Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Si@Void@C Particle
2.2. Characterization
2.3. Battery Preparations
2.4. Electrochemical Measurements
3. Results and Discussion
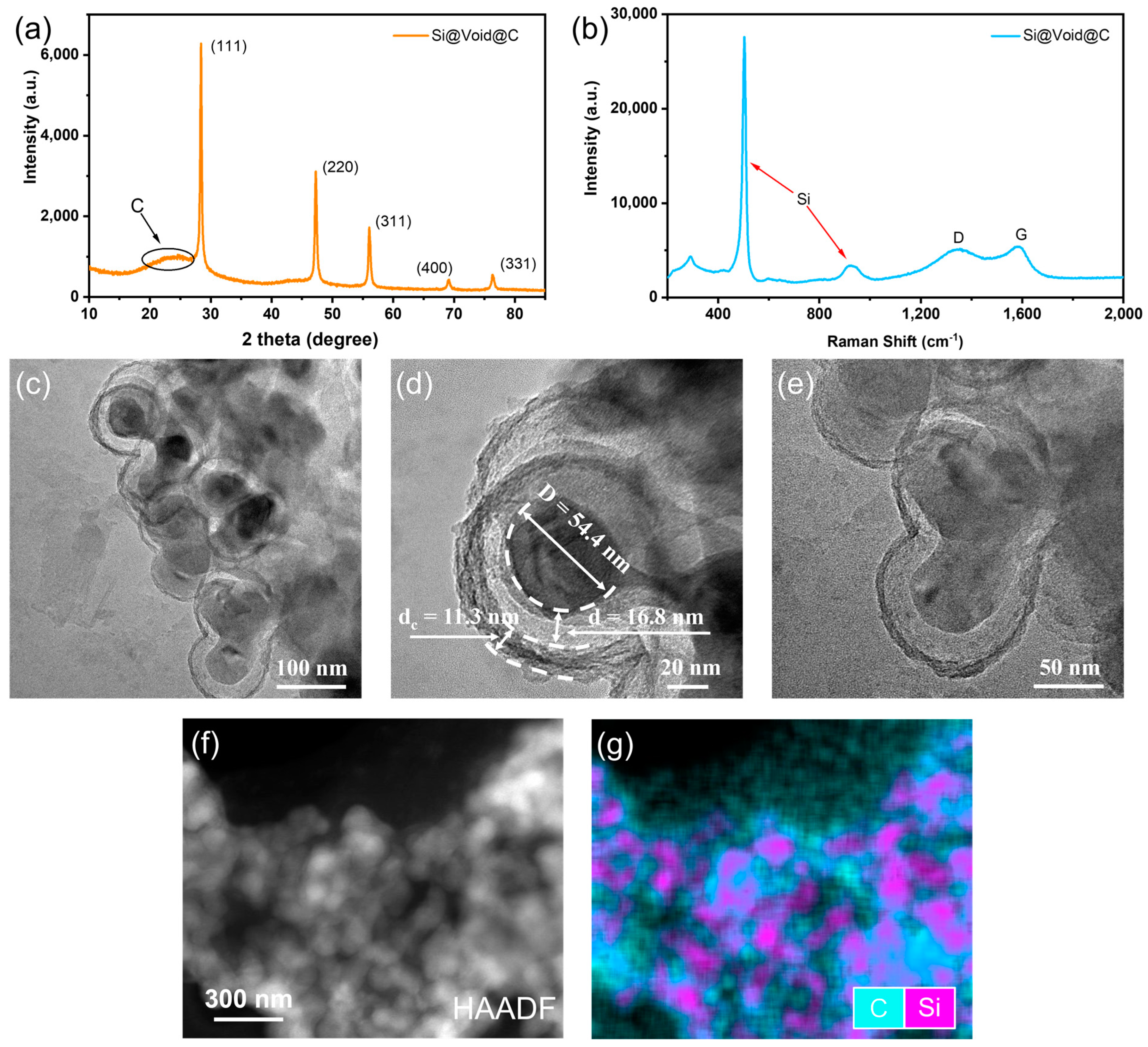
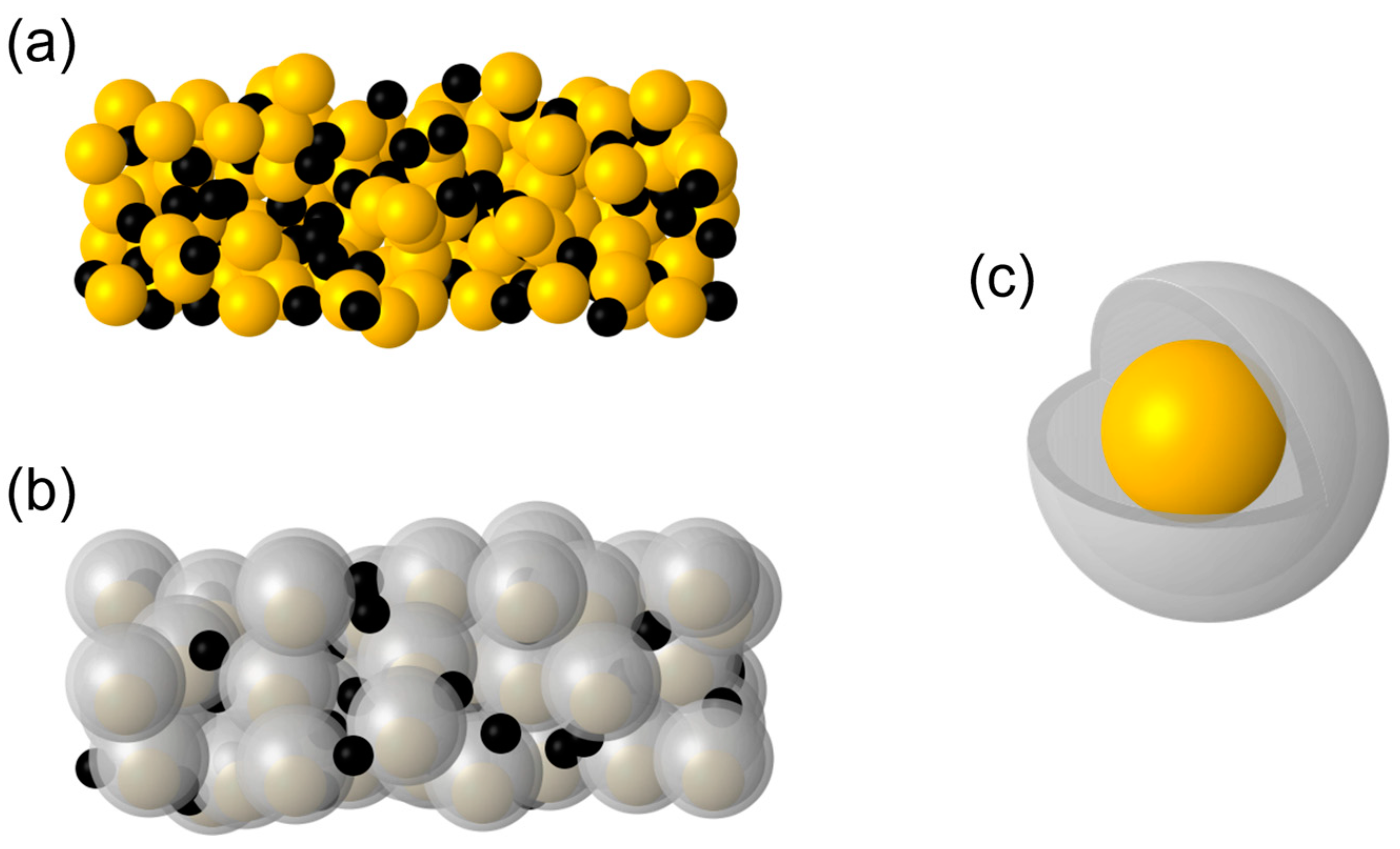
3.1. Synthesis of the Yolk-Shell Si@Void@C Particle
3.2. Yolk-Shell Structure of Si@Void@C
3.3. Electrochemical Performances of Si@Void@C Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Gong, M.; Cui, W.; Zheng, G.; Song, M. MOF-Derived Hetero-Zn/Co Hollow Core-Shell TMOs as Anode for Lithium-Ion Batteries. Coatings 2022, 12, 1487. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Lai, Y.; Yang, Z.; Yang, Q.; Liu, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Revisiting the Preparation Progress of Nano-Structured Si Anodes toward Industrial Application from the Perspective of Cost and Scalability. Adv. Energy Mater. 2022, 12, 2102181. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon Oxides: A Promising Family of Anode Materials for Lithium-Ion Batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotech. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Tang, H.; Xu, Y.; Liu, L.; Zhao, D.; Zhang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; Wang, Z. A Hollow Silicon Nanosphere/Carbon Nanotube Composite as an Anode Material for Lithium-Ion Batteries. Coatings 2022, 12, 1515. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured Silicon for High Capacity Lithium Battery Anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic Electrochemical Characterizations of Si and SiO Anodes for High-Capacity Li-Ion Batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, R.; Jiang, R.; Song, X.; Jin, Z.; Sun, L. A Review of Existing and Emerging Binders for Silicon Anodic Li-Ion Batteries. Nano Res. 2023, 16, 6736–6752. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, Y.; Zheng, X.; Or, T.; Ma, Q.; Qian, L.; Deng, Y.; Yu, A.; Li, J.; Chen, Z. Design Criteria for Silicon-Based Anode Binders in Half and Full Cells. Adv. Energy Mater. 2022, 12, 2200850. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Yuan, L.; You, C.; Wu, J.; Liu, L.; Ye, J.; Wu, Y.; Fu, L. Recent Advances in Modification Strategies of Silicon-Based Lithium-Ion Batteries. Nano Res. 2023, 16, 3781–3803. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, H.; Li, X.; Song, T.; Pei, Y.; Liu, L.; Long, B.; Wu, X.; Wang, X. Sn-Doped BiOCl Nanosheet with Synergistic H+/Zn2+ Co-Insertion for “Rocking Chair” Zinc-Ion Battery. J. Energy Chem. 2023, 81, 623–632. [Google Scholar] [CrossRef]
- Park, J.-K. Principles and Applications of Lithium Secondary Batteries; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-3-527-65042-2. [Google Scholar]
- Wolfenstine, J. Critical Grain Size for Microcracking during Lithium Insertion. J. Power Sources 1999, 79, 111–113. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-Performance Lithium-Ion Anodes Using a Hierarchical Bottom-up Approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, G.-L.; Wang, S.; Zhang, J.; Wang, J.; Zhong, W.; Tang, F.; Yang, Z.-L.; Zheng, J.; Li, X. In Situ Catalytic Growth 3D Multi-Layers Graphene Sheets Coated Nano-Silicon Anode for High Performance Lithium-Ion Batteries. Chem. Eng. J. 2019, 356, 895–903. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Dou, Y.; Cheng, N.; Cui, D.; Du, Y.; Liu, P.; Al-Mamun, M.; Zhang, S.; Zhao, H. A Yolk–Shell Structured Silicon Anode with Superior Conductivity and High Tap Density for Full Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 8824–8828. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M. Polyaniline Nanostructures and the Role of Aniline Oligomers in Their Formation. Prog. Polym. Sci. 2010, 35, 1420–1481. [Google Scholar] [CrossRef]
- Kuzmany, H.; Sariciftci, N.S. In Situ Spectro-Electrochemical Studies of Polyaniline. Synth. Met. 1987, 18, 353–358. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline: A New Concept in Conducting Polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y. Electrochemical Performance of Manganese Coordinated Polyaniline. Adv. Electron. Mater. 2019, 5, 1900816. [Google Scholar] [CrossRef]
- Molapo, K.M.; Ndangili, P.M.; Ajayi, R.F.; Mbambisa, G.; Mailu, S.M.; Njomo, N.; Masikini, M.; Baker, P.; Iwuoha, E.I. Electronics of Conjugated Polymers (I): Polyaniline. Int. J. Electrochem. Sci. 2012, 7, 11859–11875. [Google Scholar] [CrossRef]
- Lu, B.; Ma, B.; Deng, X.; Li, W.; Wu, Z.; Shu, H.; Wang, X. Cornlike Ordered Mesoporous Silicon Particles Modified by Nitrogen-Doped Carbon Layer for the Application of Li-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 32829–32839. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Chou, S.; Xu, Y.; Li, W.; Kong, B.; Dou, S.X.; Liu, H.K.; Yang, J. Critical Thickness of Phenolic Resin-Based Carbon Interfacial Layer for Improving Long Cycling Stability of Silicon Nanoparticle Anodes. Nano Energy 2016, 27, 255–264. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying Disorder in Graphite-Based Systems by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, S.; Huang, C.; Wang, X.; Cai, S.; Xiang, K.; Zhang, Y.; Xue, J. Honeycomb-like Nitrogen and Sulfur Dual-Doped Hierarchical Porous Biomass-Derived Carbon for Lithium–Sulfur Batteries. ChemSusChem 2017, 10, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; You, Y.; Xin, S.; Yin, Y.-X.; Zhang, J.; Wang, P.; Zheng, X.; Cao, F.-F.; Guo, Y.-G. Rice Husk-Derived Hierarchical Silicon/Nitrogen-Doped Carbon/Carbon Nanotube Spheres as Low-Cost and High-Capacity Anodes for Lithium-Ion Batteries. Nano Energy 2016, 25, 120–127. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, Z.; Li, J.; Meng, Y.; Huang, X. Evaluation of the Electrochemical and Expansion Performances of the Sn-Si/Graphite Composite Electrode for the Industrial Use. Energy Mater 2022, 2, 200004. [Google Scholar] [CrossRef]
- Key, B.; Morcrette, M.; Tarascon, J.-M.; Grey, C.P. Pair Distribution Function Analysis and Solid State NMR Studies of Silicon Electrodes for Lithium Ion Batteries: Understanding the (De)Lithiation Mechanisms. J. Am. Chem. Soc. 2011, 133, 503–512. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Kong, W.; Du, G.; Li, S.; Tang, Y.; Cao, J.; Lu, X.; Tan, R.; Qian, G. Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries. Batteries 2023, 9, 446. https://doi.org/10.3390/batteries9090446
Yang X, Kong W, Du G, Li S, Tang Y, Cao J, Lu X, Tan R, Qian G. Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries. Batteries. 2023; 9(9):446. https://doi.org/10.3390/batteries9090446
Chicago/Turabian StyleYang, Xiangjie, Weikang Kong, Guangyuan Du, Shilong Li, Yueyuan Tang, Jun Cao, Xueyi Lu, Rui Tan, and Guoyu Qian. 2023. "Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries" Batteries 9, no. 9: 446. https://doi.org/10.3390/batteries9090446
APA StyleYang, X., Kong, W., Du, G., Li, S., Tang, Y., Cao, J., Lu, X., Tan, R., & Qian, G. (2023). Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries. Batteries, 9(9), 446. https://doi.org/10.3390/batteries9090446






