Abstract
Leachates from electronic waste, slag dusts generated during the processing of electronic waste, sweeping jewelry, and municipal solid-waste incineration residues contain a myriad of base metals, such as aluminum (Al: 10–2000 mg/L), copper (Cu: 10–1000 mg/L), iron (Fe: 10–500 mg/L), nickel (Ni: 0.1–500 mg/L), lead (Pb: 1–500 mg/L), tin (Sn: 1–100 mg/L), and zinc (Zn: 5–500 mg/L), which are present at much higher quantities than Au (0.01–10 mg/L), which raises several drawbacks to the efficient recycling of Au with high purity using hydrometallurgical strategies. The aim of this work was to study the efficiency and selectivity of two strong basic anion exchange (DOWTM XZ-91419.00 and PurogoldTM A194) resins to recover Au from a chloride multi-metal solution containing these metals. For both resins, the adsorption kinetic and equilibrium parameters for Au(III), determined at 1.12 mol/L HCl, Eh = 1.1 V, and 25 °C, proceeded according to a pseudo-second order and a Langmuir isotherm (qmax was 0.94 and 1.70 mmol/g for DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively), respectively. Continuous adsorption experiments of Au (48 µmol/L; 2.0%) from a chloride multi-metal solution evidenced high Au retention capacity and selectivity to Au over Al, Cu, Fe, Ni, and Zn but low selectivity to Au over Ag and Sn for both resins. Concentrated (>3.3 mmol/L) and pure (>94%) Au eluates were obtained for both resins.
1. Introduction
Gold (Au) is a precious metal and it is very important because of its unique physical and chemical properties [1,2]. In the recent years, there has been a tendency for the utilization of precious metals, specifically Au, not only in traditional jewelry materials but also in new emerging applications [3,4], such as manufacturing of electronic parts and devices and corrosion-resistant materials. In electrical and electronic components, Au is used for connectors, switches, relay contacts, connecting wires, and connection strips mainly due to its corrosion resistance and electrical conductivity [1,5].
However, Au resources in the world are scarce and are mainly associated with other metal deposits [3]. In addition to that, the market values of Au are very high and are constantly rising [6]. With the growing demand of Au and reducing amount of its nature resources, it is important to recycle and reuse Au from secondary resources and industrial waste [2,4,7]. The recovery and reuse of Au is of great importance from the economic, environmental, and sustainability perspectives. Thus, the development of procedures to recovery Au from secondary resources has become an attractive and imperative issue [6].
Among the recovery methods of Au, the hydrometallurgical method is one of the most effective to recover this metal from scraps, such as electronic waste, slag dusts generated during the processing of electronic waste, and sweeping jewelry. These hydrometallurgical processes result in high volumes of solution containing low concentrations of Au together with several other metals present at high concentrations. For recovering Au from these multi-metal solutions, various purification methods such as those based on precipitation and/or solvent extraction (SX) procedures have been used. However, these strategies pose several drawbacks. For example, in the case of precipitation methods, the separation of Au is difficult and incomplete; as a consequence, several dissolution and precipitation steps are needed to achieve the purity specifications, which increases the operating costs. In the case of SX, the slow kinetics of Au extraction associated with the high risk of fire due to the use of solvents, which are highly flammable, and the high volumes of effluents generated during the SX purification process constitute serious disadvantages. These procedures definitively do not contribute to a cleaner, more efficient, and more sustainable recovery of Au from multi-metal solutions containing a low grade of Au. Therefore, the breakthrough in the development of more environmentally-friendly and highly selective Au-separation procedures is urgent.
Ion-exchange technology has been recognized as a powerful purification method and provides the possibility of enriching final Au eluate after elution if the resin has a high affinity for Au. This method has proved to be interesting from a technological and economical point of view. The low cost, easy handling, energy efficiency of the elution/regeneration process, and especially its suitability for application to solutions in which Au is present at trace levels are the main advantages [2,8,9,10]. Since cyanide has been the most widely used reagent for Au extraction from ores and secondary sources [11], the basic anion exchange resins, available on the market, have been developed for the purification of Au from cyanide solutions [1,12,13]. These resins have potentially high loading capacities and loading rates for Au, few are likely to be poisoned by organics, and they are easily regenerated [12]. However, due to the number of incidents involving cyanide, recycling industries are being pressed for substituting cyanide with other alternative strong Au complexing agents. Hydrochloric acid (HCl) offers the best prospects for commercialization and the chloride chemistry of Au is well understood. Under acidic oxidant conditions, the predominant complex ion of Au(III) species is AuCl4− and basic anion exchange resins, which contain amine functional groups, are positively charged. Thus, the adsorption of the Au complexes onto the resins could be explained by an ion-exchange interaction according to the following reaction:
where the symbol denotes the inert matrix (polymer structure) of the resins and n takes values between 1 and 3 according to the resin. When n < 3, R is replaced by hydrogen atoms (the number of hydrogen atoms is equal to 3 − n).
Presently, there is a large gap between the current scientific information available in the literature, mainly focused on the study of the ability of anion exchange resins for purifying Au using batch conditions [14,15] or column continuous experiments using mono and/or bi-metallic solutions [9,16,17], and the reality. The selective separation of Au from leachates, such as e-waste leachates, using anion exchange resins raises a much more complex issue as these leachates are multi-metal solutions containing Au together with other potential interfering metals, which, under acidic chloride media, form negative chlorocomplexes species that may compete for the anion exchange resins. The simultaneous adsorption of a considerable amount of foreign metal anions chlorocomplexes species together with AuCl4− species may affect the effective separation and recovery of Au under dynamic systems (packed bed adsorption columns). This is an open and challenging research topic that needs to be investigated in order to offer more environmentally friendly and effective solutions for recovering Au from solid waste containing various metals and constitutes the main novelty of this work. Based on all of these facts, in this work, the ability performance of two strong basic anion exchange resins to recover and purify Au from an Au chloride multi-metal solution was studied. In addition to the study of the kinetic and thermodynamic abilities of the resins, the selectivity of each resin for Au relative to other metals was evaluated under dynamic conditions, which is crucial information at the moment of choosing a resin for purifying Au from low-grade Au multi-metal leachates.
2. Results and Discussion
2.1. Batch Adsorption Kinetics and Isotherms Experiments
Envisaging the future application of the resins for recovering Au from solutions using column experiments, adsorption kinetics and isotherms were studied for both resins in order to obtain information about the properties of the resins for Au sorption.
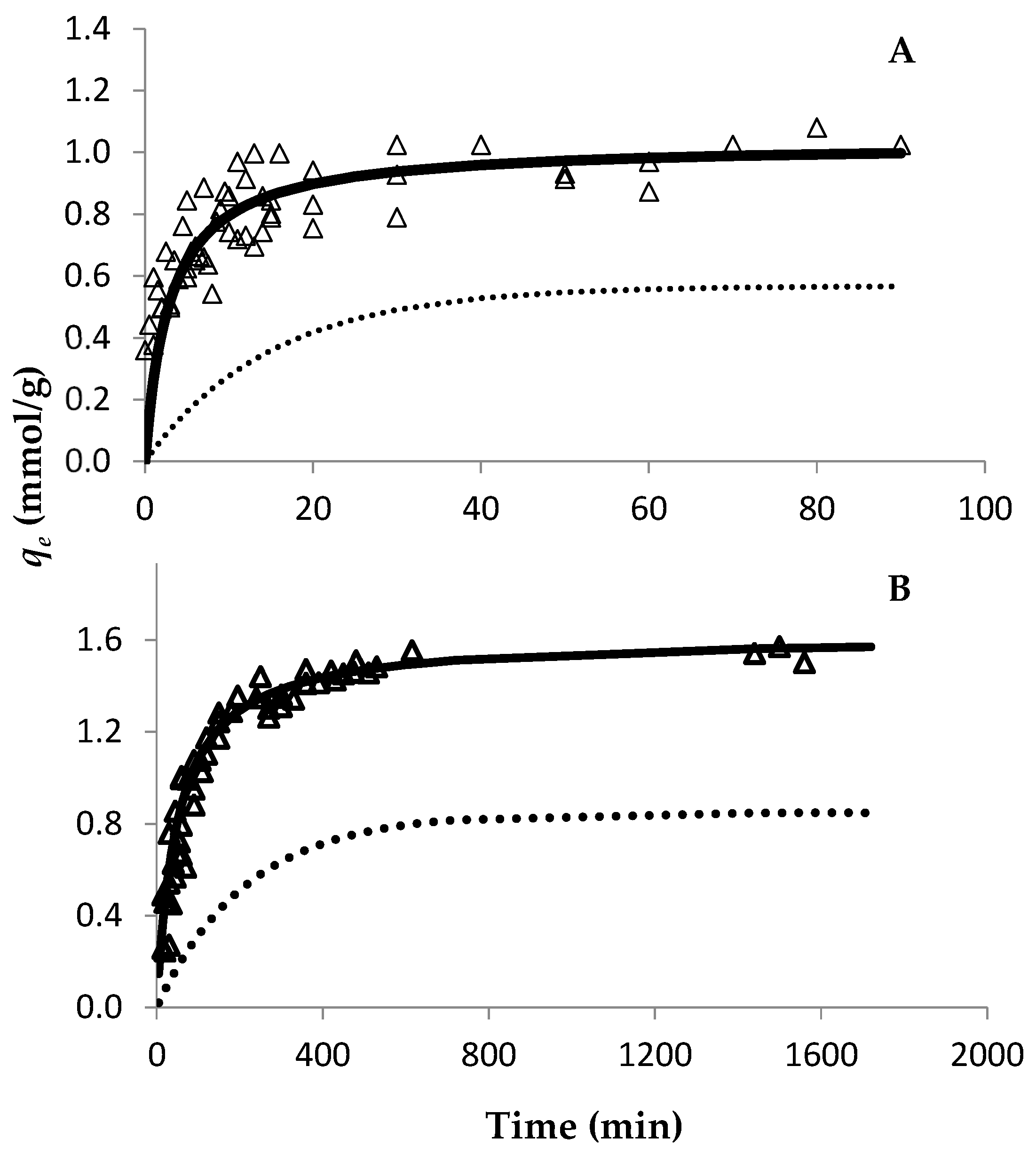
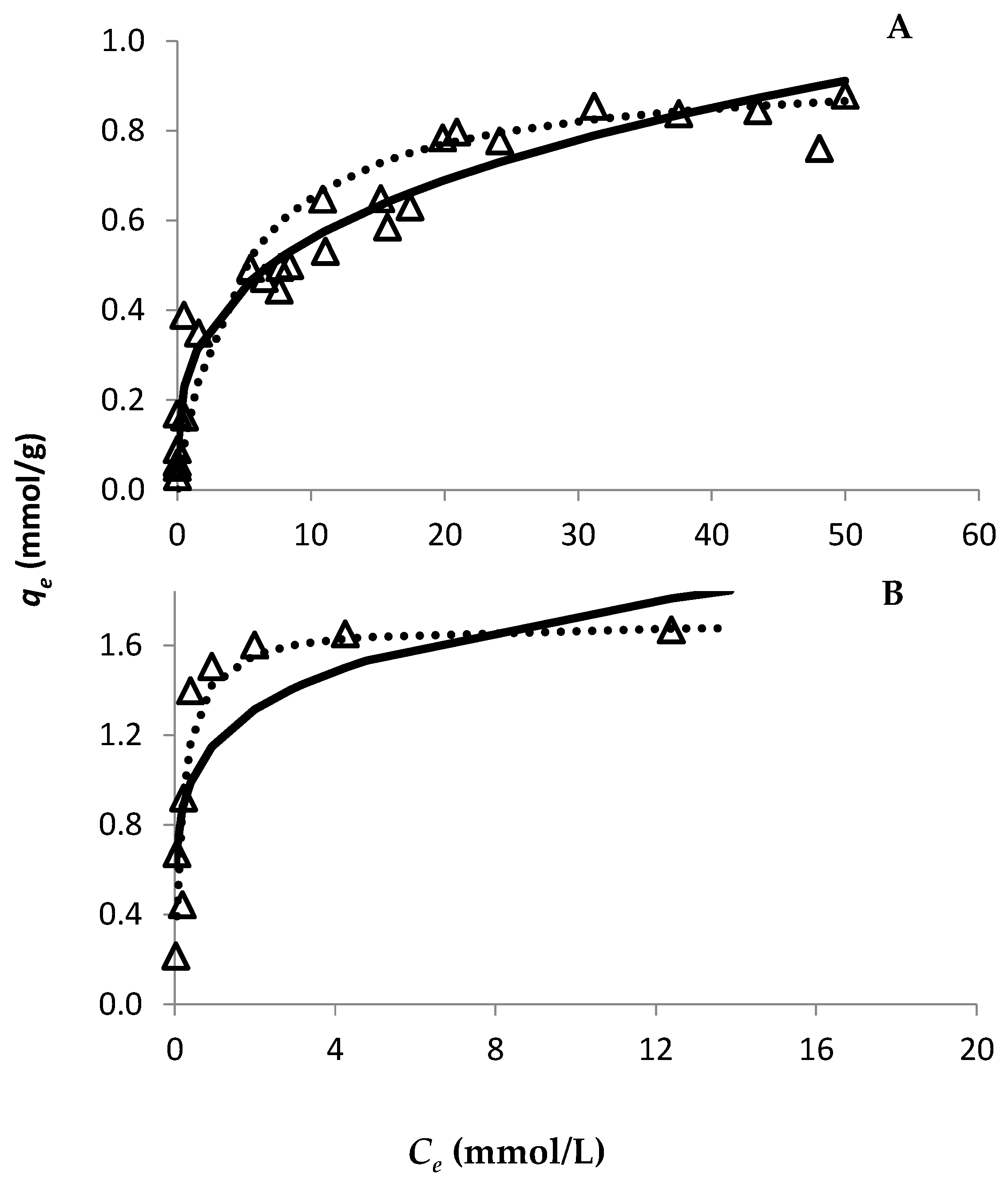
The evolution of Au retention by both resins was studied through the time using an Au concentration of 30 mmol/L (Figure 1). Since both resins are strong anionic resins, they are completely positively charged up to pH 8–9 at a particular S/L ratio. Therefore, only one S/L ratio was used to study the adsorption ability of both resins. For the PurogoldTM A194 resin, the experimental data show that approximately seven hours (420 min) were needed for the Au adsorption equilibrium to be reached (Figure 1B), while for the DOWTM XZ-91419.00 resin forty minutes were enough (Figure 1A). Pseudo-first order and type I pseudo-second order kinetic models were adjusted to the experimental points through the respective linear plots. The calculated parameters are presented in Table 1. Both models had a good correlation with the experimental data, as can be seen from the R2 values. However, even though high R2 values were achieved for the pseudo-first order kinetic model, this model did not adjust well to the experimental data, as can be seen in Figure 1. On the other hand, the type I pseudo-second order kinetic model adjusted perfectly to the experimental data (Figure 1), which shows that the kinetics of Au adsorption to the resins were better described by a pseudo-second order kinetics. For adsorption isotherms and for both resins, the variation in qe as a function of Ce is shown in Figure 2, where the Langmuir and Freundlich isotherms were adjusted to the experimental results. The parameters of each model [qmax and affinity constants (KL and KF for Langmuir and Freundlich models, respectively)] were calculated by the respective linear plots and the values are presented in Table 2. For both resins, the fitting of both models had a good correlation with the experimental data as it is evidenced by the correlation factors. However, the Langmuir model adjusted better to the experimental data (R2 values ≥ 0.993) than the Freundlich model (R2 values between 0.881 and 0.970), as evidenced in Figure 2. These results showed that the Langmuir isotherm model was more appropriate to describe the adsorption behavior of Au to both resins and suggests that the surface of the resins can be regarded as a homogeneous surface for Au adsorption. According to the Langmuir model, the maximum adsorption capacity of each resin to Au was 0.94 and 1.70 mmol/g for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively. Additionally, the values of the Langmuir constant (kL) showed a similar trend evidencing that the PurogoldTM A194 resin had the highest affinity for Au followed by the DOWTM XZ-91419.00 resin (Table 2).
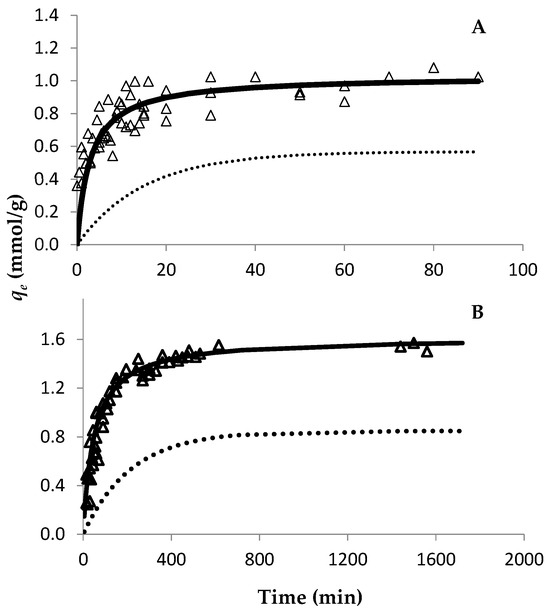
Figure 1.
Effect of Au(III) on adsorption time on the DOWTM XZ-91419.00 (A) and PurogoldTM A194 (B) resins. An Au(III) concentration of 30 mmol/L was used. Experimental conditions: 1.12 mol/L HCl at Eh = 1.1 V and 25 °C. Open triangles represent experimental data. Pseudo-first order (dotted line) and type I pseudo-second order (full line) kinetic models are represented. All points collected from two independent experiments were included in the figure.

Table 1.
Parameters for kinetic models for Au-DOWTM XZ-91419.00, Au-PurogoldTM A194, and Au-PurogoldTM S992 resins. Experimental conditions: 1.2 mol/L HCl at Eh = 1.1 V and 25 °C.
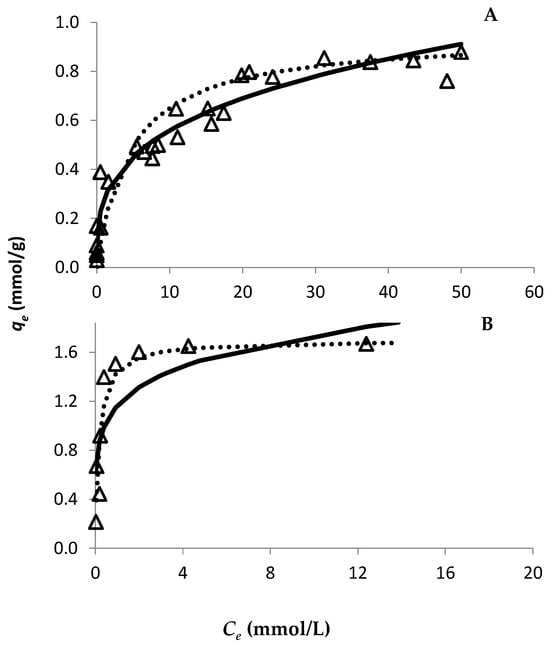
Figure 2.
Effect of Au(III) concentration on its adsorption, during 24 h on the DOWTM XZ-91419.00 (A) and PurogoldTM A194 (B) resins. Experimental conditions: 1.12 mol/L HCl at Eh = 1.1 V and 25 °C. Open triangles correspond to experimental data. The lines represent the fittings for Langmuir (dotted line) and Freundlich (full line) adsorption models. The results were obtained with points collected at least in three different assays.

Table 2.
Parameters for Langmuir and Freundlich models for Au-DOWTM XZ-91419.00 and Au-PurogoldTM A194 resins. Experimental conditions: 1.2 mol/L HCl at Eh = 1.1 V and 25 °C.
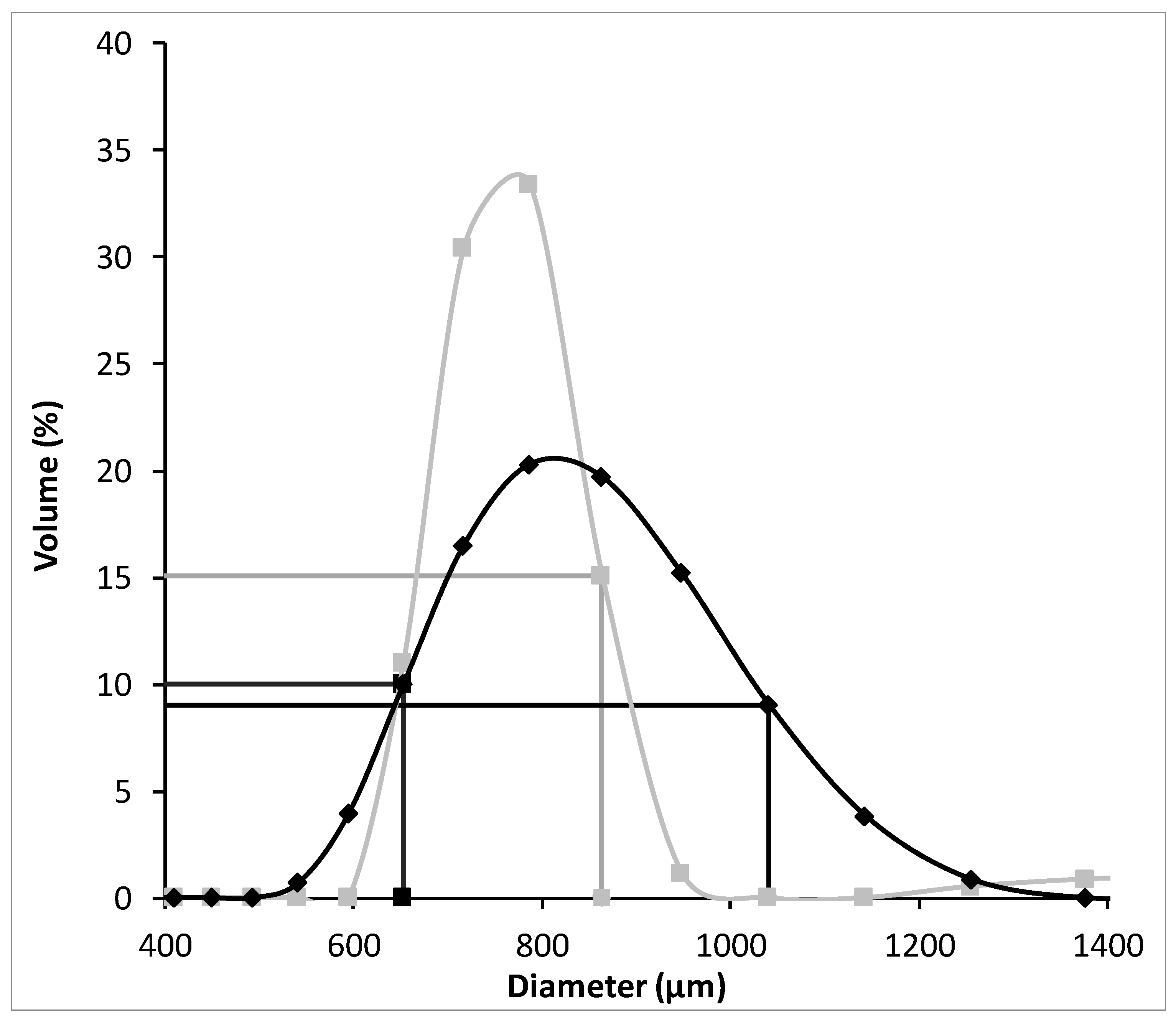
According to these results, it was possible to conclude that the DOWTM XZ-91419.00 resin has the fastest adsorption kinetics (Table 1) whereas the PurogoldTM A194 resin revealed the highest adsorption capacity and affinity for Au (Table 2). In order to understand the differences in the kinetic behavior of both resins, the porosity of both resins was evaluated. Porosity values of 58 and 20% were quantified for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively. The higher porosity observed for the DOWTM XZ-91419.00 resin may explain the faster kinetics evidenced by this resin relative to the PurogoldTM A194 resin. Additionally, the high percentage of crosslinking agent present in the case of the Purolite A194 resin relative to the DOWTM XZ-91419.00 resin, which results in an increase in the functional group density in the resin but decreases the diffusivity of AuCl4− ions through the resin [18], may also explain the lower kinetics of the Purolite A194 resin. Also, the higher bead-size uniformity observed for the DOWTM XZ-91419.00 resin relative to the PurogoldTM A194 resin (Figure 3) may contribute to the faster kinetics observed for the DOWTM XZ-91419.00 resin.
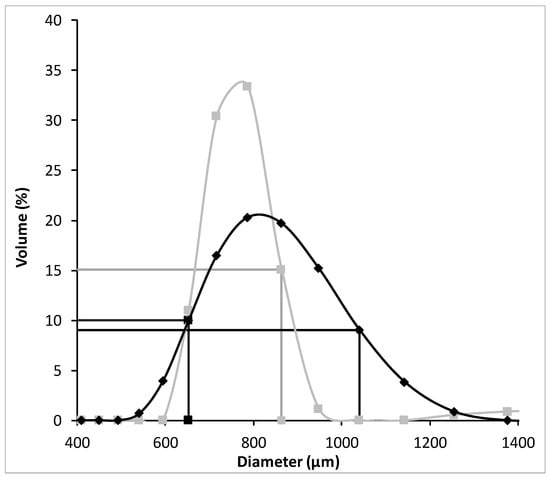
Figure 3.
Particle size distribution of the DOWTM XZ-91419.00 (■) and PurogoldTM A194 (◆) resins with emphasis on the diameter in which less than 10% and up to 90% of the particles are 653 µm and 863 µm for the DOWTM XZ-91419.00 (■) and 653 µm and 1041 µm for the PurogoldTM A194 (◆) resins.
The literature reports several studies where different polymer resins were used to recover Au from solution (Table 3). Erim et al., 2013 [4] studied the adsorption capacity of a 1,8-DAN-F blended polymer to Au; a maximum adsorption capacity of 0.60 mmol/g (119 mg/g, as described in the original paper) was achieved. Donia et al. [19] studied the ability of a magnetic adsorbent derived from chemically modified chitosan to recover Au. The maximum adsorption capacity found was 2.8 mmol/g. Pang and Yung [5] proposed the use of multiwalled carbon nanotubes and the maximum adsorption capacity was 0.47 mmol/g (93.5 mg/g, as described in the original paper). Yang et al. [20] studied the ability of cellulose acetyl derivatives to recover Au from acidic chloride solutions and cellulose acetate fibers and an adsorption capacity of 0.56 mmol/g (110 mg/g, as described in the original paper) was calculated. Fan et al. [21] prepared a resin by crosslinking the persimmon residual with formaldehyde and the maximum Au uptake was 2.6 mmol/g. A comparative analysis between the maximum adsorption capacities determined for both resins used in this work shows that they were in the range of values described in the literature for other resins studied for the same application (Table 3).

Table 3.
Values of adsorption capacity (qmax) and affinity constant (KL) for different polymer resins to Au described in the literature and those used in this work.
2.2. Column Experiments
2.2.1. Optimization of Adsorption and Elution Conditions
In electronic waste, slag dusts generated during the processing of electronic waste, sweeping jewelry, and municipal solid-waste incineration residues, Au is a minor component (usually less than 1%), when compared with the contents of the other metals [22]. Thus, all these facts together will certainly influence the selective recovery of Au from a highly complex multi-element solution. In order to predict the metal affinity series of each resin, column experiments, using a multi-metal synthetic solution (see the composition of the inlet solution in Table 4), were performed with both resins.

Table 4.
Composition of the initial multi-metal synthetic solution and the final Au solutions (eluates) after treatment with the DOWTM XZ-l91419.00 and PurogoldTM A194 resins and elution with thiourea and separation number (SN) for each metal, relative to Au.
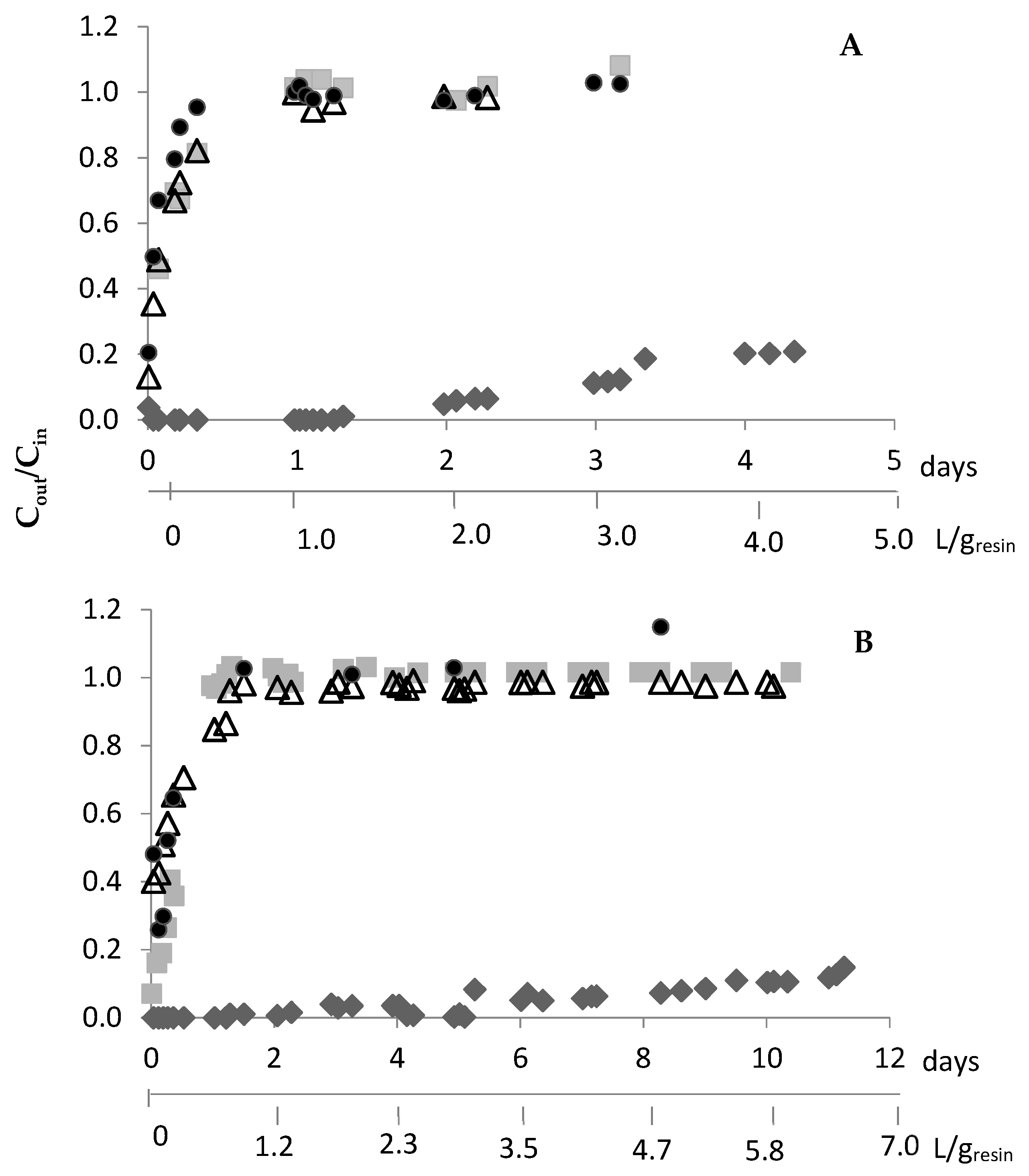
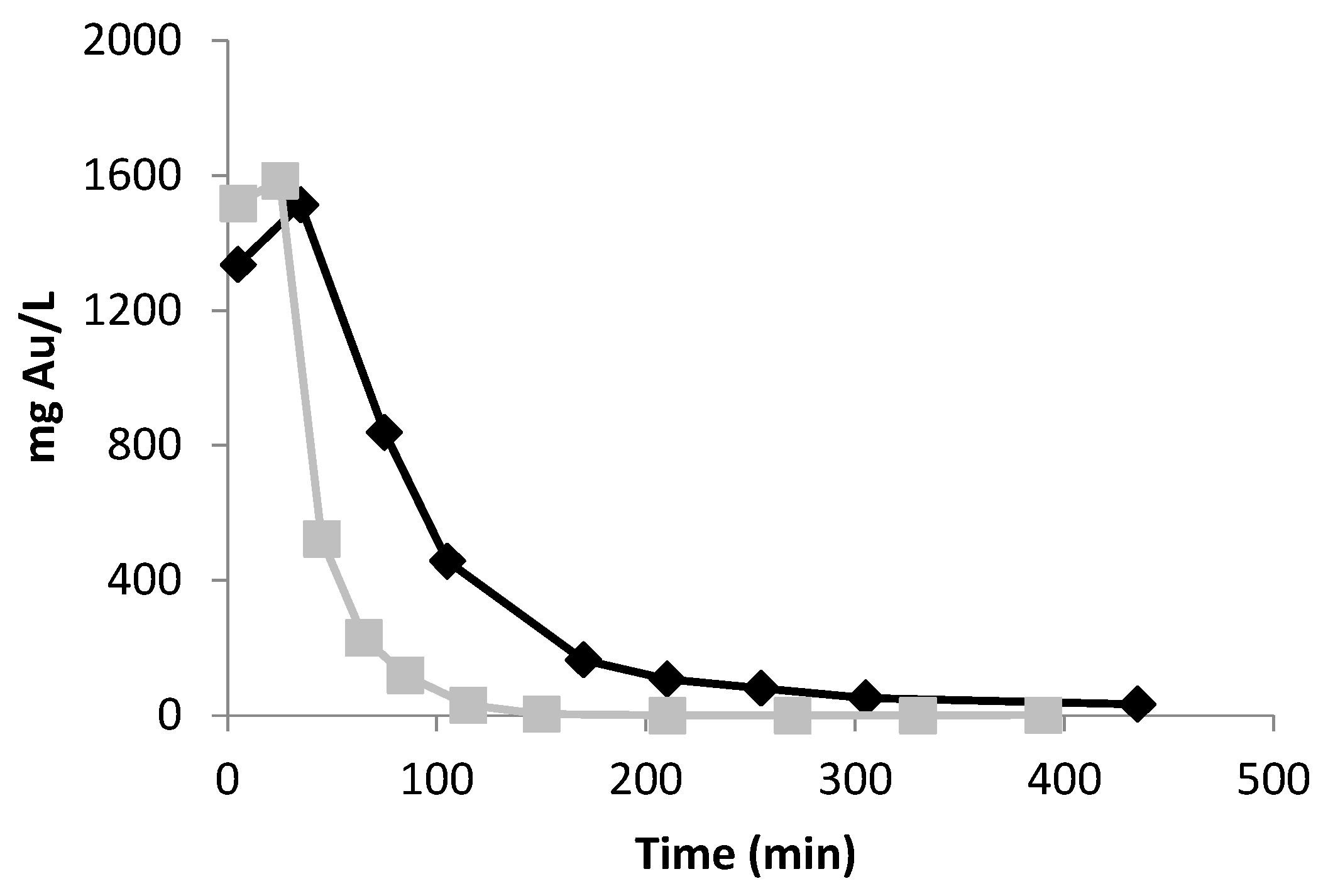
Because DOWTM XZ-91419.00 evidenced faster adsorption kinetics than the PurogoldTM A194 resin (Table 1), different adsorption kinetic profiles are expected with both resins. After preliminary experiments, continuous adsorption experiments using the following flow rates of 0.83 and 0.42 mL/(min.g) for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively, were selected (Figure 4). For both resins, the outlet concentrations of Al, Cu, Fe, and Ni were similar to the inlet concentrations since the beginning of the assays. These results pointed out that no appreciable amount of Al, Cu, Fe, and Ni was adsorbed by either resin, and they were not considered in the figure. Figure 4 evidences that both resins retained Au efficiently for a long period of time. For the DOWTM XZ-91419.00 resin, Au was retained for 3 days (72 h); after this period of time, more than 10% of Au entered the raffinate solution. After 8 h of assay, Zn was not retained by the resin and after 24 h Sn and Ag were also not retained. In the case of the PurogoldTM A194 resin, Au was retained for 10 days (223 h). After 24 h, Sn, Ag, and Zn were not retained any more. For both resins, when Au started to leave the resin, the other metals were also not retained for a long time.
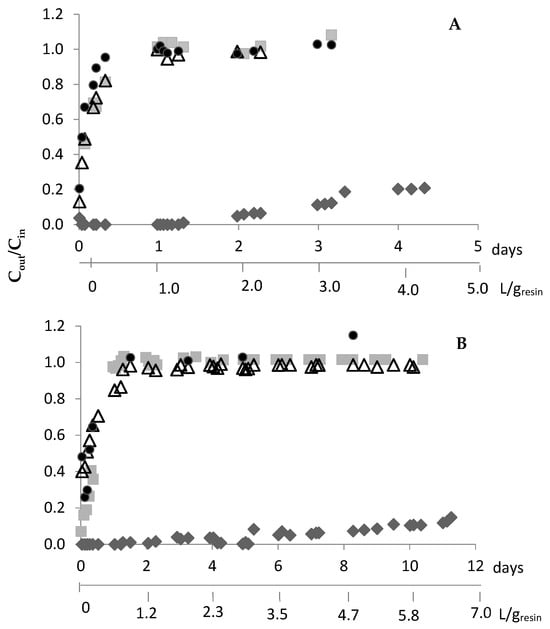
Figure 4.
Ratio between the metal concentrations in the outlet, Cout, and in the inlet solution (1.12 mol/L HCl at Eh = 1.1 V), Cin, vs. time for the DOWTM XZ-91419.00 (A) and PurogoldTM A194 (B) resins: Au (♦), Ag (■), Sn (△) and Zn (●). Flow rates of 0.83 and 0.42 mL/(min.g) were used for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively. These are examples of experiments performed two times.
The results obtained for both resins suggest that they can be interesting to selectively recover Au from the synthetic chloride multi-metal solution, and 72 and 223 h were the times defined for stopping the adsorption of Au for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively.
The adsorption step with the PurogoldTM A194 resin was slower than with the DOWTM XZ-91419.00 resin. Moreover, the PurogoldTM A194 resin allowed the treatment of 5.8 L of synthetic solution per gram of resin (Figure 4B), while 3.0 L/g was treated with the DOWTM XZ-91419.00 resin (Figure 4A). These results correlate well with the maximum adsorption capacities of both resins: the ratio of the volume of synthetic solutions treated by both resins (5.8/3.0 = 1.9) (Figure 4) is equal to the ratio between the qmax values (1.70/0.94 = 1.8) achieved by isotherm studies (Table 2).
The elution of Au adsorbed from both resins (Figure 5) was promoted using thiourea (TU). During this desorption stage, Au(III) is reduced to Au(I) and then complexed with TU [23], which is released to the bulk solution according to Equation (2):
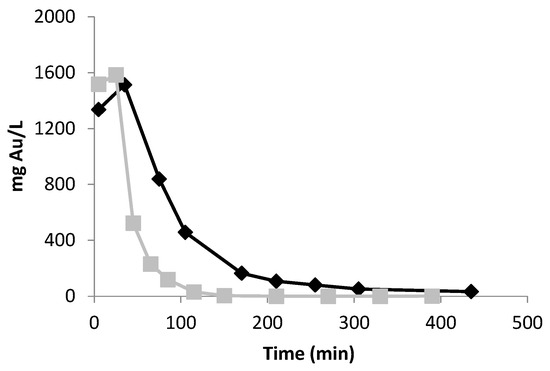
Figure 5.
Gold elution profiles for the DOWTM XZ-91419.00 (■) and PurogoldTM A194 (◆) resins with 0.5 mol/L H2SO4 and 0.1 mol/L thiourea. Flow rates of 0.83 and 0.42 mL/(min.g) were used for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively.
For this purpose, a 0.1 mol/L thiourea and 0.5 mol/L H2SO4 solution was tested using the same flow rates applied in the adsorption step. For the DOWTM XZ-91419.00 resin, the elution process was shown to be fast; after 65 and 85 min, 85 and 95% of the Au adsorbed was eluted, respectively. After 120 min, almost all of the Au was removed from the resin. On the other hand, for the PurogoldTM A194 resin, the elution was slower. After 170 min, 88% of the adsorbed Au was eluted, and after 210 min, the Au elution achieved 92%. For total Au elution, more than 420 min was needed. In order to reduce the time of elution, a higher concentration of thiourea (0.25 mol/L) was tested but no significant improvement was observed.
Based on these results, elution times of 65 and 200 min were defined for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively, since final solutions with higher concentrations of Au are achieved. To close the cycle, the direct recovery of Au from thiourea, as Au metallic, can be achieved by reduction using sodium borohydride followed by filtration (not tested in this work) and the remaining filtrate containing thiourea can be reused in another elution cycle [24]. By this way, thiourea can be reused in another elution cycle contributing to reduce the operating cost of the process.
2.2.2. Concentration and Selectivity Ability of Resins for Gold
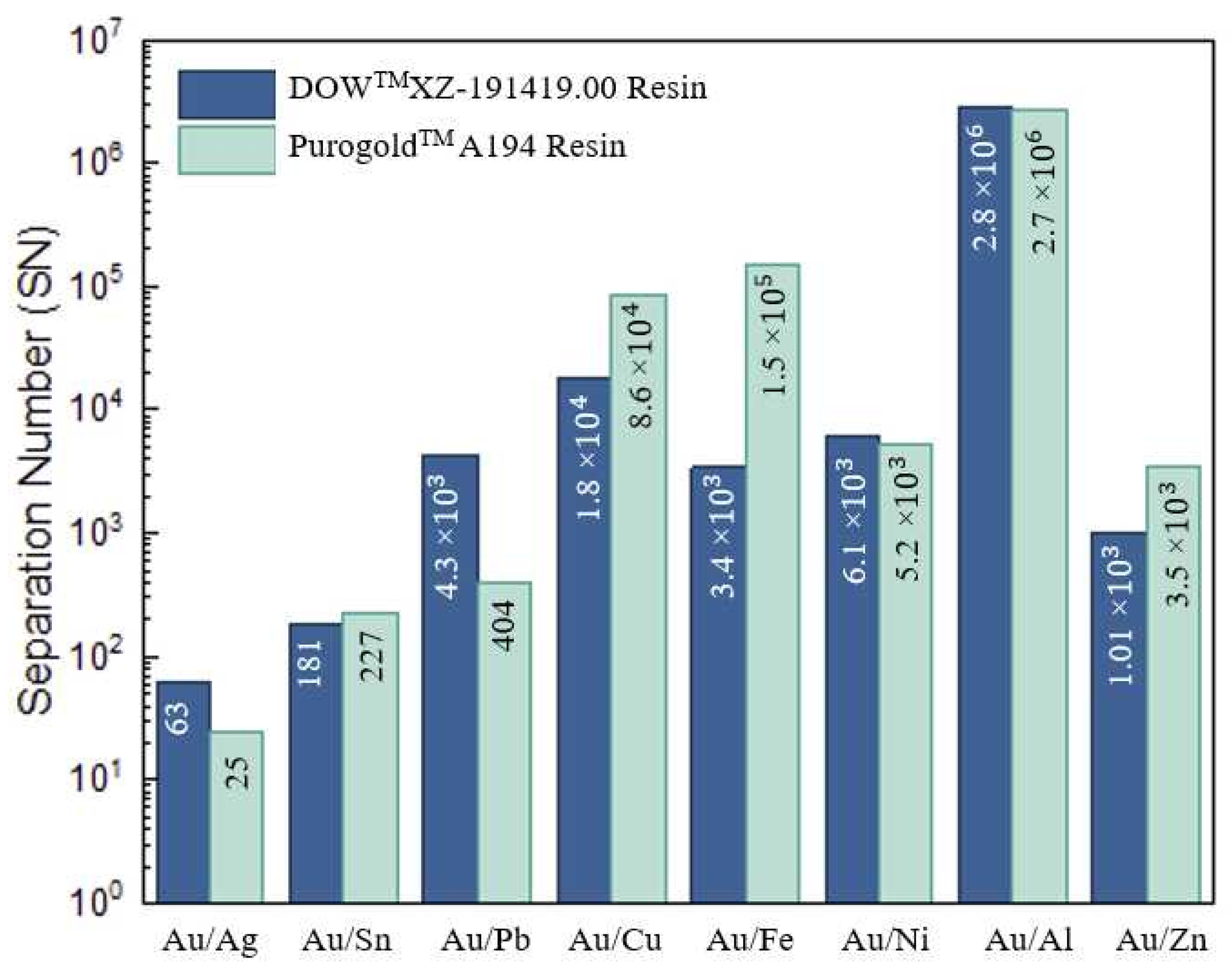
For both resins, additional experiments to evaluate the simultaneous concentration and selectivity (expressed by the separation number, SN) ability of both resins for recovering Au from a chloride multi-metal synthetic solution (Figure 6) were performed using the experimental (adsorption and elution) conditions previously optimized and described in Section 2.2.1. The metal composition of the initial chloride multi-metal synthetic solution (inlet solution), measured by AAS-FA and expressed as a percentage, as well as the chemical metal species predicted by computer chemical simulations are presented in Table 4. Additionally, the metal composition of the eluate solutions, quantified by AAS-FA and expressed as a percentage, is presented in Table 4. Moreover, the SN values for both resins are presented in Figure 6.
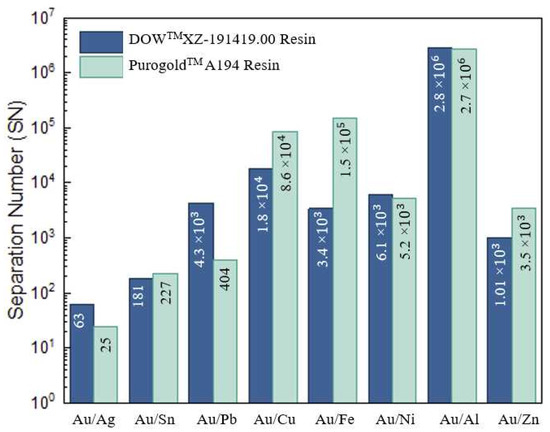
Figure 6.
Separation number for the DOWTM XZ-91419.00 and PurogoldTM A194 resins with 0.5 mol/L H2SO4 and 0.1 mol/L thiourea.
The results presented in Table 4 show the high affinity of both resins for Au and are in agreement with the previous adsorption parameters. Final solutions containing Au with high purity (94.6 and 94.1% for DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively) and concentration (3.5 and 3.3 mmol/L, respectively; the amount of Au in the final solutions increased almost 70 times when compared with the concentration in the initial solution, 48 µmol/L) were achieved (Table 4).
For the Au purified solution using the DOWTM XZ-91419.00 resin, Sn was the major (3.9%) contaminant followed by Ag (0.84%) and Fe (0.40%)—whereas for the Au eluate resulting from the PurogoldTM A194 resin, Sn was the major contaminant (3.1%), followed by Ag (2.3%) and Pb (0.44%).
Even though Cu (43.0%), Fe (24.4%), and Al (6.6%) were the major metals in the initial chloride multi-metal synthetic solution, together with Ni also present in a significant amount (3.5%), both resins evidenced a high selectivity, SN > 103, to Au over these metals (Figure 6). This behavior can be explained by the fact that no anionic chloride species of these metals are formed under the chemical conditions used (Table 4), and thus, no adsorption occurs. As a consequence, less than 0.5% of these metals was present in all final Au eluates (see the metal composition of the eluates in Table 4). On the other hand, both resins evidenced poor selectivity for Ag, SN ≤ 63, (Figure 6). As a consequence, even though Ag was a minor (1.2%) constituent in the initial chloride multi-metal solution, no significant purification occurred for the DOWTM XZ-91419.00 XZ resin (the amount of Ag was 0.84% in the eluate vs. 1.2% in the initial solution) and a concentration effect occurred when the PurogoldTM A194 resin (the amount of Ag was 2.3% in the eluate vs. 1.2% in the initial solution) was used. Moreover, for Sn (for both resins) and Pb (for PurogoldTM A194 resin), SN values lower than 404 were recorded, which denotes the lower selectivity of these resins for these metals relative to Au. This lower selectivity (Figure 6) can be explained by the adsorption of several anionic chloride complexes of these metals (Table 4), which are formed in solution, by a similar mechanism as described for Au but with less attraction to the exchange sites on the resin due to the high charge density that enhances Au’s attraction to the exchange sites on the resin, leading to a stronger interaction and preferential uptake.
Considering that chlorination is a practicable alternative for Au leaching due to the high dissolution rate [25] and the good selectivity for Au relative to other metals demonstrated by both resins in the present work, it seems plausible to anticipate that the combination of chlorination leaching and purification of Au using these two strong anionic resins presents potentialities for recovering Au with high purity from various sources, such as electronic waste, slag dusts generated during the processing of electronic waste, sweeping jewelry, and municipal solid-waste incineration residues.
3. Materials and Methods
3.1. Reagents and Analytical Techniques
Two strong basic anion exchange resins were used: DOWTM XZ-91419.00 and PurogoldTM A194. Table 5 lists some of the main physical and chemical properties of these resins. The DOWTM XZ-91419.00 and PurogoldTM A194 resins were kindly given by the DOW (Midland, MI, USA) and Purolite (Philadelphia, PA, USA) companies.

Table 5.
Physical and chemical properties of the resins studied in this work.
All chemicals used in the experiments were of analytical reagent grade. Synthetic mono-metal (containing only Au for kinetics and isotherms adsorption studies) and multi-metal solutions containing Ag, Al, Au, Cu, Fe, Ni, Pb, Sn, and Zn were prepared by solubilizing a suitable amount of the salt of each metal [HAuCl4.3H2O, CuSO4.5H2O, Ni(NO3)2·6H2O, Fe(NO3)3.9H2O, Pb(NO3)2, Al2(SO4)3·16H2O, ZnCl2, Ag2SO4, and SnCl2.2H2O] in a 1.12 mol/L HCl solution. Aqueous solutions prepared from analytical grade thiourea, SC(NH2)2, and sulfuric acid (H2SO4) (95%) were used in the elution studies. Sodium hypochlorite (NaClO) was used to raise the redox potential (Eh) of the solutions up to 1.1 V in order to stabilize Au as [AuCl4]−.
The metal (Ag, Al, Au, Cu, Fe, Ni, Pb, Sn, and Zn) concentration was measured by atomic absorption spectrometry with flame atomization (AAS-FA) in a Perkin Elmer AAnalyst 400 spectrometer (Perkin Elmer, Norwalk, CT, USA) or an Analytik JenaA NovAA350 spectrometer (Konrad-Zuse, Germany). A nitrous oxide flame was used for the determination of Al and Sn and an acetylene flame for the remaining metals.
For all solutions, the redox potential was measured with a Pt indicator electrode using a Metrohm 744 millivoltmeter (Metrohm AG, Herisau, Switzerland).
3.2. Batch Experiments
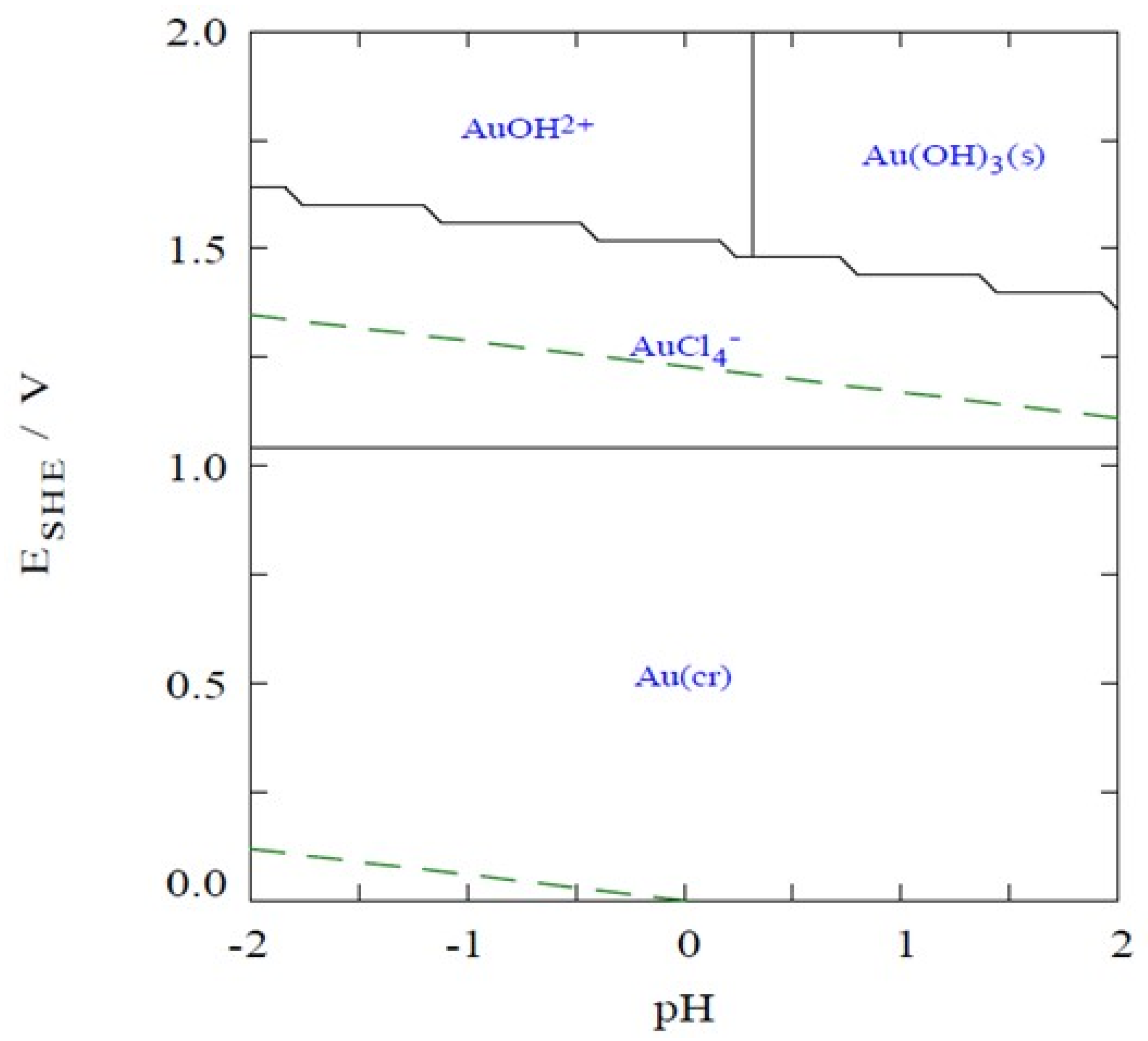
Kinetic and isotherm adsorption batch experiments were performed in solutions containing Au(III), prepared from the HAuCl4·3H2O salt, in 1.12 mol/L HCl, at an Eh of 1.1 V; under this Eh and pH conditions, all Au is present as [AuCl4]− (Figure 7). Because the DOWTM XZ-91419.00 and PurogoldTM A194 resins are strong basic anion exchange resins, they are completely positively charged up to at least pH 8–9 and present a maximum ion-exchange capacity up to this pH [26]. Therefore, only one solid/liquid (S/L) ratio of 10 g/L was used to study the adsorption ability of both resins. For this purpose, 0.5 g of wet resin was accurately weighed into a flask and 50 mL of each solution containing an adequate amount of Au(III), prepared from the HAuCl4·3H2O salt, in 1.12 mol/L HCl, at an Eh of 1.1 V was used. The flasks were shaken in a bath with temperature control (OLS200, Grant, Royston, United Kingdom) at 25 °C and 150 rotations per minute (rpm).
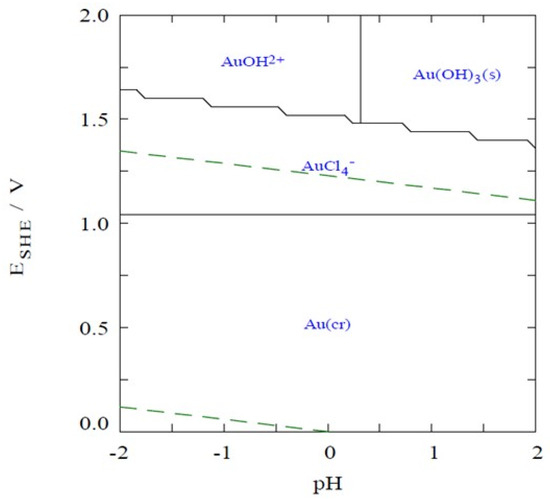
Figure 7.
Pourbaix-type diagram for the Au3+/Cl−/e− system, as function of pH. Temperature of 25 °C; [Au3+] = 48 µmol/L; [Cl−]= 1.12 mol/L.
3.2.1. Adsorption Kinetics
For kinetic studies, a synthetic 30 mmol/L Au(III) solution was used. At different intervals of time, an amount of 0.4 mL of the suspension (solution plus resin) was taken from the flask under agitation. Then, the resin was separated from the suspension by filtration and not returned back to the flask. The respective concentration of Au was measured in the filtrate by AAS-FA.
Langergren pseudo-first order and type I pseudo-second order models were fitted into the experimental points using linear regression (Equations (3) and (4), respectively):
Langergren pseudo-first order:
Type 1 pseudo-second order:
3.2.2. Adsorption Isotherms
For isotherm studies, flasks containing solutions with increasing concentrations of Au(III) were shaken during 24 h. After this period of time, each solution was separated from the resin by filtration. The concentration of Au was analyzed by AAS-FA. The quantity of metal adsorbed per mass of resin (qe) was calculated according to Equation (5), where C0 and Ce are the initial and equilibrium metal concentrations (mmol/L), respectively, m is the mass of resin (g), V is the volume of solution (L), and qe is the equilibrium adsorption capacity (mmol/g).
Langmuir and Freundlich isotherm models were tested. The Langmuir isotherm assumes adsorption on a homogeneous surface (monolayer) with a negligible interaction between adsorbed molecules and allows the estimation of the maximum metal uptake (qmax) and the Langmuir constant (kL) according to Equation (6).
The Freundlich isotherm assumes adsorption on heterogeneous surfaces. The Freundlich constant (kF) and n are Freundlich parameters that can be estimated from experimental data according to Equation (7). Values of n greater than 1 indicate favorable conditions for adsorption.
3.3. Column Experimental Conditions
For experiments performed in continuous mode, a chloride multi-metal synthetic solution was prepared in 1.12 mol/L HCl, at an Eh of 1.1 V, with the following metal compositions: 4.8 × 10−5, 5.4 × 10−5, 1.2 × 10−3, 3.2 × 10−3, 2.1 × 10−3, 2.9 × 10−4, 8.7 × 10−5, 5.9 × 10−4, and 1.1 × 10−4 mol/L of Au, Ag, Al, Cu, Fe, Ni, Pb, Sn, and Zn, respectively.
For the DOWTM XZ-91419.00 and PurogoldTM A194 resins, a precise amount of resin, equivalent to 0.5 bed volumes (1.5 mL), was placed in the chromatographic glass column (di = 6.6 mm, h = 100 mm) and wetted with water in accordance with the manufacturer’s specifications [27]. The air pockets were removed from the bed column by attaching the water line at the bottom of the column and simultaneously the resin was washed. For both resins, flow rates of 0.83 and 0.42 mL/(min.g), respectively, were used. The flow rates were controlled using a peristaltic pump MS-Reglo (Ismatec, Glattbrugg, Switzerland). Between adsorption and elution steps, the resins were washed with 30 mL of deionized water. After elution, each resin was washed with 60 mL of deionized water and reused for the next adsorption and elution cycle. All experiments were performed at room temperature and, for each experiment, at least two independent experiments were conducted.
Samples were collected from the outlet solution at different intervals of time to measure the metal concentration by AAS-FA. Elution of the retained metals was performed using two concentrations (0.1 and 0.25 mol/L) of thiourea and a fixed concentration (0.5 mol/L) of H2SO4.
For all solutions (inlet, raffinate, and eluates from both resins), the concentration of all metals (Ag, Al, Au, Cu, Fe, Ni, Pb, Sn, and Zn) was measured by AAS-FA.
For gold purification (concentration and selectivity ability of resins for gold) assays, the adsorption and elution steps had a duration of 72 h and 65 min, respectively, for the DOWTM XZ-91419.00 resin, and a duration of 223 h and 200 min, respectively, when the PurogoldTM A194 resin was used. For both resins, the elution of Au was performed with 0.1 mol/L thiourea and 0.5 mol/L H2SO4.
For each resin, the separation number (SN) for each metal, relative to Au, was calculated according to Equation (8).
3.4. Computer Chemical Simulations
The Eh–pH diagrams of dissolved Au-chloride species at 25 °C were drawn using the SPANA software (Visual Basic, Stockholm, Sweden, version 5.5.43), whereas metal chemical speciation simulations were carried out using MINEQL+ software (version 4.5) [28]. Software generates chemical equilibrium concentrations of the species considered in the model by the program reactions, based on component stability constants [29] and the total molar metal concentrations measured by AAS.
3.5. Porosity Determination
Total porosity (Φ) is defined as the fraction of the total volume occupied by pores and can be expressed as follows:
where Φ is the total porosity (%), is the density of the material without pores being considered, and is the density including the pores [30].
The determination of was carried out using a custom-developed gas picnometry method with helium as the working gas. The pycnometer consists of two containers: one designed to hold the sample and another with a known internal volume, serving as a reference. Both containers were sealed and helium was introduced into the system at a pressure of 1 bar. The initial helium volume in the reference container was recorded. Then, the valve of the container holding the sample was opened, allowing the helium to flow into it. The remaining helium volume in the reference container was then measured, and the volume of the sample container was determined by the difference. Finally, the sample was weighed, and its density was calculated by dividing the measured mass by the obtained volume [31]. The was determined using the PoreMaster Mercury-intrusion apparatus [32].
4. Conclusions
In this work, a comparative study of the ability of two strong basic anion exchange resins to adsorb and purify Au from a chloride multi-metal solution was performed.
Firstly, Au(III) adsorption kinetic and equilibrium parameters were determined at a temperature of 25 °C in 1.12 mol/L HCl at Eh 1.1 V and 25 °C using batch experiments. The Au adsorption proceeded according to a pseudo-second order model for both resins. The kinetics of Au adsorption was faster for the DOWTM XZ-91419.00 resin [K2 = 0.330 g/(mmol.min)] than for the PurogoldTM A194 resin [K2 = 0.012 g/(mmol.min)]. The experimental data fitted well to a Langmuir isotherm model, and maximum adsorption capacities of 0.94 and 1.70 were determined for the DOWTM XZ-91419.00 and PurogoldTM A194 resins, respectively.
In a second attempt, the efficiency and selective ability of the resins to retain Au(III) was evaluated by performing continuous column experiments with a synthetic chloride multi-metal solution (1.2 mol/L HCl and Eh = 1.1 V). Final Au solutions with a purity grade of at least 94% were achieved due to the high selectivity revealed by both resins to Au over Al, Cu, Fe (except for the DOWTM XZ-91419.00 resin), Pb (except for the PurogoldTM A194 resin), Ni, and Zn. However, even though eluates with similar amounts of Au (about 3.5 mmol/L) were produced for both resins, there were some differences in their composition. For the DOWTM XZ-91419.00 resin, Sn was the major contaminant, followed by Ag and Fe whereas, for the PurogoldTM A194 resin, Ag and Sn were the major contaminants.
The results obtained in this work evidence that both resins are interesting options for purifying Au from chloride multi-metal solutions containing a low grade of Au, particularly in the following conditions: (i) DOWTM XZ-91419.00 resin is suitable to purify Au from solutions containing Al, Ag, Cu, Fe, Ni, Pb, and Zn; (ii) PurogoldTM A194 resin is suitable to purify Au from solutions containing Al, Cu, Fe, Ni, Pb, and Zn.
Author Contributions
Conceptualization: H.M.V.M.S.; methodology: I.F.F.N. and M.A.D.S.; validation: I.F.F.N. and M.A.D.S.; formal analysis, I.F.F.N. and M.A.D.S.; resources: H.M.V.M.S.; original draft preparation: I.F.F.N. and H.M.V.M.S.; writing—review and editing: H.M.V.M.S.; supervision: H.M.V.M.S.; project administration: H.M.V.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by national funds through the FCT/MCTES (PIDDAC), under the project PTDC/CTA-AMB/3489/2021—RECY-SMARTE—Sustainable approaches for recycling discarded mobile phones, with DOI 10.54499/PTDC/CTA-AMB/3489/2021 (https://doi.org/10.54499/PTDC/CTA-AMB/3489/2021).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ok, Y.S.; Jeon, C. Selective adsorption of the gold–cyanide complex from waste rinse water using Dowex 21K XLT resin. J. Ind. Eng. Chem. 2014, 20, 1308–1312. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Yuan, W.; Yi, Y.; Zhao, L. Recovery of Au(III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem. Eng. J. 2016, 283, 504–513. [Google Scholar] [CrossRef]
- Ahamed, M.E.H.; Mbianda, X.Y.; Mulaba-Bafubiandi, A.F.; Marjanovic, L. Selective extraction of gold(III) from metal chloride mixtures using ethylenediamine N-(2-(1-imidazolyl)ethyl) chitosan ion-imprinted polymer. Hydrometallurgy 2013, 140, 1–13. [Google Scholar] [CrossRef]
- Erim, Ü.C.; Gülfen, M.; Aydın, A.O. Separation of gold(III) ions by 1,8-diaminonaphthalene-formaldehyde chelating polymer. Hydrometallurgy 2013, 134–135, 87–95. [Google Scholar] [CrossRef]
- Pang, S.-K.; Yung, K.-C. Prerequisites for achieving gold adsorption by multiwalled carbon nanotubes in gold recovery. Chem. Eng. Sci. 2014, 107, 58–65. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Gao, X.; Alam, S.; Inoue, K. Sustainability in the Metallurgical Industry: Chemically Modified Cellulose for Selective Biosorption of Gold from Mixtures of Base Metals in Chloride Media. Ind. Eng. Chem. Res. 2014, 53, 8565–8576. [Google Scholar] [CrossRef]
- Xiong, C.; Zhou, S.; Liu, X.; Jia, Q.; Ma, C.; Zheng, X. 2-Aminothiazole Functionalized Polystyrene for Selective Removal of Au(III) in Aqueous Solutions. Ind. Eng. Chem. Res. 2014, 53, 2441–2448. [Google Scholar] [CrossRef]
- Pilśniak-Rabiega, M.; Trochimczuk, A.W. Selective recovery of gold on functionalized resins. Hydrometallurgy 2014, 146, 111–118. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Adsorption characteristics of noble metals on the strongly basic anion exchanger Purolite A-400TL. J. Mater. Sci. 2014, 49, 6191–6202. [Google Scholar] [CrossRef][Green Version]
- Sousa, R.; Regufe, M.J.; Fiúza, A.; Leite, M.M.; Futuro, A. A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extr. Ind. Soc. 2022, 9, 101018. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Chmielarz, A. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Green, B.R.; Kotze, M.H.; Wyethe, J.P. Developments in ion exchange: The mintek perspective. JOM 2002, 54, 37–43. [Google Scholar] [CrossRef]
- Yahorava, V.; Kotze, M.H. Novel application of a gold cyanide selective resin in the PGMs industry. In Hydrometallurgy; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2014; Volume 2, pp. 269–279. [Google Scholar]
- Dicinoski, G.W.; Gahan, L.R.; Lawson, P.J.; Rideout, J.A. Application of the shrinking core model to the kinetics of extraction of gold(I), silver(I) and nickel(II) cyanide complexes by novel anion exchange resins. Hydrometallurgy 2000, 56, 323–336. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Mahandra, H.; Ramsay, J.; Ghahreman, A. Selective gold recovery from pregnant thiocyanate leach solution using ion exchange resins. Hydrometallurgy 2023, 218, 106055. [Google Scholar] [CrossRef]
- Zhang, H.; Dreisinger, D.B. The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy 2014, 72, 225–234. [Google Scholar] [CrossRef]
- Negrea, A.; Mihailescu, M.; Mosoarca, G.; Ciopec, M.; Duteanu, N.; Negrea, P.; Minzatu, V.E. Estimation on fixed-bed column parameters of breakthrough behaviors for gold recovery by adsorption onto modified/functionalized amberlite xad7. Int. J. Environ. Res. Public Health 2020, 17, 6868. [Google Scholar] [CrossRef]
- Mohebbi, A.; Abolghasemi Mahani, A.; Izadi, A. Ion exchange resin technology in recovery of precious and noble metals. In Applications of Ion Exchange Materials in Chemical and Food Industries; Inamuddin, R.T.A., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 193–258. [Google Scholar]
- Donia, A.M.; Yousif, A.M.; Atia, A.A.; Elsamalehy, M.F. Efficient adsorption of Ag(I) and Au(III) on modified magnetic chitosan with amine functionalities. Desalination Water Treat 2014, 52, 2537–2547. [Google Scholar] [CrossRef]
- Yang, J.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Application of cellulose acetate to the selective adsorption and recovery of Au(III). Carbohydr. Polym. 2014, 111, 768–774. [Google Scholar] [CrossRef]
- Fan, R.; Xie, F.; Guan, X.; Zhang, Q.; Luo, Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from e-wastes. Bioresour. Technol. 2014, 163, 167–171. [Google Scholar] [CrossRef]
- Neto, I.F.; Sousa, C.A.; Brito, M.S.; Futuro, A.M.; Soares, H.M. A simple and nearly-closed cycle process for recycling copper with high purity from end life printed circuit boards. Sep. Purif. Technol. 2016, 164, 19–27. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Adeva, P.; Alonso, M. Processing of residual gold (III) solutions via ion exchange. Gold Bull. 2005, 38, 9–13. [Google Scholar] [CrossRef]
- Farouk, T.; Awadalla, H.; Ronald, E.; Molnar, O.; Gordon, M.R. Recovery of Platinum Group Metals (PGM) from Acidic Solutions by Reudction Precpitation with Sodium Borohydride. U.S. Patent 5304233, 19 April 1994. [Google Scholar]
- Aylmore, M.G. Alternative Lixiviants to Cyanide for Leaching Gold Ores. In Gold Ore Processing: Project Development and Operations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 447–484. [Google Scholar]
- Hubicki, Z.; Kolodynska, D. Selective Removal of Heavy Metal Ions from Waters and Waste Waters Using Ion Exchange Methods. In Ion Exchange Technology; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- van Deventer, J.; Bazhko, V.; Yahorava, V. Comparison of gold selective ion exchange resins and activated carbon for the recovery of gold from copper-gold leach liquors. In Proceedings of the Alta 2014 Gold Conference, Perth, Australia, 24–31 May 2014. [Google Scholar]
- Schecher, W.D.; Mcavoy, D.C. MINEQL+: A Chemical Equilibrium Modeling System, Version 4.5 for Windows, User’s Manual, 2nd ed.; Environmental Research Software: Hallowell, ME, USA, 2003. [Google Scholar]
- Martell, A.E.; Smith, R.M. NIST SRD 46; NIST Standard Reference Database 46 Version 8.0. NIST Critically Selected Stability Constants of Metal Complexes Database. US Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2004.
- Rouquerol, J.; Rouquerol, F.; Sing, K. Assessment of Mesoporosity. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 1st ed.; Elsevier: San Diego, CA, USA, 1998; pp. 191–236. [Google Scholar]
- Columbu, S.; Mulas, M.; Mundula, F.; Cioni, R. Strategies for helium pycnometry density measurements of welded ignimbritic rocks. Measurement 2021, 173, 108640. [Google Scholar] [CrossRef]
- Mukaida, K.-I. Density Measurement of Small Porous Particles by Mercury Porosimetry. Powder Technol. 1981, 29, 99–107. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).