Abstract
With the growing demand for critical metals, extraction from secondary sources such as slag, tailings, and end-of-life materials has become inevitable. Processing from such secondary sources is described as technospheric mining. Technospheric mining is a broad term for extracting valuable resources from anthropogenic materials that are currently excluded from the material flow. The study utilises technospheric mining to extract cobalt and nickel from nickel furnace slag using organic acids such as ascorbic and citric acid. The experiments were designed using one variable at a time (OVAT) to optimise the different parameters: temperature, time, particle size, and reagent concentration. A maximum recovery of 79% Co and 80% Ni were obtained by leaching the nickel furnace slag using 0.5 mol/L citric acid and 0.5 mol/L ascorbic acid for 6 h at 80 °C. It is proposed that citric acid leaches the surface and ascorbic acid acts as a reducing agent, thereby reducing the slag matrix and leaching the metals trapped in it. The results show that treating nickel furnace slag via a mixture of organic acids is promising, as it is environmentally friendly. Retreating this material would reduce the net waste generated and aid in building a circular economy.
1. Introduction
Clean technologies, such as the electrification of vehicles, are being developed to create a low-carbon society, driving the demand for critical and strategic materials [1]. These vehicles employ certain critical and strategic materials like cobalt (Co) and nickel (Ni) as cathodes in their batteries, further increasing demand for these elements. Besides the battery industry, cobalt is used to make catalysts, dyes, pigments, and magnets and is employed in metallurgical applications such as superalloys for gas turbine engines and cemented carbides [2,3]. Nickel is a major alloying element for stainless steel, shape memory alloys, nickel-plating, and coins, and it is used as a catalyst in the hydrogenation of vegetable oils. With the increasing applications of these elements in diverse fields, there is an expected increase in demand in the upcoming years.
In nature, cobalt exists in the form of sulphide (Linnaeite, Co+2Co+32S4 and Carrollite, CuCo2S4), arsenide (Skutterudite, CoAs3), or a combination of both (Cobaltite, CoAsS) due to its chalcophile and siderophile properties [4]. Nickel usually occurs as sulphides, arsenides, silicates, and oxides, of which nearly 60% are found as laterites [5]. Majorly exploited nickel-bearing minerals include pentlandite (Fe, Ni)9S8, millerite NiS, gersdorffite NiAsS, and nickeliferous limonite FeO(OH)·nH2O [6].
The primary cobalt supply in mining and processing is geographically concentrated in the Democratic Republic of Congo (DRC) [2]. Mines in the DRC account for more than 70% of the global cobalt production, extracting orders of magnitude more than China [7]. Despite DRC being a major supplier of cobalt to the international market, its state is characterised by high governance risks. About 15 to 20% of cobalt mining in the DRC comes from artisanal mining, which appears problematic for human rights and child labour [8]. Moreover, cobalt is usually mined as a by-product of copper. This, combined with the socio-political instability and heavy geographical concentration [1], can lead to an imbalance in the supply-demand chain of cobalt [9]. This has led to a significant fear of causing a global supply shortage [2,10].
In the case of nickel, vast applications for stainless steel and other nickel-based alloys drive the demand. Albeit laterites constitute around 60% of the land-based nickel reserve, most nickel is extracted from sulphide ores. The global nickel reserves are unevenly concentrated, with Indonesia, Russia and the Philippines representing the bulk of nickel produced from natural minerals [11]. Indonesia produces over 60% of the global nickel supply [12]. The nickel prices have thus been highly dependent on the Indonesian market [12]. Certain aspects that cause price fluctuation include natural disasters, like earthquakes, typhoons and changes in government regulations [12]. One example would be the export ban on nickel ore from Indonesia, which has been an issue since 2014 [12,13]. These geopolitical risks and price fluctuations have posed continued challenges to existing operations and in bringing new operations online [11,12,13]. With nickel being classified as a critical and strategic mineral by several countries, challenges to meet global demand are well established.
In order to maintain a continuous supply of cobalt and nickel to the global markets and avoid any bottleneck situation, an alternate source for producing cobalt and nickel should be identified. Processing these elements from secondary sources such as slags, tailings, spent batteries, and discarded wastes would be an alternative [14]. Mining or processing valuable resources from a secondary source will provide access to a larger reserve volume than the estimated value from the primary sources [14,15]. The overarching term for extracting metals from secondary sources is termed technospheric mining. The technosphere is proposed as a new component of the environment along with the atmosphere, biosphere, hydrosphere, and lithosphere [16]. The technosphere includes material stocks generated by human activity that are currently excluded from material flow [16]. Technospheric mining describes extracting and recovering valuable resources from the technospheric stocks [16,17].
Johansson et al. identified that recovering metals from these stocks has received greater attention owing to environmental factors [17]. Extracting from technospheric stocks contributes to sustainable development, increases the production base to meet growing demand and diversifies supply chains in times of global environmental problems and a shortage of natural resources [14,18]. Furthermore, processing a waste stream would reduce the volume of net waste generated.
Considering the potential risks in meeting the supply chain and the technospheric streams containing significant proportions of nickel and cobalt, assessing secondary sources for viable production routes is attractive [14,16]. Conventional production of nickel is achieved through beneficiation followed by either hydrometallurgical or combined pyro- and hydrometallurgical extraction routes. Smelting is the primary technology for treating nickel sulphide concentrates, involving an oxidative step to reduce sulphur content and reject iron, resulting in a high-nickel content matte. This is then further processed to recover nickel metal or other high-value components. The primary solid waste streams generated from nickel smelting are slags composed of a solution of iron oxides and silicates along with a significant amount of Ni and Co that are rejected from the process. Even though the desired metal contents in the slag are sometimes low, the volume of waste generated, along with the growing emphasis on the circular economy, highlights the importance of processing mine waste for metal recovery. It is to be noted that an estimated 6–16 tonnes of slag are being discharged in the production of 1 tonne of nickel [19,20].
The main focus of this work is to return cobalt and nickel inventory lost to nickel furnace slag by retreating that material. Moreover, extracting from slag aligns with the principles of circular economy and contributes towards sustainability.
A few studies have been reported for extracting cobalt and nickel from slag, a technospheric stock, using pyrometallurgy, hydrometallurgy, or a combination of both and are reported below.
Yang et al. [21] utilised a matte-slag mixture from the ISAMELT furnace to recover cobalt and copper. They achieved a cobalt recovery of 98% with 2% coke and pyrite at 1260 °C for 2 h [21]. Altundogan and Tumen [22] studied the roasting of copper converter slag with Fe2(SO4)3 and then subjected it to water and sulphuric acid leaching. A comparatively higher recovery percentage of 69%, 98%, 57%, and 93% for cobalt, copper, nickel, and zinc was achieved using sulphuric acid, respectively [22]. Altundogan et al. [23] investigated the recovery of Cu, Co, Zn, and Fe from copper converter slag obtained by oxidative leaching with K2Cr2O7 and H2SO4 lixiviants. The experimental results indicated that the presence of dichromate greatly influences the extraction of metals. The process recovered 81.15% Cu, 12.0% Co, 3.15% Fe, and 10.27% Zn [23]. Anand, Das and Jena’s studies showed that using 10% furnace oil as the reducing agent yielded 80% cobalt, 82% copper, and 95% nickel by roasting the copper converting slag at 850 °C and leaching with 1.25 times the stoichiometric amount of ferric chloride for 2 h [24]. Numerous studies reported that the recovery of cobalt and nickel from nickel converter slag using pressure oxidative acid leaching with H2SO4 is promising [25,26,27]. They achieved a cobalt and nickel recovery of greater than 97%, with temperatures ranging from 200–250 °C in 80–120 min [25,26,27]. Meshram et al. used oxalic and citric acid to recover valuable metals from copper converter slags. They achieved a Co and Ni recovery of 94% and 89% using citric acid and a maximum recovery of 16% cobalt using oxalic acid [5]. Lim et al. (2023) used citric acid and hydrogen peroxide to recover Co and Ni from nickel furnace and converter slag. They achieved a recovery of 82% Ni and 91.7% Co from nickel furnace slag and 65% Ni and 99.1% Co from converter slag using the following experimental conditions: 1M citric acid, 0.5% (v/v) hydrogen peroxide, a pulp density of 2.5%, a particle size f −100 +75 µm, 400 rpm at 60 °C for 6 h [28].
Although relatively high recoveries are achieved using the above methods, it is seen that most of the techniques mentioned above utilise high energy and harsh chemicals. Also, very limited studies have been conducted to extract valuable metals using nickel slag. The study conducted by Meshram et al. (2020) [5] and Lim et al. (2023) [16] has shown promise in recovering these elements from slags using organic acids. Therefore, this study aims to investigate the potential of organic acids to recover cobalt and nickel from nickel furnace slag by studying the effect of concentration, temperature, time, and particle size.
A series of experiments were conducted by employing citric acid and ascorbic acid as leaching reagents. A description of the experimental procedure and the results obtained are discussed below.
2. Results and Discussion
2.1. Sample Characterisation
The particle size distribution indicated that the p80 of the slag was 34 µm, which was used for experimentation unless otherwise specified. The elemental composition of the slag was determined using XRF, and is presented in Table 1. For conciseness, only the elements of interest are presented. Major impurities include Si, Al and Mg.

Table 1.
Elemental composition of the slag.
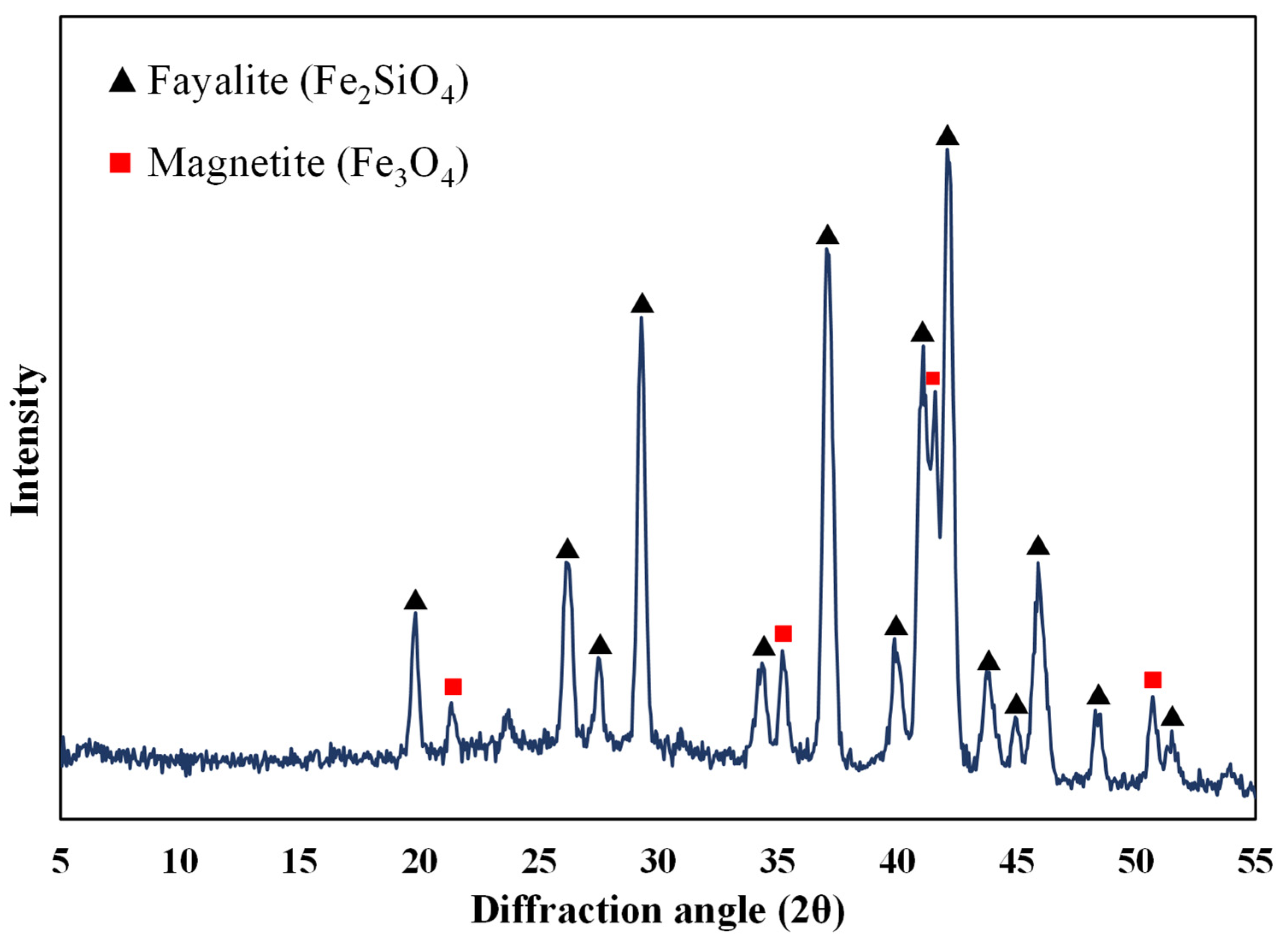
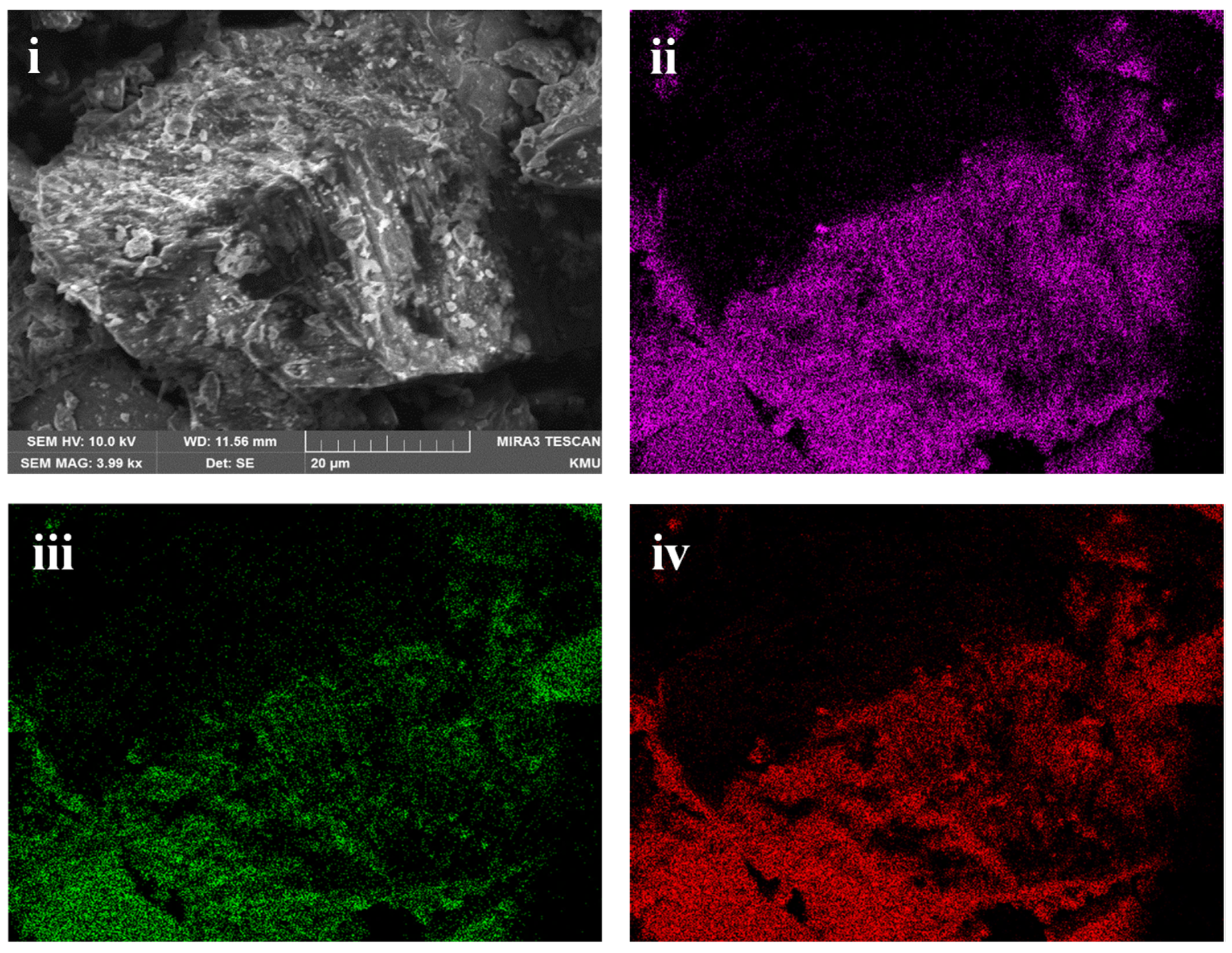
The ground sample was analysed using XRD to identify the mineralogy of the slag. Figure 1 shows the XRD analysis of the slag, and it is observed that it consists of fayalite (Fe2SiO4) and magnetite (Fe3O4). It is to be noted that the cobalt, nickel, and any other phases were not detected. It was reported by Jones et al. [29] that Co and Ni could be present either on the surface or entrained in the silicate and oxide matrix as metals or as sulphides [29]. SEM analysis of the sample also shows that silicon, iron, and oxygen are distributed throughout the slag matrix, which comprises the silicate and oxide phases (Figure 2). Previous studies on nickel slag have shown that the nickel was trapped in the matte left behind in the slag [27,28] and that Co exists as CoFe2O4 and is associated with fayalite [26,28]. TIMA analysis of the nickel furnace slag revealed that it primarily consists of fayalite, with some magnetite present [28]. Additionally, it was reported that a trace amount of godlevskite (Ni,Fe)9S8 was detected in the furnace slag [28].
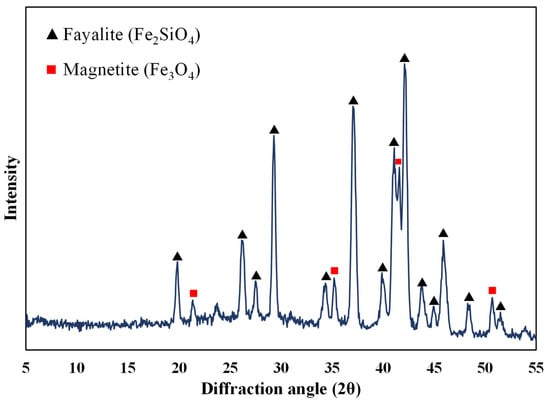
Figure 1.
XRD analysis of nickel furnace slag.
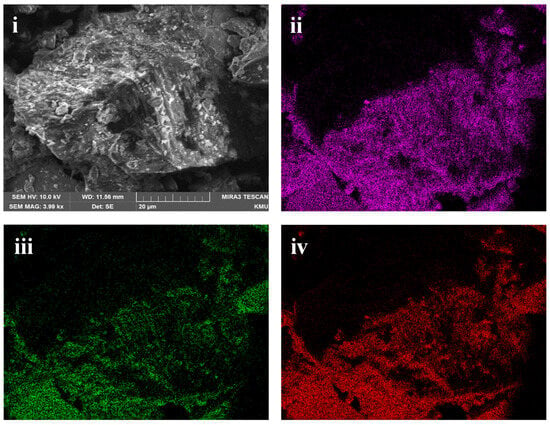
Figure 2.
Scanning electron microscope image of nickel furnace slag (i) Secondary electron image; (ii) Silicon mapping (iii) Iron mapping (iv) Oxygen mapping using SEM-EDS.
2.2. Citric Acid Leaching of Nickel Furnace Slag
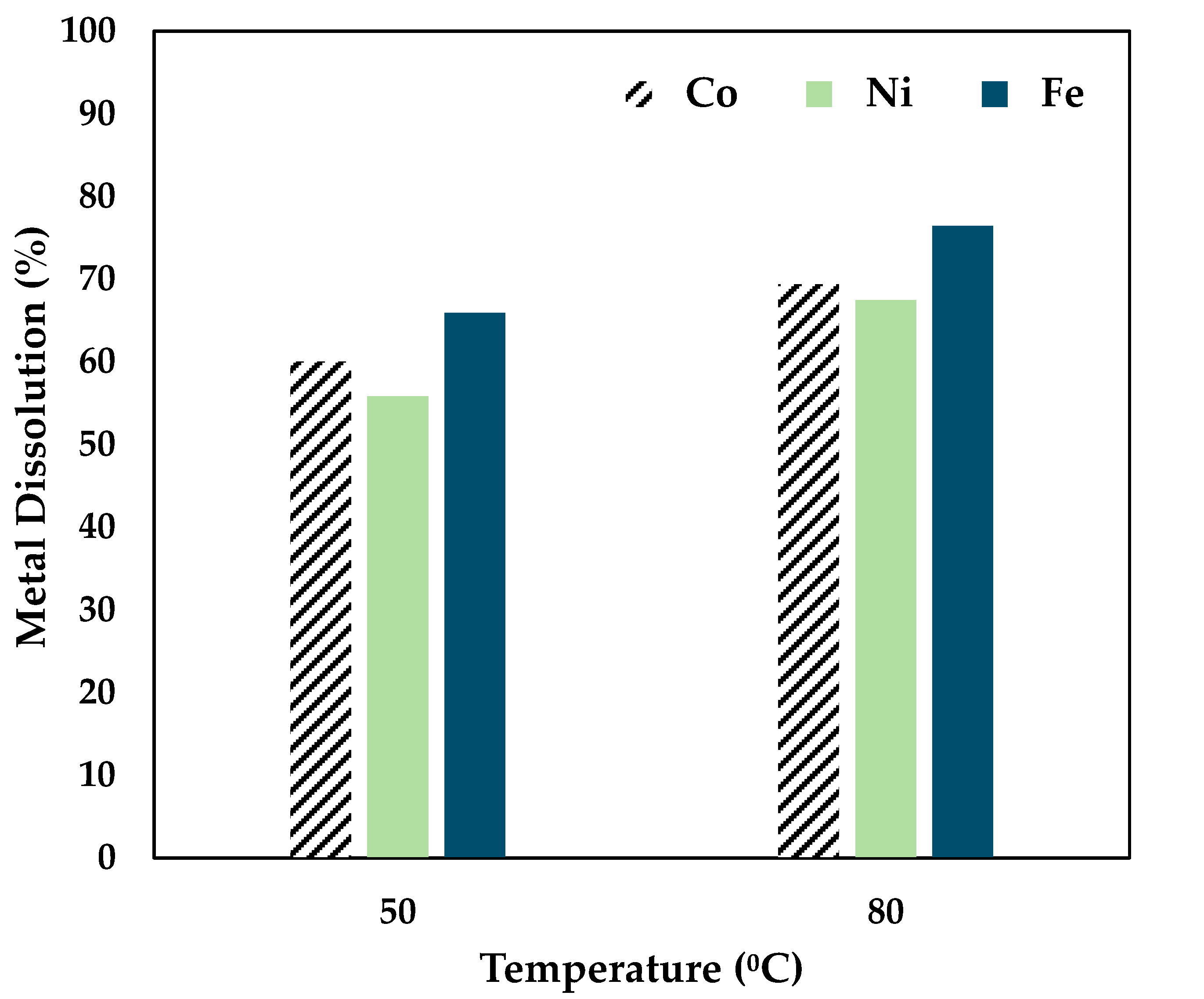
Studies carried out by Lim et al. (2023) have shown that 0.5 mol/L CA performed well in extracting Co and Ni from nickel slag [28]. Therefore, experiments were conducted to evaluate the effectiveness of leaching Co and Ni from nickel furnace slag using 0.5 mol/L CA at 50 °C and 80 °C for 6 h. The results are presented in Figure 3.
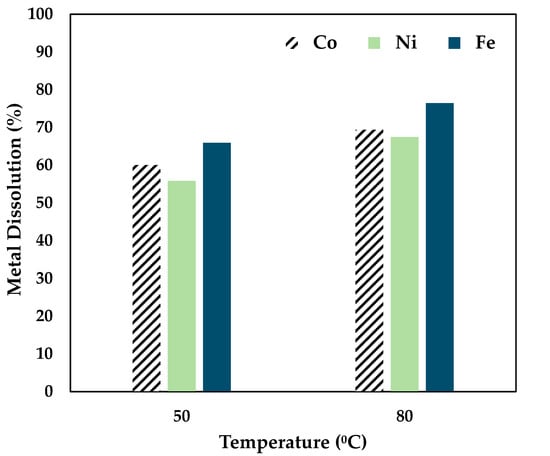
Figure 3.
Effect of citric acid on Co, Ni, and Fe dissolution (0.5 mol/L CA, 6 h, S/L ratio = 1:10, 34 µm particles, 250 rpm).
Figure 3 shows that a 60% Co, 55% Ni, and 66% Fe recovery was achieved by maintaining a temperature of 50 °C. From the data presented above, it can be seen that temperature has a considerable effect on leaching, as a recovery of 69% Co, 67% Ni, and 76% Fe was obtained at 80 °C over 6 h. Also, citric acid tends to be a strong leaching reagent for the chosen sample. Further studies were carried out by adding a strong reducing agent, ascorbic acid.
2.3. Ascorbic Acid-Assisted Citric Acid Leaching of Nickel Furnace Slag
2.3.1. Effect of Ascorbic Acid Concentration on Metal Dissolution
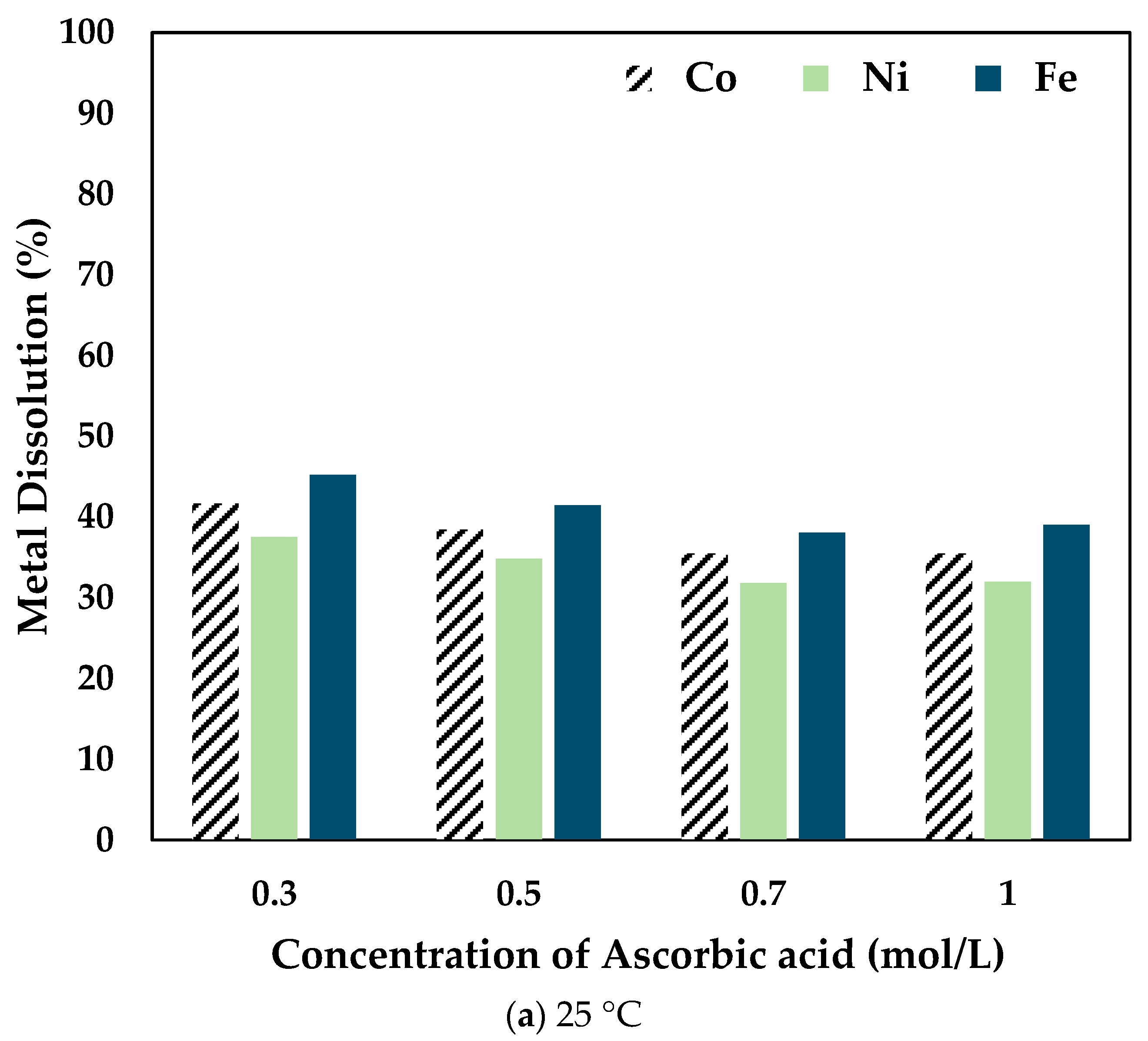
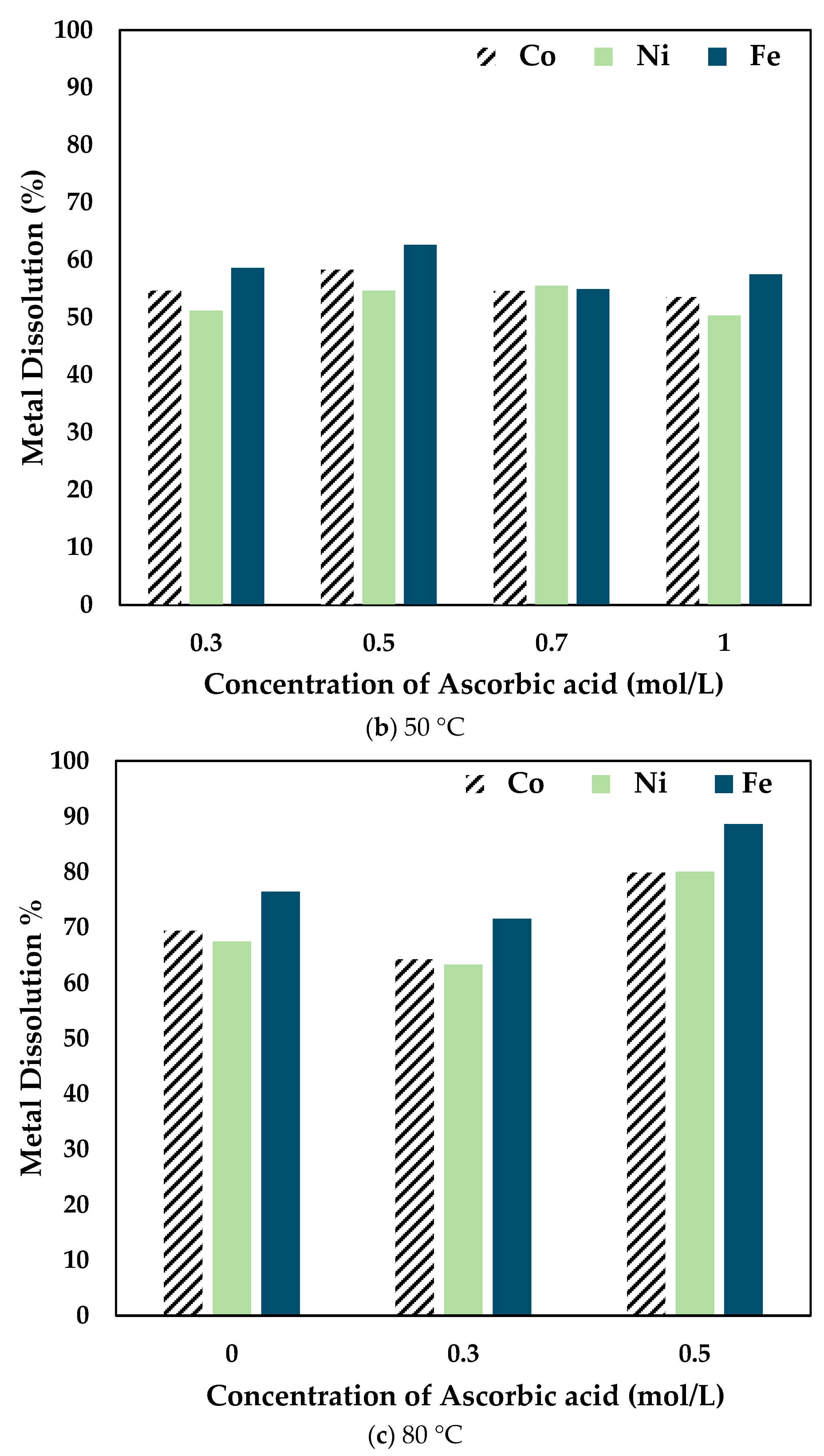
The effect of ascorbic acid concentration was investigated by leaching the slag at different temperatures for 6 h using 0.5 mol/L CA with varying concentrations of AA. The results obtained are shown in Figure 4.
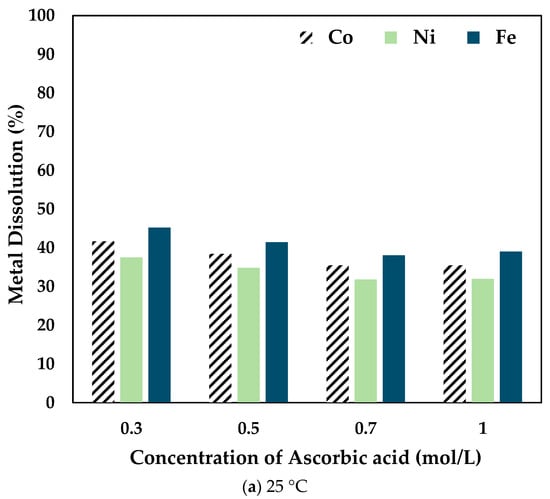
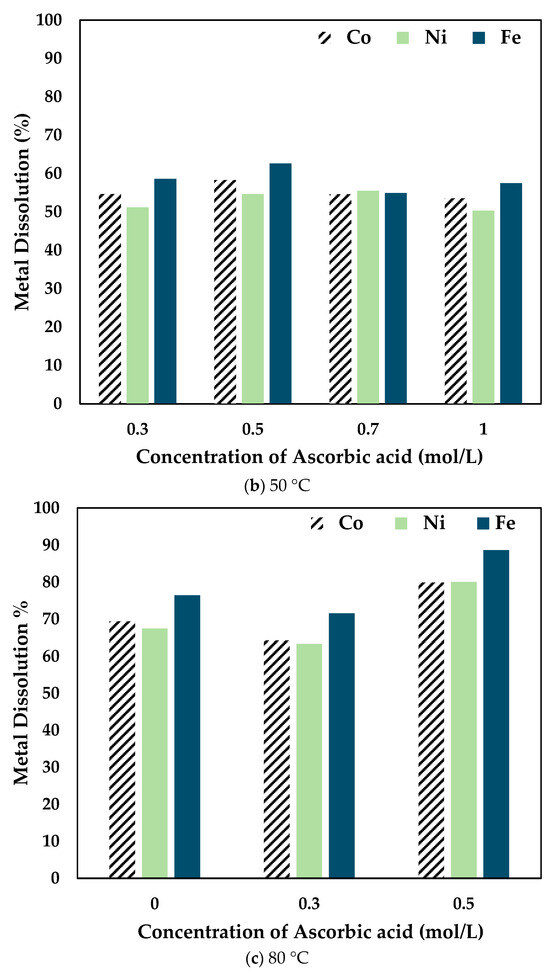
Figure 4.
Effect of ascorbic acid concentration on Co, Ni, and Fe dissolution at (a) 25 °C (b) 50 °C (c) 80 °C (0.5 mol/L CA, 6 h, S/L ratio = 1:10, particle size = 34 microns, stirring speed = 250 rpm).
At 25 °C, the recovery percentage of Co, Ni, and Fe dropped with the increase in the concentration of AA added. However, at 50 °C, the recovery percentage increased slightly at 0.5 mol/L compared to 0.3 mol/L AA. It is to be noted that the dissolution percentage for 0.7 mol/L and 1 mol/L AA concentrations was lower than the other concentrations at different temperatures. Therefore, at 80 °C, experiments were carried out only up to 0.5 mol/L AA. It was seen that the recovery percentage increased significantly when 0.5 mol/L AA was added to 0.5 mol/L CA. The drop in dissolution percentage at higher concentrations of ascorbic acid is potentially due to the increased ascorbic/citric acid ratio, thereby decreasing the activity and thus the efficacy of the citric acid. Therefore, it was concluded that the optimal combination was 0.5 mol/L AA + 0.5 mol/L CA.
2.3.2. Effect of Temperature and Time on Metal Dissolution
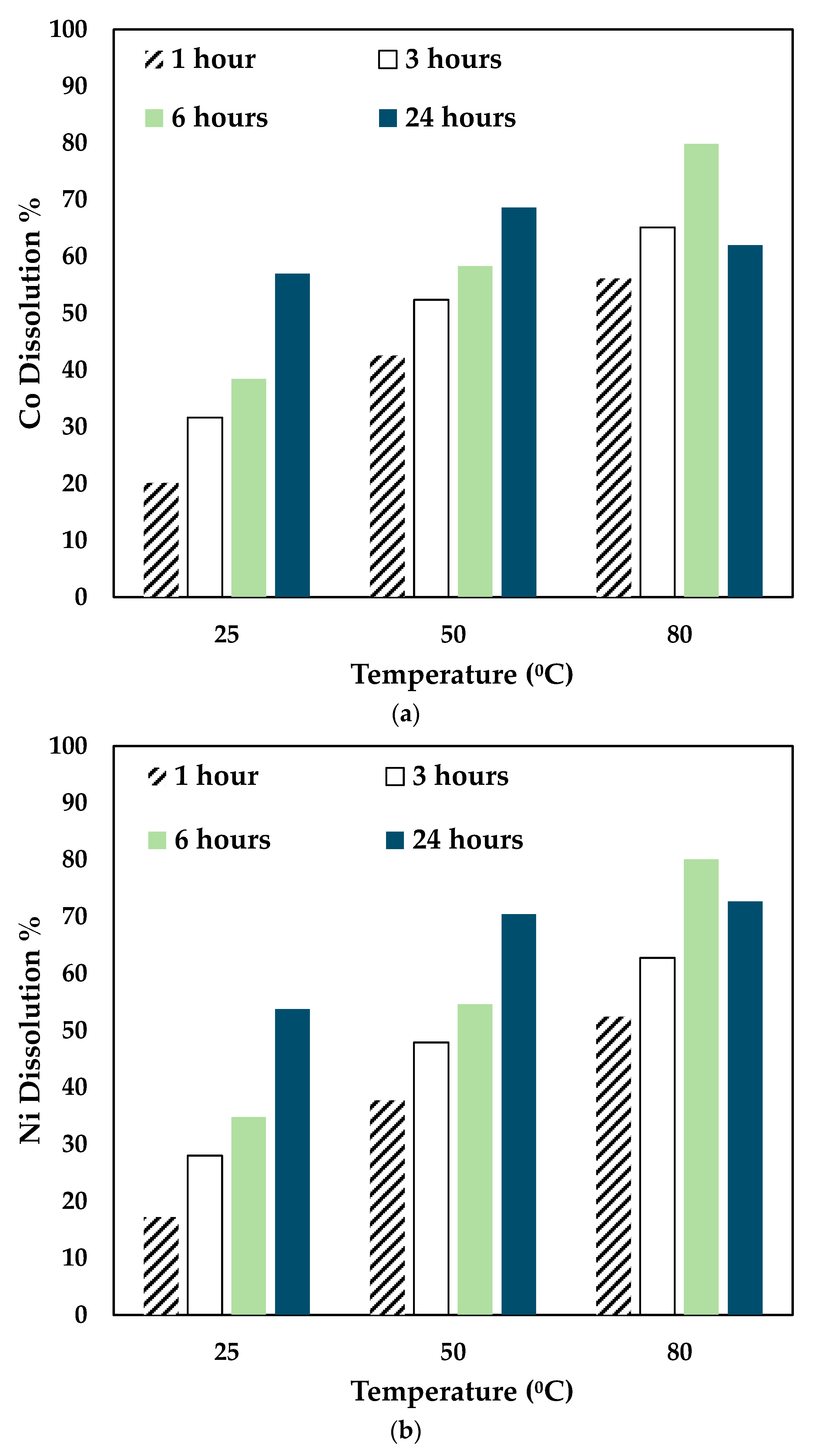
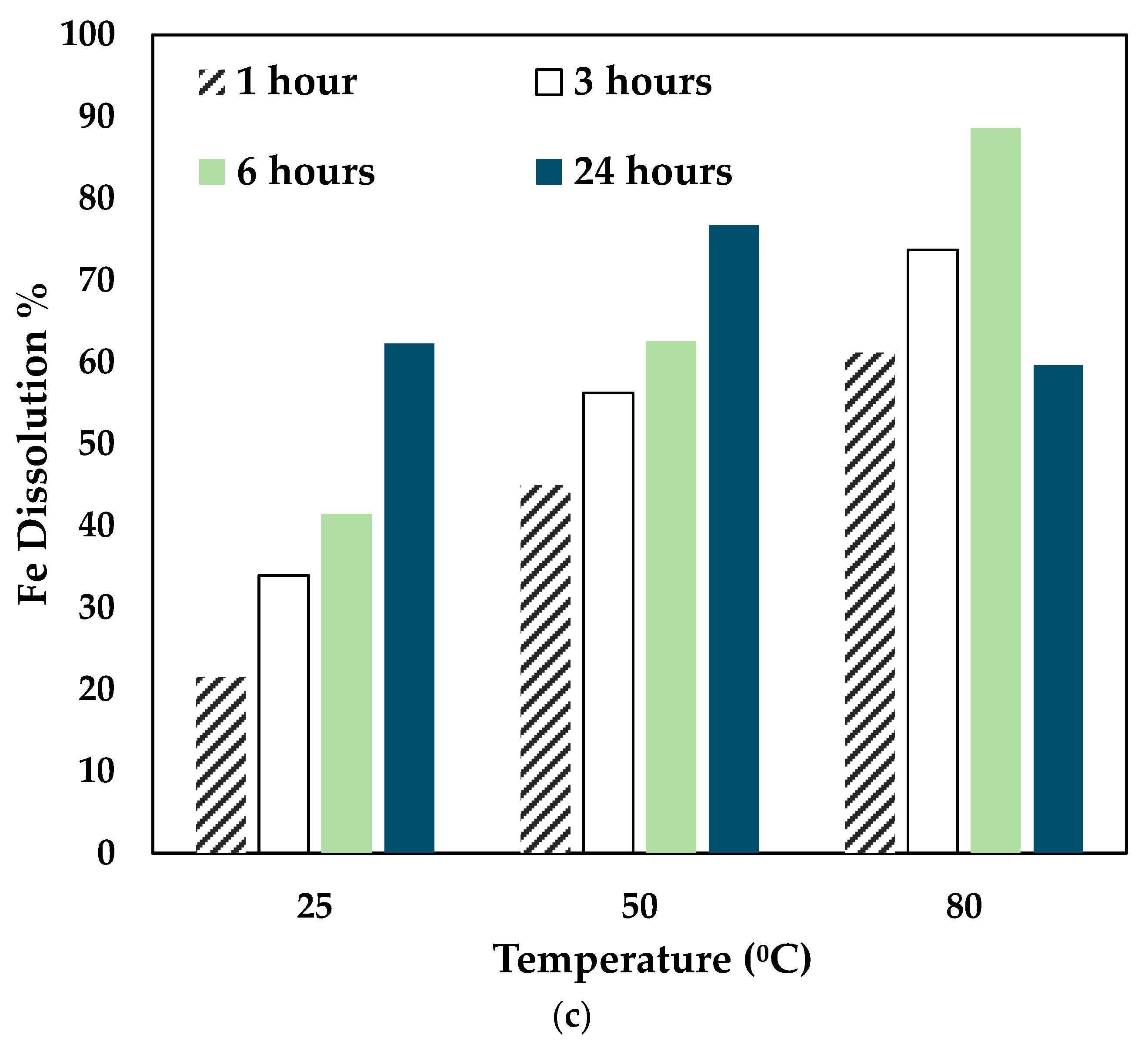
The effect of temperature and time was investigated by leaching the slag at different temperatures and times using 0.5 mol/L CA + 0.5 mol/L AA. The experiments were carried out at 25 °C, 50 °C, and 80 °C for 24 h, and the results obtained are shown in Figure 5.
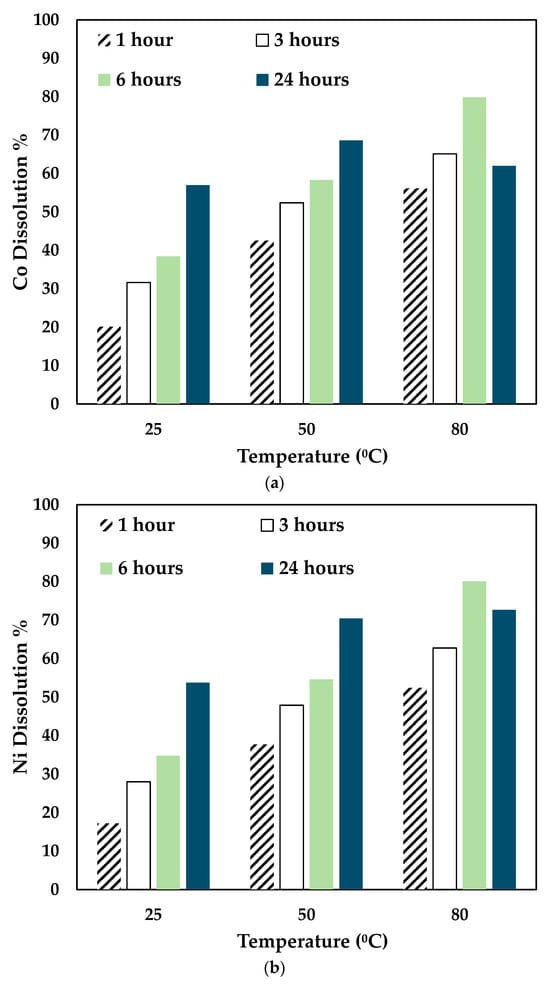
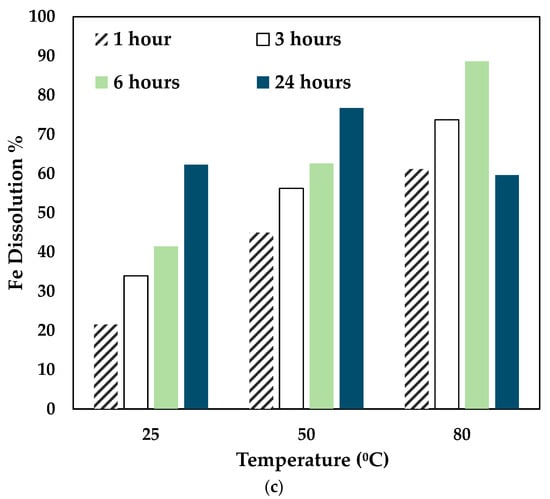
Figure 5.
Effect of temperature on (a) Co, (b) Ni, and (c) Fe dissolution (0.5 mol/L CA + 0.5 mol/L AA, S/L ratio= 1:10, particle size = 34 microns, stirring speed = 250 rpm).
With the variation of AA concentration, the temperature played a significant role. For 0.5 mol/L AA, the Co and Ni recovery increased for 25 °C and 50 °C till 24 h. However, at 80 °C, Co, Ni, and Fe recovery dropped significantly at 24 h. This shows that a mixture of 0.5 mol/L AA and 0.5 mol/L CA is suitable to operate at 80 °C for 6 h. As seen in Figure 3, with higher concentrations of AA (0.7 mol/L and 1 mol/L), the leaching efficiency of Co and Ni was relatively lesser than 0.3 mol/L and 0.5 mol/L. For 80 °C, the recovery rate increased until 6 h, and the value decreased. This is possibly due to the denaturing tendency of ascorbic acid, which worsens at 76 °C [30]. Also, Jutkus et al. observed that storing ascorbic acid at higher temperatures led to chemical instability over time [31]. Therefore, it is recommended to either run the experiment at a lower temperature or to run the experiment for a smaller duration. Based on the observations, the following operating conditions were chosen for further experimentation: 80 °C, 0.5 mol/L AA + 0.5 mol/L CA, 6 h, and 250 rpm.
2.3.3. Effect of Particle Size on Metal Recovery
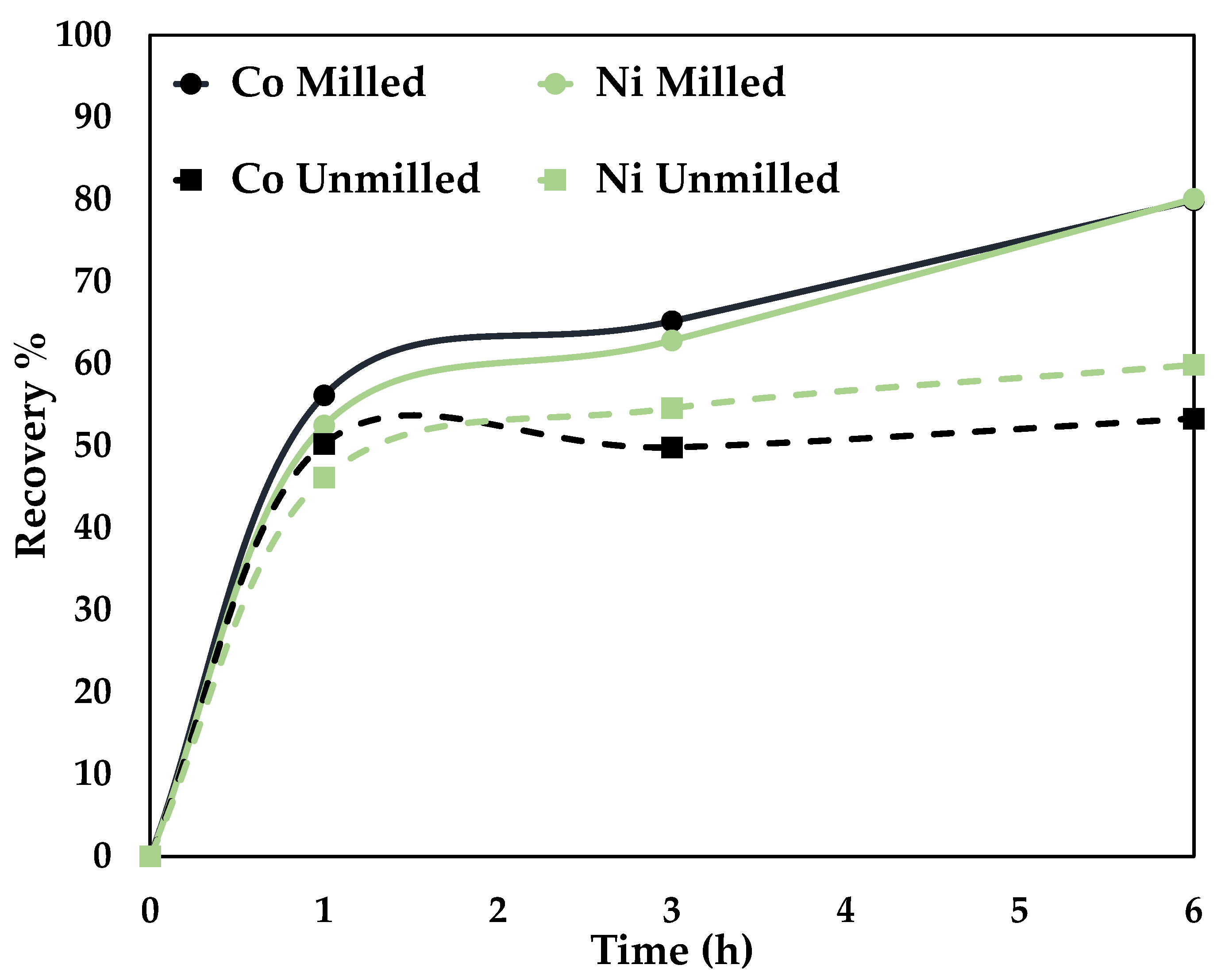
The effect of particle size on the leaching efficiency of Ni and Co was studied by using milled and unmilled particles with a p80 of 75 μm and 34 μm, respectively. The experimental conditions were: 80 °C, 0.5 mol/L AA + 0.5 mol/L CA, 250 rpm for 6 h. The results obtained are shown in Figure 6.
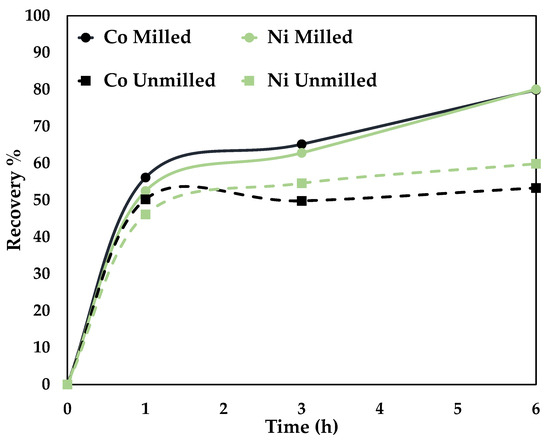
Figure 6.
Effect of particle size on Co and Ni dissolution (80 °C, 0.5 mol/L CA + 0.5 mol/L AA, S/L ratio = 1:10, stirring speed = 250 rpm).
A recovery percentage of 53% Co, 59% Ni, and 79.8% Co, 80% Ni was attained for unmilled (p80 = 75 microns) and milled (p80 = 34 microns), respectively. An optimal particle size screens the unwanted minerals and ensures the liberation of the target elements [32]. Mechanical activation was employed to reduce particle size. The disintegration by high-energy grinding increases the surface area and alters the physicochemical properties, rendering them more reactive in the subsequent processing [33]. The higher recovery percentage is due to the liberation of the target elements [32] and the increased number of particles newly exposed to the leaching conditions [33].
2.4. Leaching Mechanism of the Synergistic System
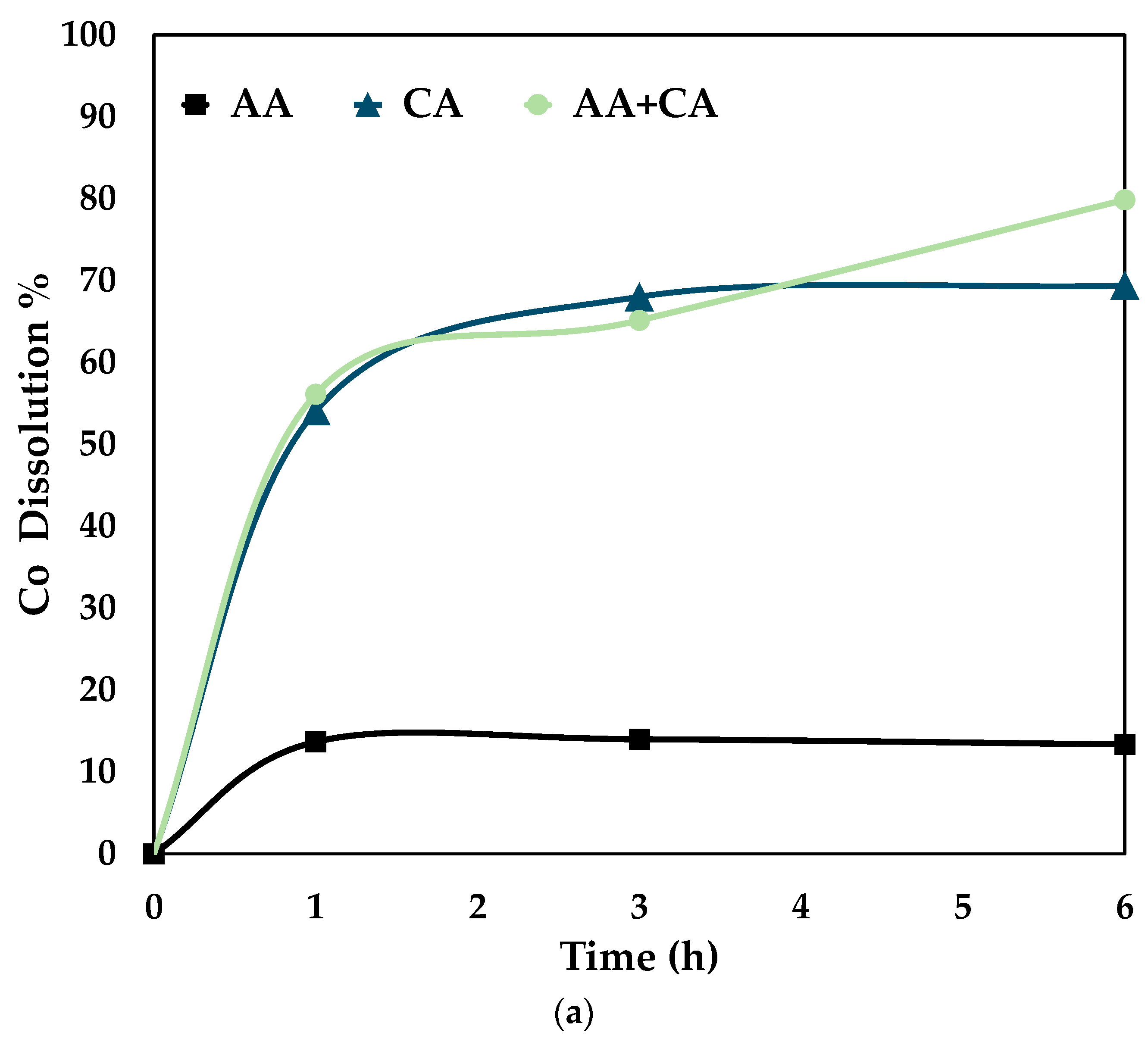
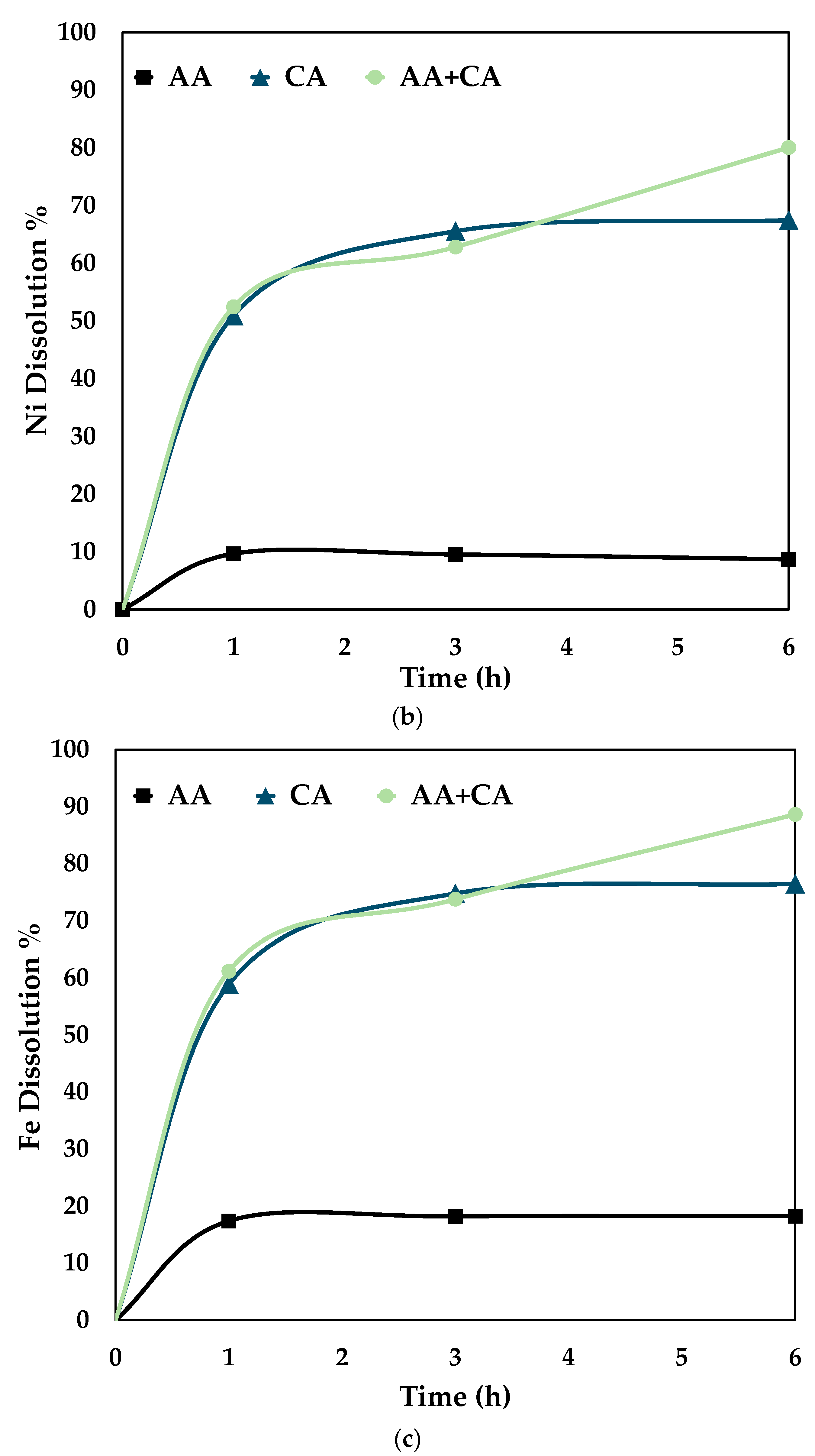
To understand the effect of the combined reagents, graphs were plotted individually for 0.5 mol/L AA, 0.5 mol/L CA, and 0.5 mol/L AA + 0.5 mol/L CA at 80 °C. The graphs were plotted for Co, Ni, and Fe recoveries under the above-said conditions, shown in Figure 7.
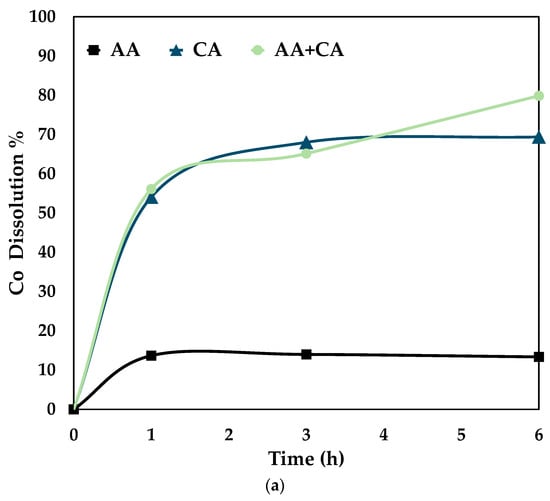
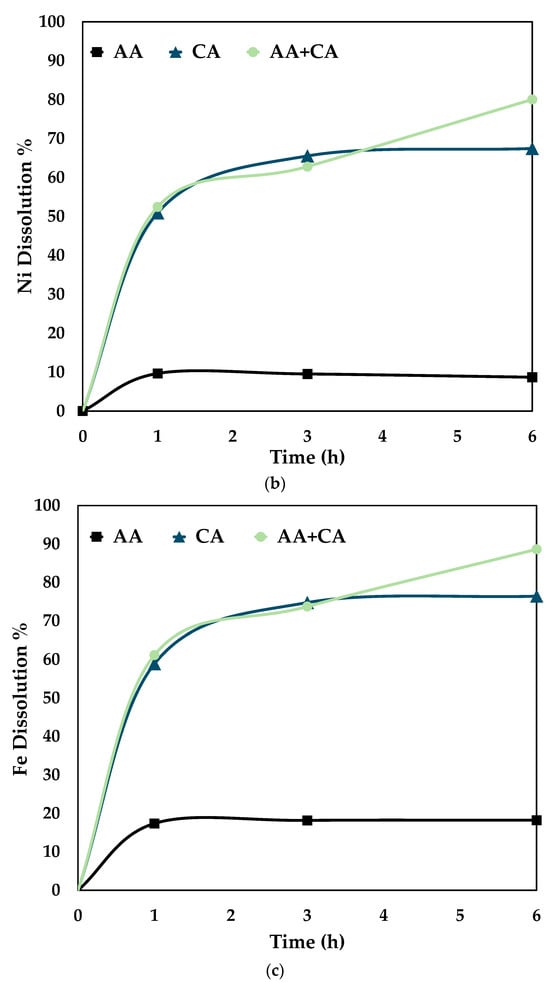
Figure 7.
Comparison of leaching efficiency (a) Co (b) Ni and (c) Fe dissolution (80 °C, particle size = 34 microns, stirring speed = 250 rpm).
Figure 7 proves that the synergistic system works better than the individual systems, i.e., only citric and ascorbic acid. It is seen from the above graphs that cobalt, nickel, and iron follow the same trend for the three different systems. This shows that it is essential to dissolve Fe by disintegrating the fayalite and magnetite matrix to ensure Co and Ni dissolution [28]. On treating the slag with either citric or ascorbic acid, it is seen that the leaching curve tends to plateau after 3 h. However, with a combination of these reagents, the recovery percentage increases till 6 h. The proposed mechanism for the synergistic system is as follows.
Citric acid provides H+ ions for protonation, which attacks the fayalite matrix, releasing ferrous ions and silicic acid. The H+ ions also facilitate the dissolution of magnetite, a mineral containing both Fe2+ and Fe3+ ions, thereby releasing ferric and ferrous ions into the solution.
Ascorbic acid, a reducing agent, converts the Fe3+ to Fe2+ ions. This reaction plays a vital role in the dissolution of magnetite, as two-thirds of the Fe content exists in the ferric state. Furthermore, ascorbic acid forms complexes with silica, promoting the breakdown of the fayalite matrix [34]. In addition, citric acid also acts as a chelating agent and forms stable complexes with both Fe2+ and Fe3+ ions.
It is evident from Figure 7 that the dissolution of iron is essential for the release of cobalt (Co) and nickel (Ni). Thus, promoting the dissolution of fayalite and magnetite matrices is necessary for efficient leaching of Co and Ni. According to Meshram et al. [5] citric acid forms complexes with both Co and Ni ions. This supports the proposed mechanism’s applicability for the extraction of these metals. The reaction equations for the mechanism are summed up in Equations (1)–(6) [5].
Fe2SiO4 + 4H+ → 2Fe2+ + H4SiO4
Fe3O4 + 8H+ → 2Fe3+ + Fe2+ + 4H2O
Fe3+ + C6H8O6 → Fe2+ + C6H6O6 + 2H+
Fe3+ + C6H8O7 → [Fe(C6H5O7)]n
Fe2+ + C6H8O7 → [Fe(C6H5O7)]n
The metals (M) reacted with citric acid to form soluble metal chelates, and the reaction was expressed as Equation (6) [5].
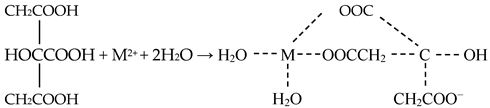
Overall, it is evident that reducing the slag matrix plays a vital role in efficiently extracting Co and Ni from slag. The combined actions of citric acid and ascorbic acid ensure the breakdown of the mineral structure and the leaching of the target metal ions.
This might be because citric acid, a strong leaching reagent, leaches the cobalt and nickel on the surface and converts them into their corresponding metal salts [5]. Meanwhile, ascorbic acid, a strong reducing agent, causes a disturbance in the slag matrix and reduces it [35]. This helps in leaching the cobalt and nickel trapped in the slag matrix. The increased iron recovery is due to the reduction of the slag matrix. Ascorbic acid also tends to reduce the metal citrate complexes. It is evident from the results that reducing the slag matrix is vital for extracting Co and Ni efficiently from the slag.
However, the decreased recovery after 6 h can be attributed to a number of reasons. The chemical instability of ascorbic acid at higher temperatures plays a major role [30,31]; this denaturing tendency of ascorbic acid causes the reprecipitation of some of the iron into the system. This, in turn, decreases the cobalt and nickel recovery as they tend to follow the same trend as iron (Figure 7). In addition, the system loses ascorbic acid due to its instability, which tends to decrease the reaction rate. It is proposed that iron reprecipitation occurs much faster than forming iron ascorbate, thereby reducing the recovery of iron, cobalt, and nickel. Based on these observations, it is said that the optimal operating conditions for this system are 6 h at 80 °C, which also helps prevent the decomposition of AA. However, further investigations are required to evaluate the extent of AA decomposition, its impact on reagent recyclability, and the overall feasibility of this approach for achieving more sustainable circular processing.
The above process not only treats waste but also provides better utilisation of resources, providing the potential to generate multiple products and thus contributing to a circular economy. The leach liquor obtained can be further processed through solvent extraction to separate Fe, Co, and Ni; however, this approach has not been tested with the specific solution obtained from this study. Given the significant concentration of Fe in the leach liquor, there is an opportunity to create a low-value iron stream that could be used for other applications. By extracting these valuable elements from furnace slag, this method enhances resource efficiency which is beneficial for nickel-producing industries globally. Additionally, it not only aids in reducing waste but also has the potential to generate an additional revenue stream.
3. Materials and Methods
3.1. Sample Preparation and Characterisation
The nickel furnace slag used in this study was obtained from a processing plant in Western Australia. The furnace slag was initially pulverised using a Rocklabs Limited Model C pulveriser. The slag was then mechanically activated using a Restch PM type 100 planetary ball mill, which had a capacity of 50 cm3. A charge-to-ball ratio of 1:10 was employed. Then, the ground sample was analysed using X-ray diffraction (XRD) (Pan Analytical, Worcestershire, UK) to identify the mineralogy of the slag. Also, Scanning Electron Microscopy–Energy Dispersive Spectroscopy (SEM-EDS) was carried out to understand the elemental distribution of the sample using an MIRA3 Tescan (Brno, Czech Republic) at John de Laeter Centre in Curtin University. The elemental composition of the ground sample was determined using X-ray fluorescence (XRF) and was conducted by Bureau Veritas Laboratory, Perth. The particle size distribution of the slag was carried out using laser sizing (Mastersizer 3000, Malvern, Worcestershire, UK). The concentration of the elements in the dissolved solution was determined using an Inductively Coupled Plasma–Optical Emission Spectroscopy (Agilent 5100 Synchronous Vertical View-SVDV, Boulder, CO, USA).
3.2. Reagent Preparation
The leaching reagents used in this study were 100% pure L-ascorbic acid (C6H8O6) (Daintree Quality Herbs, USA) and 100% anhydrous citric acid (C6H8O7) (Sigma Group Companies Pty Ltd., Perth, Australia) in a solid crystalline form. Based on the solubility of these reagents in water, the concentrations of the reagents were fixed. The individual solutions were prepared with known concentrations of these reagents in deionised water. A previous study using nickel slag indicated that 0.5 mol/L citric acid as a leaching reagent was effective at elevated temperatures [28]. Therefore, in this study, further experiments were conducted to explore the potential of the synergistic system. Thus, different ascorbic acid concentrations ranging from 0.3–1 mol/L ascorbic acid were added to 0.5 mol/L citric acid. In the upcoming sections, ascorbic and citric acid have been abbreviated as AA and CA, respectively, for ease.
3.3. Leaching Experiments
Batch leaching experiments were conducted using a three-necked round-bottom flask. The flask was fitted with a condenser on one neck and a thermometer on the other to monitor the temperature of the slurry throughout the experiment. An overhead stirrer with a collapsible impeller was fitted to ensure mixing and was set to rotate at a fixed speed of 250 rpm. The effect of variables influencing the leaching process, such as temperature, reagent concentration, particle size, and time was investigated. The reagent was taken in the three-necked round-bottom flask and placed in a heating mantle to achieve and maintain the desired reaction temperature during leaching. Once the desired temperature was attained, an aliquot of slag was weighed and added to the flask. A solid-to-liquid ratio of 1:10 was maintained. The nickel furnace slag was subjected to leaching using a mixture of ascorbic and citric acid. Solution samples were collected at 1, 3, 6, and 24 h. The pH of the solution samples were measured and recorded. The collected samples were centrifuged and vacuum filtered using a Wattman filter paper of 7–11 microns. At the end of the experiment, solid-liquid separation was done using a vacuum filter and the leach residue was washed with distilled water, which yielded a dark red-violet filtrate and a black residue. The resultant dark red solution is due to the formation of ferrous ascorbate.
4. Conclusions
In this study, experiments were carried out to determine if nickel slag is a viable source for recovering cobalt and nickel from nickel furnace slag in an environmentally benign manner using organic acids such as ascorbic and citric acid. The parameters varied were temperature, the concentration of the reagents used, time, and particle size. It was observed that the recovery of Co and Ni generally increased with temperature. Interestingly, the dissolution percentage of Co and Ni decreased at longer residence times at 80 °C and while using 0.5 mol/L of ascorbic acid and citric acid. This is plausibly due to the denaturing tendency of ascorbic acid at 76 °C, as reported by Jutkus et al. (2015) [31]. In addition, altering the physicochemical properties of the slag by mechanically activating it aided in significantly increasing the leaching efficiency of Co and Ni. A combination of the two reagents at specific concentrations attained a much higher recovery than their individual counterparts. This is because the citric acid provides H+ ions that attack the fayalite and magnetite matrices, releasing metal ions into the solution. Ascorbic acid aids in reducing these ions, while citric acid complexes with the released metal ions to enhance dissolution. Optimal recovery of 79% Co and 80% Ni was obtained by subjecting the nickel furnace slag of p80 34 µm to 0.5 mol/L citric acid and 0.5 mol/L ascorbic acid for 6 h at 80 °C. This result shows that breaking down the Fe matrix to dissolve Co and Ni is essential, as these elements are trapped in the slag matrix. The outcomes of this work highlight the potential of organic acids as effective leaching reagents owing to their capability to attain high recoveries from a low-grade material under atmospheric conditions. Moreover, extracting critical and strategic minerals from slag adds more value to the supply chain of these elements. It also enables us to reprocess the material and reduce the net waste generated, thereby contributing to sustainability and aiding in a circular economy. Additionally, a comprehensive evaluation of the process considering the supply chain of both the waste products and reagents is to be done to enhance the overall circularity of the process.
Author Contributions
Conceptualization, K.Y., R.D.A. and L.D.; Data curation, K.Y.; Formal analysis, K.Y.; Investigation, K.Y.; Methodology, K.Y. and B.L; Project administration, R.D.A.; Supervision, R.D.A. and L.D.; Visualization, K.Y., R.D.A. and L.D.; Writing—original draft, K.Y.; Writing—review & editing, R.D.A., L.D. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available upon request.
Acknowledgments
The authors thank the Western Australian School of Mines for supporting this research and Mujesira Vukancic and Lahiru Basnayaka for supplying the lab equipment and ICP analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Watari, T.; Nansai, K.; Nakajima, K. Review of Critical Metal Dynamics to 2050 for 48 Elements. Resour. Conserv. Recycl. 2020, 155, 104669. [Google Scholar] [CrossRef]
- Beatty, D.; Fu, X.; Bustamante, M.; Gaustad, G.; Babbitt, C.; Kirchain, R.; Roth, R.; Olivetti, E. Cobalt Criticality and Availability in the Wake of Increased Electric Vehicle Demand: A Short-Term Scenario Analysis. In Minerals, Metals and Materials Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 355–357. [Google Scholar]
- Ober, J.A. Geological Survey Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Kovacheva-Ninova, V.K.; Savov, G.M.; Vassileva, V.; Vutova, K.; Petrov, E.; Petrov, D. Trends in the Development of Cobalt Production. Electrotech. Electron. 2018, 53, 84–94. [Google Scholar]
- Meshram, P.; Prakash, U.; Bhagat, L.; Abhilash; Zhao, H.; van Hullebusch, E.D. Processing of Waste Copper Converter Slag Using Organic Acids for Extraction of Copper, Nickel, and Cobalt. Minerals 2020, 10, 290. [Google Scholar] [CrossRef]
- Water Resources Division; U.S. Geological Survey. Geological Survey Mineral Commodity Summaries 2015; U.S. Geological Survey: Reston, VA, USA, 2015. [Google Scholar] [CrossRef]
- US. Geological Survey. Geological Survey Mineral Commodity Summaries 2022; U.S. Geological Survey: Reston, VA, USA, 2022. [CrossRef]
- van den Brink, S.; Kleijn, R.; Sprecher, B.; Tukker, A. Identifying Supply Risks by Mapping the Cobalt Supply Chain. Resour. Conserv. Recycl. 2020, 156, 104743. [Google Scholar] [CrossRef]
- Mudd, G.M.; Weng, Z.; Jowitt, S.M.; Turnbull, I.D.; Graedel, T.E. Quantifying the Recoverable Resources of By-Product Metals: The Case of Cobalt. Ore Geol. Rev. 2013, 55, 87–98. [Google Scholar] [CrossRef]
- Shedd, K.B.; Mccullough, E.A.; Bleiwas, D.I. Global Trends Affecting the Supply Security of Cobalt. Min. Eng. 2017, 69, 37–42. [Google Scholar]
- Zheng, S.; Zhou, X.; Zhao, P.; Xing, W.; Han, Y.; Hao, H.; Luo, W. Impact of Countries’ Role on Trade Prices from a Nickel Chain Perspective: Based on Complex Network and Panel Regression Analysis. Resour. Policy 2022, 78, 102930. [Google Scholar] [CrossRef]
- Lim, B.; Kim, H.S.; Park, J. Implicit Interpretation of Indonesian Export Bans on Lme Nickel Prices: Evidence from the Announcement Effect. Risks 2021, 9, 93. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wu, T.; Zhong, H.; Su, C.W. Bubble Behaviors in Nickel Price: What Roles Do Geopolitical Risk and Speculation Play? Resour. Policy 2023, 83, 103707. [Google Scholar] [CrossRef]
- Yamini, K.; Dyer, L. Extraction of Rare Earth Elements from Low-Grade Ore and Process Waste Stream. In Proceedings of the 26th World Mining Congress, Brisbane, Australia, 26 July 2023; pp. 619–629. [Google Scholar]
- Arndt, N.T.; Fontboté, L.; Hedenquist, J.W.; Kesler, S.E.; Thompson, J.F.H.; Wood, D.G. Future Global Mineral Resources. Geochem. Perspect. 2017, 6, 1–171. [Google Scholar] [CrossRef]
- Lim, B.; Alorro, R.D. Technospheric Mining of Mine Wastes: A Review of Applications and Challenges. Sustain. Chem. 2021, 2, 686–706. [Google Scholar] [CrossRef]
- Johansson, N.; Krook, J.; Eklund, M.; Berglund, B. An Integrated Review of Concepts and Initiatives for Mining the Technosphere: Towards a New Taxonomy. J. Clean. Prod. 2013, 55, 35–44. [Google Scholar] [CrossRef]
- Krook, J.; Baas, L. Getting Serious about Mining the Technosphere: A Review of Recent Landfill Mining and Urban Mining Research. J. Clean. Prod. 2013, 55, 1–9. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, Y.; Tong, W.; Ma, H. Utilization of Nickel Slag as Raw Material in the Production of Portland Cement for Road Construction. Constr. Build. Mater. 2018, 193, 426–434. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, N.; Chen, M.; Cheng, Y. Recycling Nickel Slag by Aluminum Dross: Iron-Extraction and Secondary Slag Stabilization. ISIJ Int. 2020, 60, 602–609. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Zhang, J.; Hu, J.; Li, J.; Zhang, L.; Chen, Y.; Wang, C. Efficient Recovery of Copper and Cobalt from the Matte–Slag Mixture of ISA Furnace by Injection of Coke and Pyrite. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 3118–3126. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Tumen, F. Metal Recovery from Copper Converter Roasting with Ferric Sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Boyrazli, M.; Tumen, F. A Study on the Sulphuric Acid Leaching of Copper Converter Slag in the Presence of Dichromate. Miner. Eng. 2004, 17, 465–467. [Google Scholar] [CrossRef]
- Anand, S.; Das, R.P.; Jena, P.K. Reduction-Roasting and Ferric Chloride Leaching of Copper Converter Slag for Extracting Copper, Nickel and Cobalt Values. Hydrometallurgy 1981, 7, 243–252. [Google Scholar] [CrossRef]
- Liao, Y.L.; Shi, G.C.; Huang, F.R.; Zhang, Y. Recovery of the Valuable Metals from Complex Converter Slag at Elevated Temperature with Sulfuric Acid Solution. J. Min. Metall. Sect. B Metall. 2019, 55, 359–370. [Google Scholar] [CrossRef]
- Huang, F.; Liao, Y.; Zhou, J.; Wang, Y.; Li, H. Selective Recovery of Valuable Metals from Nickel Converter Slag at Elevated Temperature with Sulfuric Acid Solution. Sep. Purif. Technol. 2015, 156, 572–581. [Google Scholar] [CrossRef]
- Li, Y.; Perederiy, I.; Papangelakis, V.G. Cleaning of Waste Smelter Slags and Recovery of Valuable Metals by Pressure Oxidative Leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Aylmore, M.; Grimsey, D.; Alorro, R.D. Technospheric Mining of Critical and Strategic Metals from Nickel Slag—Leaching with Citric Acid and Hydrogen Peroxide. Hydrometallurgy 2023, 219, 106066. [Google Scholar] [CrossRef]
- Jones, R.T.; Hayman, D.A.; Denton, G.M. Recovery of Cobalt, Nickel, and Copper from Slags Using DC-ARC Furnace Technology. In Proceedings of the International Symposium on Challenges of Process Intensification, 35th Annual Conference of Metallurgists, Montreal, QC, Canada, 24–28 August 1996. [Google Scholar]
- Degradation of Vitamins, Probiotics and Other Active Ingredients Caused by Exposure to Heat, Water and Sunlight. Available online: https://www.nutraceuticalbusinessreview.com/news/article_page/Degradation_of_vitamins_probiotics_and_other_active_ingredients_caused_by_exposure_to_heat_water_and_sunlight/145924#:~:text=Vitamin%20C%20and%20heat,of%20Scientific%20and%20Technology%20Research.&text=The%20negative%20effects%20of%20heat,more%20at%20170%20%C2%B0F (accessed on 4 December 2023).
- Jutkus, R.A.L.; Li, N.; Taylor, L.S.; Mauer, L.J. Effect of Temperature and Initial Moisture Content on the Chemical Stability and Color Change of Various Forms of Vitamin C. Int. J. Food Prop. 2015, 18, 862–879. [Google Scholar] [CrossRef]
- Ma, N.; Houser, J.B. Recycling of Steelmaking Slag Fines by Weak Magnetic Separation Coupled with Selective Particle Size Screening. J. Clean. Prod. 2014, 82, 221–231. [Google Scholar] [CrossRef]
- Baláž, P. Mechanical Activation in Hydrometallurgy. Int. J. Miner. Process. 2003, 72, 341–354. [Google Scholar] [CrossRef]
- Fenoglio, I.; Martra, G.; Coluccia, S.; Fubini, B. Possible Role of Ascorbic Acid in the Oxidative Damage Induced by Inhaled Crystalline Silica Particles. Chem. Res. Toxicol. 2000, 13, 971–975. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).