Author Contributions
Conceptualization, B.M., J.S. and K.P.B.; methodology, B.M.; software, B.M. and J.S.; investigation, B.M. and J.S.; resources, B.M.; data curation, B.M.; writing—original draft preparation, B.M.; writing—review and editing, B.M.; visualization, B.M. and J.S.; supervision, B.M., J.S. and K.P.B.; project administration, B.M. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Cell under investigation. The pouch cell has large cell tabs on the sides, with which it can be electrically connected when testing modules. It can be seen that .
Figure 1.
Cell under investigation. The pouch cell has large cell tabs on the sides, with which it can be electrically connected when testing modules. It can be seen that .
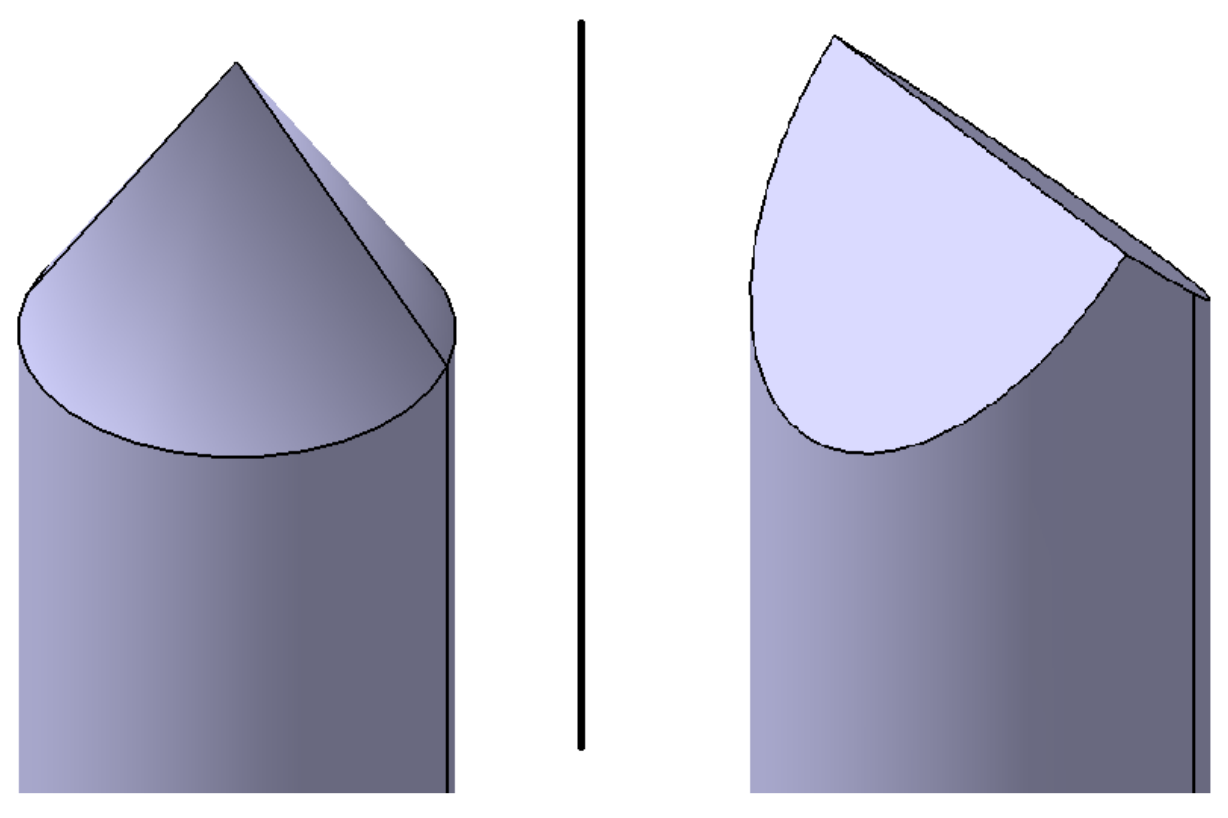
Figure 2.
Schematic view of the two nail tip shapes used, depending on the direction of nail penetration. On the (left), a conical nail tip is shown and on the (right), a wedge shaped tip.
Figure 2.
Schematic view of the two nail tip shapes used, depending on the direction of nail penetration. On the (left), a conical nail tip is shown and on the (right), a wedge shaped tip.
Figure 3.
TR testbench for test case of “Nail penetration orthogonal to electrode layer”. (Left): schematic overview of nail penetration relative to cell, (right): test bench with pouch cell pinned down with red boxes indicating the placements of sensors and .
Figure 3.
TR testbench for test case of “Nail penetration orthogonal to electrode layer”. (Left): schematic overview of nail penetration relative to cell, (right): test bench with pouch cell pinned down with red boxes indicating the placements of sensors and .
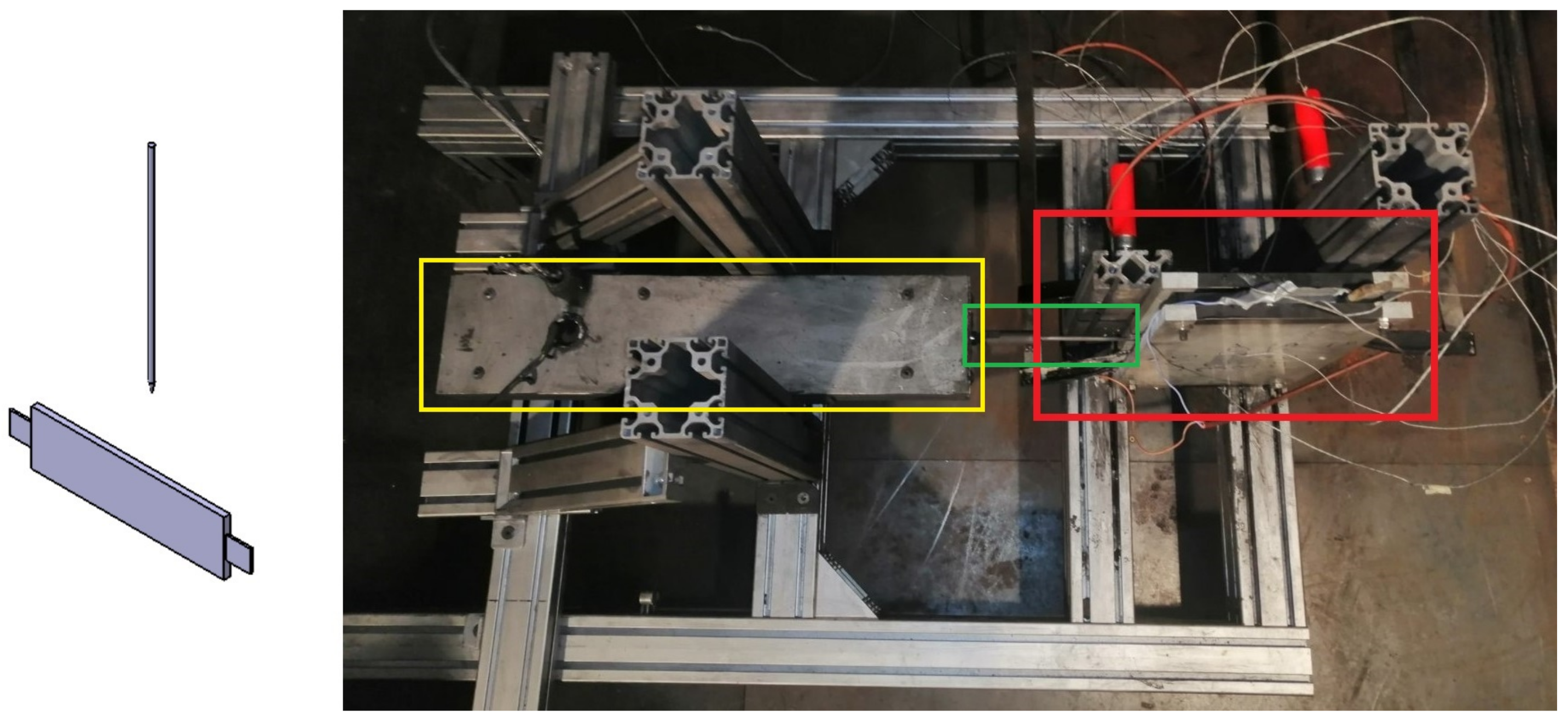
Figure 4.
TR testbench for test case of “Nail penetration parallel to electrode layer”. (Left): schematic overview of nail penetration relative to cell, (right): test bench with pouch cell clamped between two plates (red box) with nail (green box) and hydraulic system for nail penetration (yellow box).
Figure 4.
TR testbench for test case of “Nail penetration parallel to electrode layer”. (Left): schematic overview of nail penetration relative to cell, (right): test bench with pouch cell clamped between two plates (red box) with nail (green box) and hydraulic system for nail penetration (yellow box).
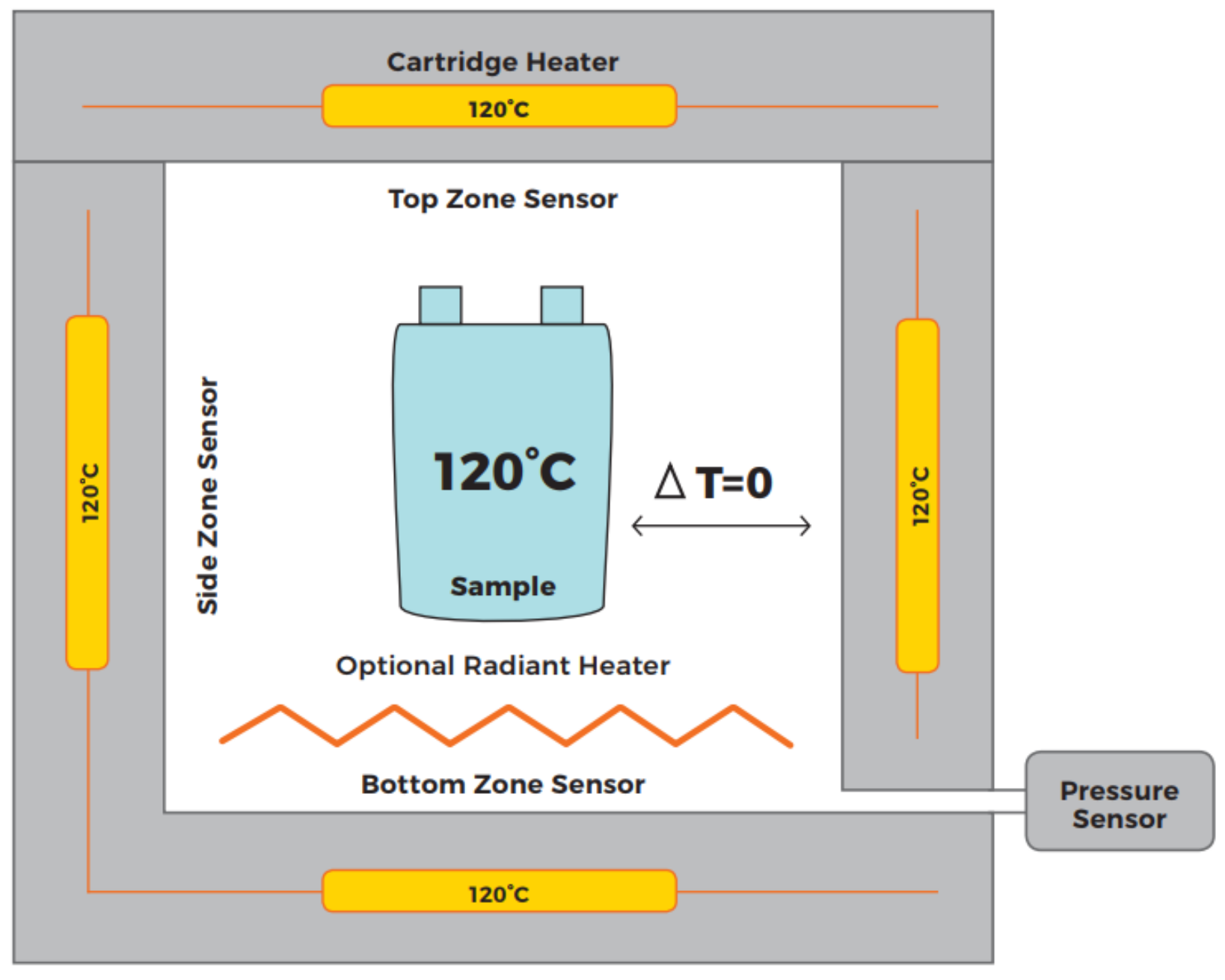
Figure 5.
Schematic overview of an ARC [
11]. The cell is in thermal equilibrium with the surrounding reaction chamber and the ARC is seeking for a temperature increase of the cell.
Figure 5.
Schematic overview of an ARC [
11]. The cell is in thermal equilibrium with the surrounding reaction chamber and the ARC is seeking for a temperature increase of the cell.
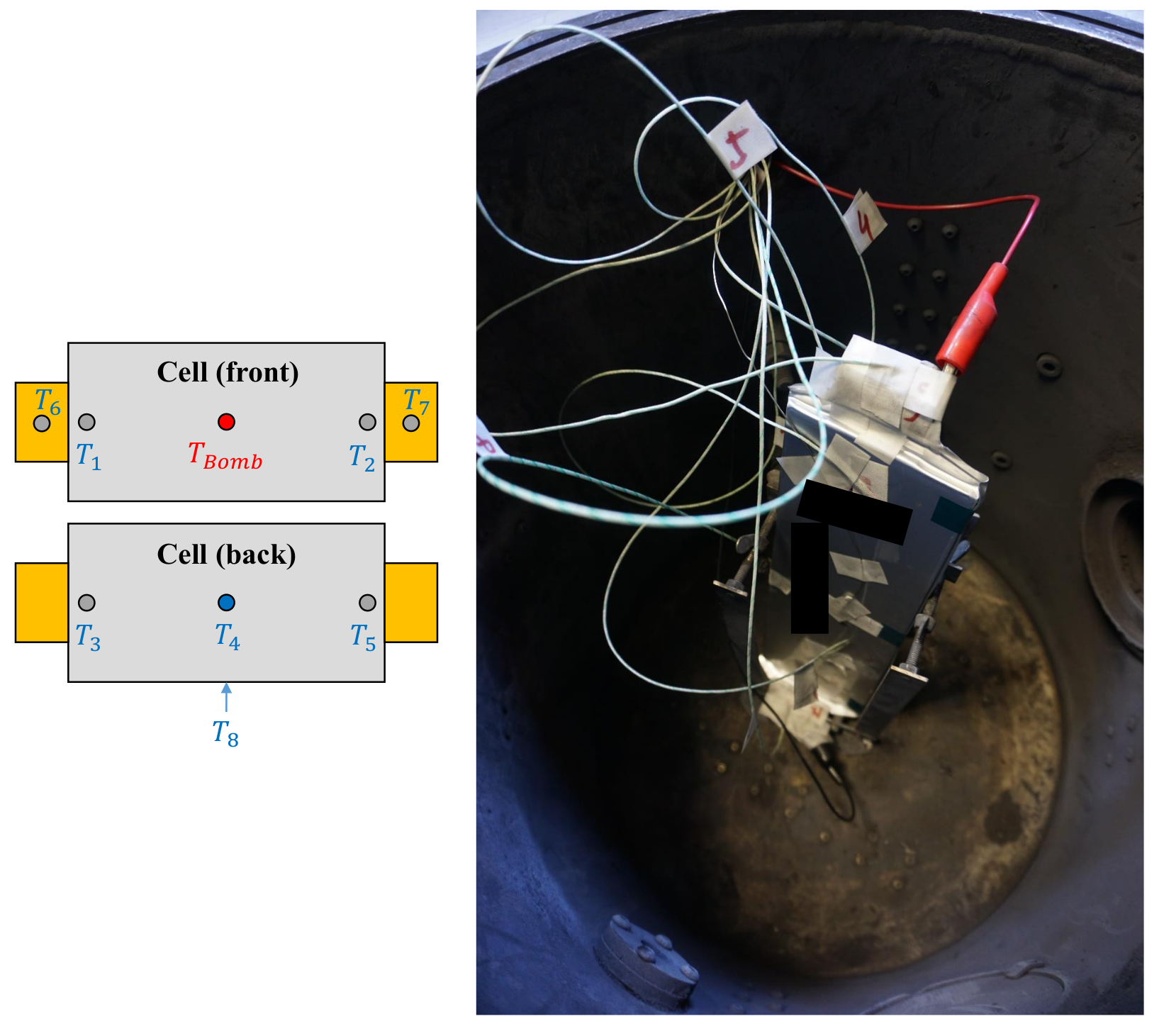
Figure 6.
(Left): Schematic view of the temperature sensor placement on the cell. (Right): View inside the ARC with the sensors attached to the cell before testing.
Figure 6.
(Left): Schematic view of the temperature sensor placement on the cell. (Right): View inside the ARC with the sensors attached to the cell before testing.
Figure 7.
Overview of the test facility: the mini-module test bench (red box) is connected via flanges to the exhaust system with a massflow meter (blue box), which allows the generated venting gas to vent into the surrounding environment.
Figure 7.
Overview of the test facility: the mini-module test bench (red box) is connected via flanges to the exhaust system with a massflow meter (blue box), which allows the generated venting gas to vent into the surrounding environment.
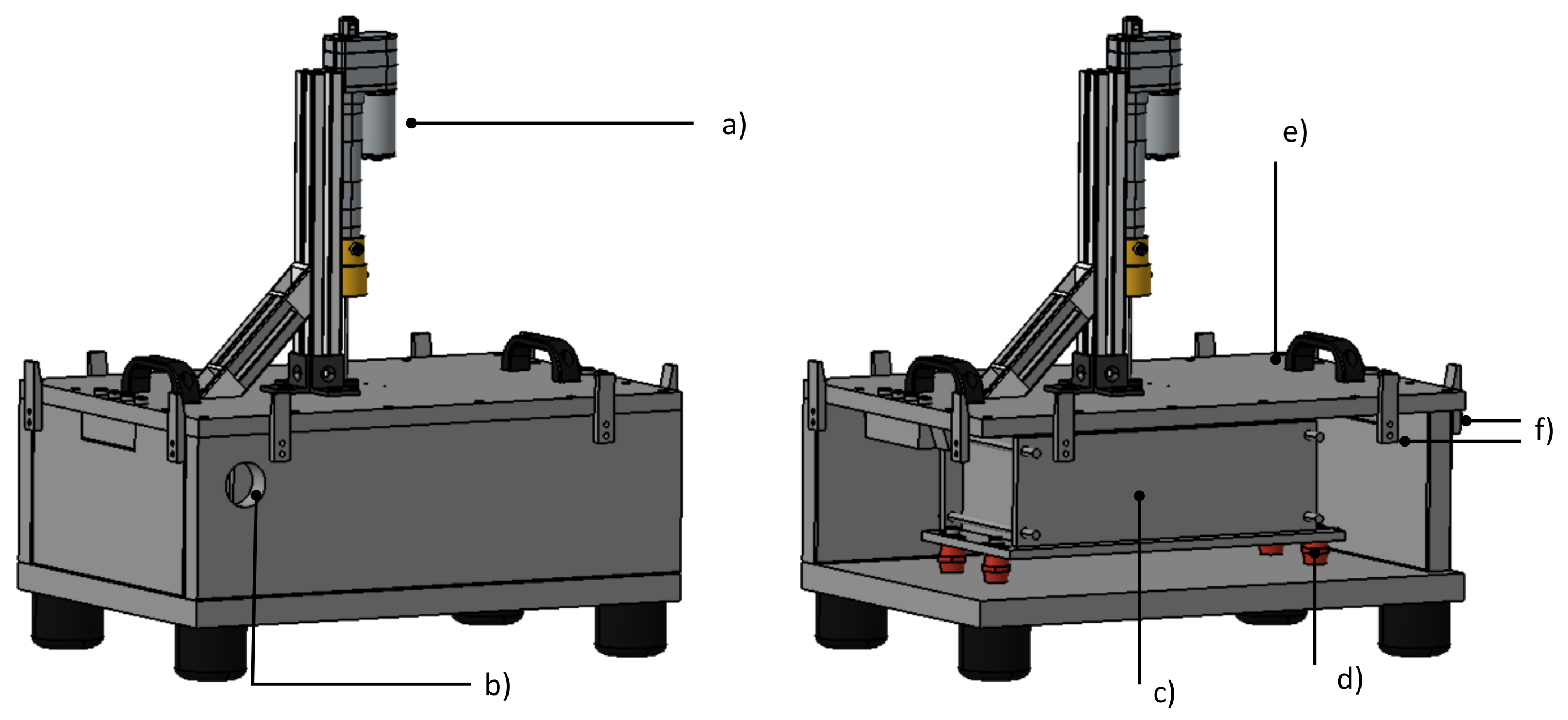
Figure 8.
CAD rendering of test bench. Left: closed test bench. Right: Placement of cell mount within the test chamber. The following components are visible: (a) Hydraulic system for nail penetration (b) Exit for exhaust line (c) Cell mount (d) Low heat conduction post insulators (e) Removable lid (f) Clamping system.
Figure 8.
CAD rendering of test bench. Left: closed test bench. Right: Placement of cell mount within the test chamber. The following components are visible: (a) Hydraulic system for nail penetration (b) Exit for exhaust line (c) Cell mount (d) Low heat conduction post insulators (e) Removable lid (f) Clamping system.
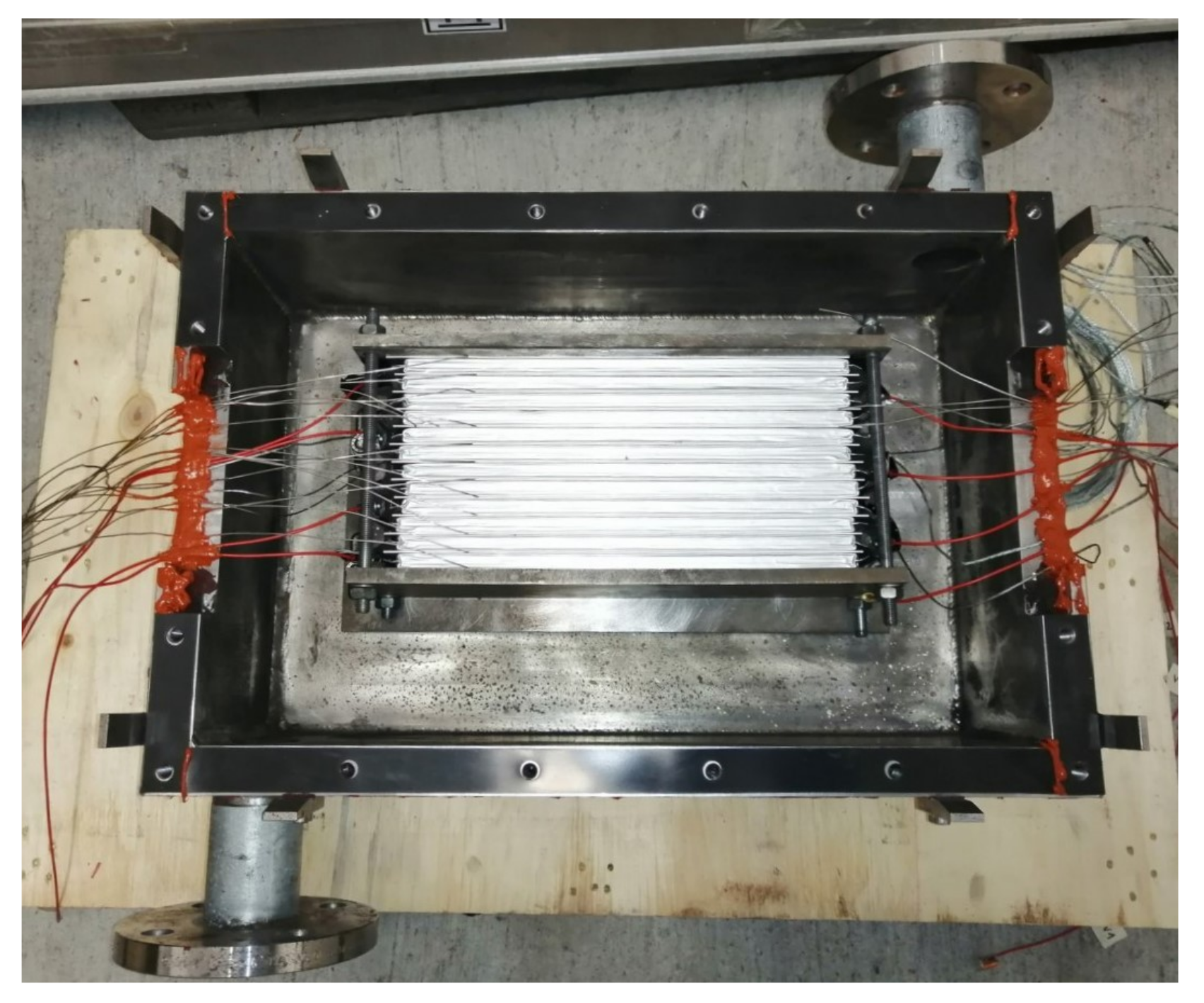
Figure 9.
View of the open test bench with a setup of 12 2p6s-connected pouch cells. The cables are routed through the sides of the box, the red Loctite sealent is already applied in this area.
Figure 9.
View of the open test bench with a setup of 12 2p6s-connected pouch cells. The cables are routed through the sides of the box, the red Loctite sealent is already applied in this area.
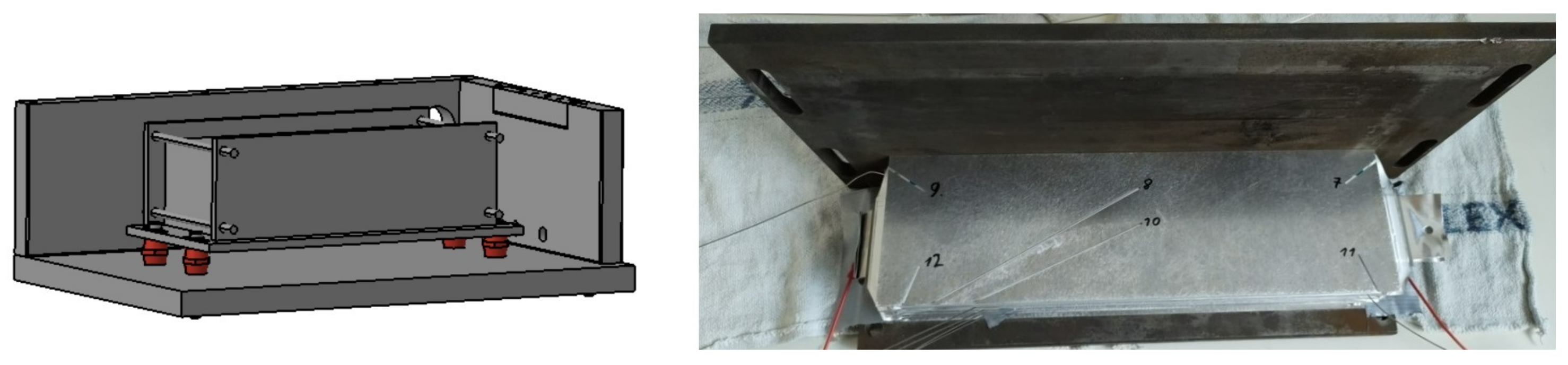
Figure 10.
(Left): Schematic overview of the cell mount connected to the test bench. (Right): Mid-assembly of the cell stack in the cell mount. The aluminium sheet between cell 4 and 5 is placed on top cell 4, the cutouts for the six temperature sensors (–) clearly visible.
Figure 10.
(Left): Schematic overview of the cell mount connected to the test bench. (Right): Mid-assembly of the cell stack in the cell mount. The aluminium sheet between cell 4 and 5 is placed on top cell 4, the cutouts for the six temperature sensors (–) clearly visible.
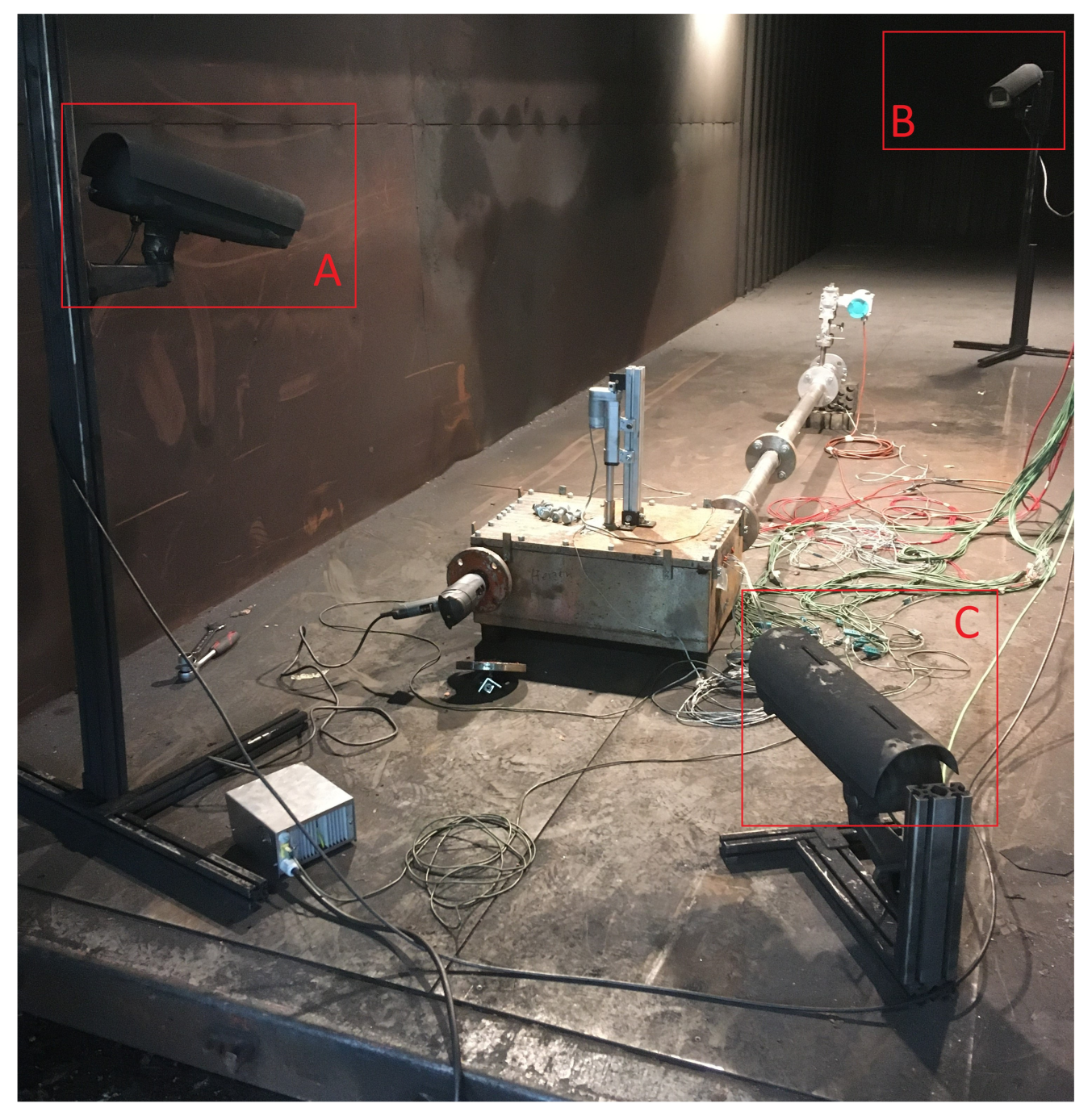
Figure 11.
Camera setup: camera A focuses on the test bench and the hydraulic system for nail penetration, camera B on the exhaust and captures the venting gas. Camera C is used in this setup to record another view angle of the test bench.
Figure 11.
Camera setup: camera A focuses on the test bench and the hydraulic system for nail penetration, camera B on the exhaust and captures the venting gas. Camera C is used in this setup to record another view angle of the test bench.
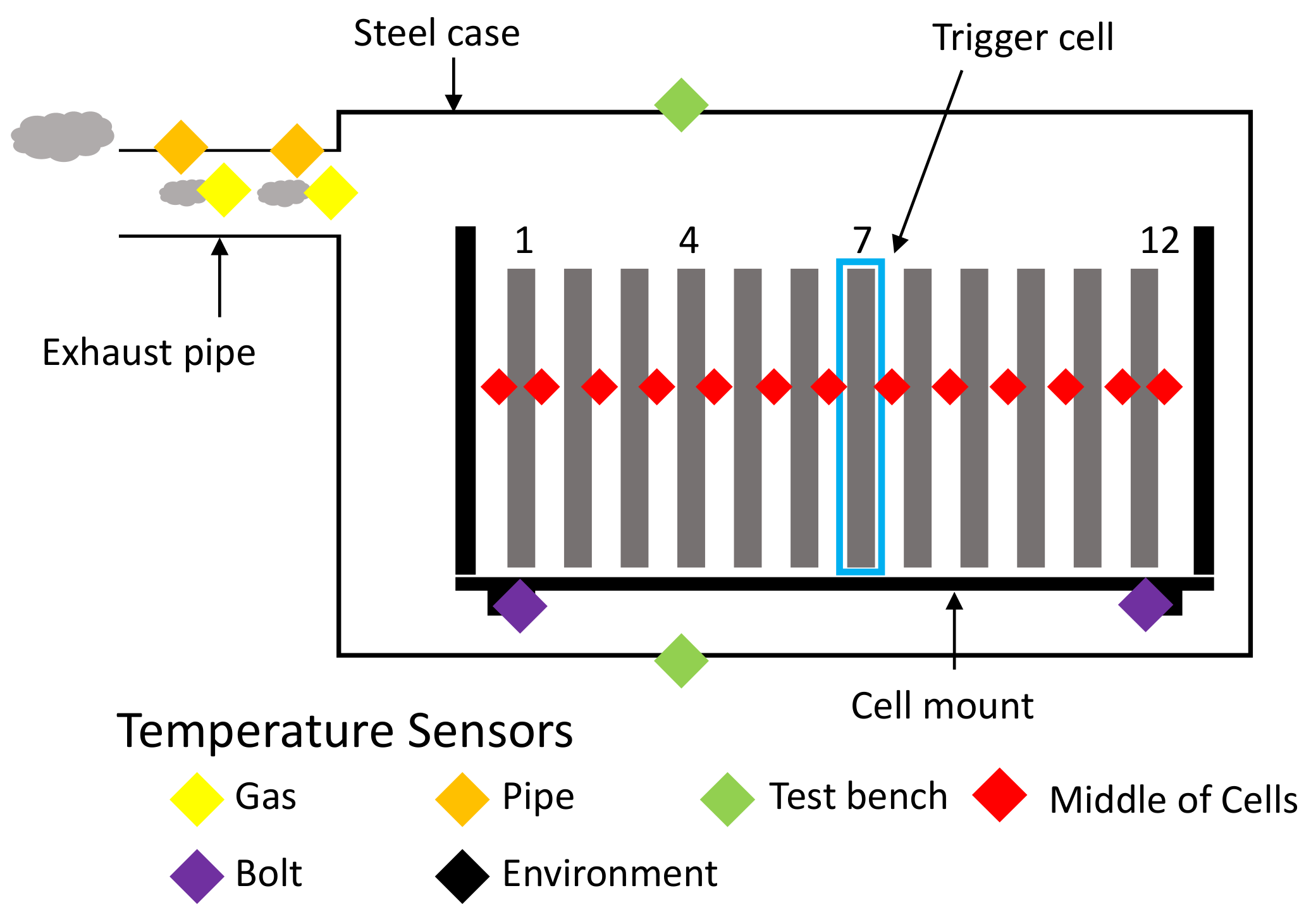
Figure 12.
Schematic overview of the temperature sensor placement across the test bench. Red: 13 centrally located between cells, respectively between cells and cell mount. Orange: On the outside of the exhaust pipe. Yellow: On the inside of the exhaust pipe. Green: Outside surface of the test bench (top and bottom). Purple: Low heat conduction post insulators. Black: Measuring ambient temperatures of the surrounding environment.
Figure 12.
Schematic overview of the temperature sensor placement across the test bench. Red: 13 centrally located between cells, respectively between cells and cell mount. Orange: On the outside of the exhaust pipe. Yellow: On the inside of the exhaust pipe. Green: Outside surface of the test bench (top and bottom). Purple: Low heat conduction post insulators. Black: Measuring ambient temperatures of the surrounding environment.
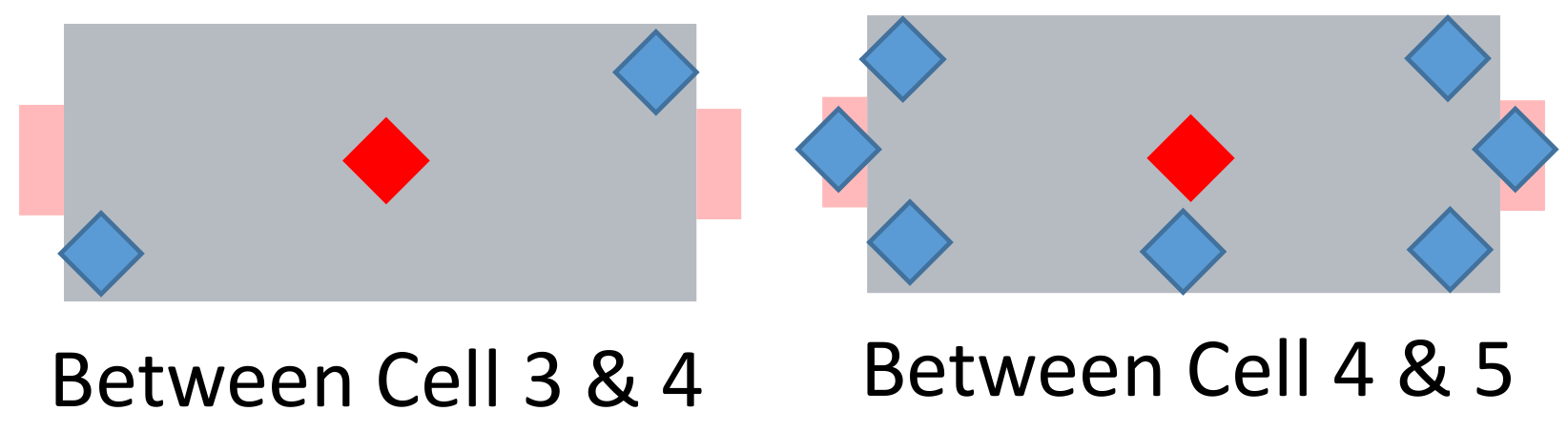
Figure 13.
Schematic view of the nicknamed “super sensor cell”. Red: two centrally located temperature sensors (see
Figure 12). Blue: additionally placed sensors.
Figure 13.
Schematic view of the nicknamed “super sensor cell”. Red: two centrally located temperature sensors (see
Figure 12). Blue: additionally placed sensors.
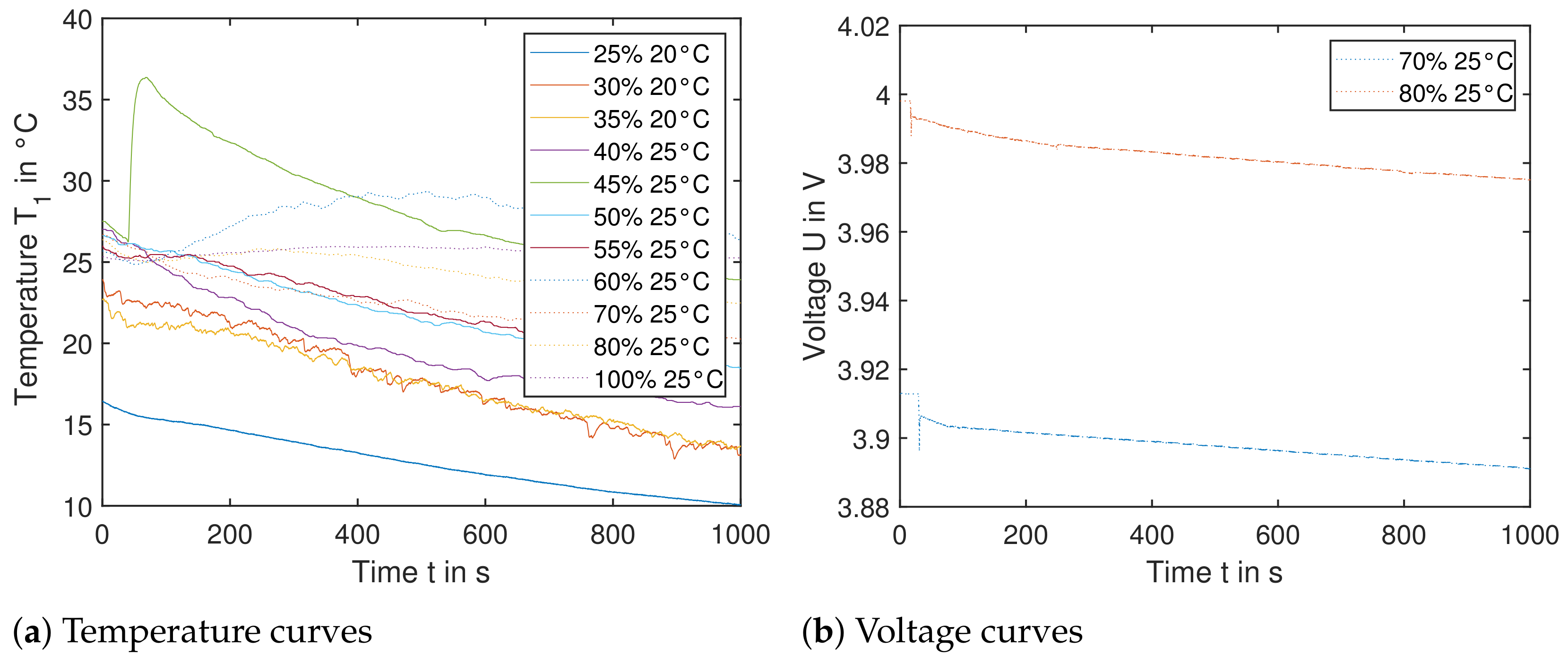
Figure 14.
Single cells triggered by nail orthogonal to electrode layers. (Left): The temperature data from sensor is shown for cells with SoC ranging from to . No significant temperature increase is recorded; therefore the cell reaction in a module would not lead to TP. (Right): The voltage U of cells with starting SoC and is shown. The sudden decrease in the voltage within the first seconds correlates with the moment, when each nail penetrates the cell. The voltage decreases slowly, leading to insignificant heat generation.
Figure 14.
Single cells triggered by nail orthogonal to electrode layers. (Left): The temperature data from sensor is shown for cells with SoC ranging from to . No significant temperature increase is recorded; therefore the cell reaction in a module would not lead to TP. (Right): The voltage U of cells with starting SoC and is shown. The sudden decrease in the voltage within the first seconds correlates with the moment, when each nail penetrates the cell. The voltage decreases slowly, leading to insignificant heat generation.
Figure 15.
Single cells triggered by nail parallel to electrode layers. (Left): The temperature data from sensor is shown for cells charged directly to a SoC of , and without cycling. A temperature increase is recorded, yet well below temperatures, where a cell’s reactions could lead to TP. (Right): The cell voltage U is decreasing as the respective cells are undergoing TR.
Figure 15.
Single cells triggered by nail parallel to electrode layers. (Left): The temperature data from sensor is shown for cells charged directly to a SoC of , and without cycling. A temperature increase is recorded, yet well below temperatures, where a cell’s reactions could lead to TP. (Right): The cell voltage U is decreasing as the respective cells are undergoing TR.
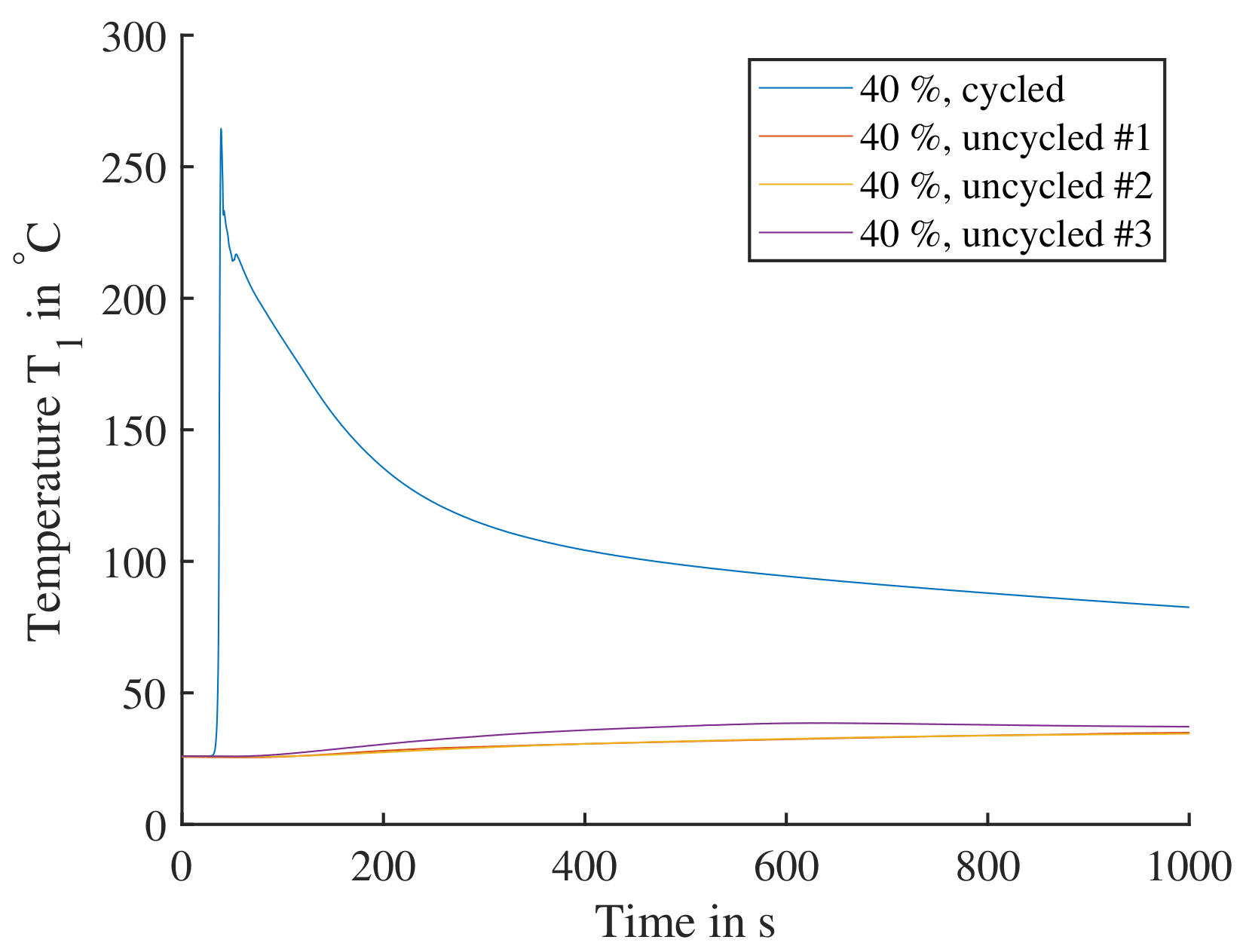
Figure 16.
Single cells triggered by nail penetration parallel to electrode layers. The cells are charged to SoC; one cell with a previous cycle is compared to three cells directly charged to without cycling. The cycled cell shows a significant reaction, where the cell reaction would lead to TP. The three uncycled cells all display a comparable insignificant reaction and would not lead to TP.
Figure 16.
Single cells triggered by nail penetration parallel to electrode layers. The cells are charged to SoC; one cell with a previous cycle is compared to three cells directly charged to without cycling. The cycled cell shows a significant reaction, where the cell reaction would lead to TP. The three uncycled cells all display a comparable insignificant reaction and would not lead to TP.
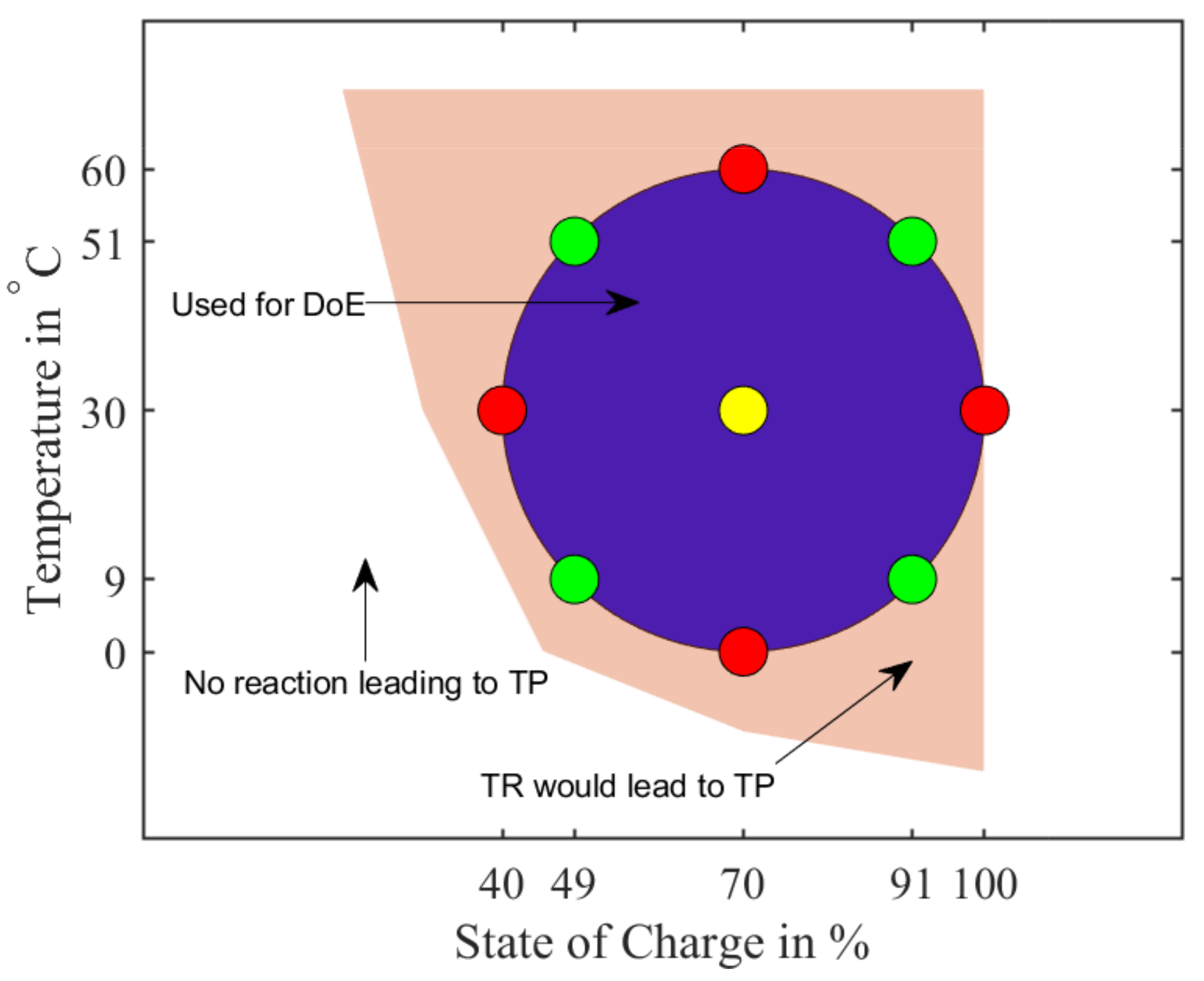
Figure 17.
Schematic view of the Design of Experiment with a central point (yellow) at and . Red markers have only one variable (T or SoC) varied in regards to the central point, whereas the green markers have both variables changed.
Figure 17.
Schematic view of the Design of Experiment with a central point (yellow) at and . Red markers have only one variable (T or SoC) varied in regards to the central point, whereas the green markers have both variables changed.
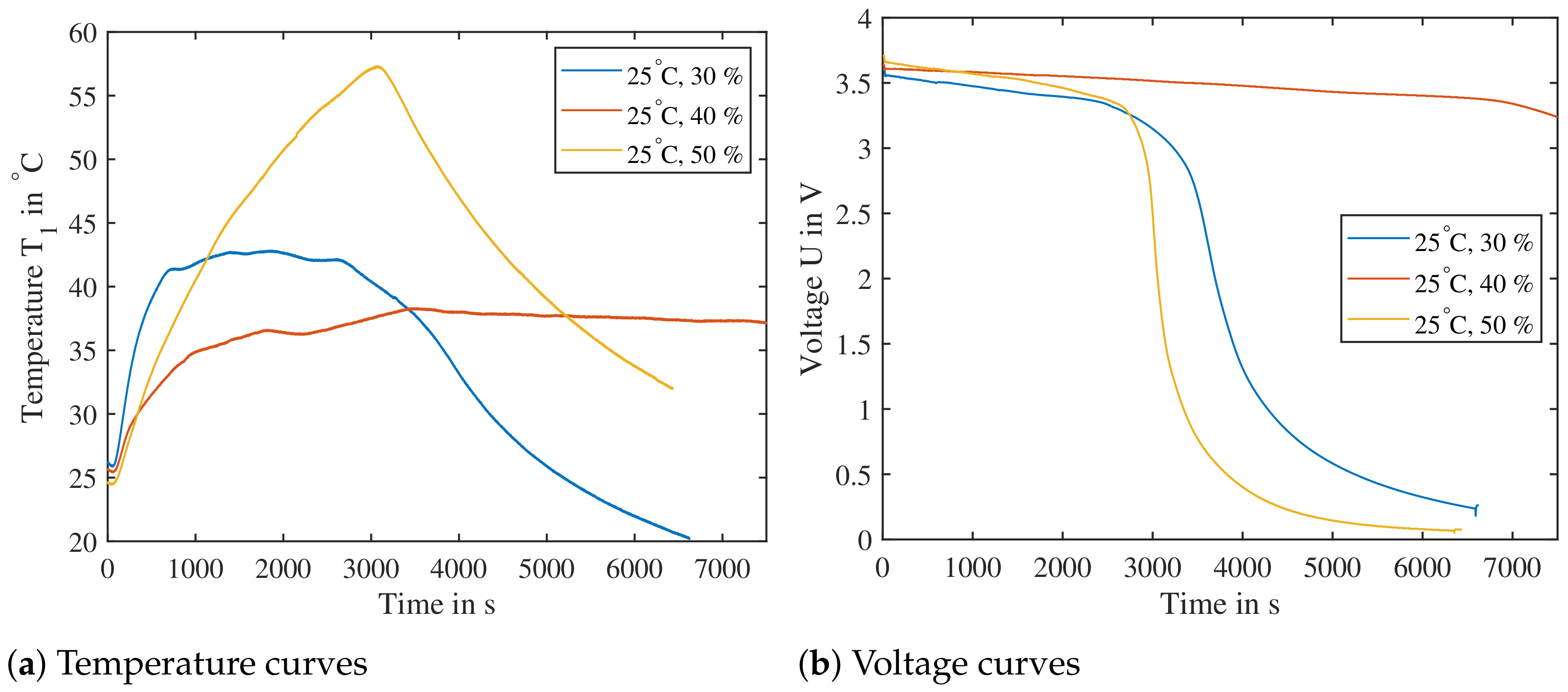
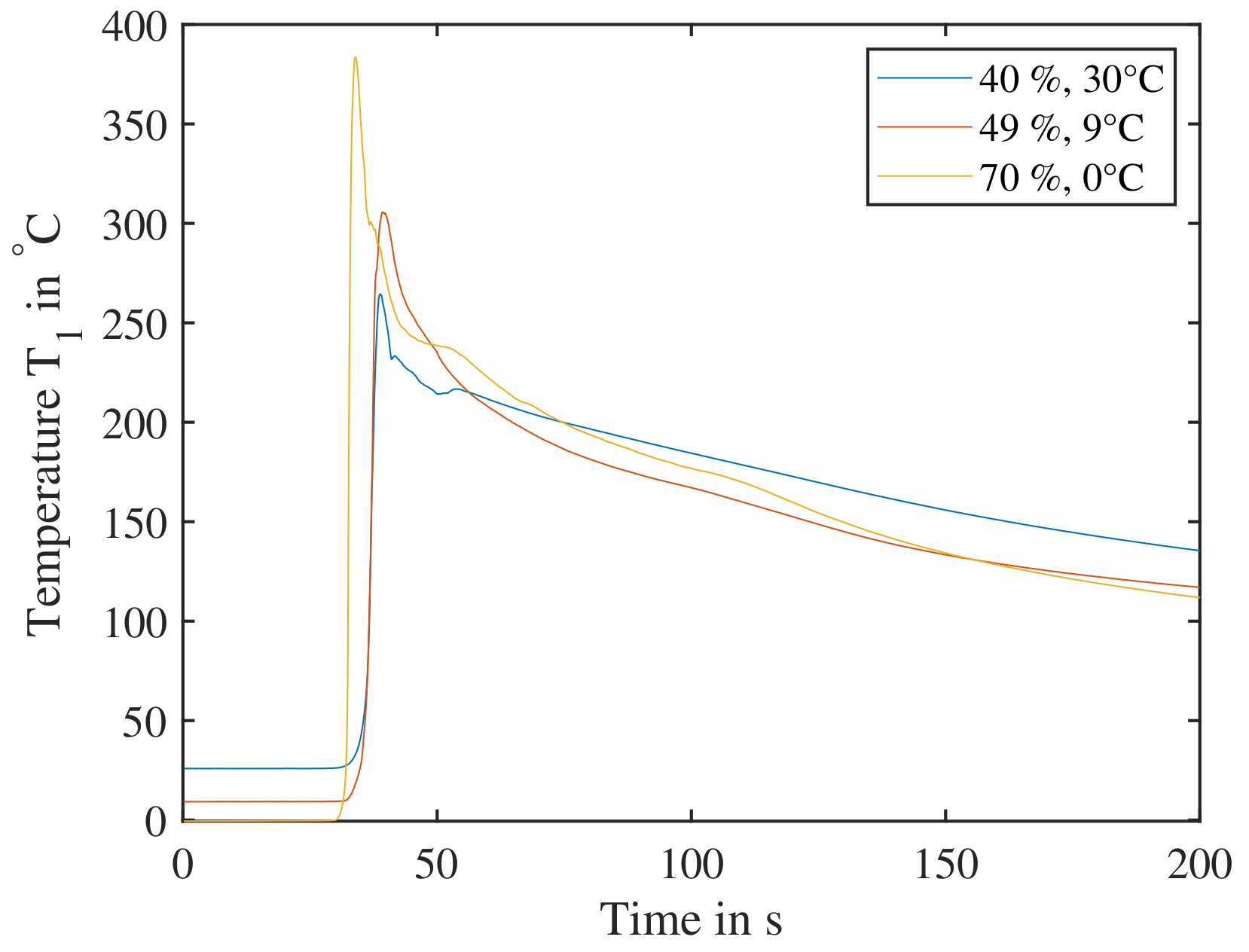
Figure 18.
Combinations of temperature/SoC from the DoE of low expected reactivity tested for their heat release. The cells are triggered parallel to the electrode layers and are subjected to a previous cycle. Cell reaction at point A ( at ), B ( at ) and C ( at ) are shown, all three clearly showing a temperature increase sufficient for triggering a propagation.
Figure 18.
Combinations of temperature/SoC from the DoE of low expected reactivity tested for their heat release. The cells are triggered parallel to the electrode layers and are subjected to a previous cycle. Cell reaction at point A ( at ), B ( at ) and C ( at ) are shown, all three clearly showing a temperature increase sufficient for triggering a propagation.
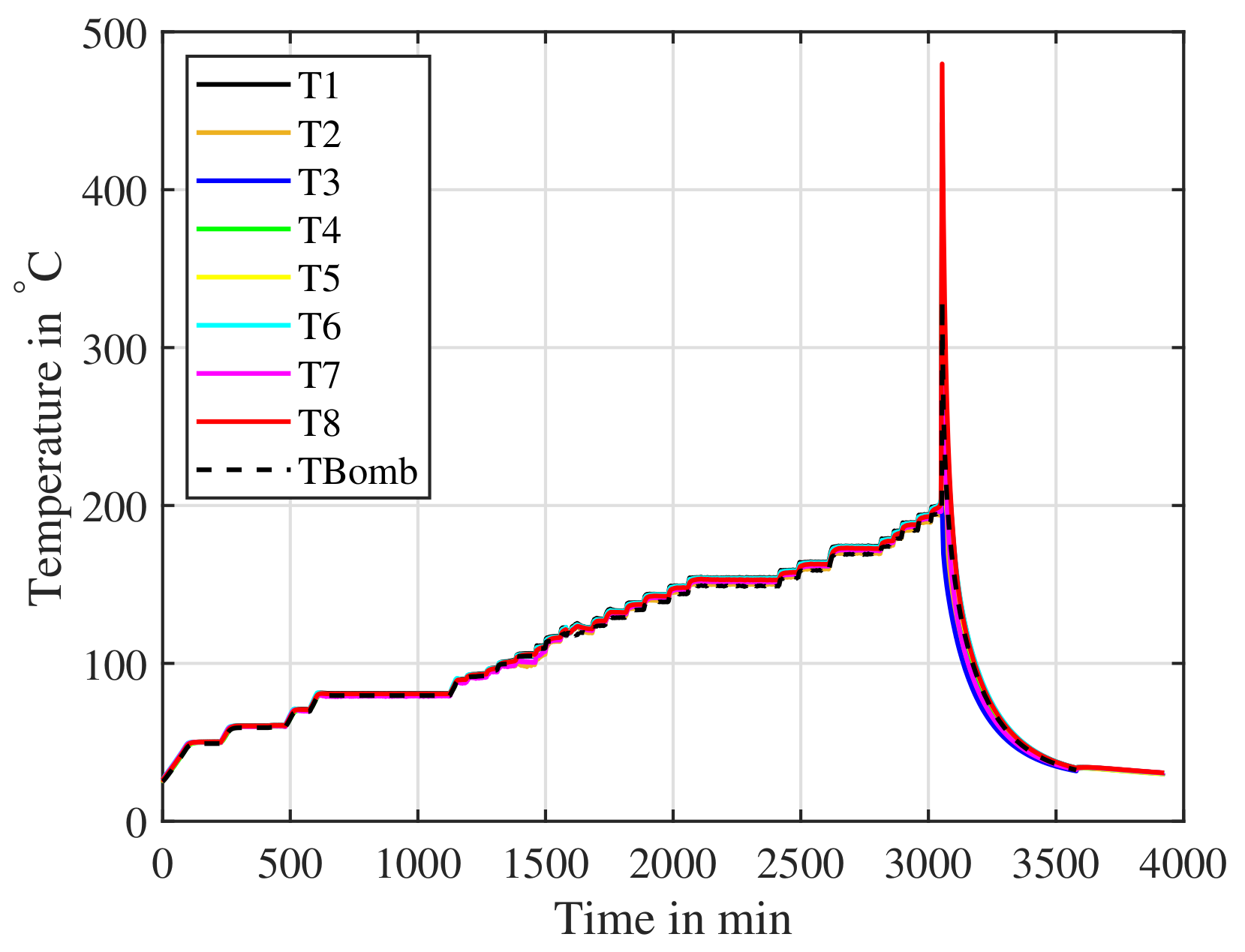
Figure 19.
A cell cycled and then charged to is placed inside an ARC. The cell is slowly heated and the ARC searches for a response from the cell. The ARC detects the thermal runaway from onwards.
Figure 19.
A cell cycled and then charged to is placed inside an ARC. The cell is slowly heated and the ARC searches for a response from the cell. The ARC detects the thermal runaway from onwards.
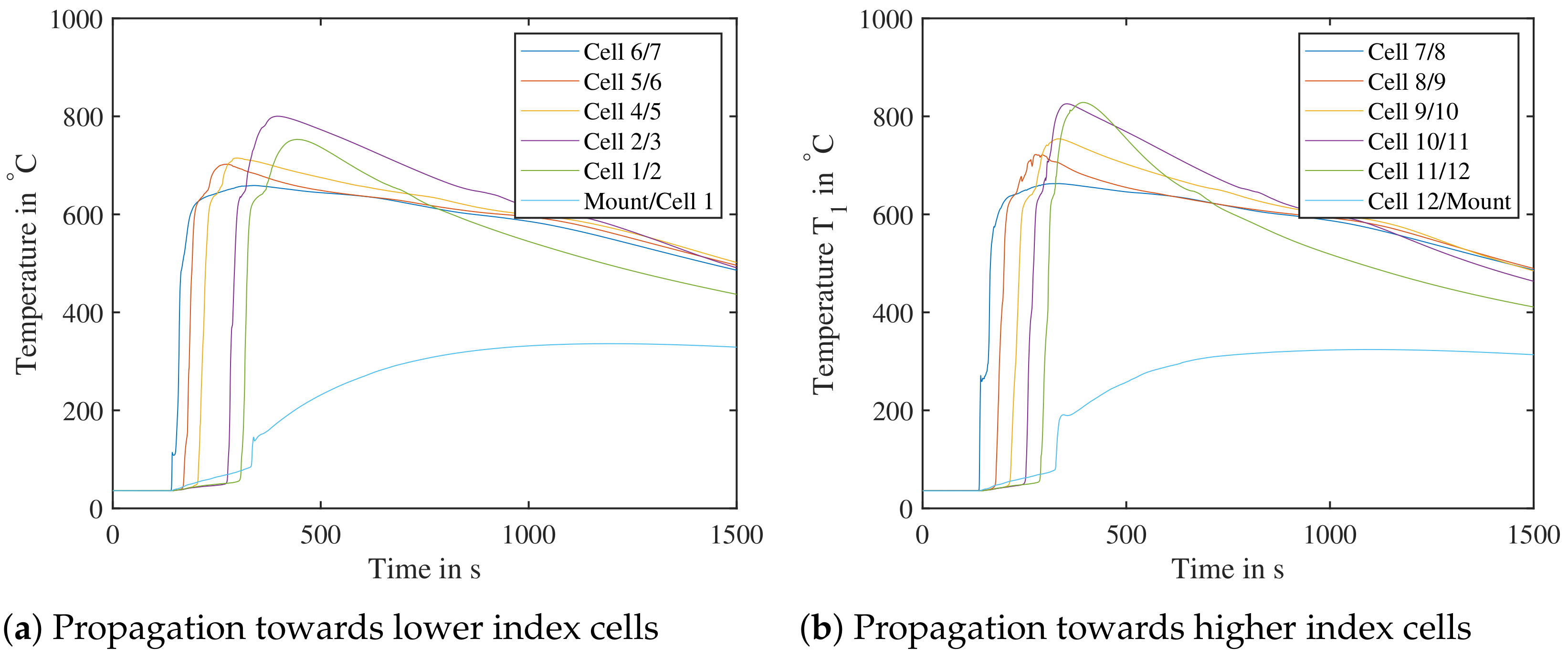
Figure 20.
Test bench validation with air, cell number 7 triggered by nail penetration. The cell pack was tested at an initial setup of SoC at . (Left): Propagation towards cells with lower index—Temperature sensor between cell 3 and 4 missing. (Right): Propagation towards cells with higher index.
Figure 20.
Test bench validation with air, cell number 7 triggered by nail penetration. The cell pack was tested at an initial setup of SoC at . (Left): Propagation towards cells with lower index—Temperature sensor between cell 3 and 4 missing. (Right): Propagation towards cells with higher index.
Figure 21.
Visualized propagation through the cell pack in air and nitrogen. Both cell packs was tested at an initial setup of SoC at . Temperature sensor 1 is “Mount/Cell 1”, sensor 2 “Cell 1/2”, etc.
Figure 21.
Visualized propagation through the cell pack in air and nitrogen. Both cell packs was tested at an initial setup of SoC at . Temperature sensor 1 is “Mount/Cell 1”, sensor 2 “Cell 1/2”, etc.
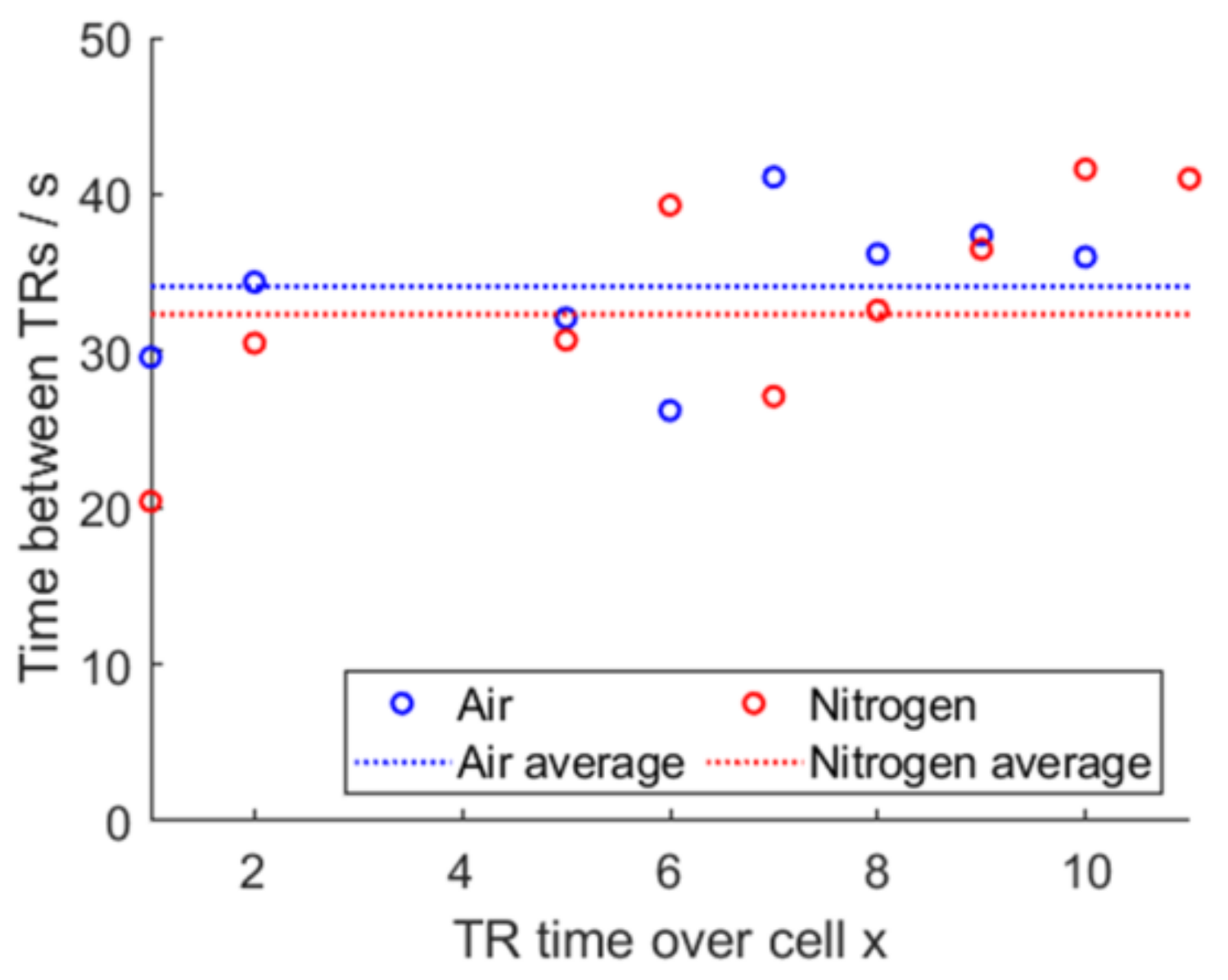
Figure 22.
Propagation time difference and averaged time, air vs. nitrogen.
Figure 22.
Propagation time difference and averaged time, air vs. nitrogen.