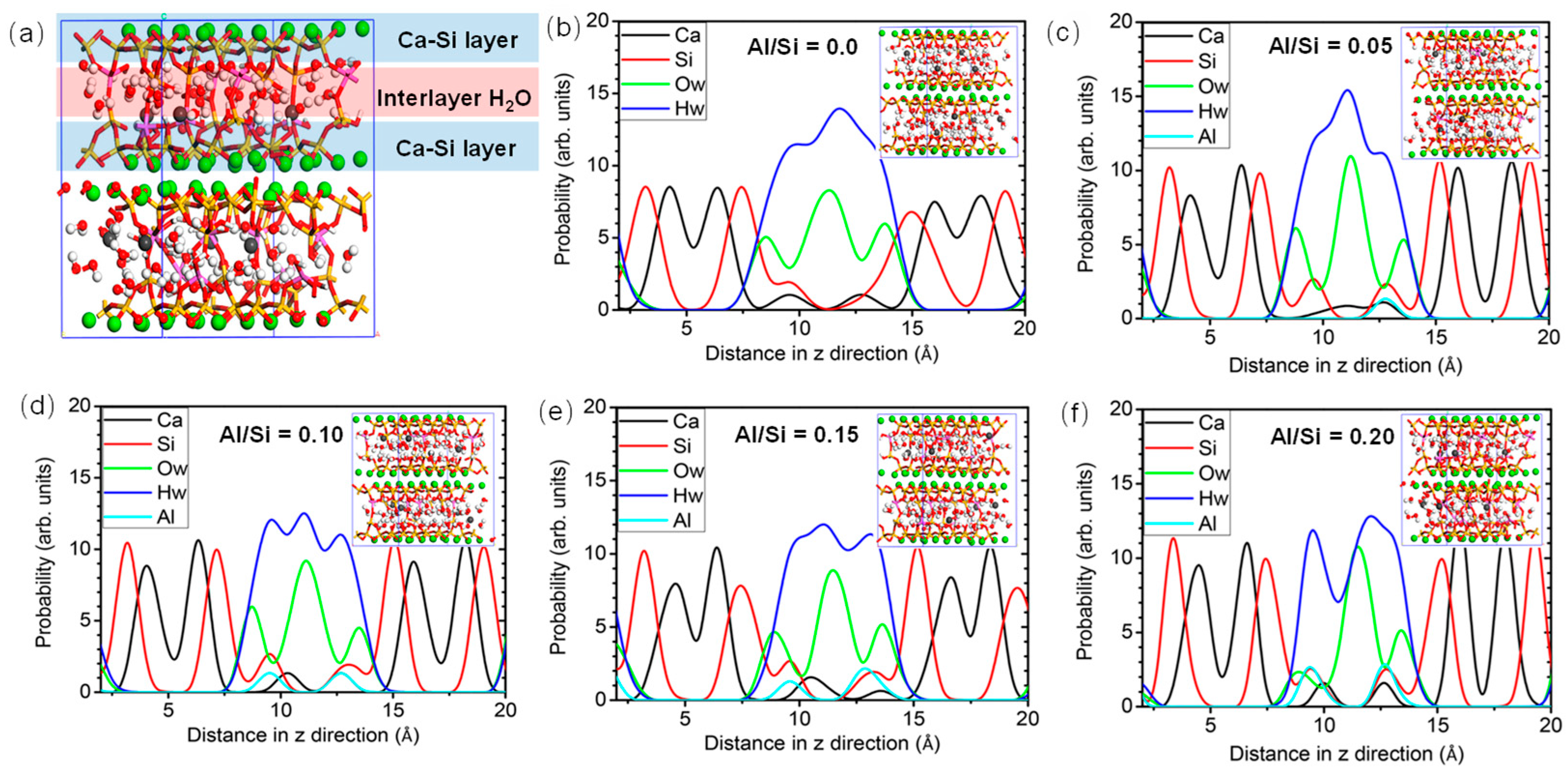
The radial distribution function (RDF) stands for the probability density of finding another atom within a given radius range. This helps to understand the distribution of other atoms around a central atom, revealing the structure and interactions between atoms. As illustrated in the Figure 3, in the short distance range (approximately 0–3 Å), the bond lengths of CASH gel are 1.63 Å for Si-O [29], 2.48 Å for Ca-O [54], and 1.88–1.90 Å for Al-O [29,55,56], while it is 0.89 Å for O-H [29,57]. At the intermediate distance range (approximately 3–5 Å), the Ca-O and Si-O overlap with the second peak within the RDF curve, indicating the formation of a branching structure [56]. The Al-O bond shows a lower peak [57], suggesting a disordered structure in the local range. Additionally, a second peak appears in the RDF of the O-H bonds corresponding to the hydrogen bond [29]. Although the introduction of Al atoms makes the Al-O-Si bonds less stable compared to Si-O-Si bonds, the Al atoms serve to connect the intermediate layers, making the molecular structure relatively disordered. In the long-distance range (>5 Å), no significant peaks are observed.
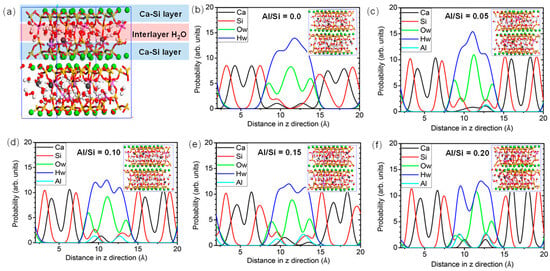
Figure 3.
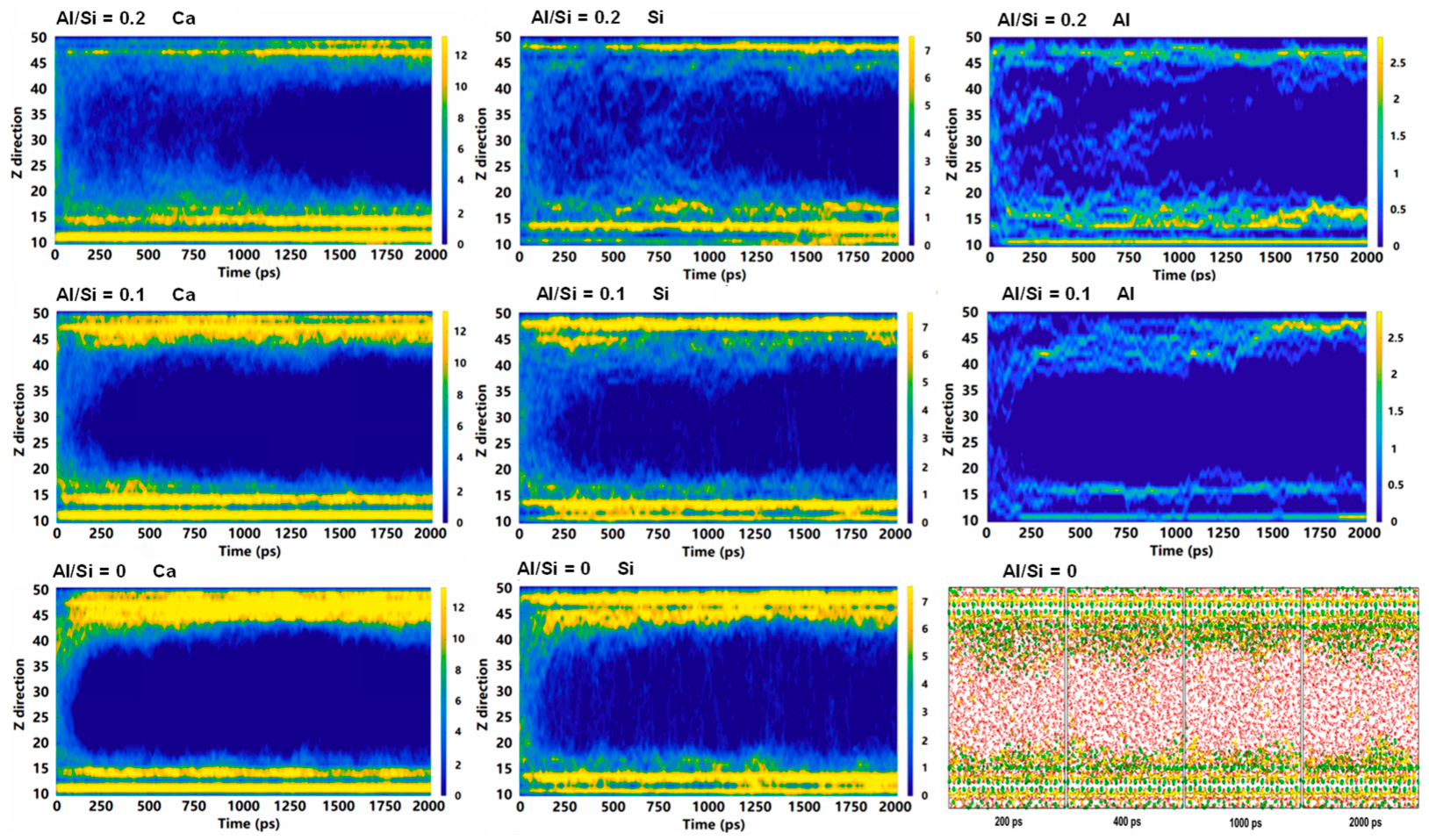
Atomic density distribution for various Al/Si ratios [58]: (a) molecular structure with Al/Si = 0.15, (b) Al/Si = 0.0, (c) Al/Si = 0.05, (d) Al/Si = 0.10, (e) Al/Si = 0.15, and (f) Al/Si = 0.20.
2.1.3. Al Content on the Evolution of Qn
The polymerization degree of CASH gel allows an understanding of the molecular structure evolution of the material, in particular the effect of Al on the polymerization degree. Qn is used to characterize the polymerization state of silicate skeletons, in which n indicates the number of bonded adjacent silicate tetrahedra. Q0, Q1, and Q2 represent monomer, dimer, and chain, respectively. Q3 and Q4 stand for branch and network [59].
- Effect of without tensile loading
In the CASH gel structure, interlayer H2O combines with Al to produce Al-OH and Al-O(H)-Si [58]. Free Al-OH/Si-OH combines with Si-OH via bridging oxygen to form T-O-T (T for Si and Al) (see Equations (1)–(3)) [43], which extends the length of the silicoaluminate chain. At low Al content (1.4 ≤ Ca/(Al + Si) < 1.7), the silicate-aluminate structure shifts from a dimer to a chain. At high Al content (0.9 < Ca/(Al + Si) < 1.4), a small percentage of the silicate-aluminate structure shifts to branched and network structures. The main chain length (MCL) increases with a growing Al/Si ratio [58,60], which quantitatively matches experimental data [61], meaning that increasing Al content improves the cohesion of the structure [53,62]. However, Q3 and Q4 completely disappear when Ca/(Al + Si) = 1.7 due to the absence of Al atoms [55]. Therefore, the increase in Al content favors the appearance of branch and network structures, but the linear structure is still the main structure (Q2 and Q3) [50,63]. Relatively high Al content is conducive to improving the polymerization degree. Dolado et al. [43] modeled CASH using monomer polymerization and showed that the presence of Al enhanced the polymerization of silicate chains at the same (Al + Si)/Ca ratio [63].
- Effect of tensile loading
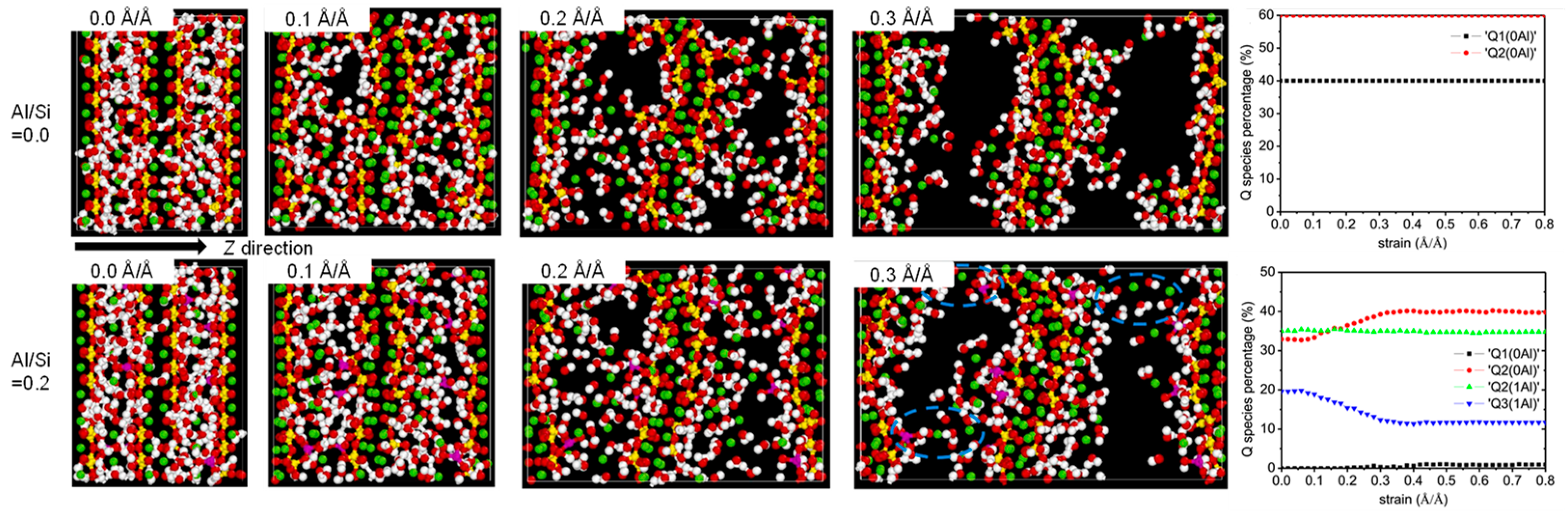
MD can simulate changes in the CASH structure under external forces. CASH undergoes chemical bond breaking and formation under external forces, resulting in constant transformation of the polymerization units. Under tensile loading in the x and y directions, the proportion of Q2 and Q3 species decreases and the proportion of Q1 species increases [29,50,56] due to the breakage of Al-Si chains. With an increasing Al/Si ratio (0 to 0.2), the proportion of Q3 in the structure increases gradually, and the proportion of Q2 + Q3 remains stable at higher strains [50]. This provides better resistance to applied loads. Figure 4 shows the structural evolution in the z direction for Al/Si = 0 and 0.2. At low strains, the Qn species ratio does not change much, except for the elongated Al-O and Si-O bonds and the stretched Al-O-Si bond angles [58]. At a higher Al/Si ratio, the high strain causes the gradual deconstruction of the Q3 species into Q2 and Q1, but the structure is not destroyed [58]. The existence of Al triggers structural rearrangement in the fractured silicate chains, leading to an increase in the percentage of Q3, thereby enabling better resistance to applied loads in the later stages of strain.
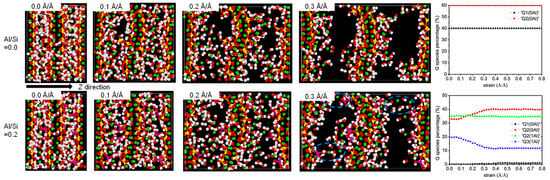
Figure 4.
Evolution of molecular structure and Qn species under z-direction tensile [50,54].
Hydration reactions accompany the whole process of Qn species evolution [56]. Under external forces, H2O moves to the silicate tetrahedra and forms hydrogen bonds [64], which promote the dissociation of H2O to produce H+ and OH−. H+ binds to ONB to generate Si-OH groups, while OH− coordinates with interlayer Ca2+ ions to generate Ca-OH groups [50]. In addition, the hydrolyzed H+ is transferred to the ONB of Al-O-Si to generate Al-OH and Al-O(H)-Si [56] instead of directly generating Al-OH and Si-OH. This implies that a structural rearrangement occurs under external forces, thus slowing down the crack development [57]. At high strains, the hydrolysis process of Si-O-Ca and Si-O-Si bonds is relatively easy (see Equations (4) and (5) [65,66]), whereas hydrolytic fracture of Al-O-Si bonds is a complex process (see Equations (6) and (7) [50]).
Therefore, by modeling CASH after obtaining effective Al/Si or Ca/(Al + Si) ratios through experiments and selecting the applicable force field and ensemble, the polymerization degree and MCL value of CASH can be predicted. This reduces the cost of NMR tests to a certain extent. Meanwhile, it can also fill the gap of not being able to obtain valuable molecular structures because the raw materials are magnetic solid wastes (e.g., steel slag powder, red mud, etc.).
2.2. Analysis of Dynamics Behavior
Dynamics analysis allows the observation and simulation of the motion and reaction processes of the particles inside CASH, which is essential for understanding the dynamic behavior of CASH.
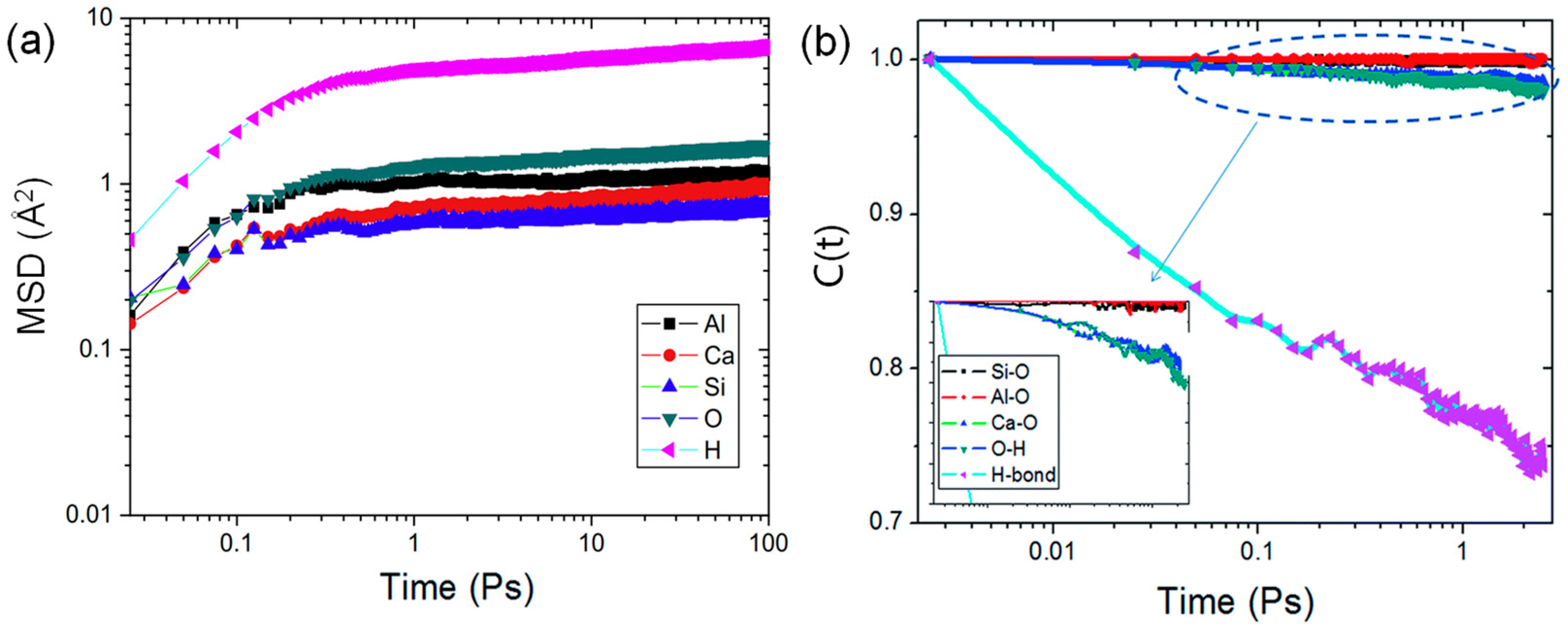
The mean square displacement (MSD) can be descriptive of an atomic dynamic property [33]. As illustrated in Figure 5a, atoms in CASH structure exhibit two dynamic phases in the MSD curves: (1) the initial ballistic jump phase, which is typically the inertial motion of atoms that is not influenced by surrounding atoms [57]; and (2) the dynamic “cage-like” phase, which exists due to collisions between atoms in a confined environment where they are constrained to move within a “cage” formed by neighboring atoms [57].
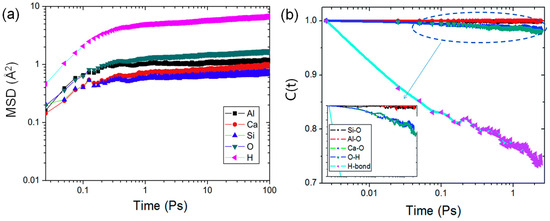
Figure 5.
Dynamics curves: (a) MSD of different atoms [57], (b) TCF curves of various bonds [56].
The diffusion ability of interlayer water molecules can indirectly indicate that the introduction of Al improves the substrate-water interactions between the CASH interlayers. Yang et al. [58] reported that H2O molecules in the interlayer are confined by a “cage” structure composed of ion-covalent and hydrogen bonds, making it difficult for them to escape [29]. As the Al/Si ratios increase, the cross-linking of the Ca−Si layer increases. The diffusion region of H2O changes from the interlayer to a discontinuous “cage” [58], therefore significantly decreasing the diffusion coefficient. Additionally, the dipole moment of H2O has a high distribution within small pores “cage”, which intensifies water-substrate interactions and further limits water diffusion [67]. Thus, the presence of Al forms a "cage-like" structure that limits the diffusion of H2O, and the restricted H2O molecules form strong hydrogen bonds with the Ca−Si layer. Furthermore, in Section 2.3.2, it was introduced that CASH has better resistance to external forces. Confined H2O molecules are more likely to undergo hydration reactions with neighboring Ca, Al, and Si atoms to resist external forces. This favors mechanical properties.
The strength of chemical bonds relies on their stability to a great extent; time-correlated function (TCF) is capable of analyzing their stability and dynamics. As indicated in Figure 5b, Hou et al. [56] reported that C(t) values of Al-O and Si-O stay near 1, indicating the aluminate-silicate local structure has good stability. The C(t) value of the Ca-O bond decreases slightly, which is caused by the cluster formation of interlayer Ca with H2O increasing the diffusion rate of Ca and leading to easy breakage of the Ca-O bond.
2.3. Prediction of Mechanical Properties
Atomic-scale prediction of mechanical properties contributes to the evaluation of mechanical properties in experiments and applications. The presence of the Al improves the mechanical properties of SCMs-cement. MD can explain the mechanism of mechanical property enhancement from the atomic scale.
2.3.1. Stress-Strain Relation
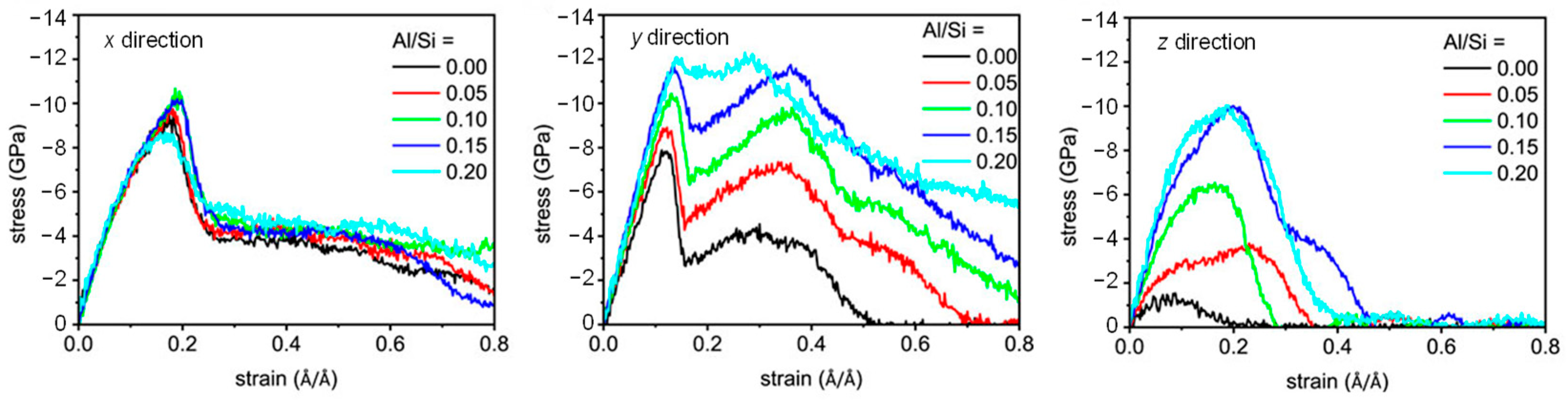
Stress-strain is capable of describing the mechanical properties of materials under external force (e.g., tensile loading). The stress-strain curve of CASH has four stages, as shown in Figure 6. Stage I, stress increases linearly as strain during the tensile loading; stage II, stress gradually increases to a maximum value, representing the tensile strength; stage III, stress rapidly decreases during the crack extension stage; and stage IV, stress slowly decreases as a strain continues to increase until the structure is damaged and the tensile process ends [29,50,53,58]. Notably, the CASH structure shows a stepped stress stage (the stress increases again) after the first drop in the y direction, which is related to the structural rearrangement of silicate-aluminates [58].
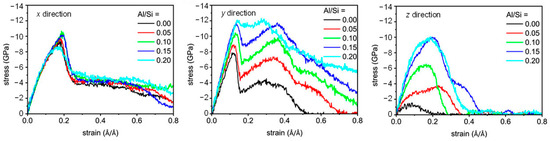
Figure 6.
Stress-strain curves with various Al/Si ratios [50].
Like CSH, the CASH structure has anisotropy as well. Cohesion in the x direction is generated by interacting the silicate with the CaO layer, while the Ca-O-Si and Si-O-Si bonds of the silicate are the source of cohesion in the y direction [50]. CASH gel has better plasticity in the y direction [56] while presenting brittleness in the z direction [53,58]. This is because the ionic covalent bonding strength of the Ca−Si chains in the y-direction is superior to the H-bond of the interlayer in the z-direction [56]. However, increasing the content of Al atoms between interlayers allows for enhancement of the mechanical properties in the z direction, achieving stress values close to or even higher than those in the x and y directions, as is shown in Table 1. This can be explained by the evolution of molecular structure under external forces in Section 2.1.2.
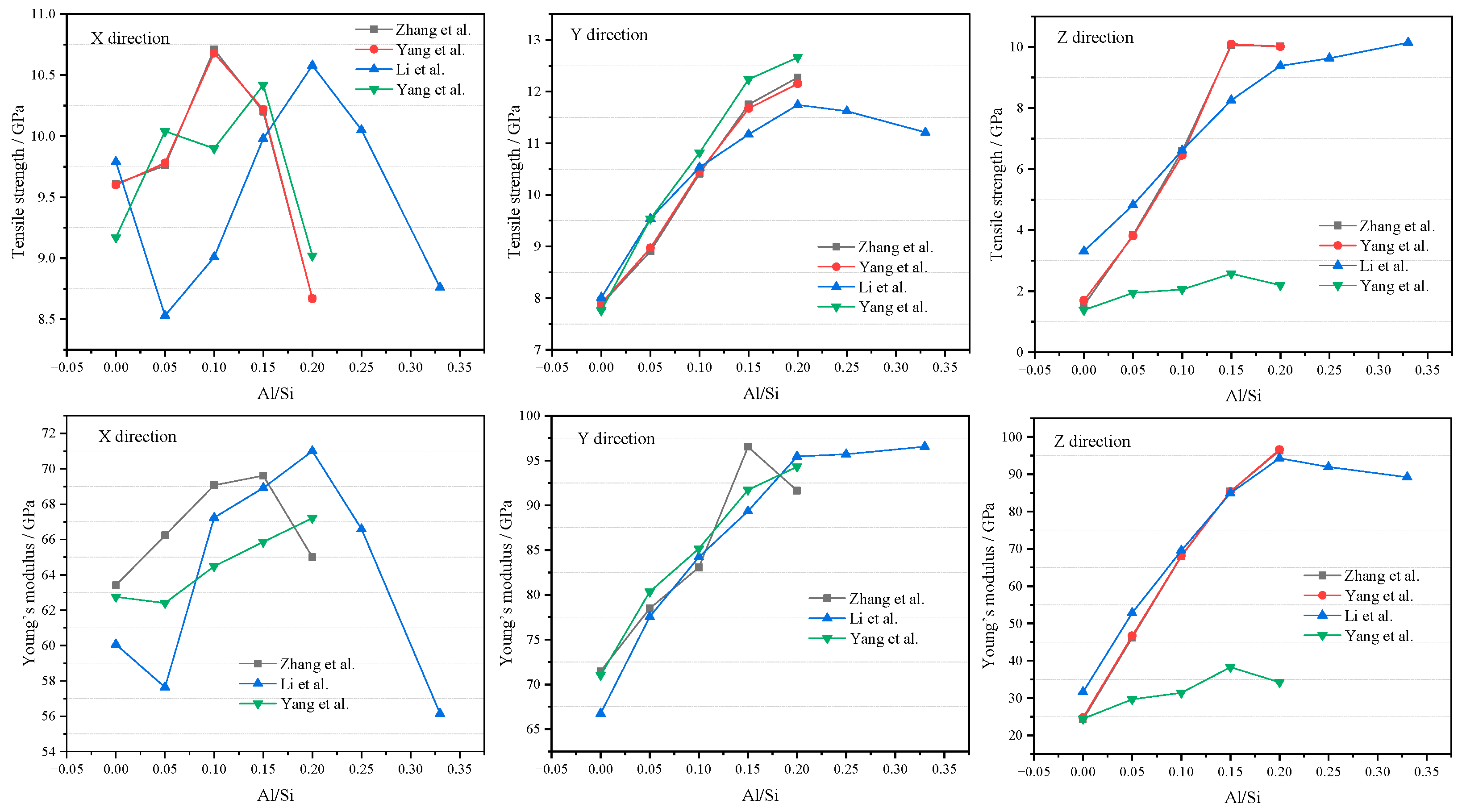
2.3.2. Tensile Strength and Young’s Modulus
The tensile strength and Young’s modulus of CASH can be predicted by MD, summarizing data from the references in Table 1. In the x direction, Young’s modulus and tensile strength show a tendency to rise and then fall by increasing the Al/Si ratio. In the y and z directions, Young’s modulus and tensile strength increase with increasing Al/Si ratio [56,58]. Figure 7 shows tensile strength and Young’s modulus (mechanical strength). In the x direction, it can be observed that mechanical strength first increases and then decreases with increasing Al content, reaching an optimal value when the Al/Si ratio is between 0.1 and 0.2. In the y direction, mechanical properties reach a maximum at Al/Si = 0.2 as Al content increases and then stabilize or decrease beyond 0.2. In the z direction, mechanical strength gradually increases with increasing Al content, which is consistent with the experimental observations obtained using high-pressure XRD technology [68], indicating that Al-induced crosslinking enhances the strength of the sample along the z direction. However, the best mechanical strength is exhibited when the Al/Si ratio is in the range of 0.15 to 0.20. Based on the above, CASH exhibits optimal mechanical properties when the Al/Si ratio is between 0.15 and 0.2. The improvement in mechanical strength is mainly attributed to the addition of aluminum, which causes cross-linking of silicoaluminates, significantly improving interlayer interactions [50,53]. At low Al content, Al atoms primarily repair defective Ca−Si sheets; at high aluminum content, a network structure forms in the interlayer region [68,69].Interestingly, when Al/Si > 0.2 (0.2~0.33), the frequent formation and fracture of hydrogen bonds between Ca−Si layers weakened the stability of the structure [54]. The tensile strength and Young’s modulus no longer increase but remain unchanged or slightly decrease. Therefore, it is better to keep Al/Si around 0.2 in cement-SCMs.
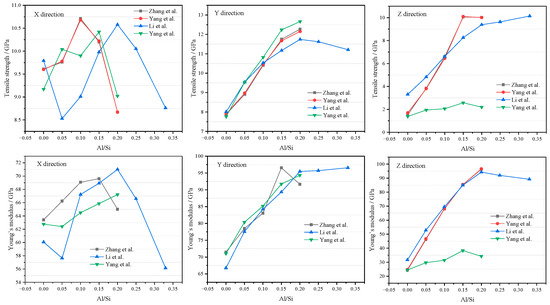
Figure 7.
Tensile strength and Young’s modulus (data source from [50,54,58,70]).

Table 1.
Parameters of MD simulation and mechanical properties in Ref.
Table 1.
Parameters of MD simulation and mechanical properties in Ref.
| Ref. | Software | Modeling Mothed | Force Field | Ensemble | Ratio | Tensile Strength/GPa | Young’s Modulus/GPa | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||||||
| [50] | LAMMPS | Al substitution for Si | CSH-FF | NPT | Al/Si = 0 | 9.61 | 7.88 | 1.54 | 63.41 | 71.48 | 24.32 |
| Al/Si = 0.05 | 9.76 | 8.91 | 3.85 | 66.24 | 78.47 | 46.22 | |||||
| Al/Si = 0.10 | 10.71 | 10.41 | 6.60 | 69.07 | 83.07 | 68.01 | |||||
| Al/Si = 0.15 | 10.20 | 11.75 | 10.06 | 69.61 | 96.54 | 85.37 | |||||
| Al/Si = 0.2 | 8.67 | 12.27 | 10.03 | 65 | 91.64 | 96.32 | |||||
| [58] | LAMMPS | Al substitution for Si | Reax-FF and CSH-FF | NPT | Al/Si = 0 | 9.6 | 7.9 | 1.7 | — | — | 24.8 |
| Al/Si = 0.05 | 9.78 | 8.97 | 3.81 | — | — | 46.7 | |||||
| Al/Si = 0.10 | 10.68 | 10.45 | 6.45 | — | — | 68.1 | |||||
| Al/Si = 0.15 | 10.22 | 11.67 | 10.10 | — | — | 85.4 | |||||
| Al/Si = 0.20 | 8.67 | 12.15 | 10.01 | — | — | 96.6 | |||||
| [56] | — | Al substitution for Ca | Reax-FF | NPT | Al/Ca = 0 | — | 6.5 | 3.25 | — | 64 | 50 |
| Al/Ca = 0.38 | — | 8.5 | 7.0 | — | 75 | 68 | |||||
| [53] | LAMMPS | Al substitution for Si | Reax-FF | water adsorption: NVT; equilibrium: NPT | Ca/(Si + Al) = 0.9 | — | 6.8 | 8.5 | — | — | — |
| Ca/(Si + Al) = 1.0 | — | 5.2 | 8.4 | — | — | — | |||||
| Ca/(Si + Al) = 1.2 | — | 4.5 | 8.0 | — | — | — | |||||
| Ca/(Si + Al) = 1.4 | — | 4.2 | 7.8 | — | — | — | |||||
| Ca/(Si + Al) = 1.7 | — | 3.1 | 6.8 | — | — | — | |||||
| [57] | — | Al substitution for Si | Reax-FF | NPT | Ca/Si = 0 | About 3.4 | 6.8 | 6.7 | — | — | — |
| Ca/Si = 0.38 | About 7.5 | 8.5 | 7.0 | — | — | — | |||||
| [54] | LAMMPS | Al substitution for Si | Reax-FF | water adsorption: NVT; equilibrium: NPT | Al/Si = 0 | 9.79 | 8.01 | 3.31 | 60.06 | 66.75 | 31.67 |
| Al/Si = 0.05 | 8.53 | 9.54 | 4.83 | 57.64 | 77.56 | 52.85 | |||||
| Al/Si = 0.10 | 9.01 | 10.53 | 6.62 | 67.24 | 84.24 | 69.54 | |||||
| Al/Si = 0.15 | 9.98 | 11.17 | 8.26 | 68.93 | 89.34 | 84.93 | |||||
| Al/Si = 0.20 | 10.58 | 11.74 | 9.39 | 71.01 | 95.45 | 94.28 | |||||
| Al/Si = 0.25 | 10.05 | 11.62 | 9.63 | 66.59 | 95.72 | 91.98 | |||||
| Al/Si = 0.33 | 8.76 | 11.21 | 10.15 | 56.15 | 96.56 | 89.23 | |||||
| [70] | LAMMPS | Al substitution for Si | Reax-FF | NPT | Al/Si = 0.20 | 9.02 | 12.66 | 2.20 | 67.22 | 94.31 | 34.22 |
3. MD Simulation for Applications
As the demand for high-performance concrete continues to grow in the field of materials science, researchers are increasingly interested in CASH gel. This material exhibits significant potential across various applications due to its unique physicochemical properties. MD simulations serve as a tool for offering an effective means to predict and analyze the properties of CASH gel in application scenarios.
3.1. Analysis of Interfacial Characteristics
Cement composites play vital roles in many fields, and the interface characteristics of cement composites affect their mechanical properties and durability. MD can reveal the interfacial interaction mechanism at the atomic level and clarify the contribution of Al to interfacial interactions.
3.1.1. CASH and Graphene Oxide
The introduction of graphene oxide (GO) can improve the properties of cement composites, e.g., mechanical properties, cracking resistance, impermeability, and durability. MD can explain the corresponding mechanism. Wang et al. [71] found that the formation of ionic bonds (Ca2+ ions with oxygen-containing functional groups on GO [72,73]) and hydrogen bonds between GO and CSH are the key factors for improved mechanical properties. Among them, the carboxyl group can form more stable chemical bonds with CSH. Mao et al. [74] found that an increase in the content of GO functional groups could enhance the mechanical properties of GO/CSH. In addition, the mechanical properties of GO/CSH could be modulated by varying the proportion of epoxy and hydroxyl groups. The way GO is present in CSH also affects the mechanical properties of the concrete. GO introduction is categorized into bridging and embedding, where bridging improves the fracture energy and serves to enhance the mechanical properties, while embedding is the opposite [75]. The orientation angle of GO is also crucial for the mechanical properties of the composites. Chen et al. [76] found the orientation angle of GO has a vital influence on the shear properties, and the shear strength increased by 23.5% when the GO orientation was 30°. It is essential to regulate the orientation and angle of the GO to fully utilize the GO.
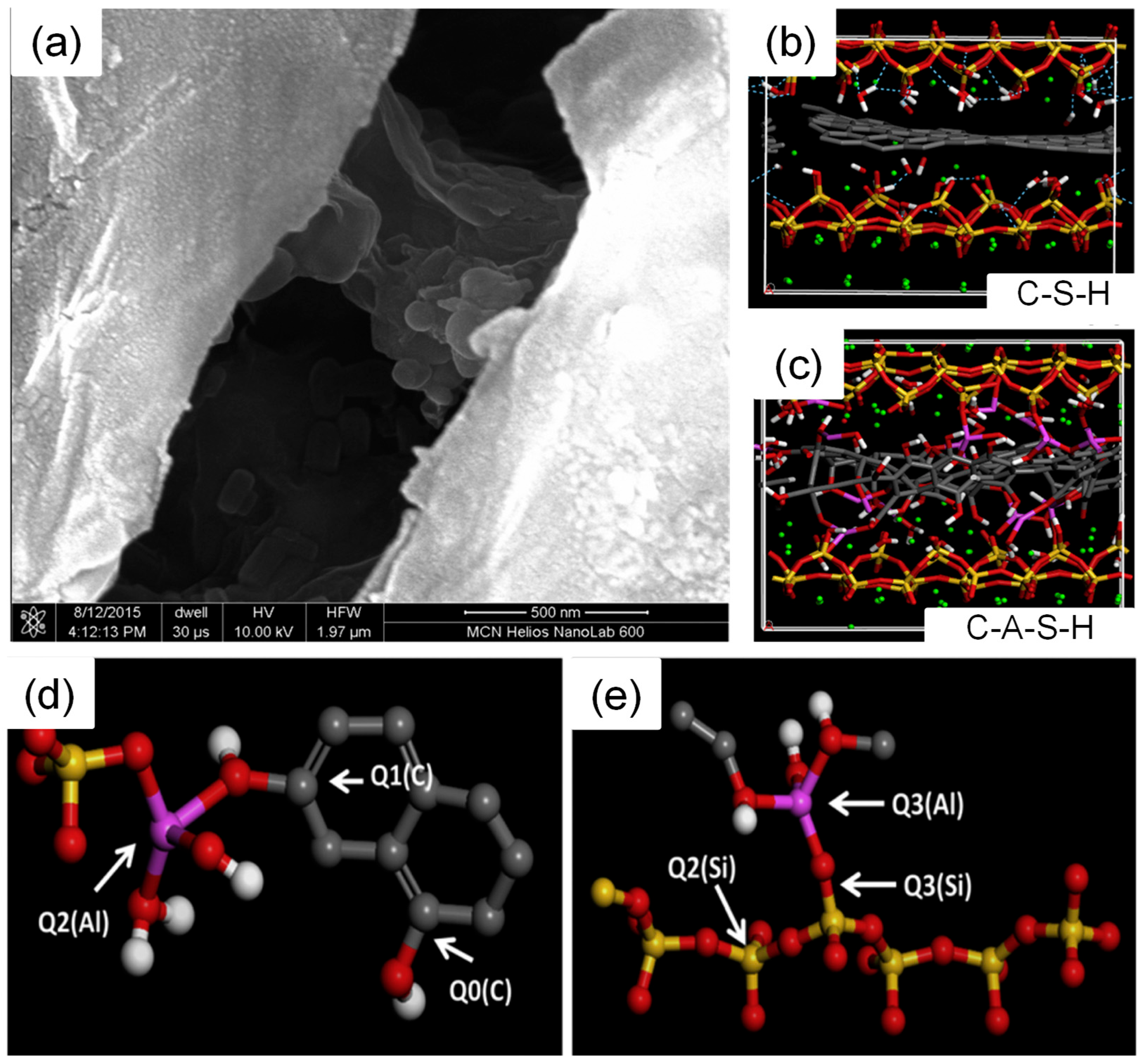
The findings of the above studies are equally applicable to the role of GO in CASH. Thus, relevant studies in CASH can be reduced accordingly, focusing more on the mode of action of Al. With the introduction of Al into CSH, CASH can improve the interaction with GO and thus enhance the mechanical properties. Hou et al. [77] studied the mechanism of GO action in cement and cement-SCMs using MD, and GO-CASH showed better mechanical properties. As illustrated in Figure 8a–c, this is due to the presence of Al atoms that can change the structure of GO from an ordered planar structure to a disordered network structure. In Figure 8d,e, Al connects silicate tetrahedra and carbon hexagonal structures, and the Al-O-C bonds formed are stable covalent bonds. Additionally, aluminum in CASH primarily promotes stress redistribution and load sharing between layers by forming new chemical bonds (Os-Al-OGO) and densifying the existing bonding network [78]. Based on the summary, the introduction of Al atoms may help alleviate the detachment of GO from CSH. However, further research is needed to validate this claim, and future studies can explore more on the MD of cement-SCMs to facilitate their application.
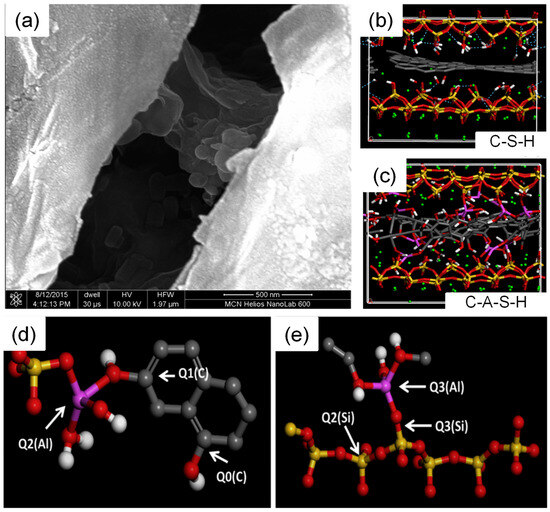
Figure 8.
Microstructure and molecular structure of GO in CSH and CASH: (a) Microstructure of GO in the concrete interlayer, (b) molecular structure of GO in the CSH interlayer, (c) molecular structure of GO in the CASH interlayer, and (d,e) formation of covalent bonds between GO and silicoaluminate chains [77]. The silicon, oxygen, hydrogen, carbon, and aluminum atoms in the molecular structure are represented by yellow, red, white, gray, and purple, respectively.
3.1.2. CASH and Fibers
Fiber reinforcement has become an effective way to address the cracking of concrete. Previous researchers have shown through numerous experiments that organic fibers (polyacrylic acid (PAA)) [79], polyvinyl alcohol (PVA) [80], polypropylene (PP) [81], and polyethylene (PE) [82] can enhance the mechanical properties of concrete and reduce concrete cracking [83].
For the fiber reinforcement mechanism, MD gives a good explanation at the atomic level. By predicting the shear strength between organic fibers and CSH, it was found that PAA fibers have the highest shear strength, followed by PVA [84]. This is because of the formation of hydrogen bonds [85], and the O-Ca-O bond formation between Ca2+ in the interlayer and the silicate chains and fibers [84]. However, due to the surface chemical inertness of organic fibers [86], it is important to improve their interfacial properties.
Surface chemical modification is an effective means of improvement. Feng et al. [87] found that PVA loaded with CaCO3 can enhance the interface interaction between CSH, attributed to the formation of chemical bonds in CaCSH-OCaCO3, thereby improving the interface bonding. Feng et al. [88] also observed that PVA modified with KH560 forms hydrogen and ionic bonds with KH560. The modified PVA forms numerous ionic bonds and Si-O-Si chemical bonds with CSH, enhancing the interfacial interaction. Lu et al. [86] utilized advanced oxidation techniques to increase the amount of OH− on the surface of PE, leading to more hydrogen bonding between PE and CSH, thereby enhancing the interfacial potential. Additionally, loading carbon nanomaterials on the surface of organic fibers is another method for interface enhancement. Lu et al. [89] showed that the oxygen of the oxygen-containing groups on GO as well as the hydrogen in the GO lattice interact with the oxygen in CSH and the hydrogen in PE via hydrogen bonds to enhance the interfacial interaction between PE and CSH. Carbon fibers, commonly used to improve the crack resistance of concrete, have inert surfaces that hinder interaction with CSH [90]. Therefore, adding GO to the surface of carbon fibers can enhance the bonding between Ca atoms in CSH and O atoms in carboxyl groups, as well as hydrogen bonding between GO and CSH [91].
The mechanism of interaction between CSH and fibers has been thoroughly elucidated, but research on the atomic-level interaction between the hydration product CASH of cement-SCMs and fibers is limited. Therefore, the mechanism of Al remains unclear and requires further investigation to fully leverage the role of SCMs in fiber-reinforced concrete. Currently, Zhang et al. [92] employed a combination of MD simulations and experimental validation to investigate the interface interaction between PVA fibers in C-(N-)A-S-H. The chemical composition analysis, elemental distribution, and fiber adhesion properties in the MD results are consistent with the experimental findings. When the Ca/(Si + Al) ratio is low and the Al/Si ratio is high, more hydrogen bonds are forming at the interface. A high Al content is beneficial for the bonding of PVA. However, when the Ca/(Si + Al) ratio is high, the presence of Ca2+ can prevent the formation of hydrogen bonds, thereby reducing the bonding strength at the interface. A high Al content is advantageous for the bonding strength of PVA. This work was conducted in the context of alkali-activated materials, and although the presence of Al phases was considered, the role of Al in this context was not elucidated.
3.1.3. CASH and Other Materials
The addition of SCMs to cement makes the original cement hydration products partially transformed from CSH to CASH gel, which will be deposited and grown on the CSH surface. However, it is very difficult to characterize this process experimentally, and MD can be a good solution to this problem. Zheng et al. [93] simulated the deposition and growth of CASH gel at a CSH interface with various Al/Si ratios. At lower Al content, the precursors were deposited on the CSH pore surfaces during the early stage of hydration. As the Al content increased, the precursors appeared within the CSH pores at the early stage of hydration, and the late-stage CASH is distributed on the surface of CSH pores. Increasing the Al content improves the early hydration rate of CASH gel, as illustrated in Figure 9. Additionally, the increase of Al content promotes the increase of polymerization at the CASH/CSH interface, increasing the mechanical properties.
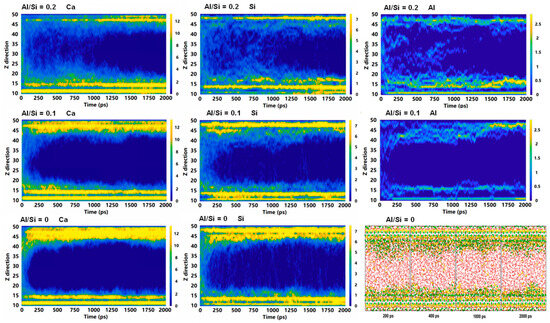
Figure 9.
The hydration process of the Ca, Si, and Al atoms in various Al/Si ratios of CASH [93].
CASH can also be used as a clay stabilizer, with the degree of solidification largely dependent on interfacial bonding. Wu et al. [94] studied the interfacial behavior of clay-CASH/NASH by using MD and illustrated that the binding energy of CASH with the clay was higher than that of NASH. This is mainly attributed to the inter-interfacial hydrogen bonding and the stabilizing effect of ionic clusters.
MD also can simulate the interfacial properties between aggregates and binders at the atomic level. Tian et al. [95] simulated the interfacial properties between CASH/NASH and steel slag aggregates. CaCO3, as a carbonation product of steel slag aggregates, favors the enhancement of interfacial interaction with CASH. Recently, carbonated steel slag aggregates have become a hot topic in the utilization of steel slag and CCUS [96], as they can greatly suppress the volume expansion of steel slag. According to this study [95], the presence of CaCO3 in carbonated steel slag aggregates has great benefits for the interfacial bonding between cementitious materials and steel slag aggregates. This provides theoretical guidance in the application of carbonated steel slag aggregates.
3.2. Ion Immobilization and Transport
CASH gel plays an essential role in the field of environmental remediation, especially in immobilizing harmful ions (including radioactive ions and heavy metal ions) and resisting seawater erosion. MD can provide insights into the mechanism of CASH immobilization of harmful ions and reveal the mechanism of transport of SO42− and Cl− ions in seawater in the CASH gel pores. This is crucial for the exploration and application of CASH gel.
3.2.1. Immobilization of Harmful Ions
The presence of radioactive ions and heavy metals threatens the safety of soil and water resources, which in turn can be harmful to human health [97]. A large number of experimental studies have shown that building cementitious materials has a good immobilization effect on radioactive ions and heavy metals [98,99,100]. The immobilization mechanism is considered to be mainly a physical or chemical process utilizing advanced experimental characterization, which is not able to clarify the bonding mechanism between atoms. MD as a tool for nanoscopic characterization can effectively solve the problem.
MD can provide additional explanations for the immobilization mechanism, such as cement immobilization of radioactive ions Cs [101,102], Sr [103], U [104], and Be [105] ions. The cause of immobilization is attributed to the substitution of the radioactive ions for the Ca ions in the CSH into the molecular structure. However, radioactive ions occupy more space than Ca2+, and the substitution of radioactive ions makes the CSH interlayer expand. This accelerated ion diffusion rate and weakened chemical bonding stability, resulting in a decrease in the mechanical properties of CSH. With the development of cement-SCMs, it is widely believed that cement-SCMs immobilize radioactive ions (e.g., Cs ions) better than cement [106,107]. The substitution of Al for Si in CASH leads to an increase in the electrostatic interactions and adsorption sites of radioactive ions, thereby reducing the migration rate of these ions [108]. Furthermore, when CASH has a low Ca/Si ratio and Al content, the solidification is more effective [109].
In addition to the immobilization of radioactive ions, cementitious material also has a good immobilization effect on heavy metal ions. Hou et al. [110] investigated the corrosion behavior of steel rebars by iron ions in concrete using MD simulation. The results indicate that the introduction of iron ions in CSH weakens the strength of Si-O bonds, reduces the polymerization of silicate chains, and consequently reduces the mechanical properties. This study provides insights into the reasons behind the decreased mechanical performance of steel rebars in corroded concrete. Furthermore, upon determining the iron ion content in the corrosive environment, MD can be employed to predict the performance of concrete. Sun et al. [111] found that Pb ions do not absorb onto the CSH surface directly, instead forming a relatively stable chemisorption. Liang et al. [112] found poor adsorption of Cu2+ by CASH compared to NASH. This is because the positively charged region of NASH is more uniform, making the positively charged regions carry a stronger negative charge. However, research on the heavy metal adsorption by CASH at the atomic level is limited. It is crucial to study whether the addition of Al phases benefits the adsorption of heavy metals in concrete, which warrants further exploration.
3.2.2. Ion Transport in Gel Pores
The durability of CASH gel is a vital indicator for judging the properties of concrete. When buildings are exposed to the marine environment, SO42− and Cl- ions are transported within the pores of CASH gel, damaging the structure and affecting the service life of the building.
Ca2+ ions are adsorbed by SO42− ions and brought into the pore solution [113]. Unstable Ca2+ in the Ca−Si layer (Coulombic or van der Waals forces) binds to SO42− to form Ca-SO4 clusters (the initial structure of gypsum). This promotes the continuous migration of interlayer calcium ions to solution [62] (decalcification phenomenon), allowing the structure to expand and crack after microcracking [114]. Compared to CSH, CASH gel has better resistance to SO42− attack. This is attributed to the increased Al/Si ratio due to the Al-Si substitution, and the formation of silicoaluminate chains with high polymerization degrees in the structure reduces the ability to decalcify.
The intrusion of Cl− is the most important cause of corrosion of reinforcement and structural damage of reinforced concrete [115]. On the experimental side, it is generally accepted [116,117,118] that the SCMs addition to the cement retards the migration of water and ions, but is only attributed to the densification of the microstructure. MD gives a new explanation. The substitution of Al for Si in the CASH structure increases the charge negativity of the oxygen atom in the silica-aluminate, causing it to adsorb more Na+. While Cl− is adsorbed on the cement surface through the formation of Na-Cl ionic pairs with Na+ [119], allowing more ionic clusters to accumulate near the interface and decreasing the transport rate of Cl−. However, Yang et al. [120] found that it is difficult for monovalent cations (Na+, K+) to immobilize Cl− ions by ion pair formation. The reason for this is that: (1) more monovalent ions need to be absorbed to compensate for the negative charge of the silicate chain compared to divalent ions (Ca2+, Mg2+). This results in increased electrostatic repulsion of chloride by CASH. (2) The relatively weak Coulombic gravitational attraction of monovalent cations towards Cl− ions leads to an increase in ion-pair instability.
Usually in seawater SO42− and Cl− coexist, and a high concentration of SO42− ions can resist the erosion of Cl− [121]. The existence of SO42− ions reduces the rate of Na+ and Cl− transport since the Na-Cl bond is not as stable as the Na-SO4 bond. The relative stability of the Na-SO4 ion pair attracts the additional ions, resulting in the formation of larger ion clusters [113], blocking the pores of CASH and thus reducing its permeability to sodium and chloride ions, as shown in Figure 10.
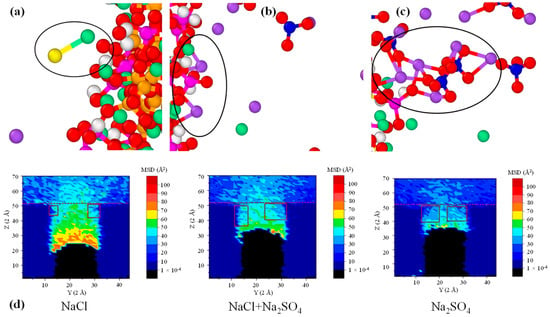
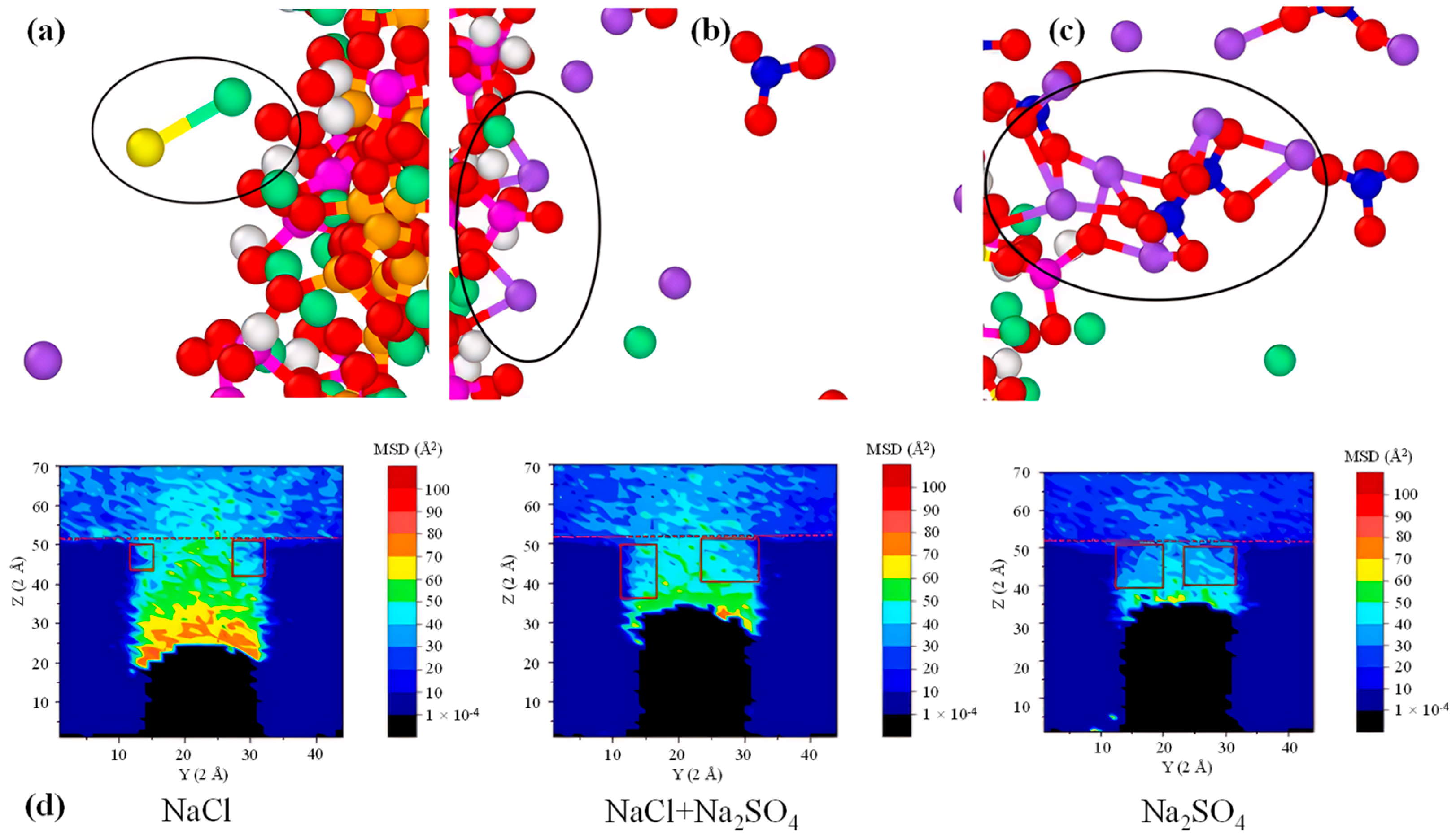
Figure 10.
CASH adsorption model: (a–c) CASH adsorption of CI− and SO42− ions to form large clusters, (d) diffusion of CI− and SO42− ions [113]. The black circles in (a), (b), and (c) represent the local structures of Cl−, Na+, and SO42− ions adsorbed on CASH gel; the red square in (d) represents the entrance to the ion channel.
3.3. Effect of Moderate and High Temperatures
It is extremely critical that temperature affects the mechanical properties of concrete. When concrete is cured under a moderate temperature (20~80 °C), the moderate temperature accelerates the hydration reaction [122,123]. Al atoms in CASH prefer to bond to bridging oxygen atoms, which leads to the conversion of the 6-coordinated Al in the silicate chain to the more stable 5- and 4-coordinated Al. Al doping helps to polymerize defective aluminate-silicate chains at moderate temperatures. Also, the stability and bonding strength of the Al atoms in the aluminosilicate chains increases with an increase in the MCL value [124]. Thus, moderate-temperature curing is very favorable to enhance the mechanical properties.
It is acknowledged that when concrete is exposed to fire, the structure is damaged and its mechanical properties are impaired to a large extent [125]. This is attributed to the expansion of the CASH interlayer at high temperatures. As illustrated in Figure 11, the expansion process disrupts the hydrogen bonding network of the interlayer water, decreases the stability of the Al-O bonds, and accelerates the depolymerization and hydrolysis reactions of CASH [29]. From Section 2.1.2, the Al atoms in CASH improve the interlayer connection and produce more high polymerization units. Therefore, compared to CSH, it is more resistant to high-temperature damage. When buildings are exposed to marine environments, high temperatures can significantly damage the durability of concrete. Although SO42− ions can capture other ions and generate large ion clusters that plug nanopores, hindering the diffusion of other ions [126]. However, the transport rate of H2O and ions (SO42− and Cl−) increases sharply at high temperatures, leading to a rapid decline in the durability of concrete.
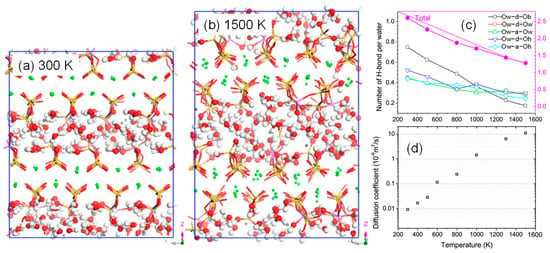
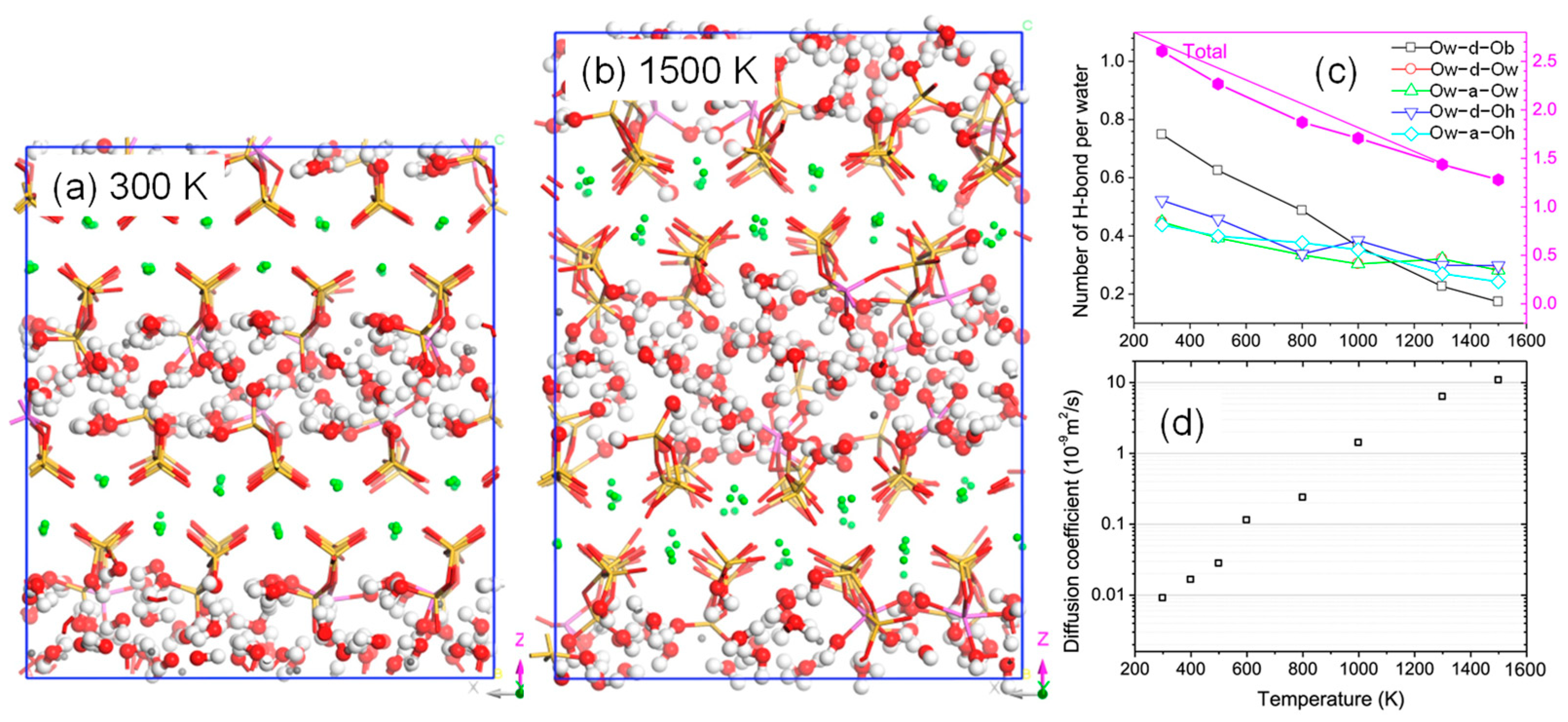
Figure 11.
Effect of high temperature on CASH: (a,b) molecular structure at 300 K and 1500 K, (c) change in the number of hydrogen bonds with temperature, and (d) diffusion coefficients of water molecules at different temperatures [29]. Note that in (c), OxY-Ox refers to the formation of hydrogen bonds, where Ox includes Ow, Ob, and Oh, with Ow referring to water molecules, Ob referring to bridging oxygen atoms, and Oh referring to hydroxyl groups; Y includes d and a, with d referring to proton donors and a referring to proton acceptors. i.e., Ow-d-Ob represents a water molecule donating a proton to form a hydrogen bond with another water molecule. In the CASH molecular structure, Si, Al, Ca, O, and H are represented by yellow, purple, gray, red, and white atoms, respectively.
4. Conclusions
The presence of the Al phase facilitates the application of cement-SCMs in concrete by improving the mechanical properties, durability, high-temperature resistance, and immobilization of radioactive ions and heavy metals. MD, as a powerful tool, can be applied to analyze the molecular structure evolution, dynamics, and mechanical properties of CASH and elucidate the form and action mechanism of Al in the CASH system. Through summarizing and evaluating previous literature, the conclusions that can be drawn are as follows:
- MD can predict the molecular structure, dynamics, and mechanical properties of CASH under different Al contents. Increasing Al content is beneficial for the formation of network structure and enhancement of polymerization when 0 < Al/Si ≤ 0.2. When Al/Si = 0.2, the crosslinking of silicoaluminate chains in the molecules is maximized, restricting atomic diffusion. At the same time, CASH exhibits optimal mechanical properties.
- Compared to CSH, the introduction of Al is advantageous for improving the interfacial properties of CASH in composite materials and the immobilization and transport effects of ions. Additionally, the presence of Al is beneficial for the polymerization of CASH in moderate-temperature environments and for resisting the destruction of CASH at high temperatures.
5. Challenges and Prospects
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript. Since MD predictions may deviate from actual experimental data, MD still needs to close the gap between simulation and experiment. The future development of MD will inevitably be combined with different simulation techniques (e.g., quantum mechanics, machine learning, finite element analysis, etc.). Cross-scale analysis from microscopic to macroscopic is realized to obtain higher simulation accuracy. Challenges and prospects in the future may include:
- Decalcification by carbonation: Concrete exposed to the atmosphere for a long period undergoes carbonation, leading to decalcification of CSH/CASH. Meanwhile, CaCO3 produced during carbonation leads to volume expansion of CSH/CASH. MD simulations can provide theoretical guidance for carbonation, decalcification, and volume expansion processes. For example, the Ca2+ migration process and CaCO3 formation in CSH/CASH gel pores.
- Ion immobilization: With the ongoing discharge of nuclear wastewater into the sea, radioactive elements will spread throughout global oceans with seawater currents, affecting the entire ecosystem. Rich Al cement-SCMs exhibit better immobilization effects. MD can provide valuable theoretical support for the immobilization of radioactive elements.
- Temperature effects: The effect of temperature on the molecular structure and properties of CASH is crucial. In addition to medium and high temperatures, whether pre-experimental samples subjected to freeze-thaw cycles, freeze-drying, and vacuum drying affect the molecular structure and properties of CASH needs to be discussed at the atomic level.
- Machine learning potential: Although the accuracy of ReaxFF of cementitious materials is widely recognized, there is a need to develop force fields better suited to CASH systems that can optimize the computational process and improve efficiency. The machine learning potential combines the advantages of speed and accuracy to simulate the CASH systems on larger time and length scales.
- Intelligent buildings: GO can form a conductive network in cement slurry, giving buildings self-sensing capabilities, which is crucial for structural health monitoring. In the future, based on machine learning, DFT simulations combined with MD and finite element simulations can analyze the molecular structure, mechanical properties, and electrical properties of composite materials from the nanoscale to the macroscale.
- Fiber and SCMs-cement: Fibers and SCMs cement: Fibers are crucial for concrete reinforcement, and MD research on SCMs cement requires an increase in the types of fibers (such as steel fibers, basalt fibers, etc.). Also, the current classical MD force field has difficulty accurately characterizing the interfacial chemical interactions between SCM cement and fibers. In the future, combining quantum mechanical bond order parameters with coarse-grained mesoscopic evolution can dynamically capture interfacial reactions from the nanometer to micrometer scale.
Author Contributions
X.J.: Conceptualization, Methodology, Investigation, Visualization, Writing—Original Draft; D.C.: Supervision, Resources; M.R.: Investigation, Validation, Writing—review and editing; A.M.: Investigation, Validation, Writing—review and editing; L.Z.: Supervision, Writing—Reviewing and Editing, Resources, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (39150174) and the Natural Science Foundation of Shandong Province (202105290004).
Data Availability Statement
There are no relevant data to share for this review.
Acknowledgments
We thank the National Natural Science Foundation of China and the Natural Science Foundation of Shandong Province for funding this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SCMs | Supplementary Cementitious Materials |
| Cement-SCMs | Cement blended with supplementary cementitious materials |
| MD | Molecular dynamics |
| LD | Linear dichroism |
| GBFS | Granulated Blast Furnace Slag |
| FA | Fly Ash |
| SF | Silica Fume |
| MSD | Mean square displacement |
| TCF | Time correlated function |
| PP | Polypropylene |
| PAA | Polyacrylic acid |
| CCUS | Carbon Capture, Utilization and Storage |
| CSH | Calcium silicate hydrate |
| CASH | Calcium silicoaluminate hydrate |
| NASH | Sodium silicoaluminate hydrate |
| GO | Graphene Oxide |
| RDF | Radial distribution function |
| MCL | Main chain length of silicon-aluminate |
| PVA | Polyvinyl alcohol |
| PE | Polyethylene |
| ONB | Non-bridging oxygen atom |
References
- Skibsted, J.; Snellings, R. Reactivity of Supplementary Cementitious Materials (SCMs) in Cement Blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Wang, T.; Medepalli, S.; Zheng, Y.; Zhang, W.; Ishida, T.; Bishnoi, S.; Hou, D.; Shi, Z. Retardation Effect of the Pozzolanic Reaction of Low-Calcium Supplementary Cementitious Materials on Clinker Hydration at Later Age: Effects of Pore Solution, Foreign Ions, and pH. Cem. Concr. Res. 2024, 177, 107416. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Li, K.; Li, M.; Lin, S.; Hao, T.; Wang, T.; Guo, Y.; Ling, Z. Investigations on the Rehydration of Recycled Blended SCMs Cement. Cem. Concr. Res. 2023, 163, 107036. [Google Scholar] [CrossRef]
- Yang, H.J.; Usman, M.; Hanif, A. Suitability of Liquid Crystal Display (LCD) Glass Waste as Supplementary Cementing Material (SCM): Assessment Based on Strength, Porosity, and Durability. J. Build. Eng. 2021, 42, 102793. [Google Scholar] [CrossRef]
- Jackson, M.D.; Chae, S.R.; Mulcahy, S.R.; Meral, C.; Taylor, R.; Li, P.; Emwas, A.-H.; Moon, J.; Yoon, S.; Vola, G.; et al. Unlocking the Secrets of Al-Tobermorite in Roman Seawater Concrete. Am. Mineral. 2013, 98, 1669–1687. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Barzgar, S.; Lothenbach, B.; Tarik, M.; Di Giacomo, A.; Ludwig, C. The Effect of Sodium Hydroxide on Al Uptake by Calcium Silicate Hydrates (CSH). J. Colloid Interface Sci. 2020, 572, 246–256. [Google Scholar] [CrossRef]
- Moreno-Juez, J.; Vegas, I.J.; Frías Rojas, M.; Vigil de la Villa, R.; Guede-Vázquez, E. Laboratory-Scale Study and Semi-Industrial Validation of Viability of Inorganic CDW Fine Fractions as SCMs in Blended Cements. Constr. Build. Mater. 2021, 271, 121823. [Google Scholar] [CrossRef]
- Rahla, K.M.; Mateus, R.; Bragança, L. Comparative Sustainability Assessment of Binary Blended Concretes Using Supplementary Cementitious Materials (SCMs) and Ordinary Portland Cement (OPC). J. Clean. Prod. 2019, 220, 445–459. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, Q.; Liu, X.; Zhang, Z.; Wei, C.; Li, Y.; Ma, S. Synergistic Preparation of High-Performance Composite Blast Furnace Slag Powder from Multiple Industrial Solid Wastes: Performance Regulation and Optimization. Constr. Build. Mater. 2024, 411, 134231. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Yan, Y.; Lothenbach, B.; Skibsted, J. Incorporation of Sodium and Aluminum in Cementitious Calcium-Alumino-Silicate-Hydrate C-(A)-S-H Phases Studied by 23Na, 27Al, and 29Si MAS NMR Spectroscopy. J. Phys. Chem. C 2021, 125, 27975–27995. [Google Scholar] [CrossRef]
- Škvára, F.; Šulc, R.; Snop, R.; Peterová, A.; Šídlová, M. Hydraulic Clinkerless Binder on the Fluid Sulfocalcic Fly Ash Basis. Cem. Concr. Compos. 2018, 93, 118–126. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Guo, J.; Zhang, L.; Yuan, N. Pressed Recycled Fly Ash and Carbide Slag: Hydration of Entirely Waste-Stream Building Components. Constr. Build. Mater. 2020, 265, 120282. [Google Scholar] [CrossRef]
- Moon, J.; Wang, Z.; Kim, M.O.; Chun, S.-C. Strength Enhancement of Alkaline Activated Fly Ash Cured at Ambient Temperature by Delayed Activation of Substituted OPC. Constr. Build. Mater. 2016, 122, 659–666. [Google Scholar] [CrossRef]
- Zhu, D.; Qiu, T.; Zhong, J.; Zeng, Q.; Fang, X. Molecular Dynamics Simulation of Aluminum Inhibited Leaching during Ion-Adsorbed Type Rare Earth Ore Leaching Process. J. Rare Earths 2019, 37, 1334–1340. [Google Scholar] [CrossRef]
- Faucon, P.; Delaye, J.M.; Virlet, J. Molecular Dynamics Simulation of the Structure of Calcium Silicate Hydrates: I. Ca4+xSi6O14+2x(OH)4−2x(H2O)2(0 ≤ x ≤ 1). J. Solid State Chem. 1996, 127, 92–97. [Google Scholar] [CrossRef]
- Tang, S.; A., H.; Chen, J.; Yu, W.; Yu, P.; Chen, E.; Deng, H.; He, Z. The Interactions between Water Molecules and C-S-H Surfaces in Loads-Induced Nanopores: A Molecular Dynamics Study. Appl. Surf. Sci. 2019, 496, 143744. [Google Scholar] [CrossRef]
- Tu, Y.; Cao, J.; Wen, R.; Shi, P.; Yuan, L.; Ji, Y.; Das, O.; Försth, M.; Sas, G.; Elfgren, L. Molecular Dynamics Simulation Study of the Transport of Pairwise Coupled Ions Confined in C-S-H Gel Nanopores. Constr. Build. Mater. 2022, 318, 126172. [Google Scholar] [CrossRef]
- Qiao, G.; Hou, D.; Li, W.; Yin, B.; Zhang, Y.; Wang, P. Molecular Insights into Migration of Heavy Metal Ion in Calcium Silicate Hydrate (CSH) Surface and Intra-CSH (Ca/Si = 1.3). Constr. Build. Mater. 2023, 365, 130097. [Google Scholar] [CrossRef]
- Hou, D.; Yang, Q.; Wang, P.; Jin, Z.; Wang, M.; Zhang, Y.; Wang, X. Unraveling Disadhesion Mechanism of Epoxy/CSH Interface under Aggressive Conditions. Cem. Concr. Res. 2021, 146, 106489. [Google Scholar] [CrossRef]
- Hou, D.; Yang, Q.; Jin, Z.; Wang, P.; Wang, M.; Wang, X.; Zhang, Y. Enhancing Interfacial Bonding between Epoxy and CSH Using Graphene Oxide: An Atomistic Investigation. Appl. Surf. Sci. 2021, 568, 150896. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Baghabra Al-Amoudi, O.S.; Obot, I.B.; Maslehuddin, M.; Ahmed, H.-R.; Saleh, T.A. Molecular Simulation of Cement-Based Materials and Their Properties. Engineering 2022, 15, 165–178. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Bonnaud, P.A.; Manzano, H. A Comprehensive Review of C-S-H Empirical and Computational Models, Their Applications, and Practical Aspects. Cem. Concr. Res. 2022, 156, 106784. [Google Scholar] [CrossRef]
- Claverie, J.; Wang, Q.; Kamali-Bernard, S.; Bernard, F. Assessment of the Reactivity and Hydration of Portland Cement Clinker Phases from Atomistic Simulation: A Critical Review. Cem. Concr. Res. 2022, 154, 106711. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Brochard, L.; Honorio, T.; Maruyama, I.; Vandamme, M. Advances in Atomistic Modeling and Understanding of Drying Shrinkage in Cementitious Materials. Cem. Concr. Res. 2021, 148, 106536. [Google Scholar] [CrossRef]
- Cho, B.H.; Chung, W.; Nam, B.H. Molecular Dynamics Simulation of Calcium-Silicate-Hydrate for Nano-Engineered Cement Composites—A Review. Nanomaterials 2020, 10, 2158. [Google Scholar] [CrossRef]
- Kunhi Mohamed, A.; Weckwerth, S.A.; Mishra, R.K.; Heinz, H.; Flatt, R.J. Molecular Modeling of Chemical Admixtures; Opportunities and Challenges. Cem. Concr. Res. 2022, 156, 106783. [Google Scholar] [CrossRef]
- Ma, H.; Hou, D.; Zhang, J. Editorial: Molecular Simulation on Cementitious Materials: From Computational Chemistry Method to Application. Front. Mater. 2021, 8, 810850. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Hou, D.; Ding, Q. Molecular Dynamics Study on Calcium Aluminosilicate Hydrate at Elevated Temperatures: Structure, Dynamics and Mechanical Properties. Mater. Chem. Phys. 2019, 233, 276–287. [Google Scholar] [CrossRef]
- Fu, J.; Kamali-Bernard, S.; Bernard, F.; Cornen, M. Comparison of Mechanical Properties of C-S-H and Portlandite between Nano-Indentation Experiments and a Modeling Approach Using Various Simulation Techniques. Compos. Part B Eng. 2018, 151, 127–138. [Google Scholar] [CrossRef]
- Wu, H.; Pan, J.; Wang, J. Molecular Dynamics Simulation Study on Dynamic Mechanical Properties of C-S-H with Diverse Ca/Si Ratios. Mater. Today Commun. 2022, 31, 103755. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.; Li, Z.; Zhu, Y. Uniaxial Tension Study of Calcium Silicate Hydrate (C–S–H): Structure, Dynamics and Mechanical Properties. Mater Struct 2015, 48, 3811–3824. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Zhu, Y.; Li, Z. Calcium Silicate Hydrate from Dry to Saturated State: Structure, Dynamics and Mechanical Properties. Acta Mater. 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Nonat, A. The Structure and Stoichiometry of C-S-H. Cem. Concr. Res. 2004, 34, 1521–1528. [Google Scholar] [CrossRef]
- Liang, Y. Nanoscale Insight into Structural Characteristics and Dynamic Properties of C-S-H after Decalcification by Reactive Molecular Dynamics Simulations. Mater. Today Commun. 2022, 33, 104684. [Google Scholar] [CrossRef]
- Kai, M.F.; Zhang, L.W.; Liew, K.M. New Insights into Creep Characteristics of Calcium Silicate Hydrates at Molecular Level. Cem. Concr. Res. 2021, 142, 106366. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Shi, J.; Luo, Q.; Jiang, J.; Hou, D. Full Process of Calcium Silicate Hydrate Decalcification: Molecular Structure, Dynamics, and Mechanical Properties. Cem. Concr. Res. 2022, 161, 106964. [Google Scholar] [CrossRef]
- Bonnaud, P.A.; Ji, Q.; Van Vliet, K.J. Effects of Elevated Temperature on the Structure and Properties of Calcium–Silicate–Hydrate Gels: The Role of Confined Water. Soft Matter 2013, 9, 6418. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Ju, J.W.; Bauchy, M. New Insights into the Mechanism Governing the Elasticity of Calcium Silicate Hydrate Gels Exposed to High Temperature: A Molecular Dynamics Study. Cem. Concr. Res. 2021, 141, 106333. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Krakowiak, K.J.; Bauchy, M.; Stewart, K.L.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S.; et al. Combinatorial Molecular Optimization of Cement Hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef]
- Hou, D.; Li, H.; Zhang, L.; Zhang, J. Nano-Scale Mechanical Properties Investigation of C-S-H from Hydrated Tri-Calcium Silicate by Nano-Indentation and Molecular Dynamics Simulation. Constr. Build. Mater. 2018, 189, 265–275. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, T.R.; Wei, P.; Li, D.W.; Han, N.X.; Xing, F.; Gan, Y.; Chen, Z. Computational Study of the Nanoscale Mechanical Properties of C-S-H Composites under Different Temperatures. Comput. Mater. Sci. 2018, 146, 42–53. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, W.; Zhou, Q.; Zhang, Y.; Liu, H.; Sant, G.; Liu, X.; Guo, L.; Bauchy, M. Precipitation of Calcium–Alumino–Silicate–Hydrate Gels: The Role of the Internal Stress. J. Chem. Phys. 2020, 153, 014501. [Google Scholar] [CrossRef] [PubMed]
- Criscenti, L.J.; Brantley, S.L.; Mueller, K.T.; Tsomaia, N.; Kubicki, J.D. Theoretical and 27Al CPMAS NMR Investigation of Aluminum Coordination Changes during Aluminosilicate Dissolution. Geochim. Cosmochim. Ac. 2005, 69, 2205–2220. [Google Scholar] [CrossRef]
- Pardal, X.; Brunet, F.; Charpentier, T.; Pochard, I.; Nonat, A. 27Al and 29Si Solid-State NMR Characterization of Calcium-Aluminosilicate-Hydrate. Inorg. Chem. 2012, 51, 1827–1836. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Effect of Alkalinity and Calcium Concentration of Pore Solution on the Swelling and Ionic Exchange of Superabsorbent Polymers in Cement Paste. Cem. Concr. Compos. 2018, 88, 150–164. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The Pore Solution of Blended Cements: A Review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Zheng, Q.; Jiang, J.; Yu, J.; Li, X.; Li, S. Aluminum-Induced Interfacial Strengthening in Calcium Silicate Hydrates: Structure, Bonding, and Mechanical Properties. ACS Sustain. Chem. Eng. 2020, 8, 2622–2631. [Google Scholar] [CrossRef]
- Hawchar, B.M.; Honorio, T. C-A-S-H Thermoelastic Properties at the Molecular and Gel Scales. J. Adv. Concr. Technol. 2022, 20, 375–388. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Yang, J.; Ding, Q.; Sun, D. Insight into the Strengthening Mechanism of the Al-Induced Cross-Linked Calcium Aluminosilicate Hydrate Gel: A Molecular Dynamics Study. Front. Mater. 2021, 7, 611568. [Google Scholar] [CrossRef]
- Manzano, H.; Dolado, J.S.; Ayuela, A. Aluminum Incorporation to Dreierketten Silicate Chains. J. Phys. Chem. B 2009, 113, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Kunhi Mohamed, A.; Moutzouri, P.; Berruyer, P.; Walder, B.J.; Siramanont, J.; Harris, M.; Negroni, M.; Galmarini, S.C.; Parker, S.C.; Scrivener, K.L.; et al. The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate. J. Am. Chem. Soc. 2020, 142, 11060–11071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, G.; Wang, C.; Zhang, Y.; Wang, P.; Yan, N. Activation Effects and Micro Quantitative Characterization of High-Volume Ground Granulated Blast Furnace Slag in Cement-Based Composites. Cem. Concr. Compos. 2020, 109, 103556. [Google Scholar] [CrossRef]
- Li, D.; Qi, Q.; Liu, Q.; Zhu, J.; Bai, Z. Uniaxial Tensile Study of Calcium Aluminosilicate Hydrate (C-A-S-H): Structure, Dynamics and Mechanical Properties. Mater. Today Commun. 2024, 38, 107854. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Ulm, F.-J.; Pellenq, R.J.-M. Evidence on the Dual Nature of Aluminum in the Calcium-Silicate-Hydrates Based on Atomistic Simulations. J. Am. Ceram. Soc. 2012, 95, 1128–1137. [Google Scholar] [CrossRef]
- Hou, D.; Li, Z.; Zhao, T. Reactive Force Field Simulation on Polymerization and Hydrolytic Reactions in Calcium Aluminate Silicate Hydrate (C–A–S–H) Gel: Structure, Dynamics and Mechanical Properties. RSC Adv. 2014, 5, 448–461. [Google Scholar] [CrossRef]
- Wan, X.; Hou, D.; Zhao, T.; Wang, L. Insights on Molecular Structure and Micro-Properties of Alkali-Activated Slag Materials: A Reactive Molecular Dynamics Study. Constr. Build. Mater. 2017, 139, 430–437. [Google Scholar] [CrossRef]
- Yang, J.; Hou, D.; Ding, Q. Structure, Dynamics, and Mechanical Properties of Cross-Linked Calcium Aluminosilicate Hydrate: A Molecular Dynamics Study. ACS Sustain. Chem. Eng. 2018, 6, 9403–9417. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, T.; Ma, H.; Li, Z. Reactive Molecular Simulation on Water Confined in the Nanopores of the Calcium Silicate Hydrate Gel: Structure, Reactivity, and Mechanical Properties. J. Phys. Chem. C 2015, 119, 1346–1358. [Google Scholar] [CrossRef]
- Hou, D.; Wu, C.; Yang, Q.; Zhang, W.; Lu, Z.; Wang, P.; Li, J.; Ding, Q. Insights on the Molecular Structure Evolution for Tricalcium Silicate and Slag Composite: From 29Si and 27Al NMR to Molecular Dynamics. Compos. Part B Eng. 2020, 202, 108401. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized Structural Description of Calcium–Sodium Aluminosilicate Hydrate Gels: The Cross-Linked Substituted Tobermorite Model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Yang, J.; Hou, D.; Zhang, G. Insight on the Mechanism of Sulfate Attacking on the Cement Paste with Granulated Blast Furnace Slag: An Experimental and Molecular Dynamics Study. Constr. Build. Mater. 2018, 169, 601–611. [Google Scholar] [CrossRef]
- Manzano, H.; Dolado, J.S.; Griebel, M.; Hamaekers, J. A Molecular Dynamics Study of the Aluminosilicate Chains Structure in Al-Rich Calcium Silicate Hydrated (C–S–H) Gels. Phys. Status Solidi 2008, 205, 1324–1329. [Google Scholar] [CrossRef]
- Aboulayt, A.; Souayfan, F.; Roziere, E.; Jaafri, R.; Cherki El Idrissi, A.; Moussa, R.; Justino, C.; Loukili, A. Alkali-Activated Grouts Based on Slag-Fly Ash Mixtures: From Early-Age Characterization to Long-Term Phase Composition. Constr. Build. Mater. 2020, 260, 120510. [Google Scholar] [CrossRef]
- Bauchy, M.; Laubie, H.; Abdolhosseini Qomi, M.J.; Hoover, C.G.; Ulm, F.-J.; Pellenq, R.J.-M. Fracture Toughness of Calcium–Silicate–Hydrate from Molecular Dynamics Simulations. J. Non-Cryst. Solids 2015, 419, 58–64. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, H.; Qiu, Y.; Zou, X.; Huang, J. A Molecular Dynamics Study on the Structure, Interfaces, Mechanical Properties, and Mechanisms of a Calcium Silicate Hydrate/2D-Silica Nanocomposite. Front. Mater. 2020, 7, 127. [Google Scholar] [CrossRef]
- Manzano, H.; Moeini, S.; Marinelli, F.; van Duin, A.C.T.; Ulm, F.-J.; Pellenq, R.J.-M. Confined Water Dissociation in Microporous Defective Silicates: Mechanism, Dipole Distribution, and Impact on Substrate Properties. J. Am. Chem. Soc. 2012, 134, 2208–2215. [Google Scholar] [CrossRef]
- Geng, G.; Myers, R.J.; Li, J.; Maboudian, R.; Carraro, C.; Shapiro, D.A.; Monteiro, P.J.M. Aluminum-Induced Dreierketten Chain Cross-Links Increase the Mechanical Properties of Nanocrystalline Calcium Aluminosilicate Hydrate. Sci. Rep. 2017, 7, 44032. [Google Scholar] [CrossRef]
- Zhang, M.; Deskins, N.A.; Zhang, G.; Cygan, R.T.; Tao, M. Modeling the Polymerization Process for Geopolymer Synthesis through Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 6760–6773. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ding, Q.; Zhao, M. Molecular Structure and Mechanical Properties of Aluminum Substituted C-S-H. J. Build. Mater. 2022, 25, 565–571. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, G.; Guo, Y.; Zhang, Y.; Hou, D.; Jin, Z.; Zhang, J.; Wang, M.; Hu, X. Molecular Dynamics Simulation of the Interfacial Bonding Properties between Graphene Oxide and Calcium Silicate Hydrate. Constr. Build. Mater. 2020, 260, 119927. [Google Scholar] [CrossRef]
- Hou, D.; Yang, T.; Tang, J.; Li, S. Reactive Force-Field Molecular Dynamics Study on Graphene Oxide Reinforced Cement Composite: Functional Group de-Protonation, Interfacial Bonding and Strengthening Mechanism. Phys. Chem. Chem. Phys. 2018, 20, 8773–8789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, T.; Jia, Y.; Hou, D.; Li, H.; Jiang, J.; Zhang, J. Molecular Dynamics Study on the Weakening Effect of Moisture Content on Graphene Oxide Reinforced Cement Composite. Chem. Phys. Lett. 2018, 708, 177–182. [Google Scholar] [CrossRef]
- Mao, S.; Yao, W. Enhancing the Mechanical Properties of Calcium Silicate Hydrate by Engineering Graphene Oxide Structures via Molecular Dynamics Simulations. Mol. Simul. 2023, 49, 351–364. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, H.; Wu, J.; Fu, G.; Feng, C.; Chen, W. Molecular Dynamics Study on the Influence of Graphene Oxide on the Tensile Behavior of Calcium Silicate Hydrate Composites. Mater. Chem. Phys. 2022, 292, 126881. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Yu, S.; Gong, C.; Wang, Z.; Gao, Y. Effects of Graphene Oxide on Shearing Performance of C–S–H Composites: A Molecular Dynamics Study. J. Mater. Sci. 2023, 58, 16972–16987. [Google Scholar] [CrossRef]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive Molecular Dynamics and Experimental Study of Graphene-Cement Composites: Structure, Dynamics and Reinforcement Mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Qiao, G.; Wang, P.; Hou, D.S.; Jin, Z.; Li, S. Reactive Molecular Dynamic Simulation of Graphene Oxide Enhanced Calcium Silicate Aluminate Hydrate. J. Chin. Ceram. Soc. 2023, 51, 1154–1164. [Google Scholar] [CrossRef]
- Gao, C.; Tang, J.; Meng, Z.; Chu, Y.; Huang, J.; Han, F.; Liu, J. Enhancement in Toughness of Cement Pastes by Chitosan Modified with Polyacrylic Acid (CS/PAA): Microstructure Evolution and Molecular Dynamics. J. Build. Eng. 2023, 79, 107822. [Google Scholar] [CrossRef]
- Qiu, J.; Lim, X.-N.; Yang, E.-H. Fatigue-Induced Deterioration of the Interface between Micro-Polyvinyl Alcohol (PVA) Fiber and Cement Matrix. Cem. Concr. Res. 2016, 90, 127–136. [Google Scholar] [CrossRef]
- Wu, B.; Qiu, J. Enhancing the Hydrophobic PP Fiber/Cement Matrix Interface by Coating Nano-AlOOH to the Fiber Surface in a Facile Method. Cem. Concr. Compos. 2022, 125, 104297. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Oh, T.; Chun, B. Highly Ductile Ultra-Rapid-Hardening Mortar Containing Oxidized Polyethylene Fibers. Constr. Build. Mater. 2021, 277, 122317. [Google Scholar] [CrossRef]
- Haddad, R.H.; Smadi, M.M. Role of Fibers in Controlling Unrestrained Expansion and Arresting Cracking in Portland Cement Concrete Undergoing Alkali–Silica Reaction. Cem. Concr. Res. 2004, 34, 103–108. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, G.; Zhang, Y.; Hou, D.; Zhang, J.; Wang, M.; Wang, X.; Hu, X. Molecular Dynamics Simulation Study on Interfacial Shear Strength between Calcium-Silicate-Hydrate and Polymer Fibers. Constr. Build. Mater. 2020, 257, 119557. [Google Scholar] [CrossRef]
- Chen, N.; Li, M.-Y. Interfacial Interaction, Bonding Energy and Configuration Transformation of Calcium Silicate Hydrate/Graphene Oxide Coupled by Grain Boundary. Appl. Phys. A 2022, 128, 722. [Google Scholar] [CrossRef]
- Lu, Z.; Yin, R.; Yao, J.; Zhao, B. Experimental and Molecular Dynamic Study on the Interfacial Strengthening Mechanism of PE Fiber/Cement Mortar Using Advanced Oxidation Processes. Constr. Build. Mater. 2021, 309, 125144. [Google Scholar] [CrossRef]
- Yong, F.; Yuan, L.; Chen, Z.; Dajing, Q.; Chao, W.; PeiYan, W. Nano-CaCO 3 Enhances PVA Fiber-Matrix Interfacial Properties: An Experimental and Molecular Dynamics Study. Mol. Simul. 2022, 48, 1378–1392. [Google Scholar] [CrossRef]
- Yong, F.; Weijian, W.; Siqi, W. PVA Fiber/Cement-Based Interface in Silane Coupler KH560 Reinforced High Performance Concrete – Experimental and Molecular Dynamics Study. Constr. Build. Mater. 2023, 395, 132184. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, J.; Yao, J.; Hou, D. Experimental and Molecular Modeling of Polyethylene Fiber/Cement Interface Strengthened by Graphene Oxide. Cem. Concr. Compos. 2020, 112, 103676. [Google Scholar] [CrossRef]
- Quan, G.; Wu, Y.; Zhang, Y.; Xiao, L.; Liu, Y. Bio-Inspired Metal Ion Coordination Cross-Linking Synergistic Strategy to Enhance the Interfacial Properties of Carbon Fiber Composites. Polym. Compos. 2024, 45, 2202–2214. [Google Scholar] [CrossRef]
- Min, B.; Chen, G.; Sun, Y.; Li, K.; Chen, X.; Wang, Z. Enhancing the Fracture Properties of Carbon Fiber-Calcium Silicate Hydrate Interface through Graphene Oxide. Mater. Des. 2024, 241, 112916. [Google Scholar] [CrossRef]
- Zhang, S.; Duque-Redondo, E.; Kostiuchenko, A.; Dolado, J.S.; Ye, G. Molecular Dynamics and Experimental Study on the Adhesion Mechanism of Polyvinyl Alcohol (PVA) Fiber in Alkali-Activated Slag/Fly Ash. Cem. Concr. Res. 2021, 145, 106452. [Google Scholar] [CrossRef]
- Zheng, H.; Duan, Y.; Li, M.; Hou, D.; Wang, P.; Chen, J.; Li, S. Reaction Molecular Dynamics Study of Calcium Alumino-Silicate Hydrate Gel in the Hydration Deposition Process at the Calcium Silicate Hydrate Interface: The Influence of Al/Si. J. Build. Eng. 2024, 86, 108823. [Google Scholar] [CrossRef]
- Wu, D.; Cao, K.; Chen, K.; Mao, N. Interfacial Characteristics and Mechanical Behavior of Geopolymer Stabilizers with Clay Mineral: A Molecular Dynamics Study. Appl. Clay Sci. 2024, 250, 107286. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Z.; Liu, H.; Zheng, W.; Tang, X.; Gui, Z. Interfacial Characteristics and Mechanical Behaviors of Geopolymer Binder with Steel Slag Aggregate: Insights from Molecular Dynamics. J. Clean. Prod. 2022, 362, 132385. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, G.; Schollbach, K.; Brouwers, H.J.H. Development of Cement-Free Bio-Based Cold-Bonded Lightweight Aggregates (BCBLWAs) Using Steel Slag and Miscanthus Powder via CO2 Curing. J. Clean. Prod. 2021, 322, 129105. [Google Scholar] [CrossRef]
- Long, Q.; Yan, H.; Wu, H.; Qiu, S.; Zhou, X.; Qiu, T. Influence Mechanism of Leaching Agent Anions on the Leaching of Aluminium Impurities in Ionic-Type Rare Earth Ores: A DFT Simulation Combined with Experimental Verification. Sep. Purif. Technol. 2025, 354, 128768. [Google Scholar] [CrossRef]
- Berra, M.; Ippolito, N.M.; Mangialardi, T.; Paolini, A.E.; Piga, L. Leaching Test Procedure for Assessing the Compliance of the Chemical and Environmental Requirements of Hardened Woody Biomass Fly Ash Cement Mixtures. Waste Manag. 2019, 90, 10–16. [Google Scholar] [CrossRef]
- Jacques, D.; Phung, Q.T.; Perko, J.; Seetharam, S.C.; Maes, N.; Liu, S.; Yu, L.; Rogiers, B.; Laloy, E. Towards a Scientific-Based Assessment of Long-Term Durability and Performance of Cementitious Materials for Radioactive Waste Conditioning and Disposal. J. Nucl. Mater. 2021, 557, 153201. [Google Scholar] [CrossRef]
- Liang, K.; Wang, X.Q.; Chow, C.L.; Lau, D. A Review of Geopolymer and Its Adsorption Capacity with Molecular Insights: A Promising Adsorbent of Heavy Metal Ions. J. Environ. Manag. 2022, 322, 116066. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Wang, M.; Yin, B.; Hou, D.; Zhang, Y. Atomistic Insights into Cesium Chloride Solution Transport through the Ultra-Confined Calcium–Silicate–Hydrate Channel. Phys. Chem. Chem. Phys. 2019, 21, 11892–11902. [Google Scholar] [CrossRef] [PubMed]
- Duque-Redondo, E.; Kazuo, Y.; López-Arbeloa, I.; Manzano, H. Cs-137 Immobilization in C-S-H Gel Nanopores. Phys. Chem. Chem. Phys. 2018, 20, 9289–9297. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Pellenq, R.J.-M.; Yildiz, B. Docking 90Sr Radionuclide in Cement: An Atomistic Modeling Study. Phys. Chem. Earth 2014, 70–71, 39–44. [Google Scholar] [CrossRef]
- Androniuk, I.; Kalinichev, A.G. Molecular Dynamics Simulation of the Interaction of Uranium (VI) with the C–S–H Phase of Cement in the Presence of Gluconate. Appl. Geochem. 2020, 113, 104496. [Google Scholar] [CrossRef]
- Androniuk, I.; Çevirim-Papaioannou, N.; Altmaier, M.; Gaona, X. Molecular Dynamics Study of the Beryllium Interaction with C-S-H Phases. J. Phys. Chem. C 2023, 127, 1798–1807. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, R.; Muhammad, F.; Yu, L.; Shiau, Y.; Li, D. Solidification/Stabilization of Chromite Ore Processing Residue Using Alkali-Activated Composite Cementitious Materials. Chemosphere 2017, 168, 300–308. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Z.; Deng, Y.; Shi, C.; Zhang, Z. Advances in Immobilization of Radionuclide Wastes by Alkali Activated Cement and Related Materials. Cem. Concr. Compos. 2022, 126, 104377. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Yamada, K.; Dolado, J.S.; Manzano, H. Microscopic Mechanism of Radionuclide Cs Retention in Al Containing C-S-H Nanopores. Comput. Mater. Sci. 2021, 190, 110312. [Google Scholar] [CrossRef]
- Evans, N.D.M. Binding Mechanisms of Radionuclides to Cement. Cem. Concr. Res. 2008, 38, 543–553. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, W.; Sun, J.; Zhang, J. Structure, Dynamics and Mechanical Properties Evolution of Calcium-Silicate-Hydrate Induced by Fe Ions: A Molecular Dynamics Study. Constr. Build. Mater. 2021, 287, 122875. [Google Scholar] [CrossRef]
- Sun, M.; Hu, K.; Zhang, Y.; Wei, G.; Rong, H. Molecular Simulation and Experimental Investigation on Enhancement of Microbe Cement in Municipal Solid Waste Incineration Fly Ash. Front. Mater. 2022, 9, 1013580. [Google Scholar] [CrossRef]
- Liang, K.; Yang, G.; Wang, X.Q.; Chow, C.L.; Lau, D. Development of Effective Porous Geopolymer Adsorbent with High Strength for Copper(II) Ion Removal. J. Clean. Prod. 2024, 449, 141752. [Google Scholar] [CrossRef]
- Tu, Y.; Wen, R.; Yu, Q.; Cao, J.; Ji, Y.; Sas, G.; Elfgren, L. Molecular Dynamics Study on Coupled Ion Transport in Aluminum-Doped Cement-Based Materials. Constr. Build. Mater. 2021, 295, 123645. [Google Scholar] [CrossRef]
- Feng, P.; Garboczi, E.J.; Miao, C.; Bullard, J.W. Microstructural Origins of Cement Paste Degradation by External Sulfate Attack. Constr. Build. Mater. 2015, 96, 391–403. [Google Scholar] [CrossRef]
- Zhou, A.; Chow, C.L.; Lau, D. Structural Behavior of GFRP Reinforced Concrete Columns under the Influence of Chloride at Casting and Service Stages. Compos. Part B Eng. 2018, 136, 1–9. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljković, M.; Zuo, Y.; Ye, G. A Review on the Durability of Alkali-Activated Fly Ash/Slag Systems: Advances, Issues, and Perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I. Durability Assessment of Alkali Activated Slag (AAS) Concrete. Mater. Struct. 2012, 45, 1425–1437. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S.J. Influence of Fly Ash on the Water and Chloride Permeability of Alkali-Activated Slag Mortars and Concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Hou, D.; Li, T.; Wang, P. Molecular Dynamics Study on the Structure and Dynamics of NaCl Solution Transport in the Nanometer Channel of CASH Gel. ACS Sustain. Chem. Eng. 2018, 6, 9498–9509. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ding, Q.; Wang, A.; Hou, D. Influence of Associated Cations on the Transport and Adsorption of Chloride Ions in the Nano-Channel of Calcium Aluminosilicate Hydrate Gel: A Molecular Dynamics Study. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2022, 37, 963–976. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Tang, L.; Li, X. Resistance of Concrete against Combined Attack of Chloride and Sulfate under Drying–Wetting Cycles. Constr. Build. Mater. 2016, 106, 650–658. [Google Scholar] [CrossRef]
- Olmeda, J. Radium Retention by Blended Cement Pastes and Pure Phases (C-S-H and C-A-S-H Gels)_ Experimental Assessment and Modelling Exercises. Appl. Geochem. 2019, 105, 45–54. [Google Scholar] [CrossRef]
- Dharmawardhana, C.C.; Misra, A.; Ching, W.-Y. Theoretical Investigation of C-(A)-S-H(I) Cement Hydrates. Constr. Build. Mater. 2018, 184, 536–548. [Google Scholar] [CrossRef]
- Hou, D.; Wu, C.; Yang, Q.; Wang, P.; Ding, Q. Temperature Effect of Hydration and Microstructure of Tricalcium Silicate–Slag Powder Hydrated Composites: An Experimental and Molecular Dynamics Investigation. Acs Sustain. Chem. Eng. 2021, 9, 13773–13787. [Google Scholar] [CrossRef]
- Ma, H.; Wu, C. Mechanical and Microstructural Properties of Alkali-Activated Fly Ash-Slag Material under Sustained Moderate Temperature Effect. Cem. Concr. Compos. 2022, 134, 104744. [Google Scholar] [CrossRef]
- Rongjia, W.; Yanqiu, C.; Tong, G.; Lei, Y.; Tongfang, W.; Qian, Y.; Yongming, T.; Gabriel, S.; Lennart, E. Effects of Temperature on Ion Transport in C–A–S–H Gel Nanopores: Insights from Molecular Dynamics Simulations. J. Mater. Sci. 2022, 57, 18437–18455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).