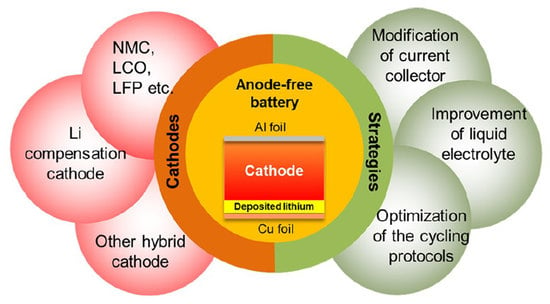
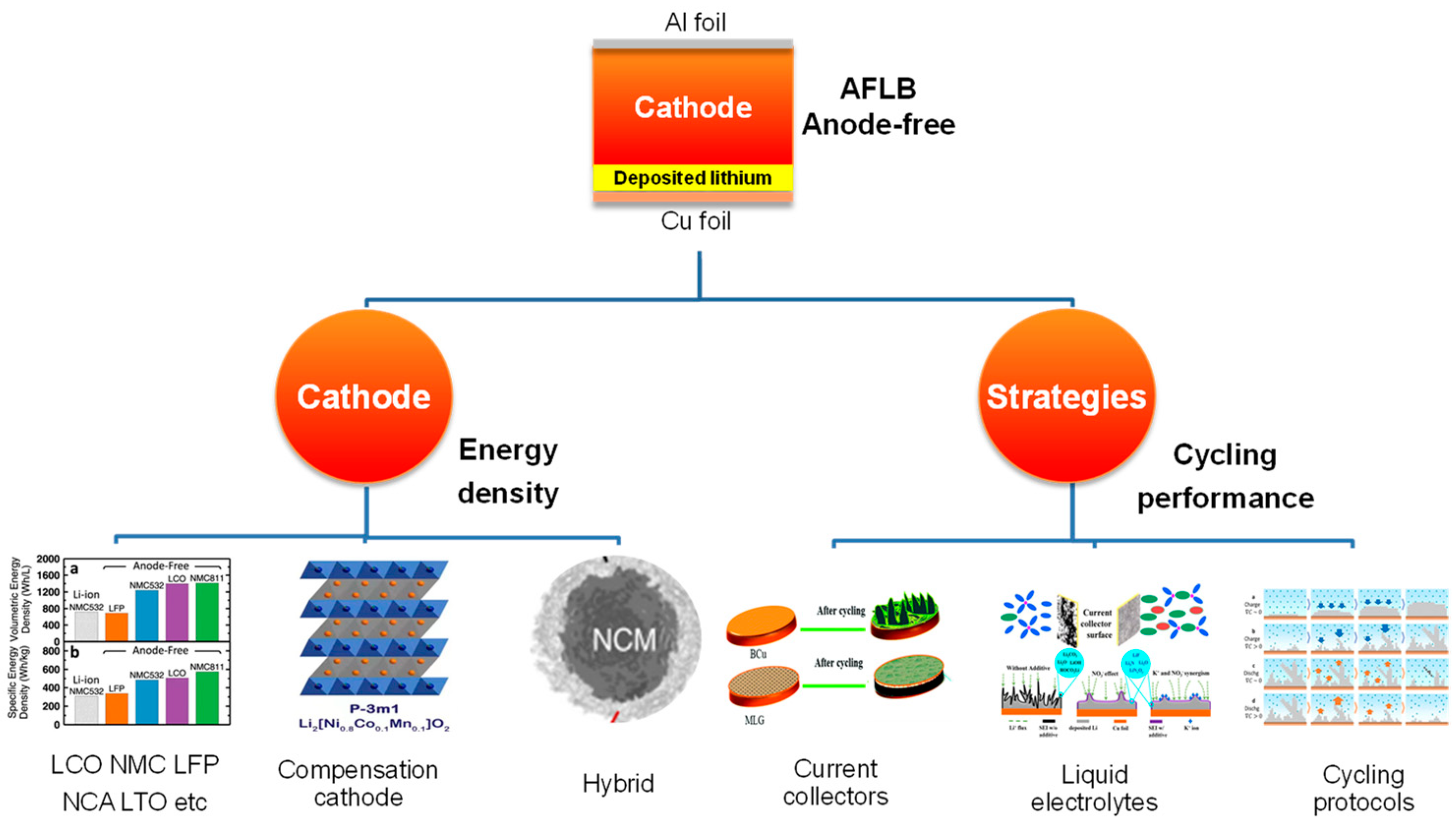
Designs of Anode-Free Lithium-Ion Batteries
Abstract
1. Introduction
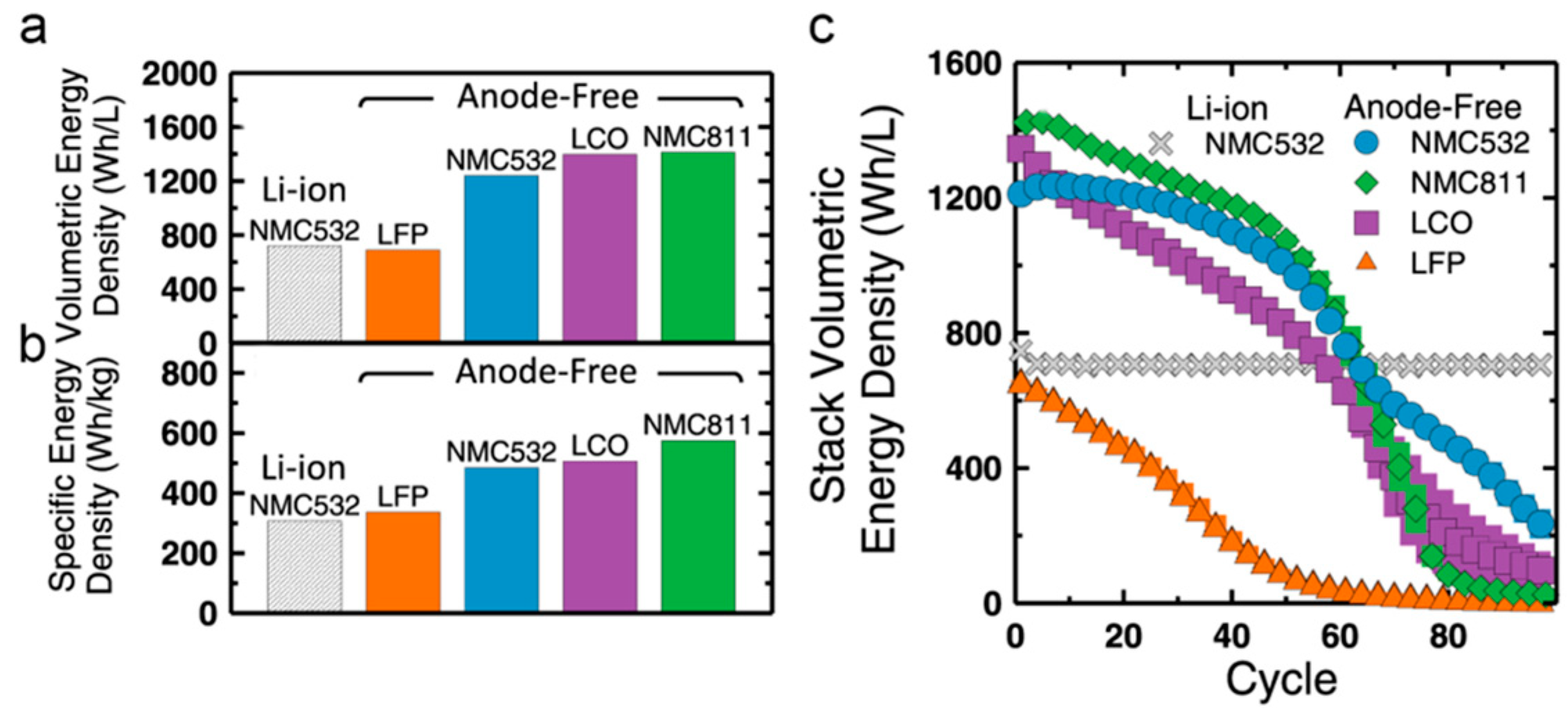
2. Cathodes of Anode-Free Lithium-ion Batteries
3. Strategies Applied to AFLB and Improvement
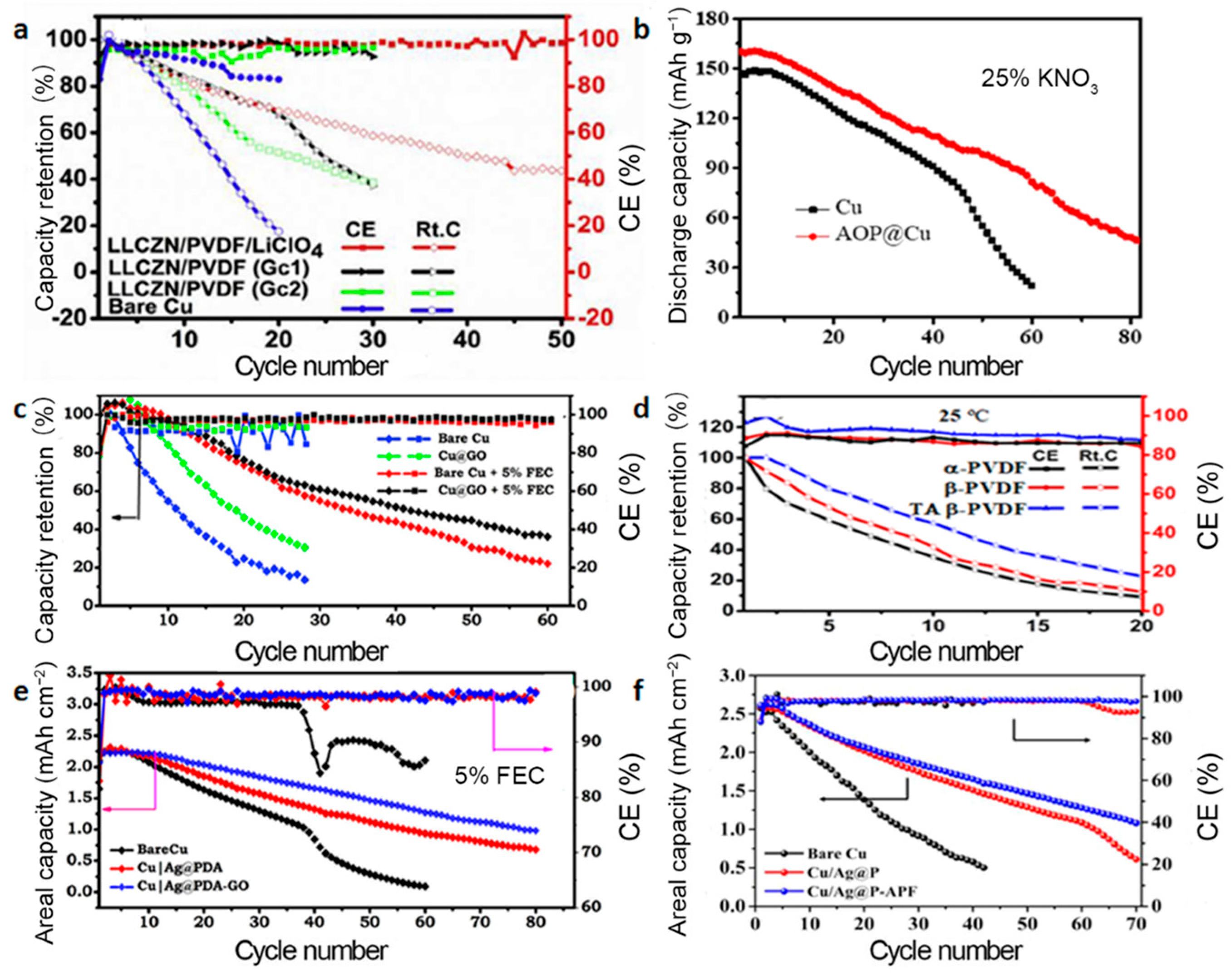
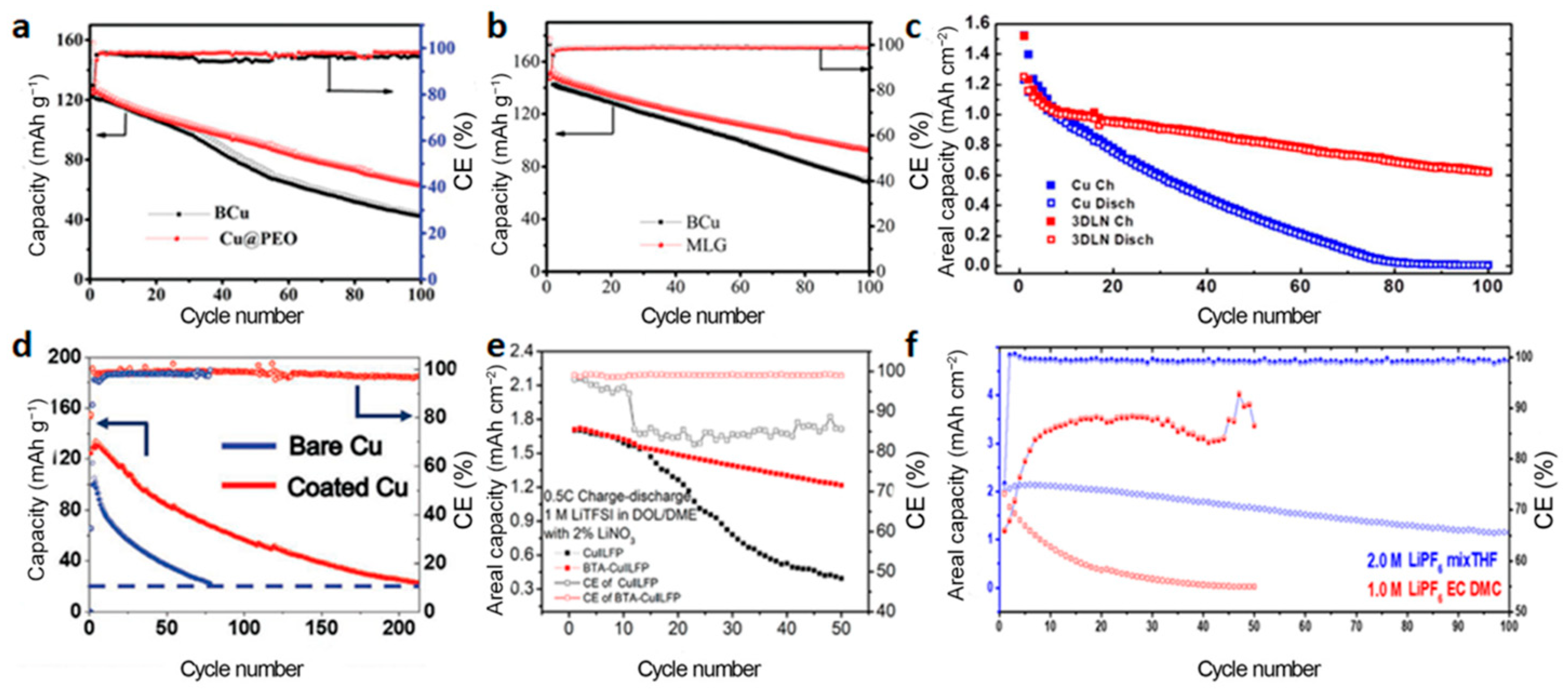
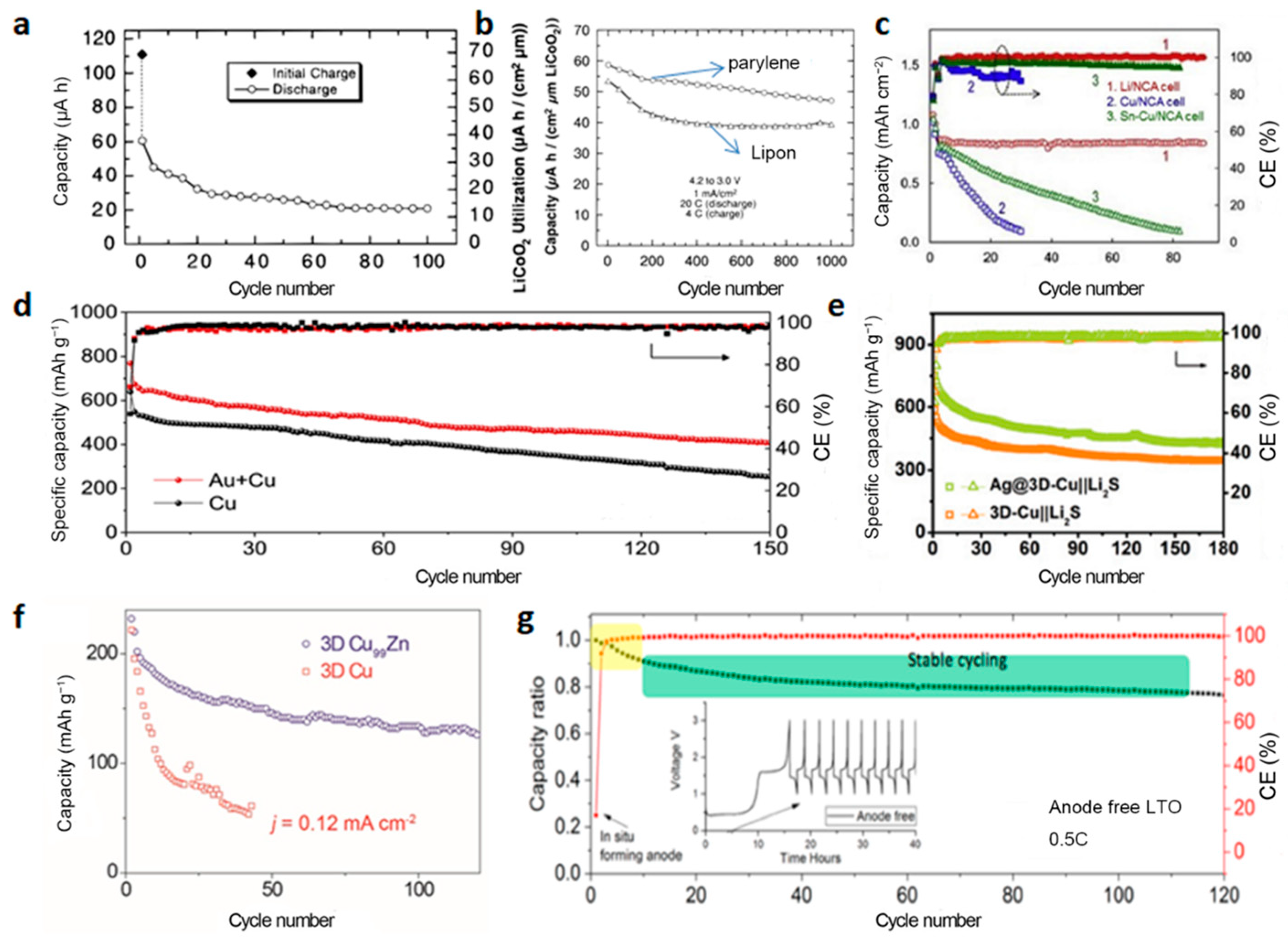
3.1. Modification of the Current Collectors
3.1.1. NMC Cathode
3.1.2. LFP Cathode
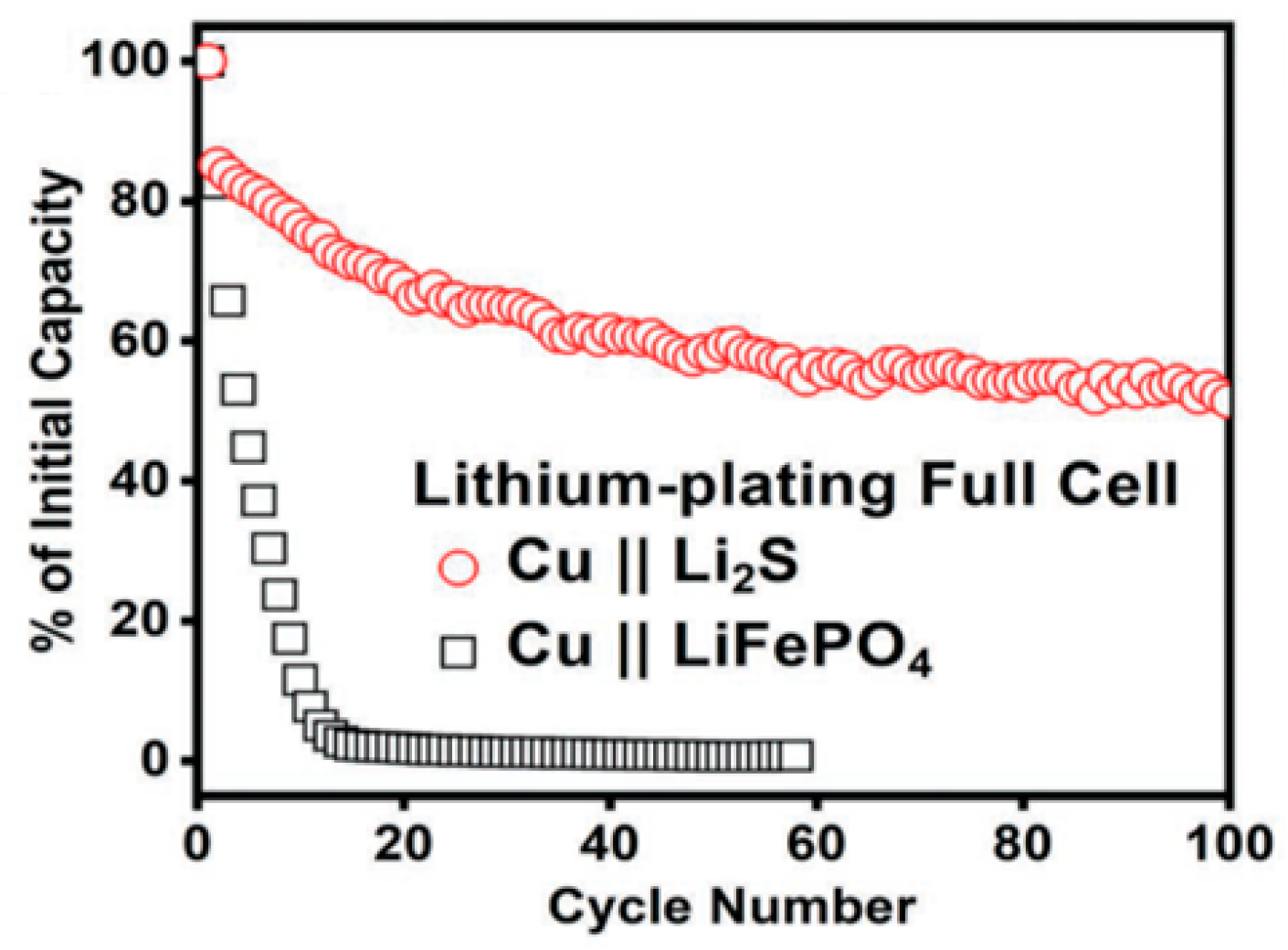
3.1.3. Other Cathodes
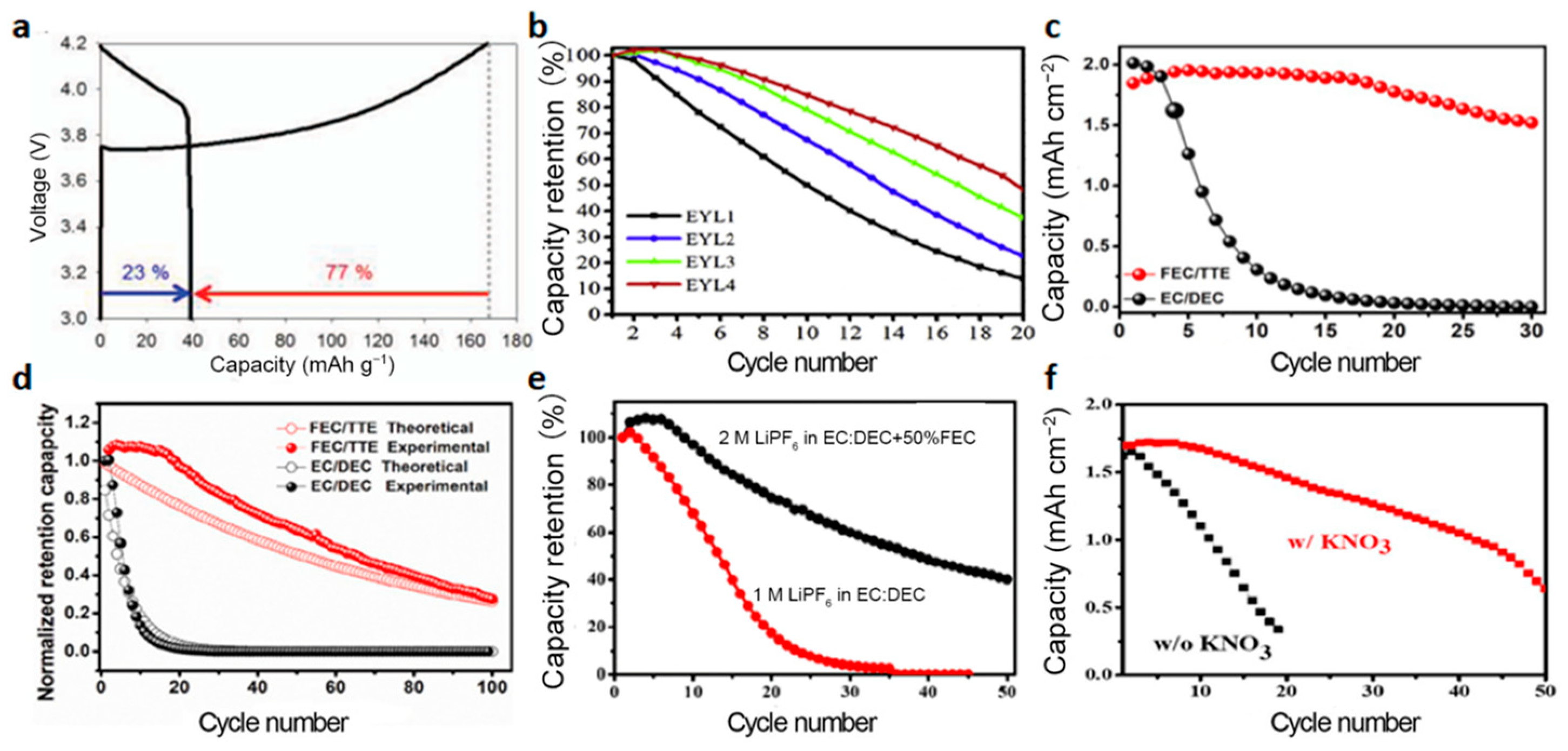
3.2. Improvement of the Liquid Electrolytes
3.2.1. NMC Cathode
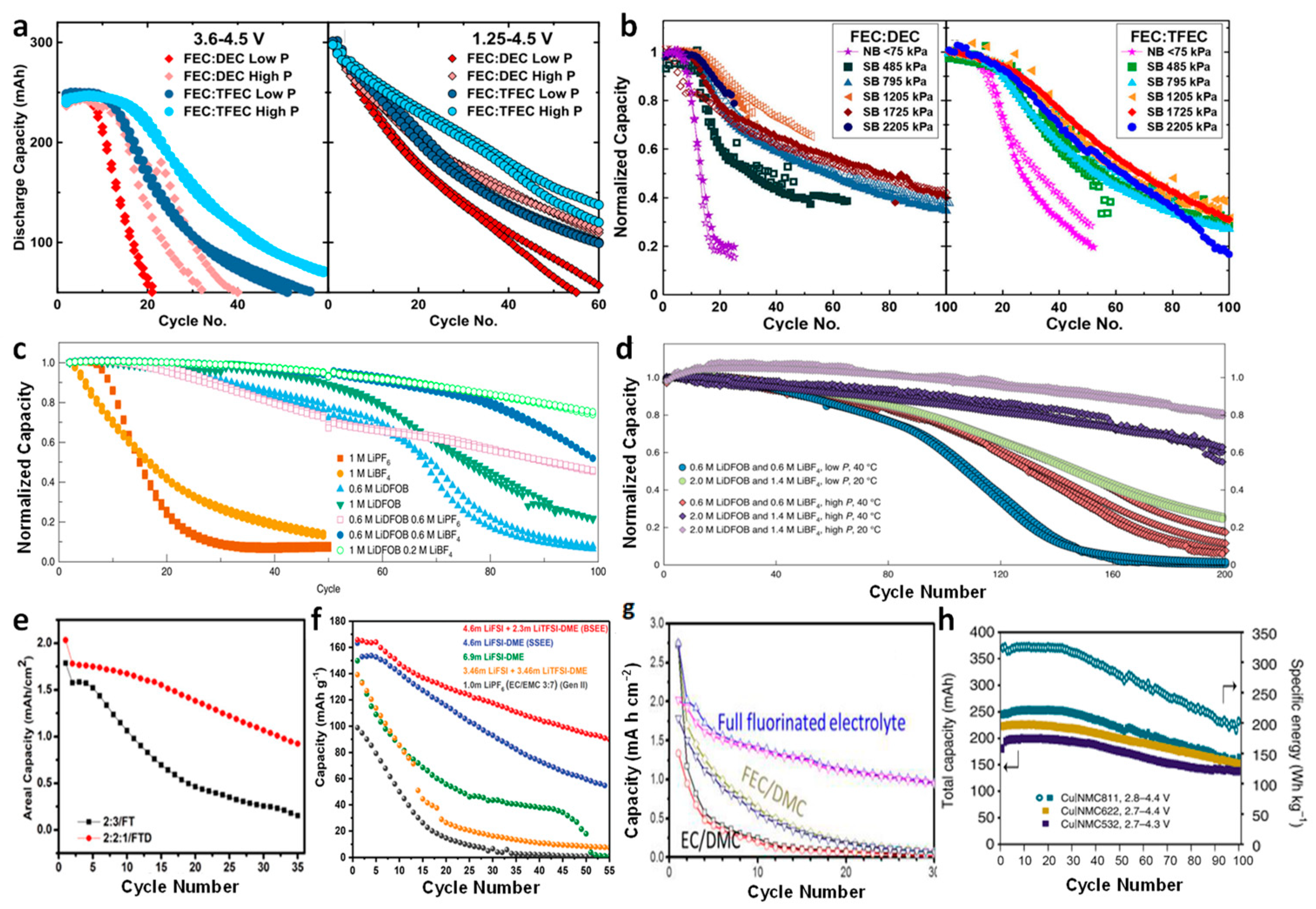
3.2.2. LFP Cathode
3.2.3. Other Cathodes
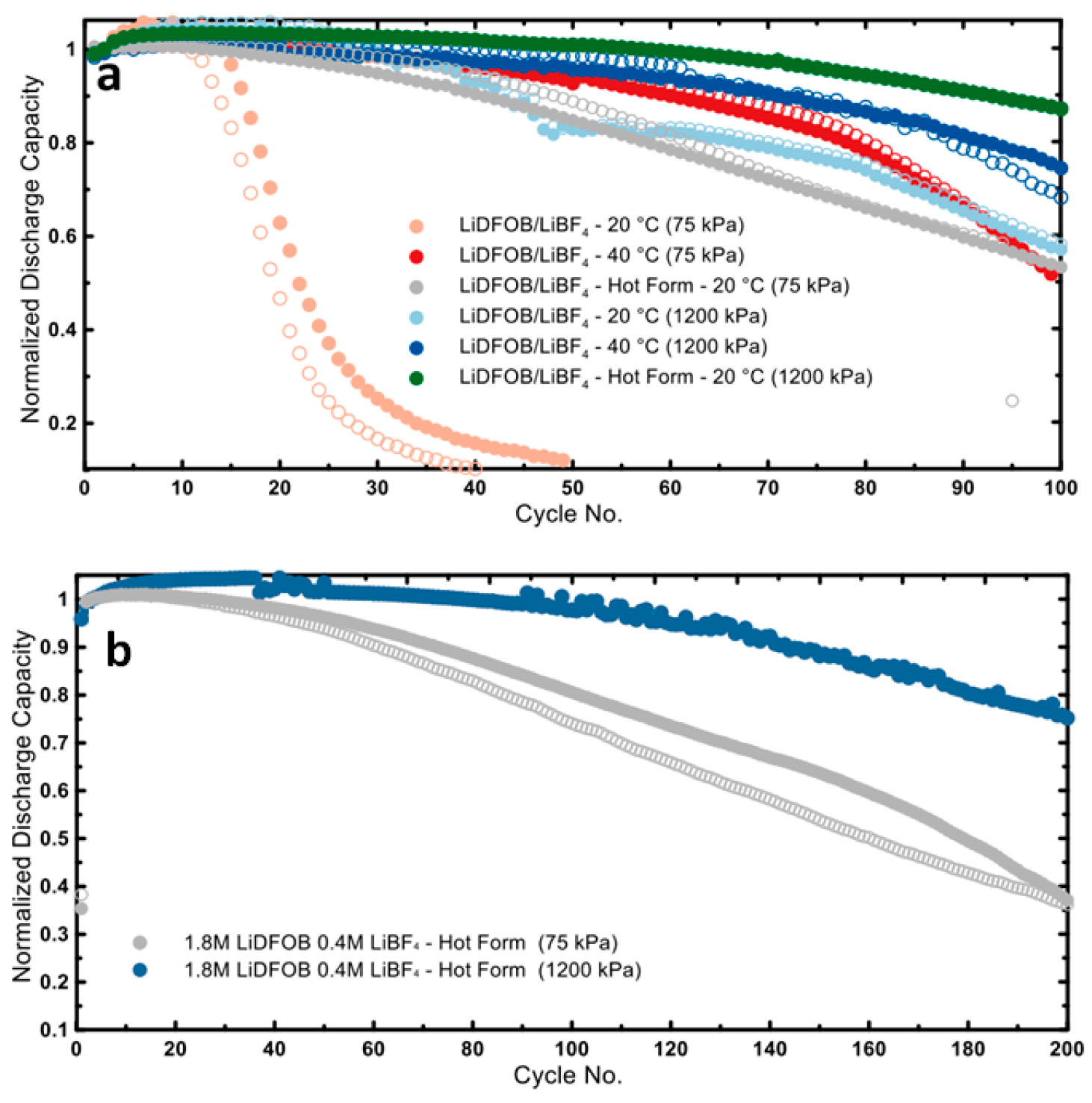
3.3. Optimization of the Cycling Protocols
4. Future Prospects
- (1)
- Separators are always ignored in the previously reported works. Besides coating layers and SEI, separators are important obstructions to prevent mossy and dendritic growth which could lead to short circuits. The optimization of the separator is also essential for prolonging the cycle lifespan.
- (2)
- The key scientific issues such as the SEI formation and its evolution mechanism, the dynamic performance of lithium ions, as well as the role of the electrolyte functional group desire further probing.
- (3)
- Most experiments only exist at the laboratory level with coin cells. They help solve problems from the point of mechanism. However, the direct transfer of these strategies to scaled-up pouch cells with the energy density calculation appears not successful in most cases. Much more effort should be devoted to the practical use.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Liu, Y.Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2017, 3, 16–21. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.N.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.Y.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Horpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Heubner, C.; Maletti, S.; Auer, H.; Hüttl, J.; Voigt, K.; Lohrberg, O.; Nikolowski, K.; Partsch, M.; Michaelis, A. From lithium-metal toward anode-free solid-state batteries: Current developments, issues, and challenges. Adv. Funct. Mater. 2021, 31, 2106608. [Google Scholar] [CrossRef]
- Woo, J.-J.; Maroni, V.A.; Liu, G.; Vaughey, J.T.; Gosztola, D.J.; Amine, K.; Zhang, Z. Symmetrical impedance study on inactivation induced degradation of lithium electrodes for batteries beyond lithium-ion. J. Electrochem. Soc. 2014, 161, A827–A830. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-free full cells: A pathway to high-energy density lithium-metal batteries. Adv. Energy Mater. 2020, 11, 2000804. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, P.; Yang, W.; Wei, Y.; Xiao, J.; Deng, L.; Gong, Y. Large-scale modification of commercial copper foil with lithiophilic metal layer for Li metal battery. Small 2020, 16, e1905620. [Google Scholar] [CrossRef]
- Tong, Z.; Bazri, B.; Hu, S.F.; Liu, R.S. Interfacial chemistry in anode-free batteries: Challenges and strategies. J. Mater. Chem. A 2021, 9, 7396–7406. [Google Scholar] [CrossRef]
- Hagos, T.M.; Bezabh, H.K.; Huang, C.-J.; Jiang, S.-K.; Su, W.-N.; Hwang, B.J. A powerful protocol based on anode-free cells combined with various analytical techniques. Acc. Chem. Res. 2021, 54, 4474–4485. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Tang, X.; Zhang, M.; Bai, M.; Ma, Y. Challenges, strategies, and prospects of the anode-free lithium metal batteries. Adv. Energy Sustain. Res. 2022, 3, 2100197. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Jiang, H.; Xiong, S.; Feng, J.; Qian, Y. Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 2020, 78, 105344. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Wang, J.; Abudula, A.; Guan, G. Anode-free rechargeable lithium metal batteries: Progress and prospects. Energy Storage Mater. 2020, 32, 386–401. [Google Scholar] [CrossRef]
- Jo, C.H.; Sohn, K.S.; Myung, S.T. Feasible approaches for anode-free lithium-metal batteries as next generation energy storage systems. Energy Storage Mater. 2023, 57, 471–496. [Google Scholar] [CrossRef]
- Wu, B.L.; Chen, C.G.; Raijmakers, L.H.J.; Liu, J.; Danilov, D.L.; Notten, P.H.L. Li-growth and SEI engineering for anode-free Li-metal rechargeable batteries: A review of current advances. Energy Storage Mater. 2023, 57, 508–539. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; deGooyer, J.; Coon, M.; Aiken, C.P.; Simunovic, Z.; Metzger, M.; Dahn, J.R. Different positive electrodes for anode-free lithium metal cells. J. Electrochem. Soc. 2022, 169, 040517. [Google Scholar] [CrossRef]
- Lin, L.; Qin, K.; Zhang, Q.; Gu, L.; Suo, L.; Hu, Y.S.; Li, H.; Huang, X.; Chen, L. Li-rich Li2[Ni0.8Co0.1Mn0.1]O2 for anode-free lithium metal batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 8289–8296. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, H.; Chang, Z.; Deng, H.; Li, X.; Zhou, H. A high-energy-density and long-life initial-anode-free lithium battery enabled by a Li2O sacrificial agent. Nat. Energy 2021, 6, 653–662. [Google Scholar] [CrossRef]
- Assegie, A.A.; Chung, C.C.; Tsai, M.C.; Su, W.N.; Chen, C.W.; Hwang, B.J. Multilayer-graphene-stabilized lithium deposition for anode-Free lithium-metal batteries. Nanoscale 2019, 11, 2710–2720. [Google Scholar] [CrossRef] [PubMed]
- Hagos, T.T.; Thirumalraj, B.; Huang, C.J.; Abrha, L.H.; Hagos, T.M.; Berhe, G.B.; Bezabh, H.K.; Cherng, J.; Chiu, S.F.; Su, W.N.; et al. Locally concentrated LiPF6 in a carbonate-based electrolyte with fluoroethylene carbonate as a diluent for anode-free lithium metal batteries. ACS Appl. Mater. Interfaces 2019, 11, 9955–9963. [Google Scholar] [CrossRef] [PubMed]
- Sahalie, N.A.; Assegie, A.A.; Su, W.-N.; Wondimkun, Z.T.; Jote, B.A.; Thirumalraj, B.; Huang, C.-J.; Yang, Y.-W.; Hwang, B.-J. Effect of bifunctional additive potassium nitrate on performance of anode free lithium metal battery in carbonate electrolyte. J. Power Sources 2019, 437, 226912. [Google Scholar] [CrossRef]
- Louli, A.J.; Coon, M.; Genovese, M.; deGooyer, J.; Eldesoky, A.; Dahn, J.R. Optimizing cycling conditions for anode-free lithium metal cells. J. Electrochem. Soc. 2021, 168, 020515. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Su, L.; Charalambous, H.; Cui, Z.; Manthiram, A. High-efficiency, anode-free lithium-metal batteries with a close-packed homogeneous lithium morphology. Energy Environ. Sci. 2022, 15, 843–854. [Google Scholar] [CrossRef]
- Tu, Z.; Zachman, M.J.; Choudhury, S.; Khan, K.A.; Zhao, Q.; Kourkoutis, L.F.; Archer, L.A. Stabilizing protic and aprotic liquid electrolytes at high-bandgap oxide interphases. Chem. Mater. 2018, 30, 5655–5662. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, C.; Cai, N.; Wang, M. Ag coated 3D-Cu foam as a lithiophilic current collector for enabling Li2S-based anode-free batteries. Chem. Commun. 2021, 57, 3708–3711. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. A lithium–sulfur cell based on reversible lithium deposition from a Li2S cathode host onto a hostless-anode substrate. Adv. Energy Mater. 2018, 8, 1801556. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, J.; Chen, X.; Yuan, L.; Li, Z.; Huang, Y. Li2S-based anode-free full batteries with modified Cu current collector. Energy Storage Mater. 2020, 30, 179–186. [Google Scholar] [CrossRef]
- Nanda, S.; Bhargav, A.; Manthiram, A. Anode-free, lean-electrolyte lithium-sulfur batteries enabled by tellurium-stabilized lithium deposition. Joule 2020, 4, 1121–1135. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.Y.; Li, R.S.; Gao, L.B.; Luo, J.Y. Dendrite-free Li metal anode by lowering deposition interface energy with Cu99Zn alloy coating. Energy Storage Mater. 2018, 14, 143–148. [Google Scholar] [CrossRef]
- Lin, L.; Qin, K.; Li, M.; Hu, Y.-s.; Li, H.; Huang, X.; Chen, L.; Suo, L. Spinel-related Li2Ni0.5Mn1.5O4 cathode for 5-V anode-free lithium metal batteries. Energy Storage Mater. 2022, 45, 821–827. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, B.; Zhu, Z.; He, Y.; Wei, Z.; Wei, J.; Jiang, H.; Hu, Y.; Li, C. Multifunctional lithium compensation agent based on carbon edges catalysis and its application in anode-free lithium batteries. Chem. Eng. J. 2023, 458, 141411. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fan, X.L.; Wang, C.S. An in-situ enabled lithium metal battery by plating lithium on a copper current collector. Electrochem. Commun. 2018, 89, 23–26. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, H.J.; Yoo, Y.; Lim, H.D.; Park, J.W.; Lee, Y.J.; Ha, Y.C.; Lee, S.M.; Kim, B.G. Stable cycling of all-solid-state batteries with sacrificial cathode and lithium-free indium layer. Adv. Funct. Mater. 2021, 32, 2108203. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Umh, H.N.; Park, J.; Yeo, J.; Jung, S.; Nam, I.; Yi, J. Lithium metal anode on a copper dendritic superstructure. Electrochem. Commun. 2019, 99, 27–31. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Hu, P.; Li, Z. Facile one-step heat treatment of Cu foil for stable anode-free Li metal batteries. Molecules 2023, 28, 548. [Google Scholar] [CrossRef]
- Lo, C.A.; Chang, C.C.; Tsai, Y.W.; Jiang, S.K.; Hwang, B.J.; Mou, C.Y.; Wu, H.L. Regulated Li electrodeposition behavior through mesoporous silica thin film in anode-free lithium metal batteries. ACS Appl. Energy Mater. 2021, 4, 5132–5142. [Google Scholar] [CrossRef]
- Weldeyohannes, H.H.; Abrha, L.H.; Nikodimos, Y.; Shitaw, K.N.; Hagos, T.M.; Huang, C.-J.; Wang, C.-H.; Wu, S.-H.; Su, W.-N.; Hwang, B.J. Guiding lithium-ion flux to avoid cell’s short circuit and extend cycle life for an anode-free lithium metal battery. J. Power Sources 2021, 506, 230204. [Google Scholar] [CrossRef]
- Abrha, L.H.; Zegeye, T.A.; Hagos, T.T.; Sutiono, H.; Hagos, T.M.; Berhe, G.B.; Huang, C.-J.; Jiang, S.-K.; Su, W.-N.; Yang, Y.-W.; et al. Li7La2.75Ca0.25Zr1.75Nb0.25O12@LiClO4 composite film derived solid electrolyte interphase for anode-free lithium metal battery. Electrochim. Acta 2019, 325, 134825. [Google Scholar] [CrossRef]
- Sahalie, N.A.; Wondimkun, Z.T.; Su, W.-N.; Weret, M.A.; Fenta, F.W.; Berhe, G.B.; Huang, C.-J.; Hsu, Y.-C.; Hwang, B.J. Multifunctional properties of Al2O3/polyacrylonitrile composite coating on Cu to suppress dendritic growth in anode-free Li-metal battery. ACS Appl. Energy Mater. 2020, 3, 7666–7679. [Google Scholar] [CrossRef]
- Wondimkun, Z.T.; Beyene, T.T.; Weret, M.A.; Sahalie, N.A.; Huang, C.-J.; Thirumalraj, B.; Jote, B.A.; Wang, D.; Su, W.-N.; Wang, C.-H.; et al. Binder-free ultra-thin graphene oxide as an artificial solid electrolyte interphase for anode-free rechargeable lithium metal batteries. J. Power Sources 2020, 450, 227589. [Google Scholar] [CrossRef]
- Abrha, L.H.; Nikodimos, Y.; Weldeyohannes, H.H.; Hagos, T.T.; Wang, D.Y.; Huang, C.J.; Jiang, S.K.; Wu, S.H.; Su, W.N.; Tsai, M.C.; et al. Effects of a thermally electrochemically activated β-PVDF fiber on suppression of Li dendrite growth for anode-free batteries. ACS Appl. Energy Mater. 2021, 4, 3240–3248. [Google Scholar] [CrossRef]
- Wondimkun, Z.T.; Tegegne, W.A.; Jiang, S.K.; Huang, C.J.; Sahalie, N.A.; Weret, M.A.; Hsu, J.Y.; Hsieh, P.L.; Huang, Y.S.; Wu, S.H.; et al. Highly-lithiophilic Ag@PDA-GO film to suppress dendrite formation on Cu substrate in anode-free lithium metal batteries. Energy Storage Mater. 2021, 35, 334–344. [Google Scholar] [CrossRef]
- Temesgen, N.T.; Tegegne, W.A.; Shitaw, K.N.; Fenta, F.W.; Nikodimos, Y.; Taklu, B.W.; Jiang, S.-K.; Huang, C.-J.; Wu, S.-H.; Su, W.-N.; et al. Mitigating dendrite formation and electrolyte decomposition via functional double layers coating on copper current collector in anode-free lithium metal battery. J. Taiwan Inst. Chem. Eng. 2021, 128, 87–97. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Thijs, M.; Ooms, F.G.B.; Ganapathy, S.; Wagemaker, M. High dielectric barium titanate porous scaffold for efficient Li metal cycling in anode-free cells. Nat. Commun. 2021, 12, 6536. [Google Scholar] [CrossRef]
- Lin, L.; Suo, L.; Hu, Y.-s.; Li, H.; Huang, X.; Chen, L. Epitaxial induced plating current-collector lasting lifespan of anode-free lithium metal battery. Adv. Energy Mater. 2021, 11, 2003709. [Google Scholar] [CrossRef]
- Assegie, A.A.; Cheng, J.H.; Kuo, L.M.; Su, W.N.; Hwang, B.J. Polyethylene oxide film coating enhances lithium cycling efficiency of an anode-free lithium-metal battery. Nanoscale 2018, 10, 6125–6138. [Google Scholar] [CrossRef]
- Liu, H.; Yue, X.; Xing, X.; Yan, Q.; Huang, J.; Petrova, V.; Zhou, H.; Liu, P. A scalable 3D lithium metal anode. Energy Storage Mater. 2019, 16, 505–511. [Google Scholar] [CrossRef]
- Chen, W.; Salvatierra, R.V.; Ren, M.; Chen, J.; Stanford, M.G.; Tour, J.M. Laser-induced silicon oxide for anode-free lithium metal batteries. Adv. Mater. 2020, 32, e2002850. [Google Scholar] [CrossRef]
- Kang, T.; Zhao, J.; Guo, F.; Zheng, L.; Mao, Y.; Wang, C.; Zhao, Y.; Zhu, J.; Qiu, Y.; Shen, Y.; et al. Dendrite-free lithium anodes enabled by a commonly used copper antirusting agent. ACS Appl. Mater. Interfaces 2020, 12, 8168–8175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.; Pollard, T.P.; Fan, X.; Borodin, O.; Wang, C. Electrolyte design for Li metal-free Li batteries. Mater. Today 2020, 39, 118–126. [Google Scholar] [CrossRef]
- Li, Q.; Pan, H.Y.; Li, W.J.; Wang, Y.; Wang, J.Y.; Zheng, J.Y.; Yu, X.Q.; Li, H.; Chen, L.Q. Homogeneous interface conductivity for lithium dendrite-free anode. ACS Energy Lett. 2018, 3, 2259–2266. [Google Scholar] [CrossRef]
- Tamwattana, O.; Park, H.; Kim, J.; Hwang, I.; Yoon, G.; Hwang, T.-h.; Kang, Y.-S.; Park, J.; Meethong, N.; Kang, K. High-dielectric polymer coating for uniform lithium deposition in anode-free lithium batteries. ACS Energy Lett. 2021, 6, 4416–4425. [Google Scholar] [CrossRef]
- Neudecker, B.J.; Dudney, N.J.; Bates, J.B. “Lithium-free” thin-film battery with in situ plated Li anode. J. Electrochem. Soc. 2000, 147, 517–523. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fan, X.; Wang, C. A tin-plated copper substrate for efficient cycling of lithium metal in an anode-free rechargeable lithium battery. Electrochim. Acta 2017, 258, 1201–1207. [Google Scholar] [CrossRef]
- Huang, W.Z.; Zhao, C.Z.; Wu, P.; Yuan, H.; Feng, W.E.; Liu, Z.Y.; Lu, Y.; Sun, S.; Fu, Z.H.; Hu, J.K.; et al. Anode-free solid-state lithium batteries: A review. Adv. Energy Mater. 2022, 12, 2201044. [Google Scholar] [CrossRef]
- Hagos, T.M.; Berhe, G.B.; Hagos, T.T.; Bezabh, H.K.; Abrha, L.H.; Beyene, T.T.; Huang, C.-J.; Yang, Y.-W.; Su, W.-N.; Dai, H.; et al. Dual electrolyte additives of potassium hexafluorophosphate and tris (trimethylsilyl) phosphite for anode-free lithium metal batteries. Electrochim. Acta 2019, 316, 52–59. [Google Scholar] [CrossRef]
- Hagos, T.T.; Su, W.-N.; Huang, C.-J.; Thirumalraj, B.; Chiu, S.-F.; Abrha, L.H.; Hagos, T.M.; Bezabh, H.K.; Berhe, G.B.; Tegegne, W.A.; et al. Developing high-voltage carbonate-ether mixed electrolyte via anode-free cell configuration. J. Power Sources 2020, 461, 228053. [Google Scholar] [CrossRef]
- Genovese, M.; Louli, A.J.; Weber, R.; Hames, S.; Dahn, J.R. Measuring the coulombic efficiency of lithium metal cycling in anode-free lithium metal batteries. J. Electrochem. Soc. 2018, 165, A3321–A3325. [Google Scholar] [CrossRef]
- Louli, A.J.; Genovese, M.; Weber, R.; Hames, S.G.; Logan, E.R.; Dahn, J.R. Exploring the impact of mechanical pressure on the performance of anode-free lithium metal cells. J. Electrochem. Soc. 2019, 166, A1291–A1299. [Google Scholar] [CrossRef]
- Weber, R.; Genovese, M.; Louli, A.J.; Hames, S.; Martin, C.; Hill, I.G.; Dahn, J.R. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 2019, 4, 683–689. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; Weber, R.; Genovese, M.; Coon, M.; deGooyer, J.; Deng, Z.; White, R.T.; Lee, J.; Rodgers, T.; et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Jote, B.A.; Beyene, T.T.; Sahalie, N.A.; Weret, M.A.; Olbassa, B.W.; Wondimkun, Z.T.; Berhe, G.B.; Huang, C.-J.; Su, W.-N.; Hwang, B.J. Effect of diethyl carbonate solvent with fluorinated solvents as electrolyte system for anode free battery. J. Power Sources 2020, 461, 228102. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Pollard, T.P.; Wang, X.; Lee, J.Z.; Zhang, M.; Wynn, T.; Ding, M.; Borodin, O.; Meng, Y.S.; et al. Bisalt ether electrolytes: A pathway towards lithium metal batteries with Ni-rich cathodes. Energy Environ. Sci. 2019, 12, 780–794. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.C.; et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Eldesoky, A.; Louli, A.J.; Benson, A.; Dahn, J.R. Cycling performance of NMC811 anode-free pouch cells with 65 different electrolyte formulations. J. Electrochem. Soc. 2021, 168, 120508. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.-G. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Brown, Z.L.; Jurng, S.; Lucht, B.L. Investigation of the lithium solid electrolyte interphase in vinylene carbonate electrolytes using Cu||LiFePO4 cells. J. Electrochem. Soc. 2017, 164, A2186–A2189. [Google Scholar] [CrossRef]
- Rodriguez, R.; Loeffler, K.E.; Edison, R.A.; Stephens, R.M.; Dolocan, A.; Heller, A.; Mullins, C.B. Effect of the electrolyte on the cycling efficiency of lithium-limited cells and their morphology studied through in situ optical imaging. ACS Appl. Energy Mater. 2018, 1, 5830–5835. [Google Scholar] [CrossRef]
- Beyene, T.T.; Bezabh, H.K.; Weret, M.A.; Hagos, T.M.; Huang, C.J.; Wang, C.H.; Su, W.N.; Dai, H.; Hwang, B.J. Concentrated dual-salt electrolyte to stabilize Li metal and increase cycle life of anode free Li-metal batteries. J. Electrochem. Soc. 2019, 166, A1501–A1509. [Google Scholar] [CrossRef]
- Beyene, T.T.; Jote, B.A.; Wondimkun, Z.T.; Olbassa, B.W.; Huang, C.J.; Thirumalraj, B.; Wang, C.H.; Su, W.N.; Dai, H.; Hwang, B.J. Effects of concentrated salt and resting protocol on solid electrolyte interface formation for improved cycle stability of anode-free lithium metal batteries. ACS Appl. Mater. Interfaces 2019, 11, 31962–31971. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.L.; Heiskanen, S.; Lucht, B.L. Using triethyl phosphate to increase the solubility of LiNO3 in carbonate electrolytes for improving the performance of the lithium metal anode. J. Electrochem. Soc. 2019, 166, A2523–A2527. [Google Scholar] [CrossRef]
- Nilsson, V.; Kotronia, A.; Lacey, M.; Edström, K.; Johansson, P. Highly concentrated LiTFSI-EC electrolytes for lithium metal batteries. ACS Appl. Energy Mater. 2019, 3, 200–207. [Google Scholar] [CrossRef]
- Rodriguez, R.; Edison, R.A.; Stephens, R.M.; Sun, H.-H.; Heller, A.; Mullins, C.B. Separator-free and concentrated LiNO3 electrolyte cells enable uniform lithium electrodeposition. J. Mater. Chem. A 2020, 8, 3999–4006. [Google Scholar] [CrossRef]
- Zhou, C.; Samson, A.J.; Garakani, M.A.; Thangadurai, V. Communication—Anode-free lithium metal batteries: A case study of compression effects on coin cell performance. J. Electrochem. Soc. 2021, 168, 060532. [Google Scholar] [CrossRef]
- Genovese, M.; Louli, A.J.; Weber, R.; Martin, C.; Taskovic, T.; Dahn, J.R. Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 2019, 166, A3342–A3347. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Pan, J.; Zhang, D.; Tang, Y.; Tai, Z.; Liu, Y.; Gao, H.; Huang, F. Designs of Anode-Free Lithium-Ion Batteries. Batteries 2023, 9, 381. https://doi.org/10.3390/batteries9070381
Zhao P, Pan J, Zhang D, Tang Y, Tai Z, Liu Y, Gao H, Huang F. Designs of Anode-Free Lithium-Ion Batteries. Batteries. 2023; 9(7):381. https://doi.org/10.3390/batteries9070381
Chicago/Turabian StyleZhao, Pei, Jun Pan, Dongqi Zhang, Yufeng Tang, Zhixin Tai, Yajie Liu, Hong Gao, and Fuqiang Huang. 2023. "Designs of Anode-Free Lithium-Ion Batteries" Batteries 9, no. 7: 381. https://doi.org/10.3390/batteries9070381
APA StyleZhao, P., Pan, J., Zhang, D., Tang, Y., Tai, Z., Liu, Y., Gao, H., & Huang, F. (2023). Designs of Anode-Free Lithium-Ion Batteries. Batteries, 9(7), 381. https://doi.org/10.3390/batteries9070381