Efficiency Determination of Water Lily (Eichhornia crassipes) Fiber Delignification by Electrohydrolysis Using Different Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass and Reagents
2.1.1. Heat Treatment
2.1.2. Chemical Treatment (Acid and Alkaline)
2.2. Electrohydrolysis
2.3. Hybrid Electrohydrolysis
3. Characterization
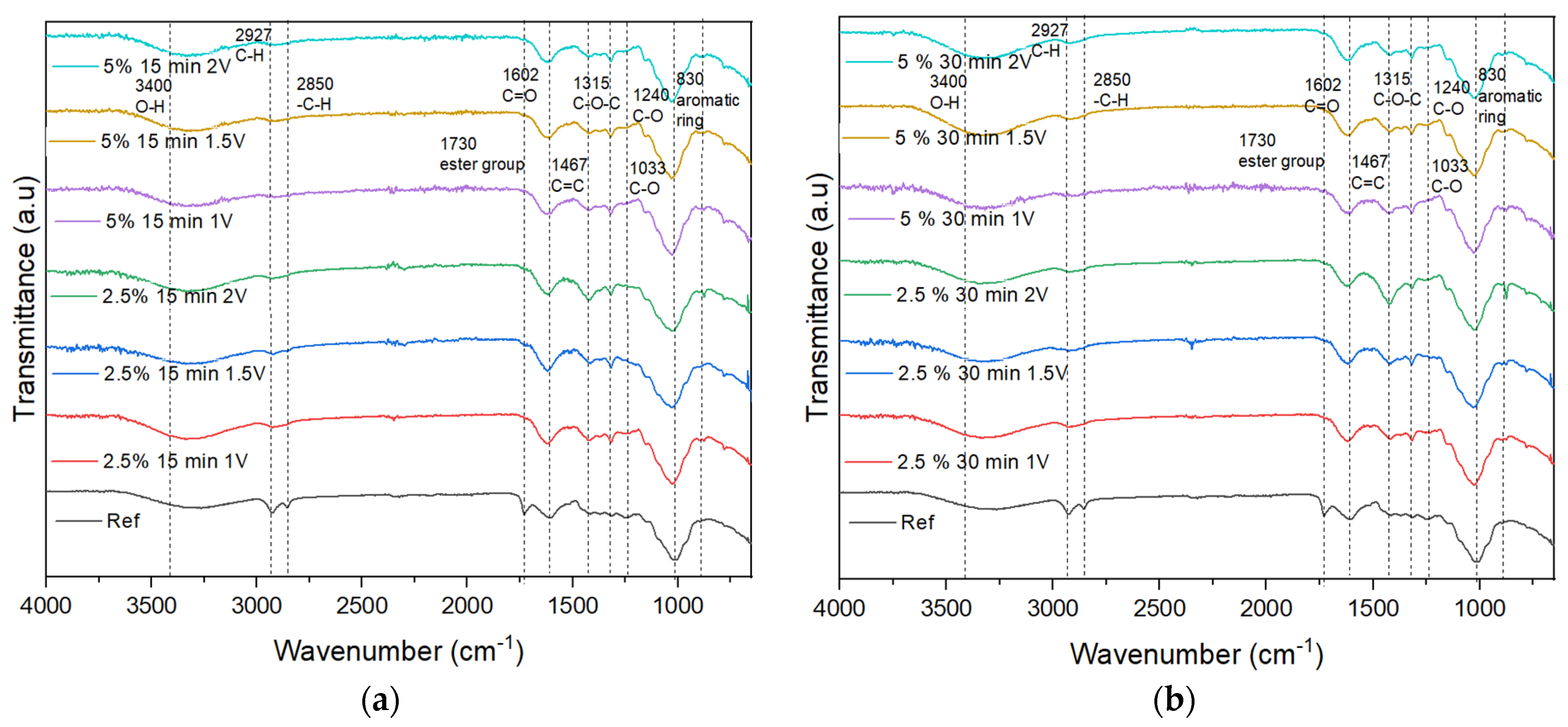
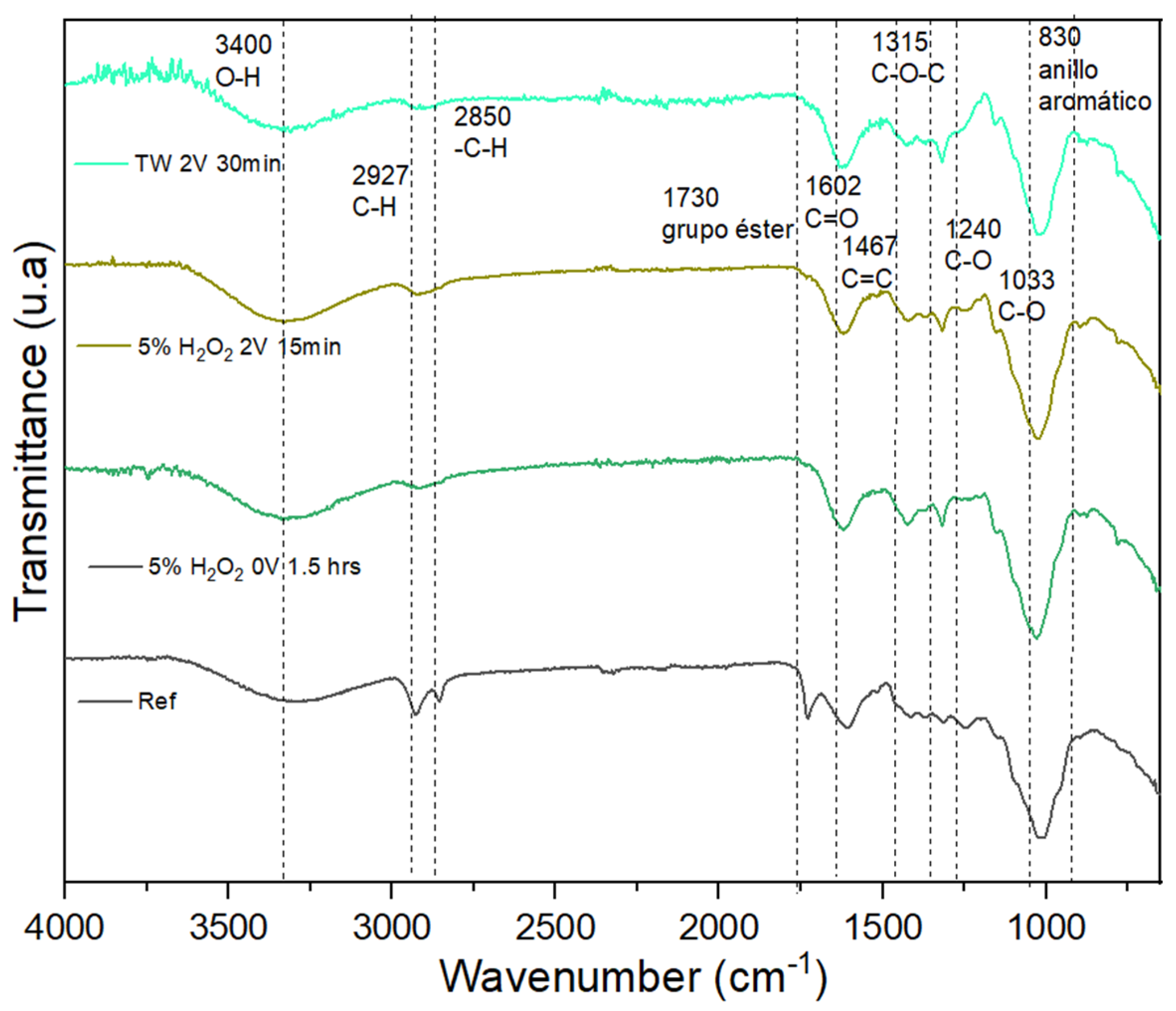
3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
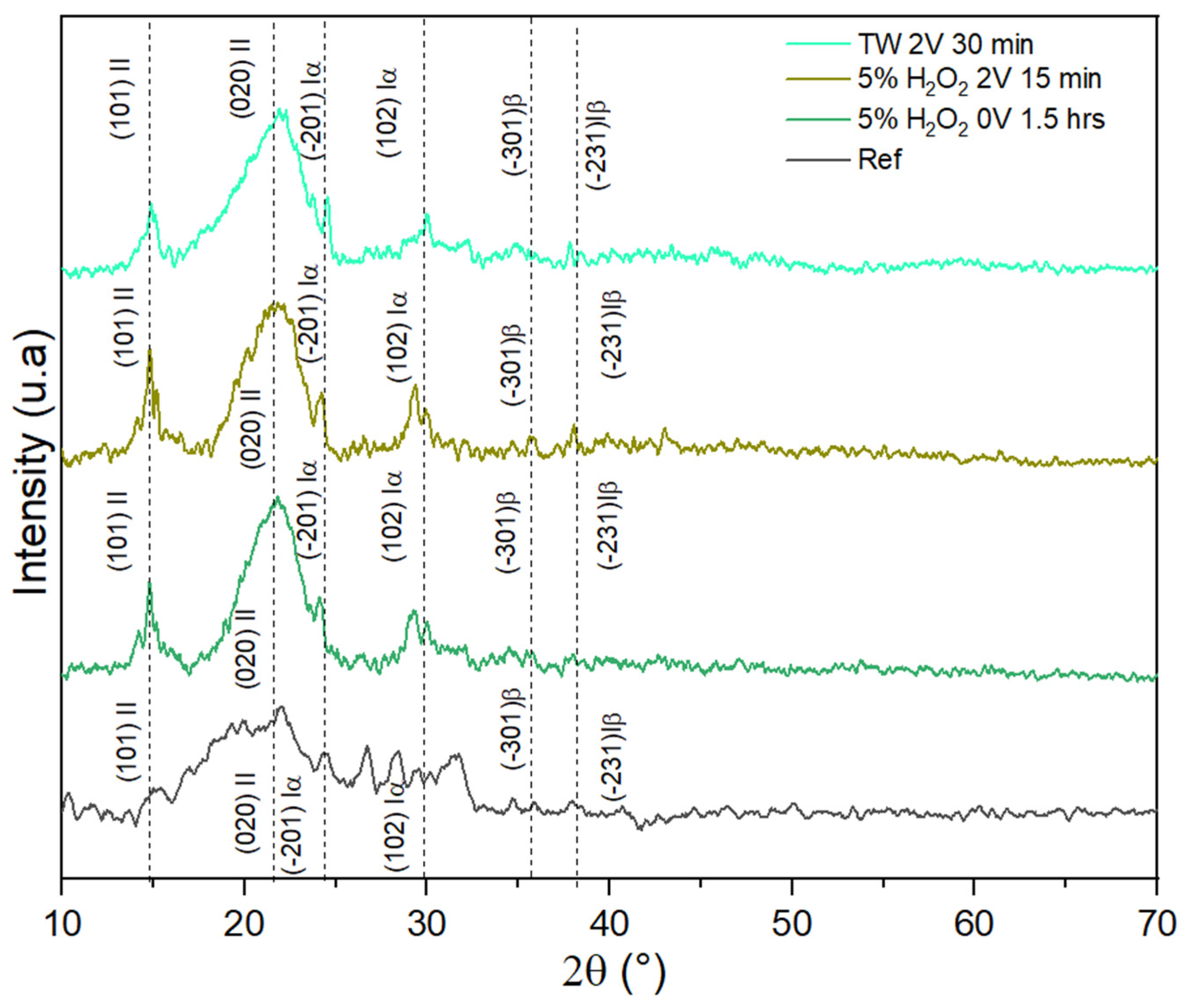
3.2. X-Ray Diffraction (XRD)
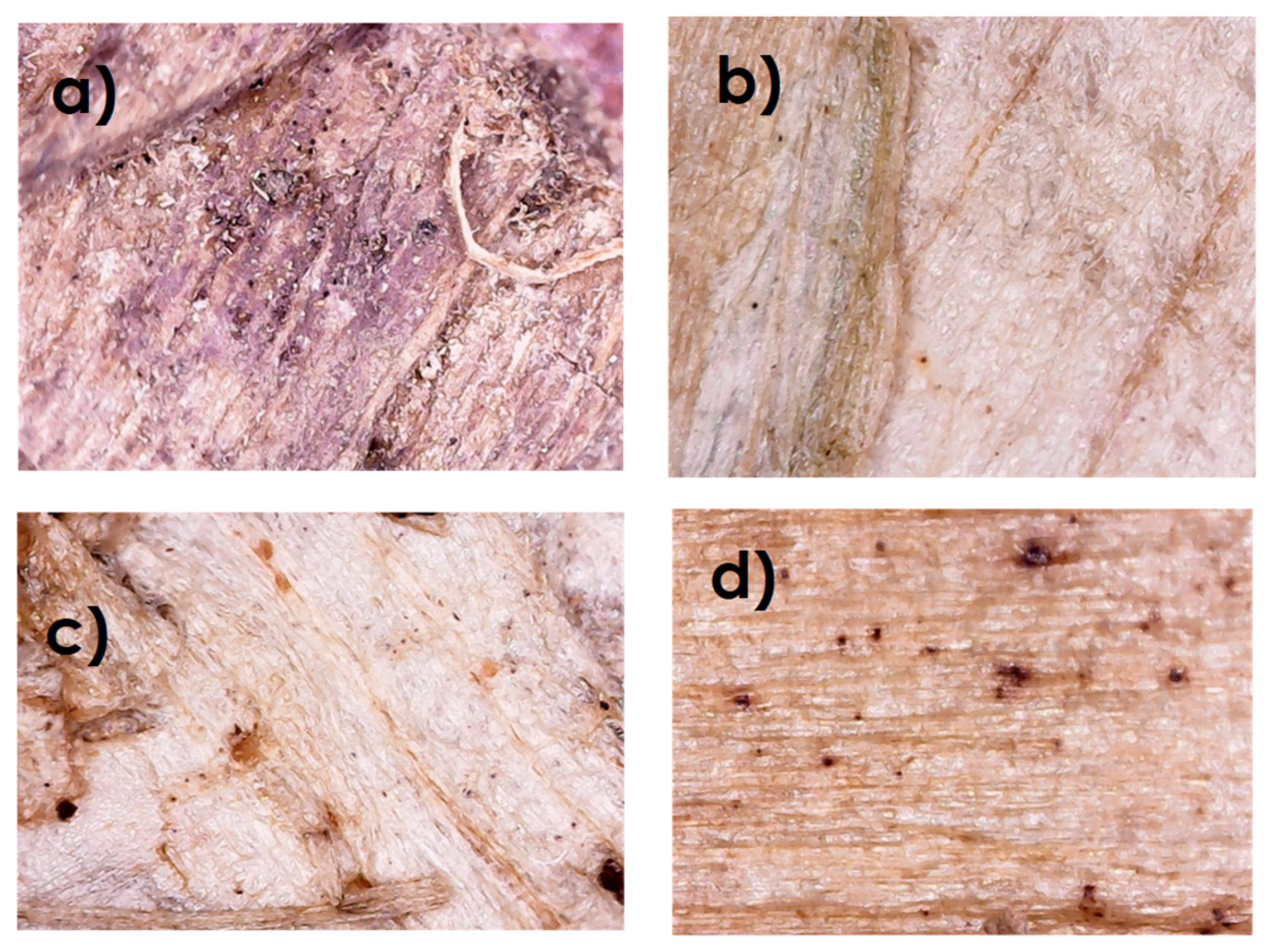
3.3. Optical Microscopy (OM)
4. Results and Discussions
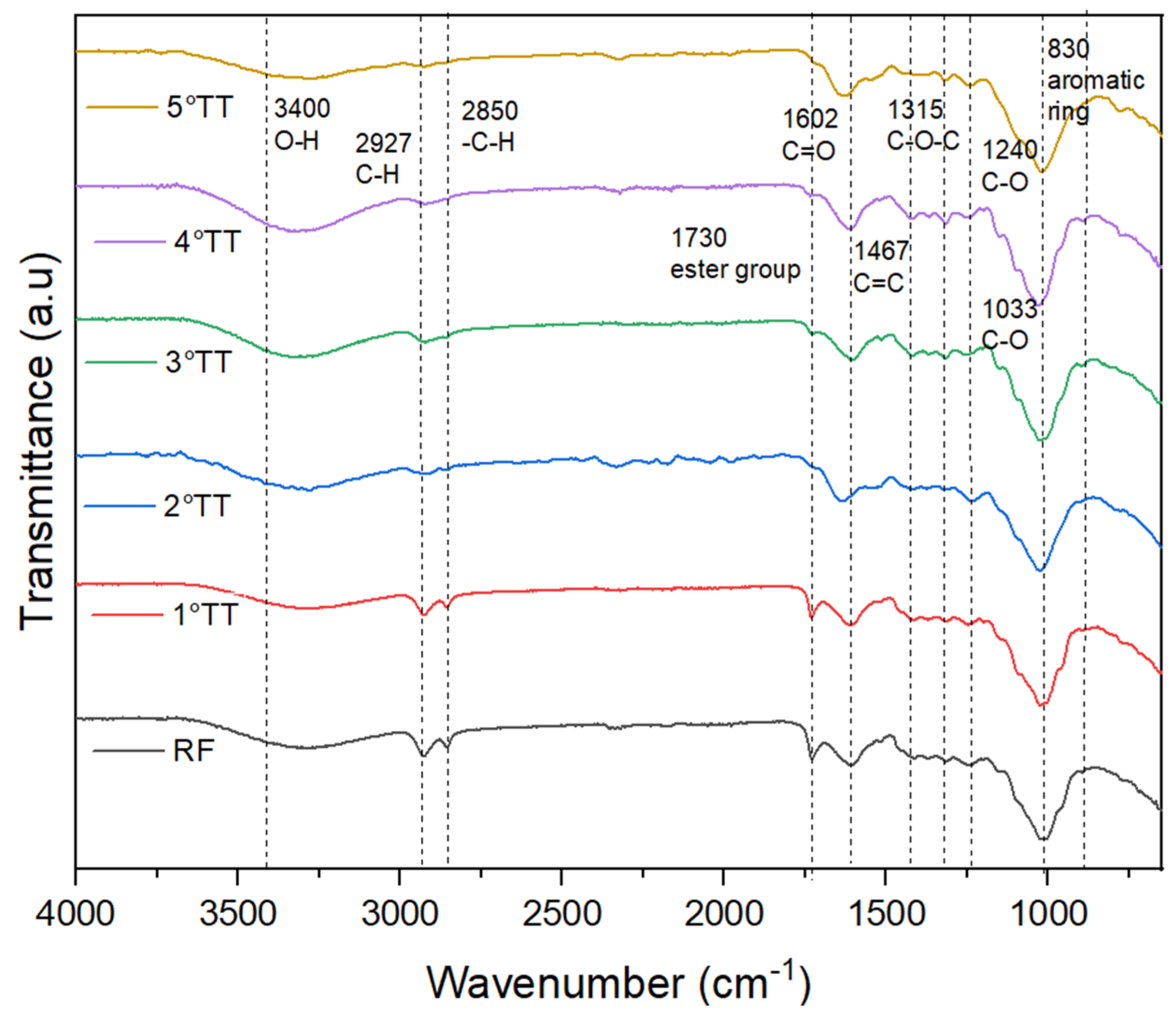
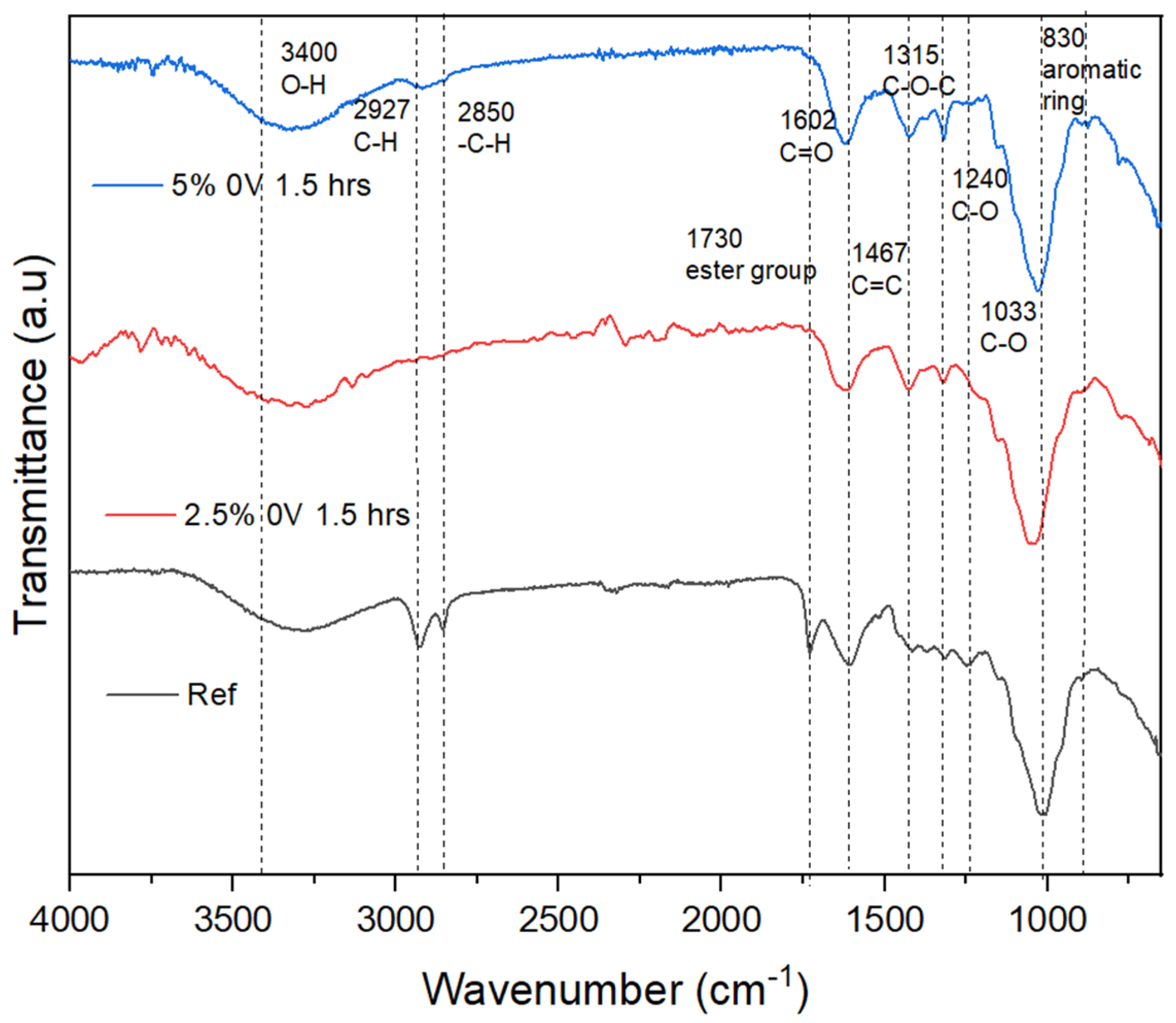
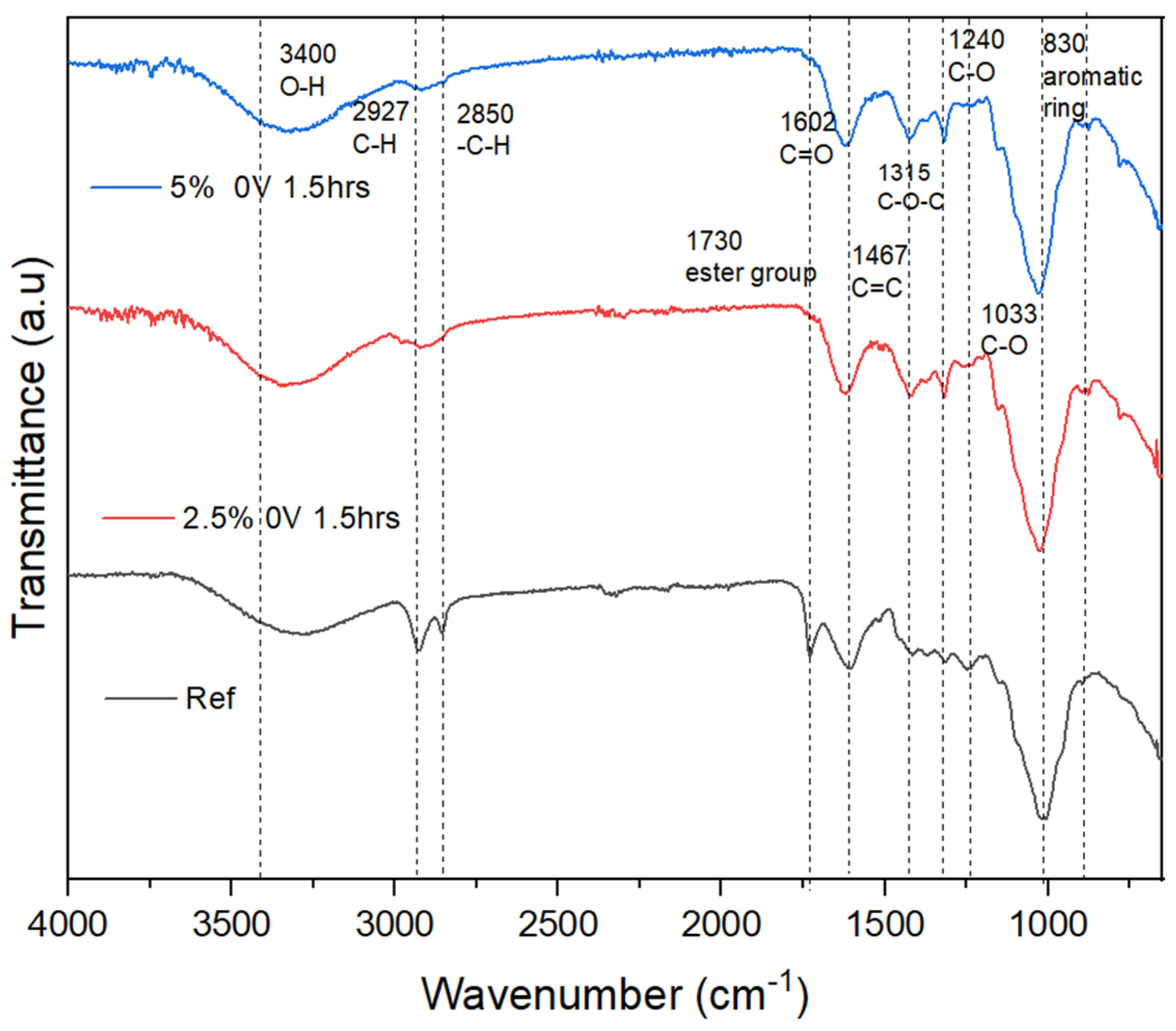
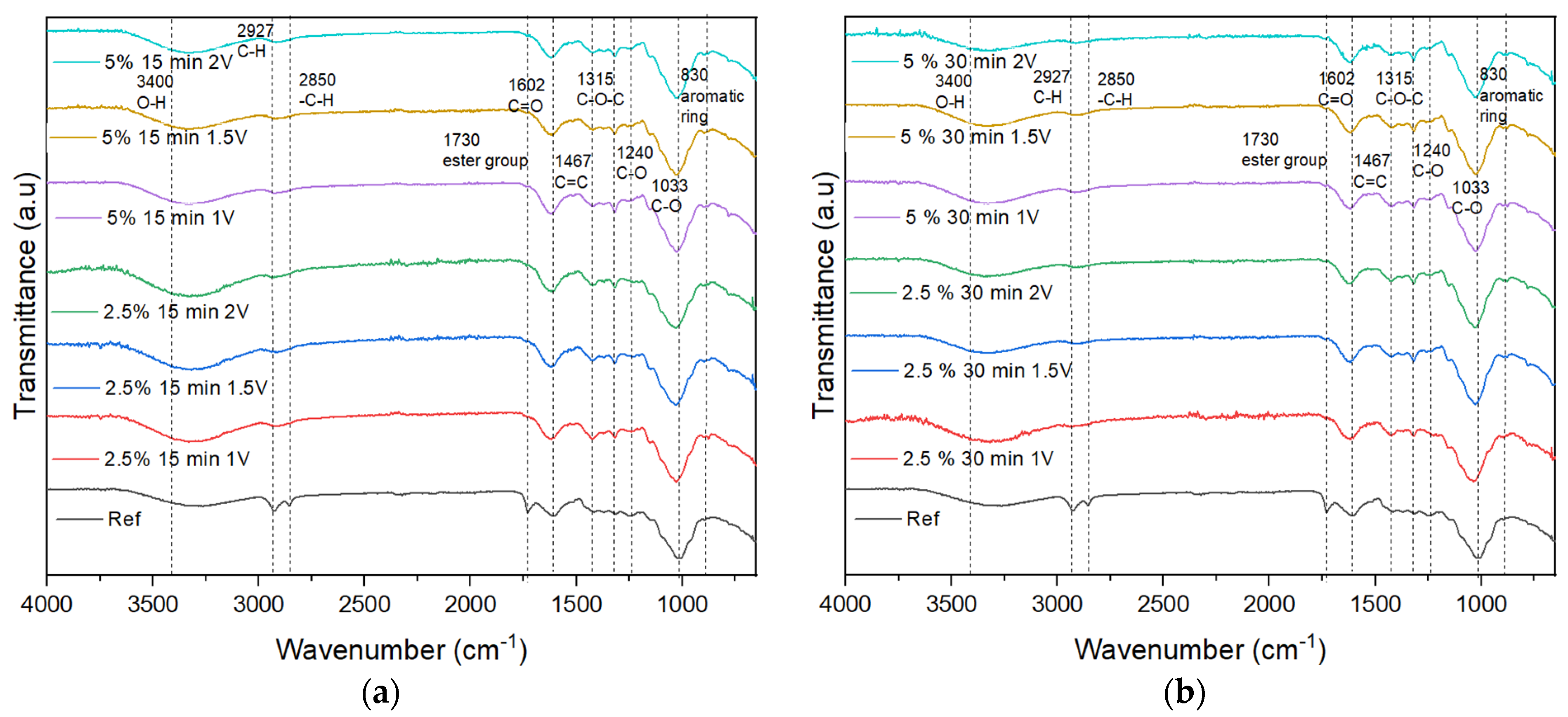
4.1. FT-IR
4.2. Heat Treatment
4.3. Chemical Treatment (Acid and Alkaline)
4.4. Electrohydrolysis
4.5. Hybrid Electrohydrolysis
4.6. Calculation of Delignification Efficiency
4.7. XRD
4.8. Optical Microscopy (OM)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veluchamy, C.; Raju, V.W.; Kalamdhad, A.S. Prerequisite—An electrohydrolysis pretreatment for anaerobic digestion of lignocellulose waste material. Bioresour. Technol. 2017, 235, 274–280. [Google Scholar] [CrossRef]
- Margarita, Z.-D.C.L.; Osney, P.-O.; Antonio, R.-R.P.; María, Z.-D.C.B.; Geraldo, L. Potencialidades del bagazo para la obtención de etanol frente a la generación de electricidad. Ing. Investig. Tecnol. 2015, 16, 407–418. [Google Scholar] [CrossRef]
- Romero, R.G.; Onofre, E.; Espíndola, A.C. Espíndola Delignification and characterization of Tule (Typha Dominguensis) for its potential use in the production of green concrete. J. Phys. Conf. Ser. 2024, 2699, 012002. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Banthia, N. Banthia Plant-based natural fibre reinforced cement composites: A review. Cem. Concr. Compos. 2016, 68, 96–108. [Google Scholar] [CrossRef]
- Sellami, A.; Merzoud, M.; Amziane, S. Amziane Improvement of mechanical properties of green concrete by treatment of the vegetals fibers. Constr. Build. Mater. 2013, 47, 1117–1124. [Google Scholar] [CrossRef]
- Ren, G.; Yao, B.; Ren, M.; Gao, X. Utilization of natural sisal fibers to manufacture eco-friendly ultra-high performance concrete with low autogenous shrinkage. J. Clean. Prod. 2022, 332, 130105. [Google Scholar] [CrossRef]
- Ali, M.; Liu, A.; Sou, H.; Chouw, N. Mechanical and dynamic properties of coconut fibre reinforced concrete. Constr. Build. Mater. 2012, 30, 814–825. [Google Scholar] [CrossRef]
- dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Martín Sendra, A. Estudio comparativo de fibras naturales para reforzar hormigón. Bachelor’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2020. [Google Scholar]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.S.; Goud, V.V. Electrohydrolysis pretreatment methods to enhance the methane production from anaerobic digestion of rice straw using graphite electrode. Renew. Energy 2019, 142, 1–10. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, H.; Wang, L.; Shi, Y.; He, Y.; Cui, T. Radiative cooling performance analysis of cooling wood with three delignification processes for building applications. Energy Build. 2024, 312, 114185. [Google Scholar] [CrossRef]
- Torres, R.S.; Bustamante, E.O.; Cecilia, A.; Flores, E.; Guadalupe, R.; Guzmán, R. Physico-Chemical and Electrochemical Delignification as a Pretreatment of Lignocellulosic Biomass. Cellul. Chem. Technol. 2024, 58, 737–746. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef] [PubMed]
- More, A.; Elder, T.; Jiang, Z. A review of lignin hydrogen peroxide oxidation chemistry with emphasis on aromatic aldehydes and acids. Holzforschung 2021, 75, 806–823. [Google Scholar] [CrossRef]
- Cao, W.; Sun, C.; Liu, R.; Yin, R.; Wu, X. Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresour. Technol. 2012, 111, 215–221. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Liang, M.; Guo, Z.; Cai, Y.; Zhu, C.; Wang, Z.; Wang, S.; Xu, J.; Ying, H. Physical–Chemical–Biological Pretreatment for Biomass Degradation and Industrial Applications: A Review. Waste 2024, 2, 451–473. [Google Scholar] [CrossRef]
- Sanchez-Torres, A.C.E.-F.R.; Onofre-Bustamante, E. Trends in the Addition of PET and Natural Fibers to the Concrete—Steel Reinforcement System. Civ. Eng. Res. J. 2021, 12, 555841. [Google Scholar] [CrossRef]
- Veluchamy, C.; Raju, V.W.; Kalamdhad, A.S. Electrohydrolysis pretreatment for enhanced methane production from lignocellulose waste pulp and paper mill sludge and its kinetics. Bioresour. Technol. 2018, 252, 52–58. [Google Scholar] [CrossRef]
- Bhat, M.I.; Shahi, N.C.; Lohani, U.C.; Singh, S.; Sidique, Q.; Sirohi, R. Microwave irradiation assisted intensive and quick delignification of lignocellulosic biomass, and confirmation by spectral, morphological and crystallinity characterization. Bioresour. Technol. 2022, 351, 127029. [Google Scholar] [CrossRef]
- Debnath, B.; Duarah, P.; Purkait, M.K. Microwave-assisted quick synthesis of microcrystalline cellulose from black tea waste (Camellia sinensis) and characterization. Int. J. Biol. Macromol. 2023, 244, 125354. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Wood modification by heat treatment: A review. BioResources 2008, 4, 370–404. [Google Scholar] [CrossRef]
- Ruiz, E.J.; Ortega-Borges, R.; Jurado, J.L.; Chapman, T.W.; Meas, Y. Simultaneous Anodic and Cathodic Production of Sodium Percarbonate in Aqueous Solution. Electrochem. Solid State Lett. 2009, 12, E1. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, S.; Zhang, Q.; Wang, K.; Liang, P.; Huang, X. Coupling anodic and cathodic reactions using an electrocatalytic dual-membrane system actuates ultra-efficient degradation with regulable mechanisms. Water Res. 2023, 233, 119741. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Belda, J.; Kost, H.-J.; Magomajew, J.; Sperling, R.A.; Wernig, P. Peroxodicarbonate: Electrosynthesis and first directions to green industrial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100341. [Google Scholar] [CrossRef]
- Tiwari, Y.M.; Sarangi, S.K. Characterization of raw and alkali treated cellulosic Grewia Flavescens natural fiber. Int. J. Biol. Macromol. 2022, 209, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.J.; Meas, Y.; Ortega, R.; Jurado, J.L. Electrochemical Syntheses of Sodium Percarbonate in Aqueous Solution. ECS Trans. 2007, 3, 29–35. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Sangeetha, K. Investigation of Physical, Chemical and Structural Characterization of Eichhornia crassipes Fiber. In International Conference on Information Engineering, Management and Security 2017; Association of Scientists, Developers and Faculties: London, UK, 2016; pp. 92–96. Available online: https://www.researchgate.net/publication/318701964_Investigation_of_Physical_Chemical_and_Structural_Characterization_of_Eichhornia_crassipes_Fiber (accessed on 24 June 2025).
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Biological Characterization and Instrumental Analytical Comparison of Two Biorefining Pretreatments for Water Hyacinth (Eichhornia crassipes) Biomass Hydrolysis. Sustainability 2021, 13, 245. [Google Scholar] [CrossRef]
- Arivendan, A.; Jappes, W.; Thangiah, J. Study on characterization of water hyacinth ( Eichhornia crassipes ) novel natural fi ber as reinforcement with epoxy polymer matrix material for lightweight applications. J. Ind. Text. 2022, 51, 8157–8174. [Google Scholar] [CrossRef]
- Abdelrahman, N.; Galiwango, E. Klason Method: An Effective Method for Isolation of Lignin Fractions from Date Palm Biomass Waste. J. Food Process Eng. 2018, 57, 46–58. [Google Scholar]
- Behera, S.; Behera, B.C.; Thatoi, H. Valorization of Lignin Based Nanoparticle from Grass Biomass: An Overview on Synthesis, Characterization and Application. Asian J. Biol. 2023, 17, 34–46. [Google Scholar] [CrossRef]
- Pesti, G.M. A new analytical procedure to replace the outdated Weende proximal feed ingredient analysis paradigm is long overdue. Anim. Prod. Sci. 2024, 64, AN24176. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Yu, T. Kappa statistic considerations in evaluating inter-rater reliability between two raters: Which, when and context matters. BMC Cancer 2023, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- Kostryukov, S.G.; Malov, N.A.; Masterova, Y.Y.; Matyakubov, K.B.; Konushkin, I.A.; Savrasov, K.V.; Pynenkov, A.A.; Khluchina, N.A. On the Possibility of Quantitative Determination of Lignin and Cellulose in Plant Materials Using IR Spectroscopy. Russ. J. Bioorganic Chem. 2023, 49, 1628–1635. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Rebello, N.S. Students’ understanding and application of the area under the curve concept in physics problems. Phys. Rev. Spec. Top. Phys. Educ. Res. 2011, 7, 010112. [Google Scholar] [CrossRef]
- Wang, N.; Xu, A.; Liu, K.; Zhao, Z.; Li, H.; Gao, X. Performance of green solvents in microwave-assisted pretreatment of lignocellulose. Chem. Eng. J. 2024, 482, 148786. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Tyagi, V.K.; Kazmi, A.; Ojha, C.S.P. Hydrothermal and thermal-acid pretreatments of wheat straw: Methane yield, recalcitrant formation, process inhibition, kinetic modeling. Energy 2023, 283, 129083. [Google Scholar] [CrossRef]




| Medium | Polarization (V) | Concentration (%) | Time (min) |
|---|---|---|---|
| Na2H3CO6 | 1, 1.5, 2 | 2.5–5 | 15–30 |
| H2O2 | 1, 1.5, 2 | 2.5–5 | 15–30 |
| Sample | Removal Rate (%) | Removal Rate (%) | Increase Rate (%) | Average Delignification Rate (%) | Std. Dev. |
|---|---|---|---|---|---|
| 5% H2O2, 0 V, 1.5 h | 70.60 | 77.61 | 14.62 | 72.94 | ±0.0037 |
| 5% H2O2, 2 V, 15 min | 78.01 | 92.14 | 23.44 | 82.72 | ±0.0167 |
| TW, 2 V, 30 min | 79.73 | 91.89 | 22.45 | 83.78 | ±0.0102 |
| Corn stover (microwave irradiation) [38] | 74.89 | 75.01 | - | 74.95 | - |
| Wheat straw (hydrothermal) [39] | 39.01 | 53.40 | - | 46.21 | - |
| Wheat straw (distilled water) [10] | 54.80 | 22.40 | - | 38.60 | - |
| Sample | Crystallinity Degree Xc | 2θ (°) | Interplanar Distances d(Å) | Crystallite Size D(Å) |
|---|---|---|---|---|
| WLF | 30.4 ± 2.2 | 22.05 | 8.149 | 255.789 |
| 5% H2O2, 0 V, 1.5 h | 90.2 ± 1.1 | 21.59 | 4.111 | 22.446 |
| 5% H2O2, 2 V, 15 min | 93.4 ± 1.2 | 21.538 | 4.041 | 25.488 |
| TW, 2 V, 30 min | 95 ± 1 | 21.59 | 4.028 | 25.589 |
| Sample | Ra | Average | Rq | Average | ||||
|---|---|---|---|---|---|---|---|---|
| Reference | 0.0719 | 0.0755 | 0.0668 | 0.0714 | 0.0952 | 0.0953 | 0.0864 | 0.0923 |
| 5% H2O2, 0 V, 1.5 h | 0.0368 | 0.0340 | 0.0324 | 0.0344 | 0.0466 | 0.0439 | 0.0434 | 0.0446 |
| 5% H2O2, 2 V, 15 min | 0.0377 | 0.0379 | 0.0397 | 0.0384 | 0.0580 | 0.0534 | 0.0556 | 0.0557 |
| TW, 2 V, 30 min | 0.0328 | 0.0391 | 0.0236 | 0.0318 | 0.0433 | 0.0498 | 0.0318 | 0.0416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Torres, R.; Bustamante, E.O.; López, T.P.; Espindola-Flores, A.C. Efficiency Determination of Water Lily (Eichhornia crassipes) Fiber Delignification by Electrohydrolysis Using Different Electrolytes. Recycling 2025, 10, 130. https://doi.org/10.3390/recycling10040130
Sanchez-Torres R, Bustamante EO, López TP, Espindola-Flores AC. Efficiency Determination of Water Lily (Eichhornia crassipes) Fiber Delignification by Electrohydrolysis Using Different Electrolytes. Recycling. 2025; 10(4):130. https://doi.org/10.3390/recycling10040130
Chicago/Turabian StyleSanchez-Torres, R., E. Onofre Bustamante, T. Pérez López, and A. C. Espindola-Flores. 2025. "Efficiency Determination of Water Lily (Eichhornia crassipes) Fiber Delignification by Electrohydrolysis Using Different Electrolytes" Recycling 10, no. 4: 130. https://doi.org/10.3390/recycling10040130
APA StyleSanchez-Torres, R., Bustamante, E. O., López, T. P., & Espindola-Flores, A. C. (2025). Efficiency Determination of Water Lily (Eichhornia crassipes) Fiber Delignification by Electrohydrolysis Using Different Electrolytes. Recycling, 10(4), 130. https://doi.org/10.3390/recycling10040130





