New Strategy for Upcycling Marine Plastic Waste Through the Development of a Diamine-Functionalized Poly(ethylene terephthalate) Compatibilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
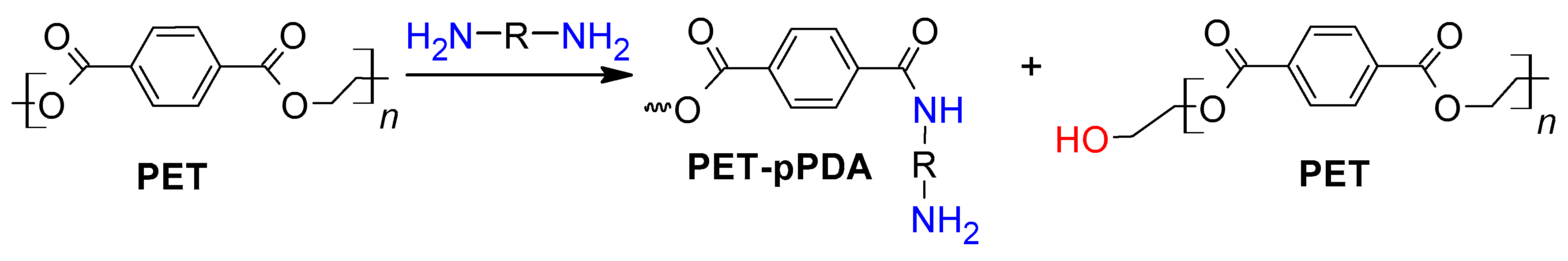
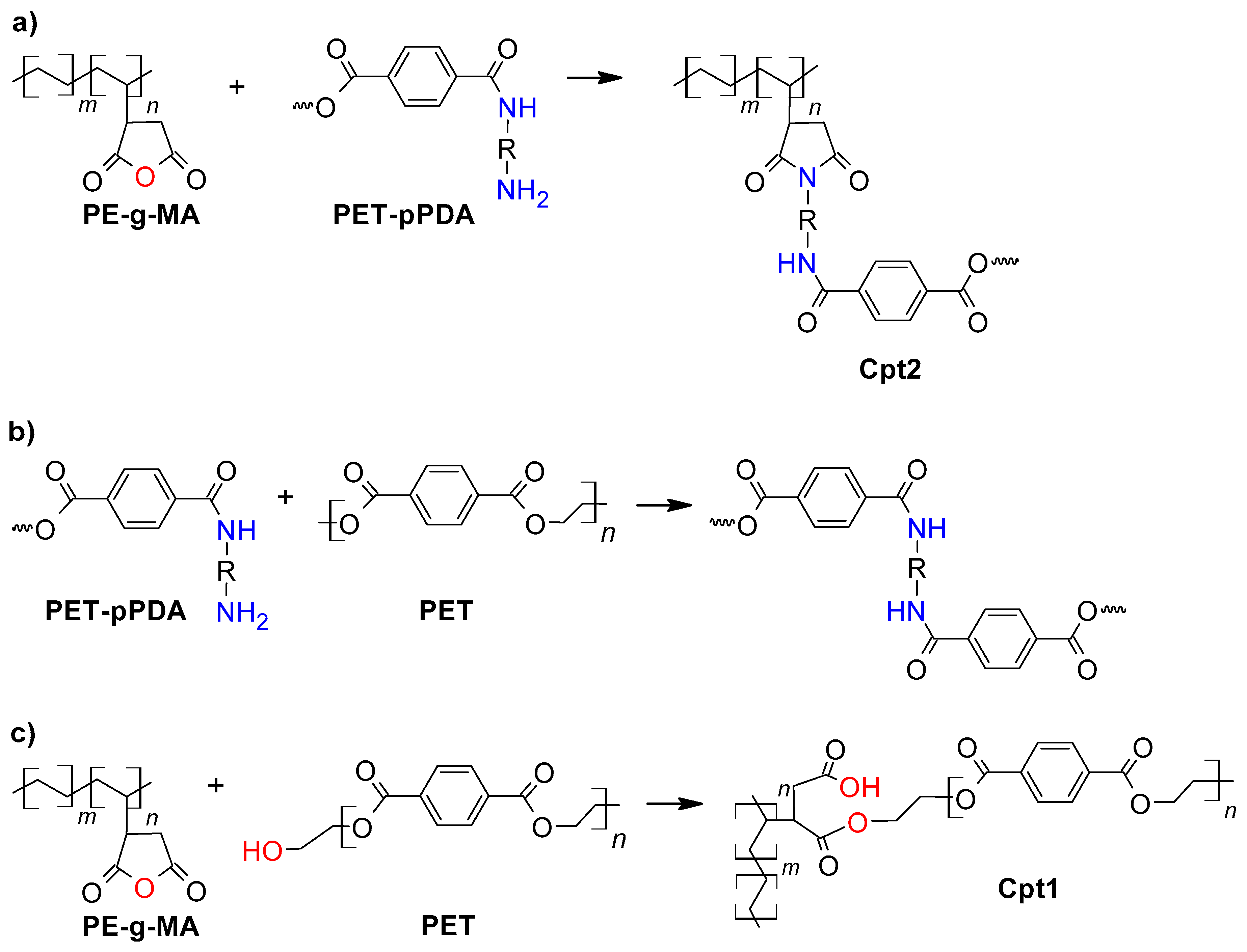
2.2. PET Functionalization and Specimen Preparation
2.3. Compatibilization of LDPE/PET Blend
2.4. Characterization
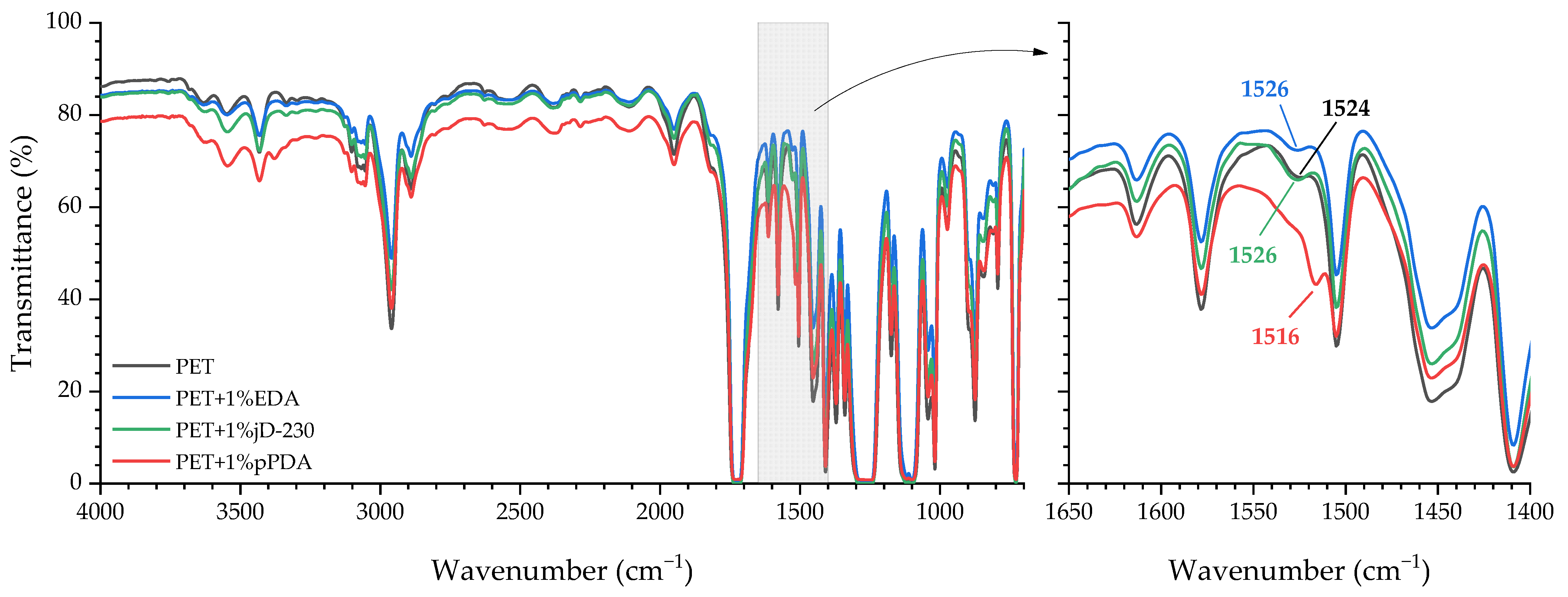
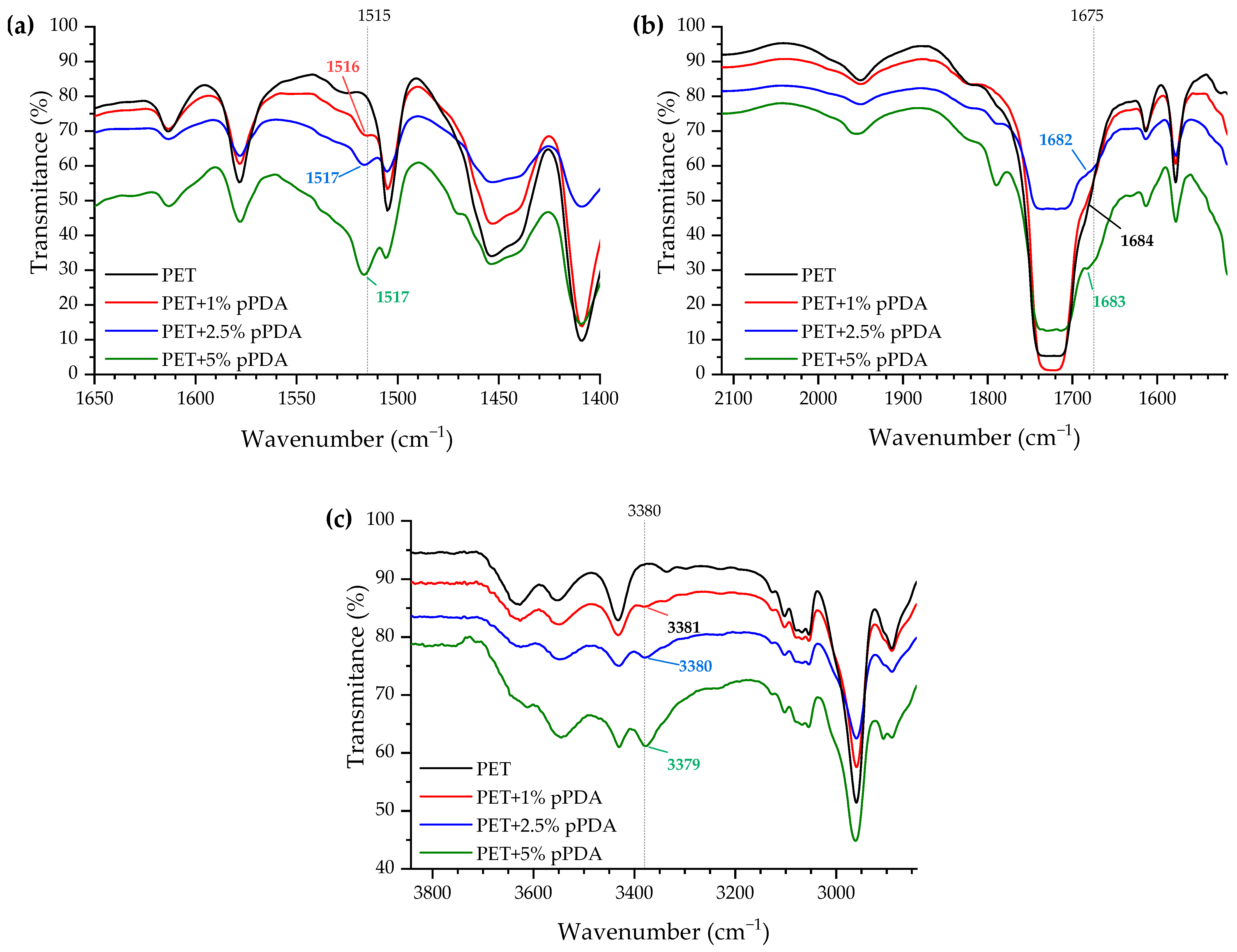
2.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Rheological Measurements
2.4.5. Dynamic Mechanical Analysis (DMA)
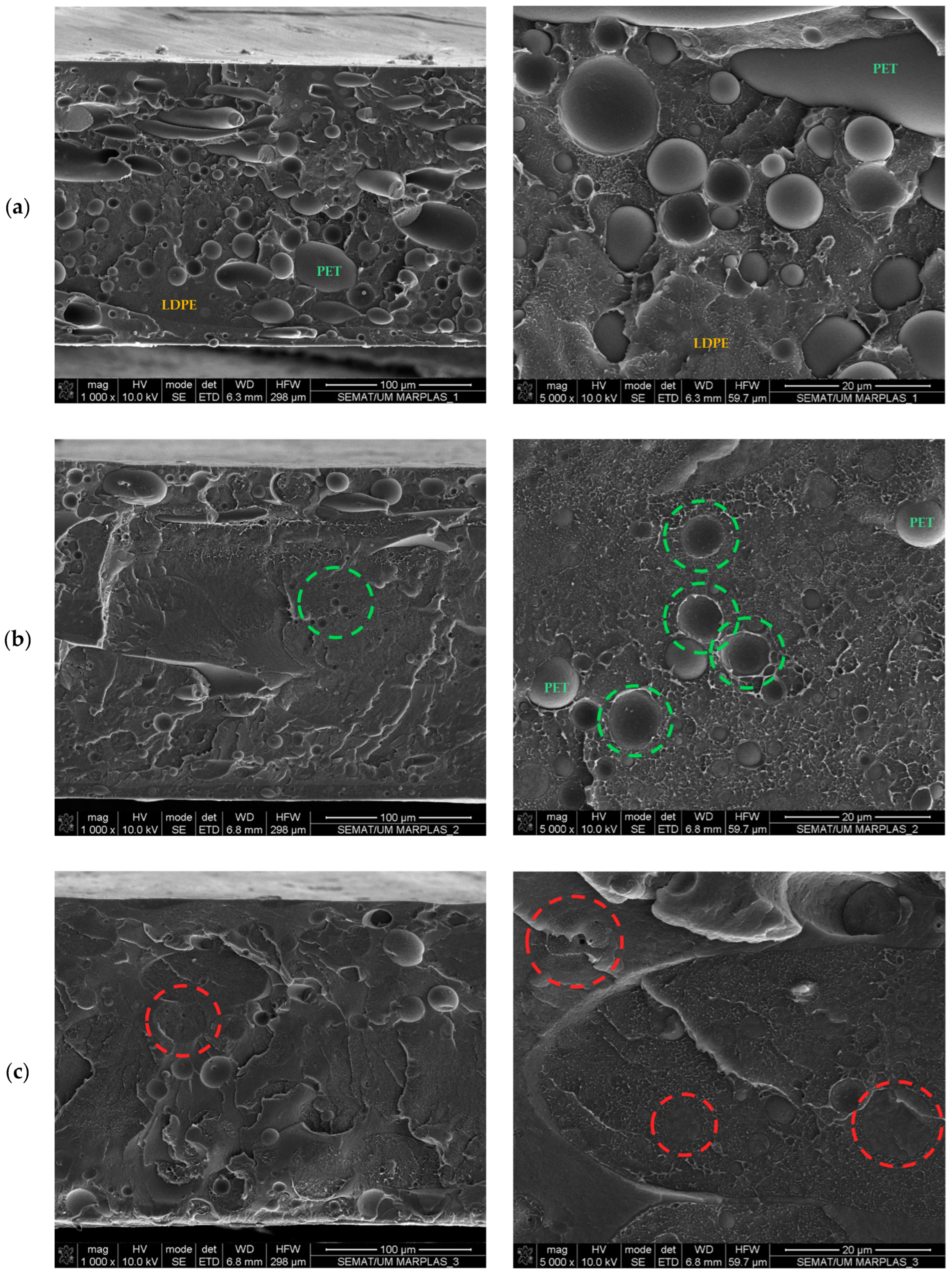
2.4.6. Field Emission Gun-Scanning Electron Microscopy (FEG-SEM)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kučera, F.; Petruš, J.; Žídek, J.; Poláček, P.; Šimonek, M. An Approach on Reactive Processing of Plastic Waste. Polym. Eng. Sci. 2022, 62, 4100–4114. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, L.; Iudici, N.; Oliva, L. Synthesis of Well-Defined Polypropylene-Graft-Polystyrene and Relationship between Structure and the Ability to Compatibilize the Polymeric Blends. Macromolecules 2005, 38, 4894–4900. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Liu, K.; Mun, S.C.; Ghazaryan, G.; Senger, C.T.; Ellison, C.J.; Macosko, C.W.; Peterson, T.H.; Mukhopadhyay, S.; Thurber, C.M. Accelerating the Coupling of Maleated Polyolefins with Polyesters via Tin Compounds. Macromolecules 2019, 52, 8359–8366. [Google Scholar] [CrossRef]
- Champagne, M.F.; Huneault, M.A.; Roux, C.; Peyrel, W. Reactive Compatibilization of Polypropylene/Polyethylene Terephthalate Blends. Polym. Eng. Sci. 1999, 39, 976–984. [Google Scholar] [CrossRef]
- Chen, S.-C.; Zhang, L.-H.; Zhang, G.; Zhong, G.-C.; Li, J.; Zhang, X.-M.; Chen, W.-X. An Investigation and Comparison of the Blending of LDPE and PP with Different Intrinsic Viscosities of PET. Polymers 2018, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Kalfoglou, N.K.; Skafidas, D.S.; Kallitsis, J.K.; Lambert, J.-C.; Van der Stappen, L. Comparison of Compatibilizer Effectiveness for PET/HDPE Blends. Polymer 1995, 36, 4453–4462. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical Recycling: Compatibilization of Mixed Thermoplastic Wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Guo, W.; Wu, C. Effects of Different Types of Polyethylene on the Morphology and Properties of Recycled Poly(Ethylene Terephthalate)/Polyethylene Compatibilized Blends. Polym. Adv. Technol. 2011, 22, 1851–1858. [Google Scholar] [CrossRef]
- Ha, C.-S.; Park, H.-D.; Kim, Y.; Kwon, S.-K.; Cho, W.-J. Compatibilizer in Polymer Blends for the Recycling of Plastics Waste I: Preliminary Studies on 50/50 Wt% Virgin Polyblends. Polym. Adv. Technol. 1996, 7, 483–492. [Google Scholar] [CrossRef]
- Fasce, L.; Seltzer, R.; Frontini, P.; Pita, V.J.R.; Pacheco, E.B.A.V.; Dias, M.L. Mechanical and Fracture Characterization of 50:50 HDPE/PET Blends Presenting Different Phase Morphologies. Polym. Eng. Sci. 2005, 45, 354–363. [Google Scholar] [CrossRef]
- Nomura, K.; Peng, X.; Kim, H.; Jin, K.; Kim, H.J.; Bratton, A.F.; Bond, C.R.; Broman, A.E.; Miller, K.M.; Ellison, C.J. Multiblock Copolymers for Recycling Polyethylene–Poly(Ethylene Terephthalate) Mixed Waste. ACS Appl. Mater. Interfaces 2020, 12, 9726–9735. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, C.; Keum, J.; Chen, J.; Dial, B.E.; Wang, Y.; Tsai, W.-Y.; Bras, W.; Saito, T.; Bowland, C.C.; et al. Upcycling of Semicrystalline Polymers by Compatibilization: Mechanism and Location of Compatibilizers. RSC Adv. 2022, 12, 10886–10894. [Google Scholar] [CrossRef]
- Yousfi, M.; Soulestin, J.; Vergnes, B.; Lacrampe, M.F.; Krawczak, P. Morphology and Mechanical Properties of PET/PE Blends Compatibilized by Nanoclays: Effect of Thermal Stability of Nanofiller Organic Modifier. J. Appl. Polym. Sci. 2013, 128, 2766–2778. [Google Scholar] [CrossRef]
- Papadopoulou, C.P.; Kalfoglou, N.K. Comparison of Compatibilizer Effectiveness for PET/PP Blends: Their Mechanical, Thermal and Morphology Characterization. Polymer 2000, 41, 2543–2555. [Google Scholar] [CrossRef]
- Kalfoglou, N.K.; Skafidas, D.S.; Sotiropoulou, D.D. Compatibilization of Blends of Poly(Ethylene Terephthalate) and Linear Low Density Polyethylene with the Ionomer of Poly(Ethylene-Co-Methacrylic Acid). Polymer 1994, 35, 3624–3630. [Google Scholar] [CrossRef]
- Friedrich, K.; Evstatiev, M.; Fakirov, S.; Evstatiev, O.; Ishii, M.; Harrass, M. Microfibrillar Reinforced Composites from PET/PP Blends: Processing, Morphology and Mechanical Properties. Compos. Sci. Technol. 2005, 65, 107–116. [Google Scholar] [CrossRef]
- Akshaya, E.M.; Palaniappan, R.; Sowmya, C.F.; Rasana, N.; Jayanarayanan, K. Properties of Blends from Polypropylene and Recycled Polyethylene Terephthalate Using a Compatibilizer. Mater. Proc. 2020, 24, 359–368. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–56. ISBN 978-3-319-16510-3. [Google Scholar]
- Hetemi, D.; Pinson, J. Functionalization of Polymers by Hydrolysis, Aminolysis, Reduction, Oxidation, and Some Related Reactions. In Surface Modification of Polymers; Wiley-VCH: Weinheim, Germany, 2019; pp. 211–240. ISBN 978-3-527-81924-9. [Google Scholar]
- Lepoittevin, B.; Costa, L.; Pardoue, S.; Dragoé, D.; Mazerat, S.; Roger, P. Hydrophilic PET Surfaces by Aminolysis and Glycopolymer Brushes Chemistry. J. Polym. Sci. Part Polym. Chem. 2016, 54, 2689–2697. [Google Scholar] [CrossRef]
- Xu, T.; Li, Z.; Ju, X.; Tang, H.; Xiang, W. Chemical Degradation of Waste PET and Its Application in Wood Reinforcement and Modification. ACS Omega 2023, 8, 30550–30562. [Google Scholar] [CrossRef] [PubMed]
- Kárpáti, L.; Ganyecz, Á.; Nagy, T.; Hamar, G.; Banka, E.; Kállay, M.; Vargha, V. Synthesis and Characterization of Isophorondiamine-Based Oligoamides: Catalytic Effect of Amides during the Curing of Epoxy Resins. Polym. Bull. 2020, 77, 4655–4678. [Google Scholar] [CrossRef]
- Hoang, C.N.; Dang, Y.H. Aminolysis of Poly(Ethylene Terephthalate) Waste with Ethylenediamine and Characterization of α,ω-Diamine Products. Polym. Degrad. Stab. 2013, 98, 697–708. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent Advances in Chemical Recycling of Polyethylene Terephthalate Waste into Value Added Products for Sustainable Coating Solutions—Hope vs. Hype. Mater. Adv. 2022, 3, 1974–1992. [Google Scholar] [CrossRef]
- Heidari, S.; Tahvildari, K. Preparation and Characterization of Diols and Polyols Based on Aminolysis of Poly (Ethylene Terephthalate) Wastes with Alkanolamines. J. Appl. Chem. Res. 2013, 7, 33–42. [Google Scholar]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Spychaj, T.; Fabrycy, E.; Spychaj, S.; Kacperski, M. Aminolysis and Aminoglycolysis of Waste Poly(Ethylene Terephthalate). J. Mater. Cycles Waste Manag. 2001, 3, 24–31. [Google Scholar] [CrossRef]
- Aminolysis of Polyesters for Cracking and Structure Clarifying: A Review—Rabiei—2022—Polymers for Advanced Technologies—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/pat.5837 (accessed on 12 April 2023).
- Todd, A.D.; McEneany, R.J.; Topolkaraev, V.A.; Macosko, C.W.; Hillmyer, M.A. Reactive Compatibilization of Poly(Ethylene Terephthalate) and High-Density Polyethylene Using Amino-Telechelic Polyethylene. Macromolecules 2016, 49, 8988–8994. [Google Scholar] [CrossRef]
- Kamatani, H.; Konagaya, S.; Nakamura, Y. Effect of Phosphoric Acid on the Polycondensation of Bis(2-Hydroxyethyl) Terephthalate Catalyzed by Sb(III) Compounds. Polym. J. 1980, 12, 125–130. [Google Scholar] [CrossRef]
- ASTM D3418; A Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM: Philadelphia, PA, USA, 2021.
- Mehta, A.; Gaur, U.; Wunderlich, B. Equilibrium Melting Parameters of Poly(Ethylene Terephthalate). J. Polym. Sci. Polym. Phys. Ed. 1978, 16, 289–296. [Google Scholar] [CrossRef]
- Gao, M.; Jiao, Q.; Cui, W.; Feng, C.; Zhao, Y.; Xiang, A.; Mu, X.; Ma, L. Preparation of PET/LDH Composite Materials and Their Mechanical Properties and Permeability for O2. Polym. Eng. Sci. 2019, 59, E366–E371. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. IR Spectroscopy. Structure Determination of Organic Compounds: Tables of Spectral Data, Springer: Berlin/Heidelberg, Germany, 2020; 307–373ISBN 978-3-662-62439-5. [Google Scholar]
- Badía, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal Analysis as a Quality Tool for Assessing the Influence of Thermo-Mechanical Degradation on Recycled Poly(Ethylene Terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Litmanovich, A.D.; Platé, N.A.; Kudryavtsev, Y.V. Reactions in Polymer Blends: Interchain Effects and Theoretical Problems. Prog. Polym. Sci. 2002, 27, 915–970. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated Cationic Polymerization of Monofunctional Benzoxazine. J. Polym. Sci. Part Polym. Chem. 2003, 41, 3320–3328. [Google Scholar] [CrossRef]
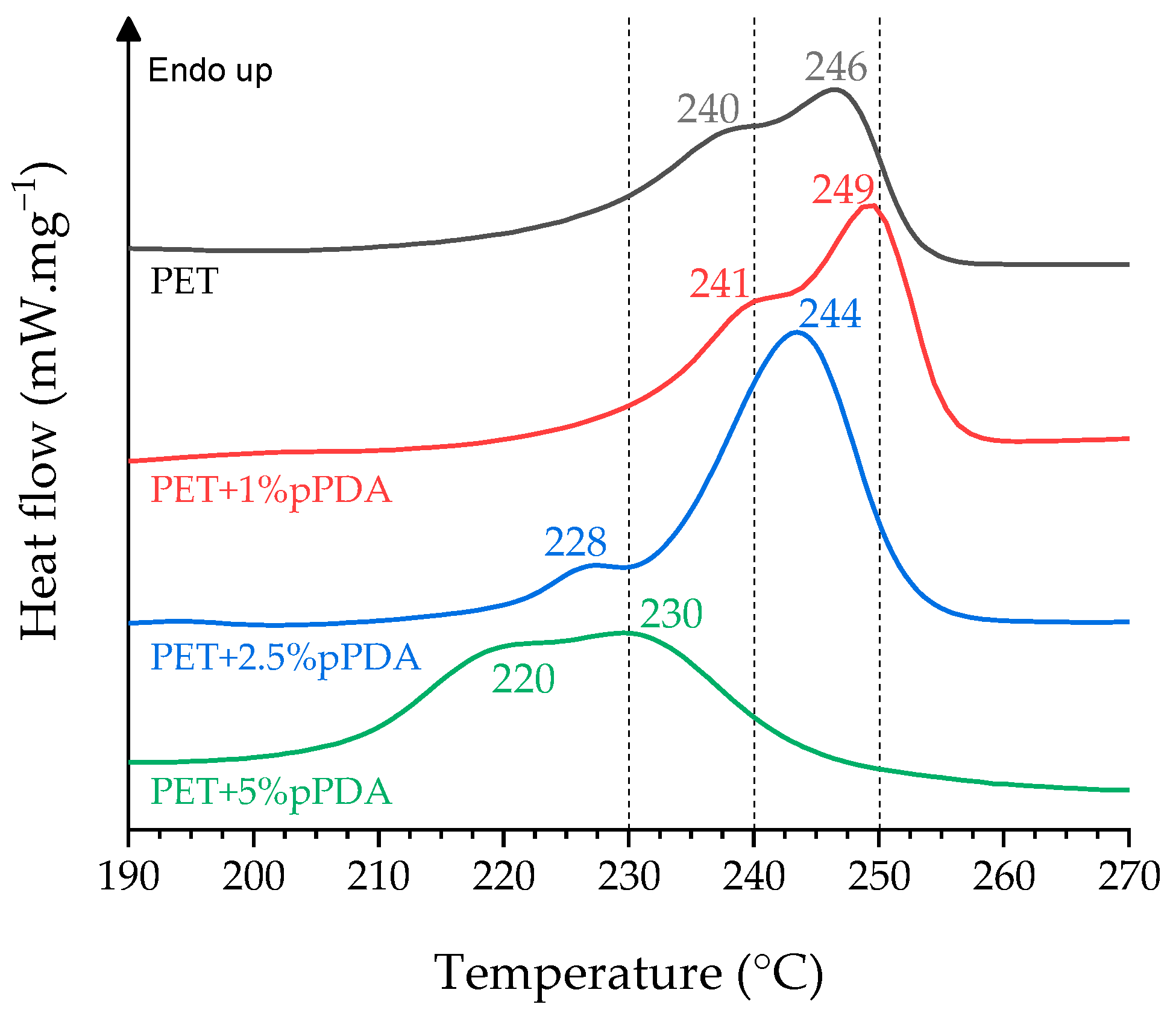


| Material | Tg (°C) | Tm1 (°C) | Tm2 (°C) | ∆Hm (J∙g−1) | Xc (%) |
|---|---|---|---|---|---|
| PET | 82 | 240 | 246 | 37 | 26 |
| PET+1%EDA | 85 | 242 | 249 | 35 | 25 |
| PET+1%pPDA | 81 | 241 | 249 | 40 | 29 |
| PET+2.5%pPDA | 90 | 228 | 244 | 42 | 30 |
| PET+5%pPDA | 84 | 220 | 230 | 38 | 27 |
| PET+1%jD230 | 83 | 240 | 248 | 41 | 29 |
| Material | LDPE Fraction | PET Fraction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | Tc (°C) | ∆Hm (J∙g−1) | Xc (%) | Tm (°C) | Tc (°C) | Tg (°C) | ∆Hm (J∙g−1) | Xc (%) | |
| PET | - | - | - | - | 240/246 | 187 | 82 | 37 | 26 |
| LDPE | 116 | 96 | 115 | 39 | - | - | - | - | - |
| LDPE/PET | 109 | 96 | 64 | 22 | 242/249 | 194 | - | 16 | 11 |
| LDPE/PET+9%Cpt1 | 114 | 100 | 64 | 22 | -/247 | 210 | - | 15 | 11 |
| LDPE/PET+9%Cpt2 | 108 | 98 | 62 | 21 | -/249 | 184 | - | 19 | 13 |
| MAPE | 122 | 100 | 68 | 23 | - | - | - | - | - |
| Cpt1 | 120 | 104 | 64 | 22 | -/250 | 213 | - | 20 | 15 |
| Cpt2 | 120 | 103 | 64 | 22 | -/249 | 192 | - | 19 | 14 |
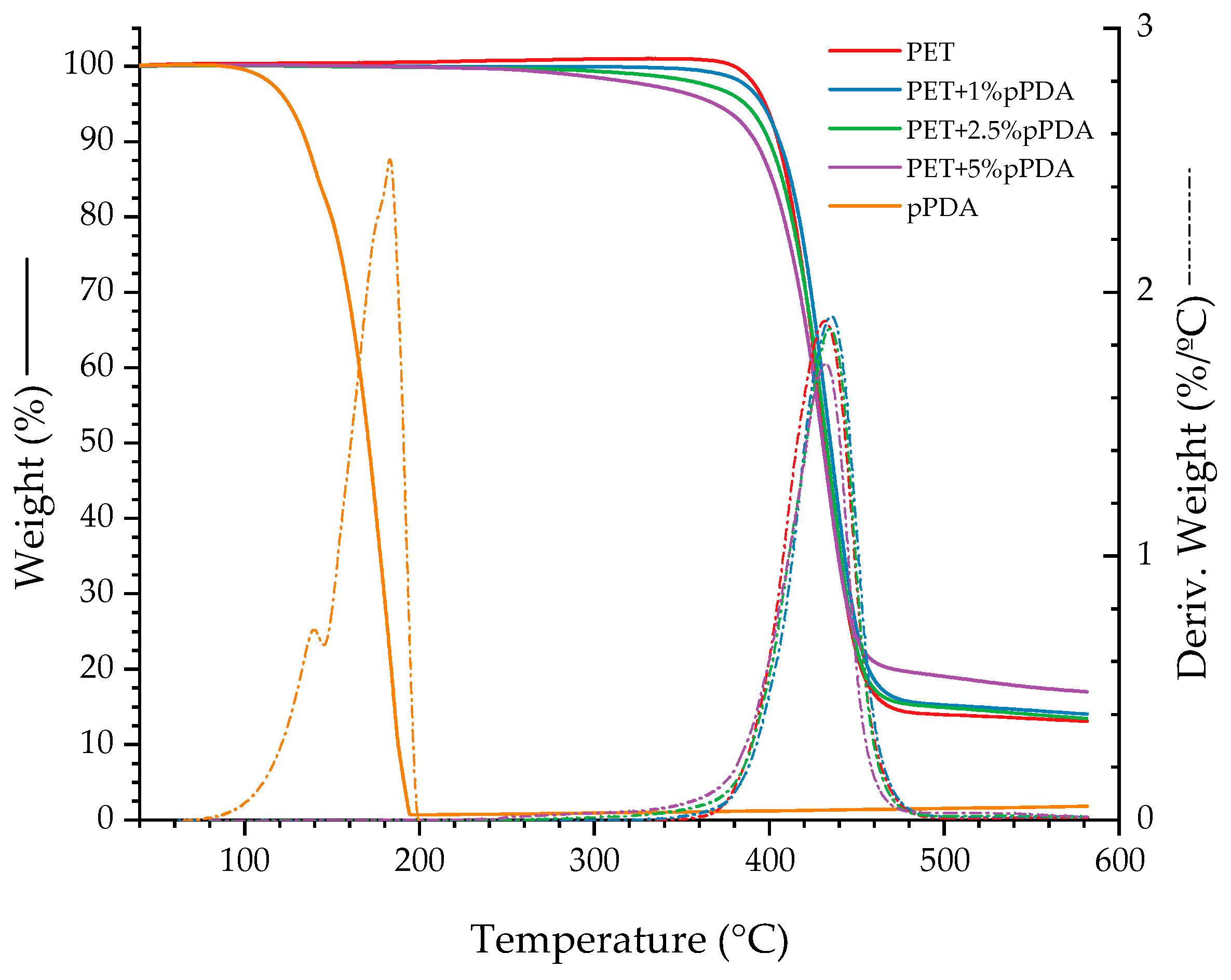
| Material | Tonset (°C) | Tmax (°C) | Tfinal (°C) | Residue (%) |
|---|---|---|---|---|
| PET | 404 | 430 | 500 | 0 |
| PET+1%pPDA | 409 | 436 | 500 | 14 |
| PET+2.5%pPDA | 407 | 435 | 500 | 13 |
| PET+5%pPDA | 404 | 432 | 500 | 17 |
| pPDA | 153 | 177 | 200 | 1 |
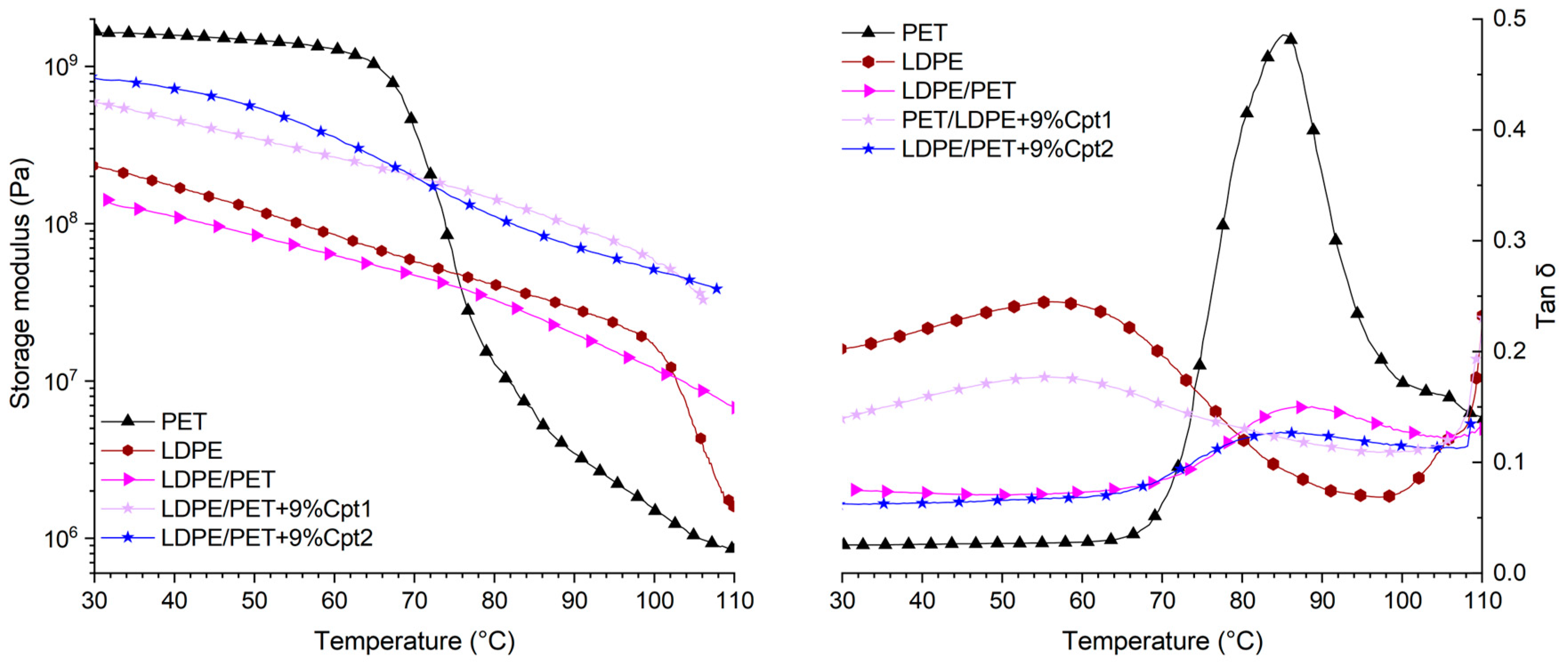
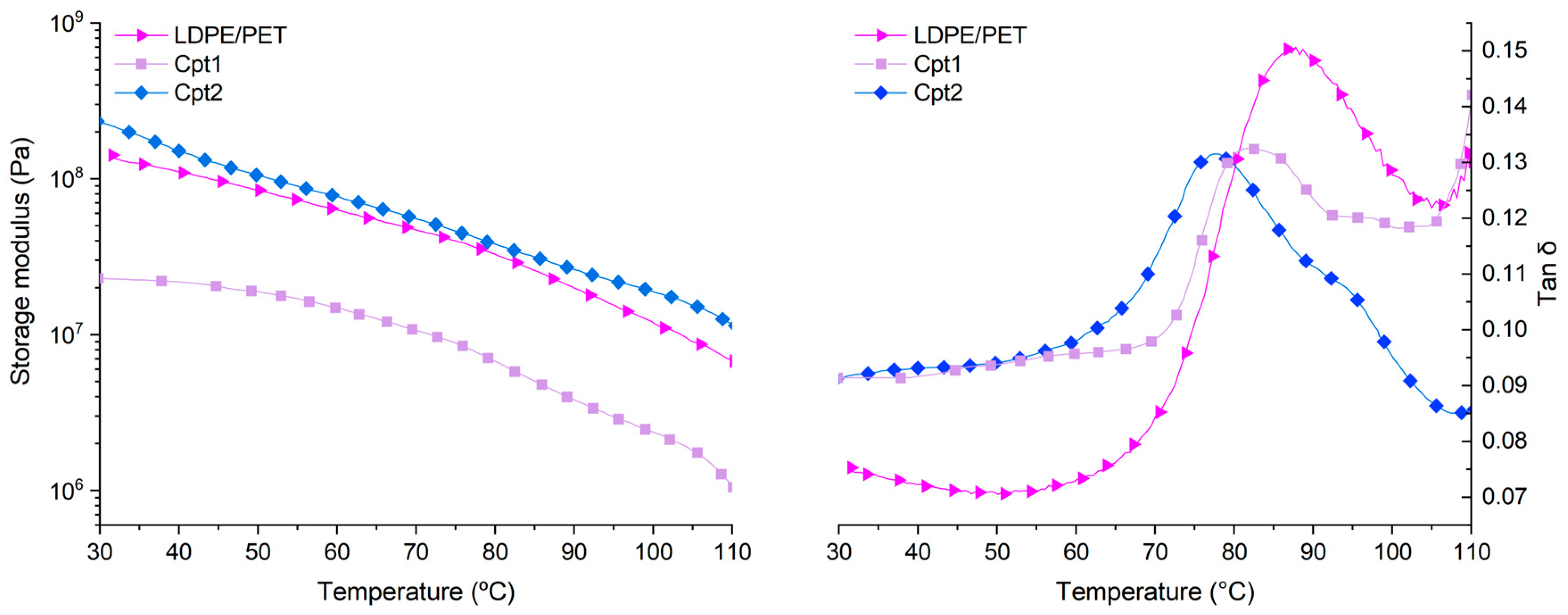
| Material | E’30°C (MPa) | E’100°C (MPa) | Tg at tan δ peak (°C) | Tan δ |
|---|---|---|---|---|
| PET | 1636.8 | 1.5 | 85.1 | 0.486 |
| LDPE | 231.3 | 18.4 | - | - |
| LDPE/PET | 141.0 | 11.8 | 86.8 | 0.151 |
| LDPE/PET+9%Cpt1 | 587.2 | 56.3 | - | - |
| LDPE/PET+9%Cpt2 | 822.0 | 49.9 | 84.8 | 0.127 |
| Cpt1 | 22.9 | 2.3 | 82.0 | 0.132 |
| Cpt2 | 233.6 | 18.6 | 77.3 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.V.; Cestari, S.P.; Cruz, V.; Castro, M.C.R.; Machado, A.V. New Strategy for Upcycling Marine Plastic Waste Through the Development of a Diamine-Functionalized Poly(ethylene terephthalate) Compatibilizer. Recycling 2025, 10, 82. https://doi.org/10.3390/recycling10030082
Rodrigues PV, Cestari SP, Cruz V, Castro MCR, Machado AV. New Strategy for Upcycling Marine Plastic Waste Through the Development of a Diamine-Functionalized Poly(ethylene terephthalate) Compatibilizer. Recycling. 2025; 10(3):82. https://doi.org/10.3390/recycling10030082
Chicago/Turabian StyleRodrigues, Pedro V., Sibele P. Cestari, Vasco Cruz, M. Cidália R. Castro, and Ana Vera Machado. 2025. "New Strategy for Upcycling Marine Plastic Waste Through the Development of a Diamine-Functionalized Poly(ethylene terephthalate) Compatibilizer" Recycling 10, no. 3: 82. https://doi.org/10.3390/recycling10030082
APA StyleRodrigues, P. V., Cestari, S. P., Cruz, V., Castro, M. C. R., & Machado, A. V. (2025). New Strategy for Upcycling Marine Plastic Waste Through the Development of a Diamine-Functionalized Poly(ethylene terephthalate) Compatibilizer. Recycling, 10(3), 82. https://doi.org/10.3390/recycling10030082








