Ultramicroporous N-Doped Activated Carbon Materials for High Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of AC-N
2.2. Physicochemical Characterization of AC-N
2.3. Electrochemical Measurements
2.3.1. Preparation of Electrodes
Ink Preparation
Ink Deposition on Glassy Carbon Rod
2.3.2. Electrochemical Measurements
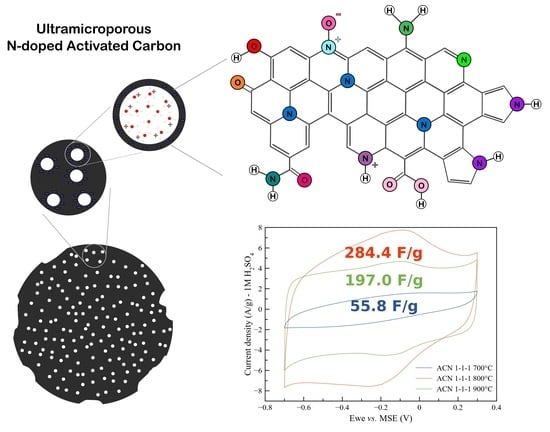
3. Results
3.1. Synthesis of AC-N Samples
3.2. Physicochemical Characterization of AC Samples
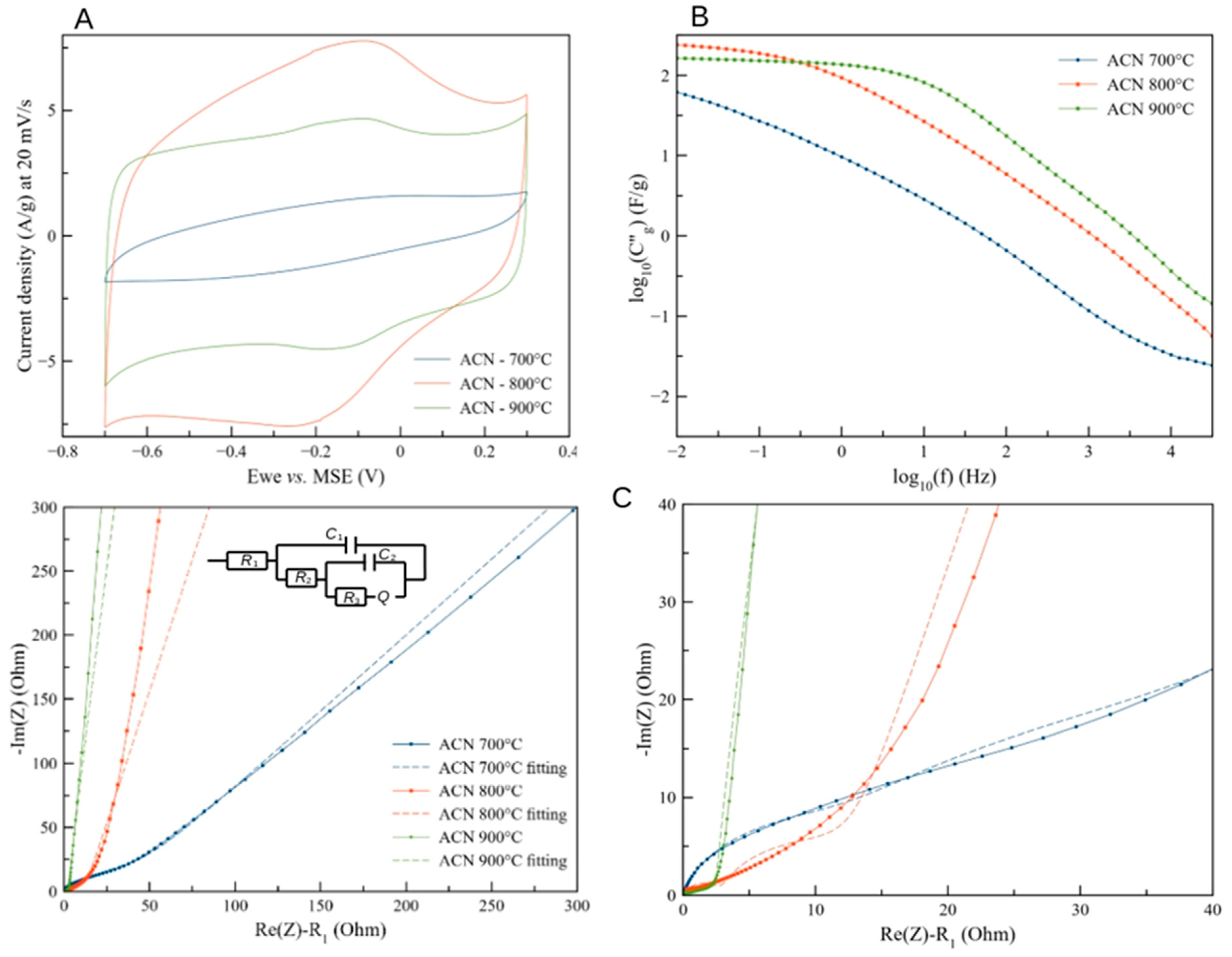
3.3. Electrochemical Properties of AC Samples
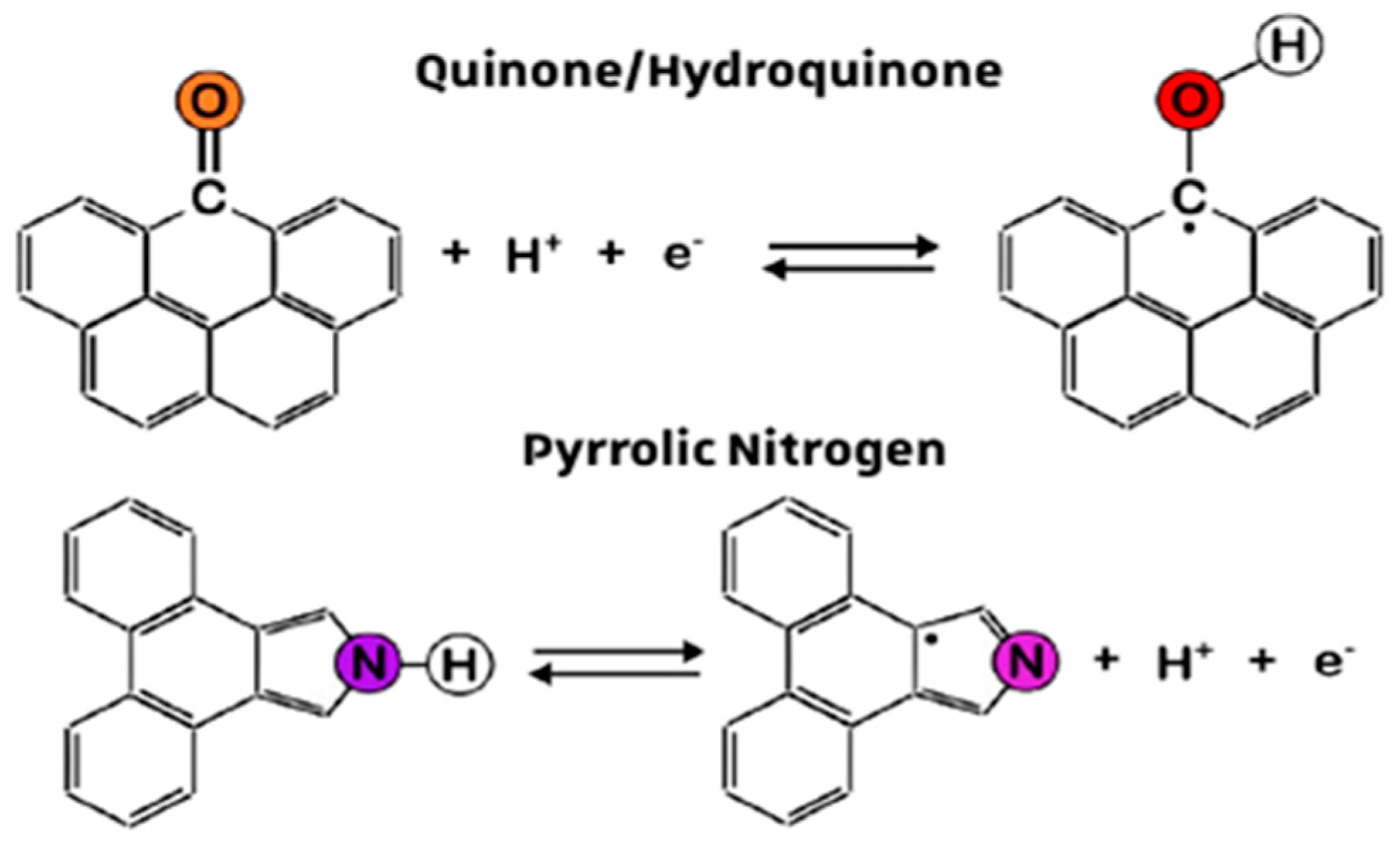
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Libich, J.; Maca, J.; Vondrak, J.; Cech, O.; Sedlarikova, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, S.; Wang, L.; Li, C.; Meng, F.; Wang, N.; Zhou, S.; Xiong, Y.; Wang, Z.; Wu, Y.; et al. Recent Advances in Porous Carbon Materials as Electrodes for Supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef]
- Zallouz, S.; Pronkin, S.; Le Meins, J.-M.; Pham-Huu, C.; Ghimbeau, C.M. New development in carbon-based electrodes and electrolytes for enhancement of supercapacitor performance and safety. In Renewable Energy Production and Distribution Solutions and Opportunities; Elsevier Academic Press: Amsterdam, The Netherlands, 2023; pp. 353–408. [Google Scholar]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Capacitive energy storage in nanostructured carbon-electrolyte systems. Acc. Chem. Res. 2013, 46, 1094. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Ma, L.; Shen, X.; Tong, H. Recent design and control of carbon materials for supercapacitors. J. Mater. Sci. 2021, 56, 1919–1942. [Google Scholar] [CrossRef]
- Zahir, N.; Magri, P.; Luo, W.; Gaumet, J.J.; Pierrat, P. Recent Advances on Graphene Quantum Dots for Electrochemical Energy Storage Devices. Energy Environ. Mater. 2022, 5, 201–214. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based Materials for Supercapacitor Electrodes—A Review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Noked, M.; Soffer, A.; Aurbach, D.; Noked, M.; Soffer, A.; Aurbach, D. The electrochemistry of activated carbonaceous materials: Past, present, and future. J. Solid State Electrochem. 2011, 15, 1563–1578. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Nunes, M.; Rocha, I.M.; Fernandes, D.M.; Mestre, A.S.; Moura, C.N.; Carvalho, A.P.; Pereira, M.F.R.; Freire, C. Sucrose-derived activated carbons: Electron transfer properties and application as oxygen reduction electrocatalysts. RSC Adv. 2015, 5, 102919–102931. [Google Scholar] [CrossRef]
- Evans, M.J.B.; Halliop, E.; MacDonald, J.A.F. The production of chemically-activated carbon. Carbon 1999, 37, 269–274. [Google Scholar] [CrossRef]
- Susanti, R.F.; Arie, A.A.; Kristianto, H.; Erico, M.; Kevin, G.; Devianto, H. Activated carbon from citric acid catalyzed hydrothermal carbonization and chemical activation of salacca peel as potential electrode for lithium ion capacitor’s cathode. Ionics 2019, 25, 3915–3925. [Google Scholar] [CrossRef]
- Lei, Q.; Kannan, S.; Raghavan, V. Uncatalyzed and acid-aided microwave hydrothermal carbonization of orange peel waste. Waste Manag. 2021, 126, 106–118. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zheng, M.; Xiao, Y.; Hu, H.; Liang, Y.; Liu, Y.; Dong, H. Preparation of High-Performance Porous Carbon Materials by Citric Acid-Assisted Hydrothermal Carbonization of Bamboo and Their Application in Electrode Materials. Energy Fuels 2022, 36, 9303–9312. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Frackowiak, E.; Lota, G.; Machnikowski, J.; Vix-Guterl, C.; Béguin, F. Optimisation of supercapacitors using carbons with controlled nanotexture and nitrogen content. Electrochim. Acta 2006, 51, 2209–2214. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science. 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Shafizadeh, F. Mechanism of thermal degradation of sucrose. A preliminary study. Aust. J. Chem. 1978, 31, 1825. [Google Scholar] [CrossRef]
- Lee, J.W.; Thomas, L.C.; Jerrell, J.; Feng, H.; Cadwallader, K.R.; Schmidt, S.J. Investigation of Thermal Decomposition as the Kinetic Process That Causes the Loss of Crystalline Structure in Sucrose Using a Chemical Analysis Approach (Part II). J. Agric. Food Chem. 2011, 59, 702–712. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Kusumah, S.S.; Subyakto; Umemura, K. Modification of novel bio-based adhesive made from citric acid and sucrose by ZnCl2. Int. J. Adhes. Adhes. 2021, 108, 102866. [Google Scholar] [CrossRef]
- Illingworth, J.M.; Rand, B.; Williams, P.T. Understanding the mechanism of two-step, pyrolysis-alkali chemical activation of fibrous biomass for the production of activated carbon fibre matting. Fuel Process. Technol. 2022, 235, 107348. [Google Scholar] [CrossRef]
- Bogeat, A.B. Understanding and Tuning the Electrical Conductivity of Activated Carbon: A State-of-the-Art Review. Crit. Rev. Solid State Mater. Sci. 2021, 46, 1–37. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Maslova, O.A.; Ammar, M.R.; Guimbretière, G.; Rouzaud, J.-N.; Simon, P. Determination of crystallite size in polished graphitized carbon by Raman spectroscopy. Phys. Rev. B 2012, 86, 134205. [Google Scholar] [CrossRef]
- Fang, B.; Wei, Y.-Z.; Suzuki, K.; Kumagai, M. Surface modification of carbonaceous materials for EDLCs application. Electrochim. Acta 2005, 50, 3616–3621. [Google Scholar] [CrossRef]
- Weinberg, N.L.; Weinberg, H.R. Electrochemical oxidation of organic compounds. Chem. Rev. 1968, 68, 449–523. [Google Scholar] [CrossRef]
- Roth, H.G.; Romero, N.A.; Nicewicz, D.A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723. [Google Scholar] [CrossRef]
- Pronkin, S.N.; Shokina, N.Y.; Pham-Huu, C. Redox transitions in pseudocapacitor materials: Critera and ruling factors. In Redox Chemistry—From Molecules to Energy Storage; Intech Open: London, UK, 2022; pp. 1–22. Available online: https://cdn.intechopen.com/pdfs/81487.pdf (accessed on 22 August 2023).
- Keiser, H.; Beccu, K.D.; Gutjahr, M.A. Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen. Electrochim. Acta 1976, 21, 539–543. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Bograchev, D.A.; Rychagov, A.Y.; Sosenkin, V.E.; Chaika, M.Y. Supercapacitors with carbon electrodes. Energy efficiency: Modeling and experimental verification. J. Solid State Electrochem. 2015, 19, 2771–2779. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Bograchev, D.A.; Mikhalin, A.A.; Bagotsky, V.S. Supercapacitor carbon electrodes with high capacitance. J. Solid State Electrochem. 2014, 18, 1351–1363. [Google Scholar] [CrossRef]

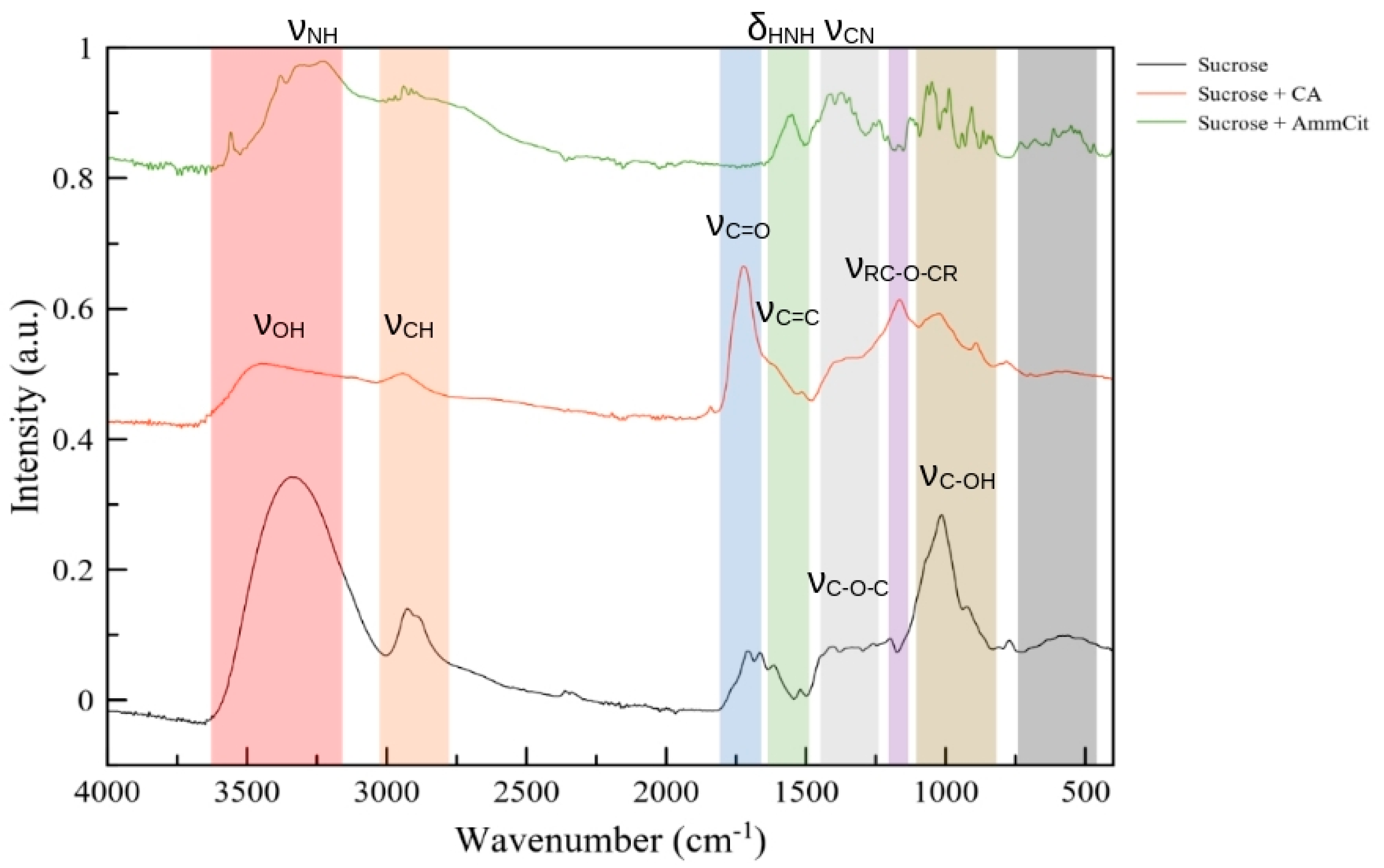


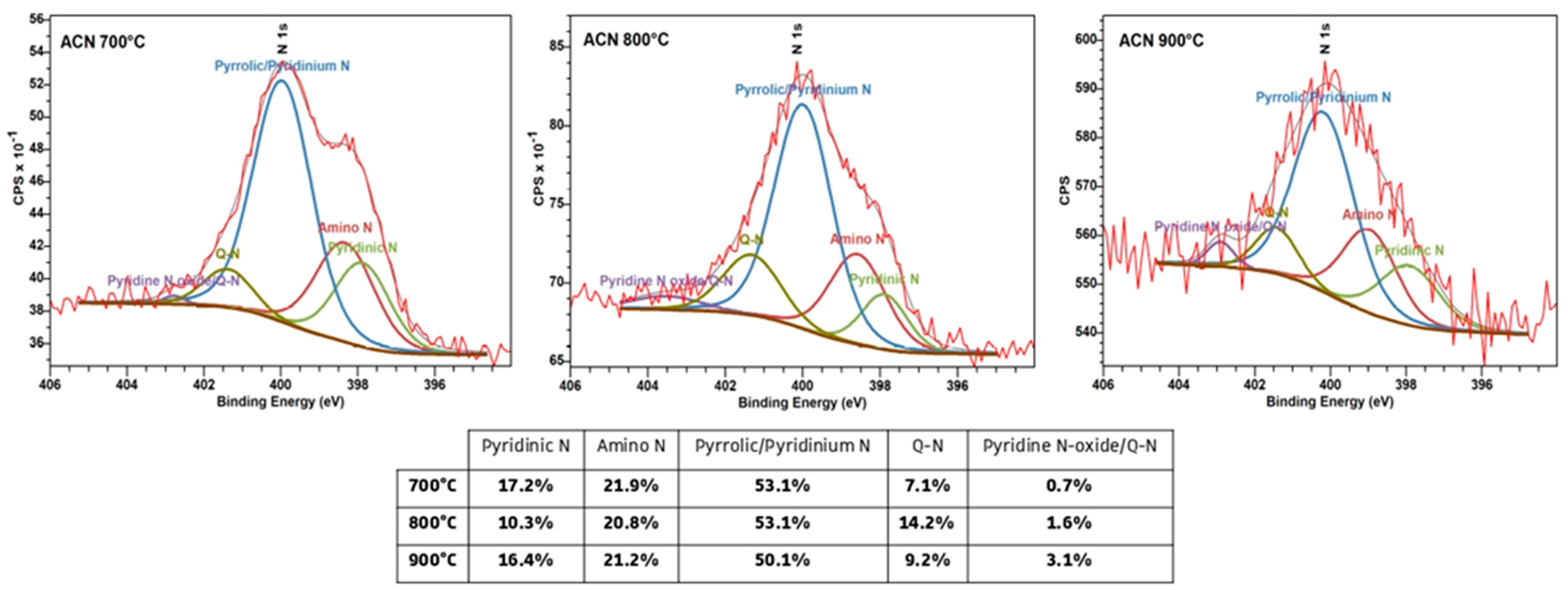

| Sample Name | Precursors | Activation Temperature, °C | SSA, m2/g | x(O), x(N), at.% | ρ, kOhm. cm | Cg, F/g (2 A/g, 1 M H2SO4) |
|---|---|---|---|---|---|---|
| AC-800 | Sucrose | 800 | 10 | 30.4/- | 0.61 | 3.2 |
| AC-C-800 | Sucrose + Citric acid | 800 | 130 | 15.9/- | 0.30 | 75 |
| AC-N-700 | Sucrose + (NH4)3Cit | 600 | 271 | 11.8/7.3 | 19.5 | 44.2 |
| AC-N-800 | Sucrose + (NH4)3Cit | 700 | 776 | 10.8/3.3 | 0.19 | 320.9 |
| AC-N-900 | Sucrose + (NH4)3Cit | 800 | 1379 | 8.6/1.5 | 0.009 | 208.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakoç, T.; Ba, H.; Phuoc, L.T.; Bégin, D.; Pham-Huu, C.; Pronkin, S.N. Ultramicroporous N-Doped Activated Carbon Materials for High Performance Supercapacitors. Batteries 2023, 9, 436. https://doi.org/10.3390/batteries9090436
Karakoç T, Ba H, Phuoc LT, Bégin D, Pham-Huu C, Pronkin SN. Ultramicroporous N-Doped Activated Carbon Materials for High Performance Supercapacitors. Batteries. 2023; 9(9):436. https://doi.org/10.3390/batteries9090436
Chicago/Turabian StyleKarakoç, Taylan, Housseinou Ba, Lai Truong Phuoc, Dominique Bégin, Cuong Pham-Huu, and Sergey N. Pronkin. 2023. "Ultramicroporous N-Doped Activated Carbon Materials for High Performance Supercapacitors" Batteries 9, no. 9: 436. https://doi.org/10.3390/batteries9090436
APA StyleKarakoç, T., Ba, H., Phuoc, L. T., Bégin, D., Pham-Huu, C., & Pronkin, S. N. (2023). Ultramicroporous N-Doped Activated Carbon Materials for High Performance Supercapacitors. Batteries, 9(9), 436. https://doi.org/10.3390/batteries9090436