Recent Advances in All-Solid-State Lithium–Oxygen Batteries: Challenges, Strategies, Future
Abstract
1. Introduction
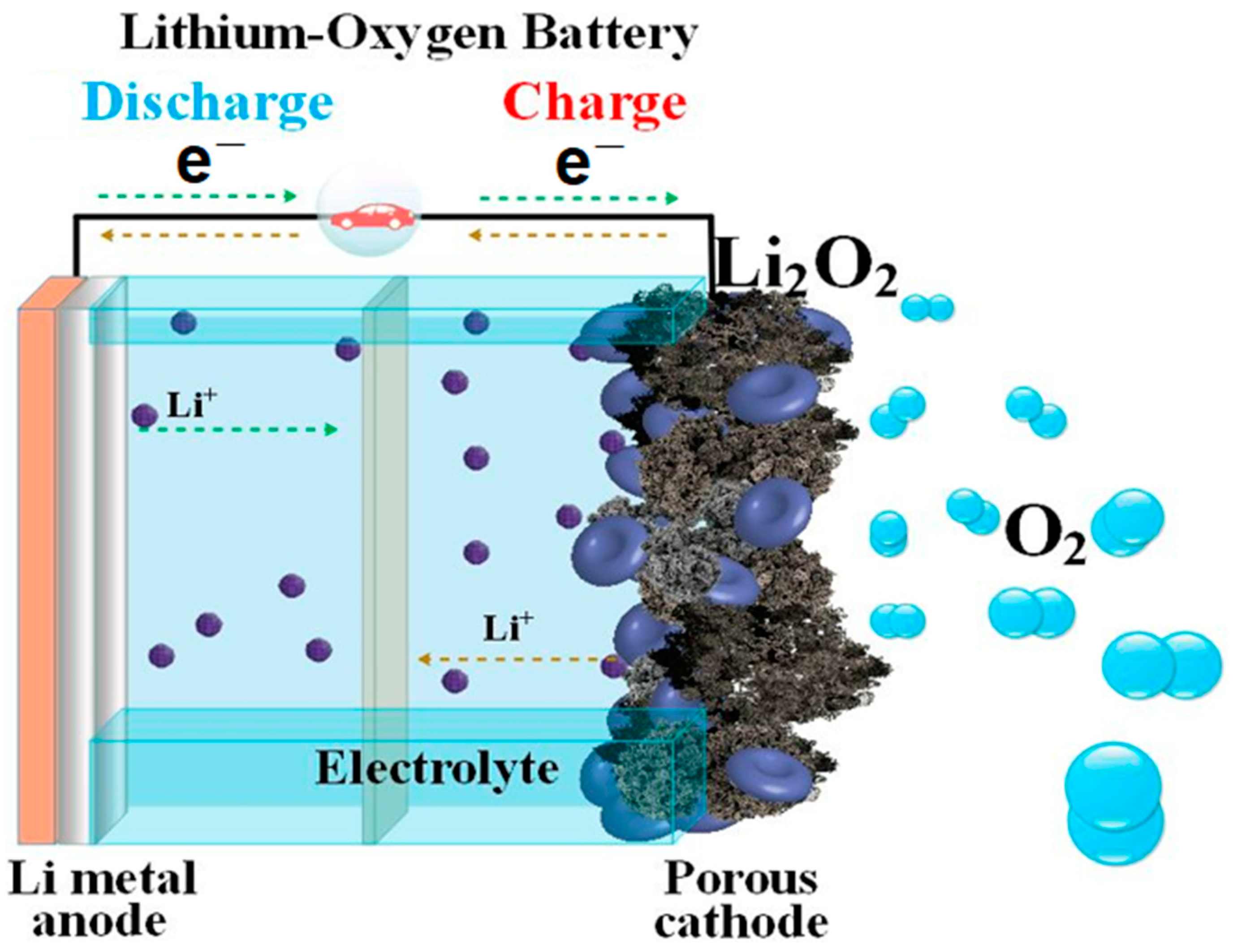
1.1. The Principal Operation of Lithium–Oxygen Batteries
1.2. Challenges in Lithium–Oxygen Batteries
2. All-Solid-State Lithium–Oxygen Batteries
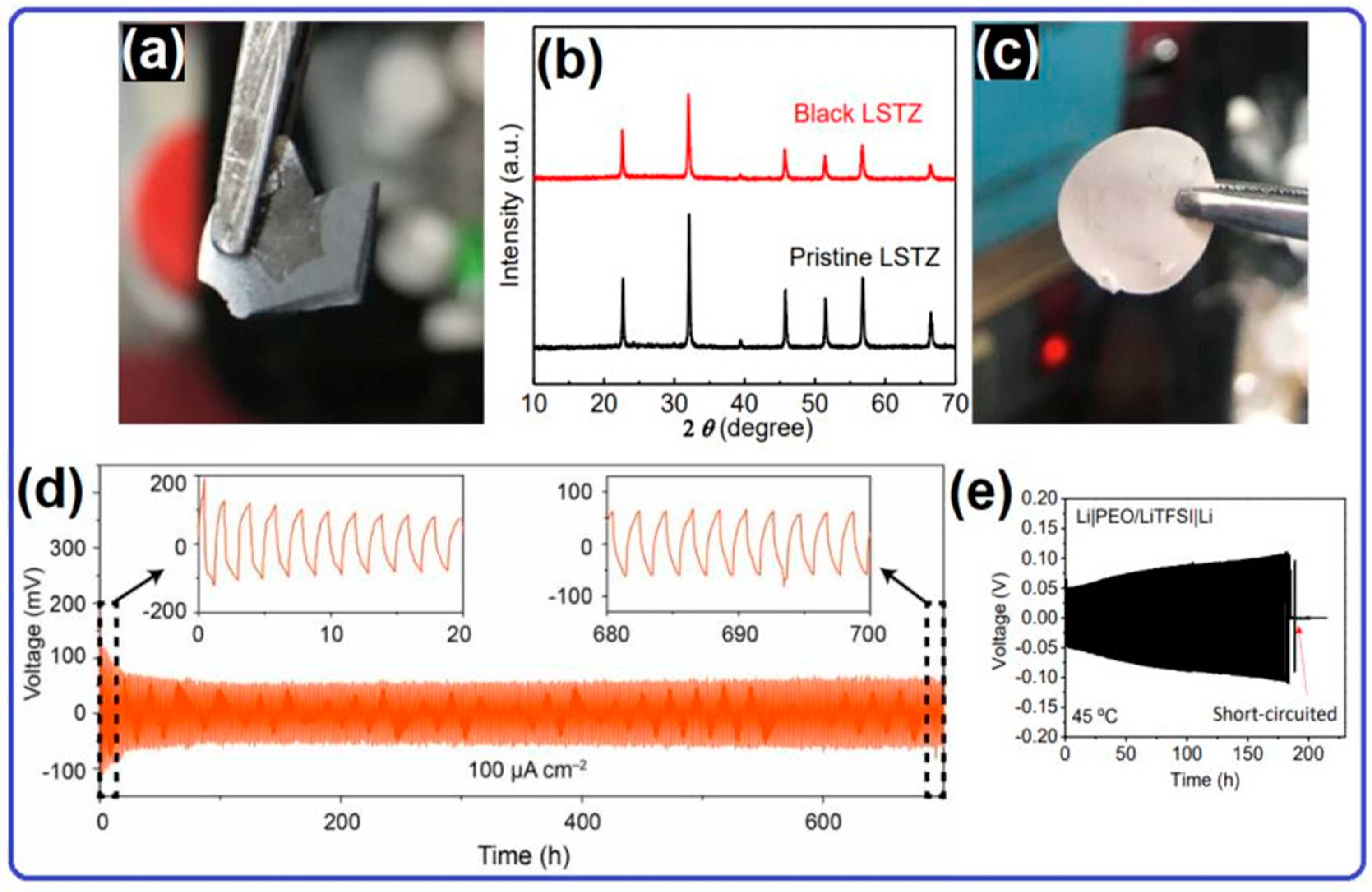
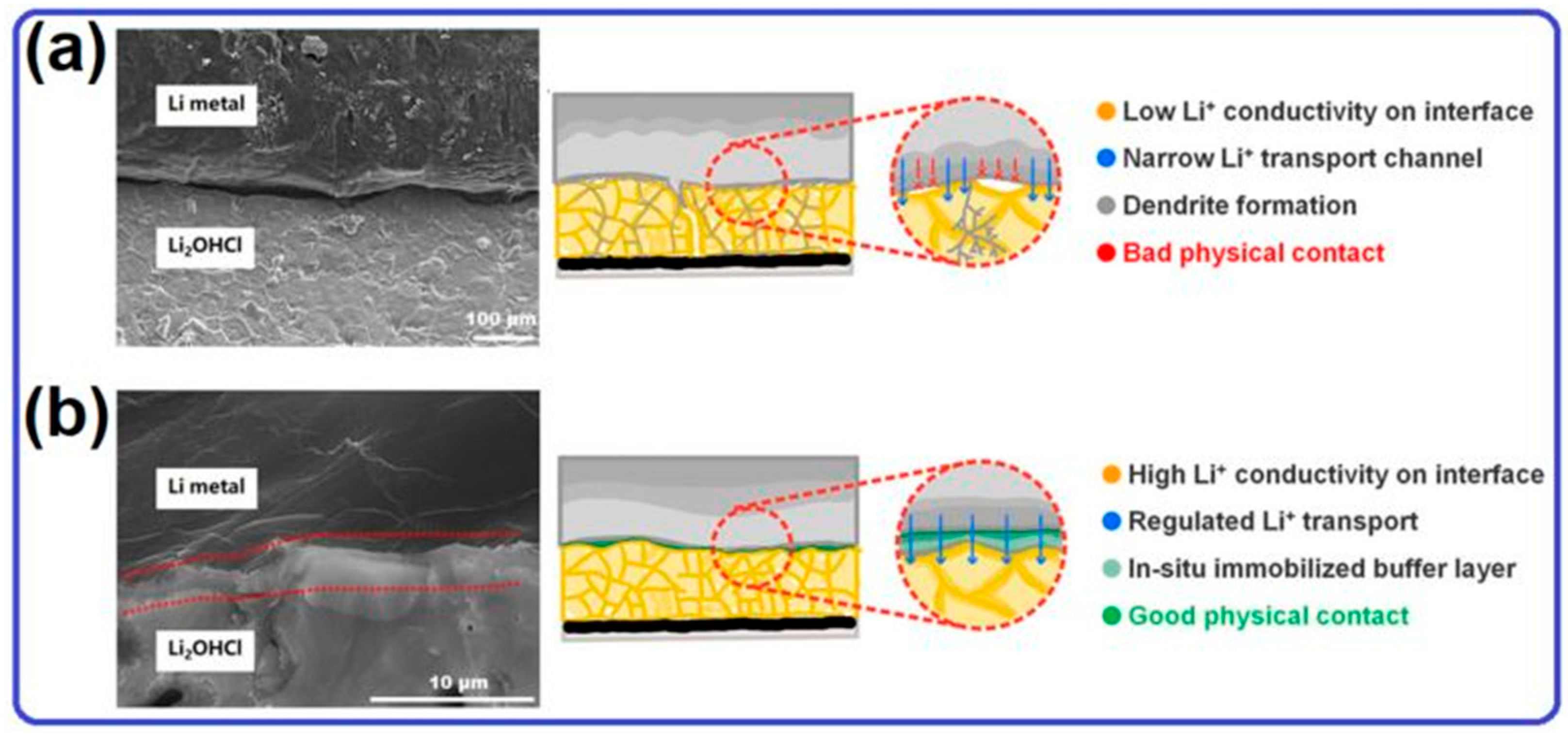
2.1. Lithium Metal Anode for Solid-State Lithium–Oxygen Batteries
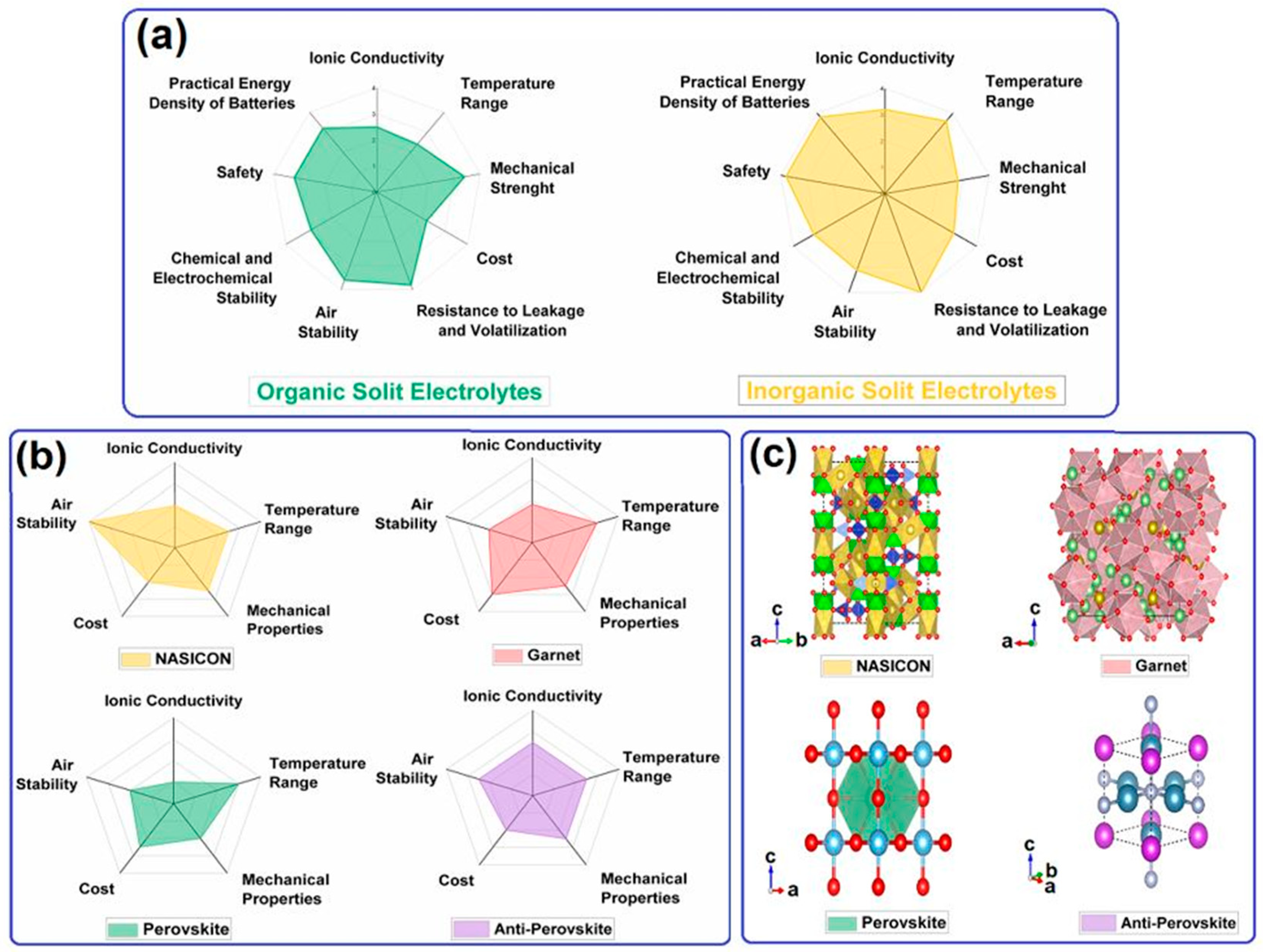
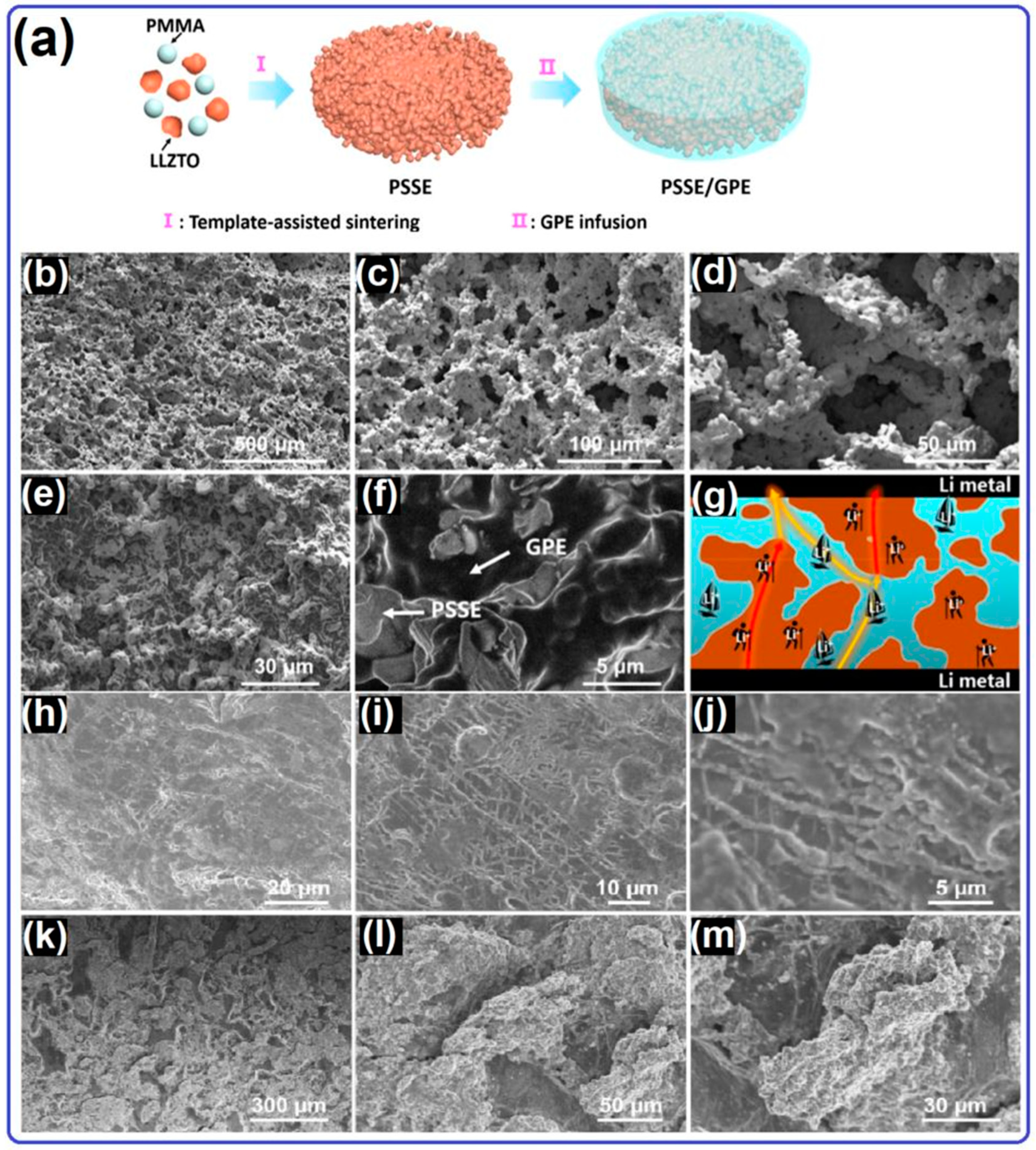
2.2. Solid Electrolyte for Lithium–Oxygen Batteries

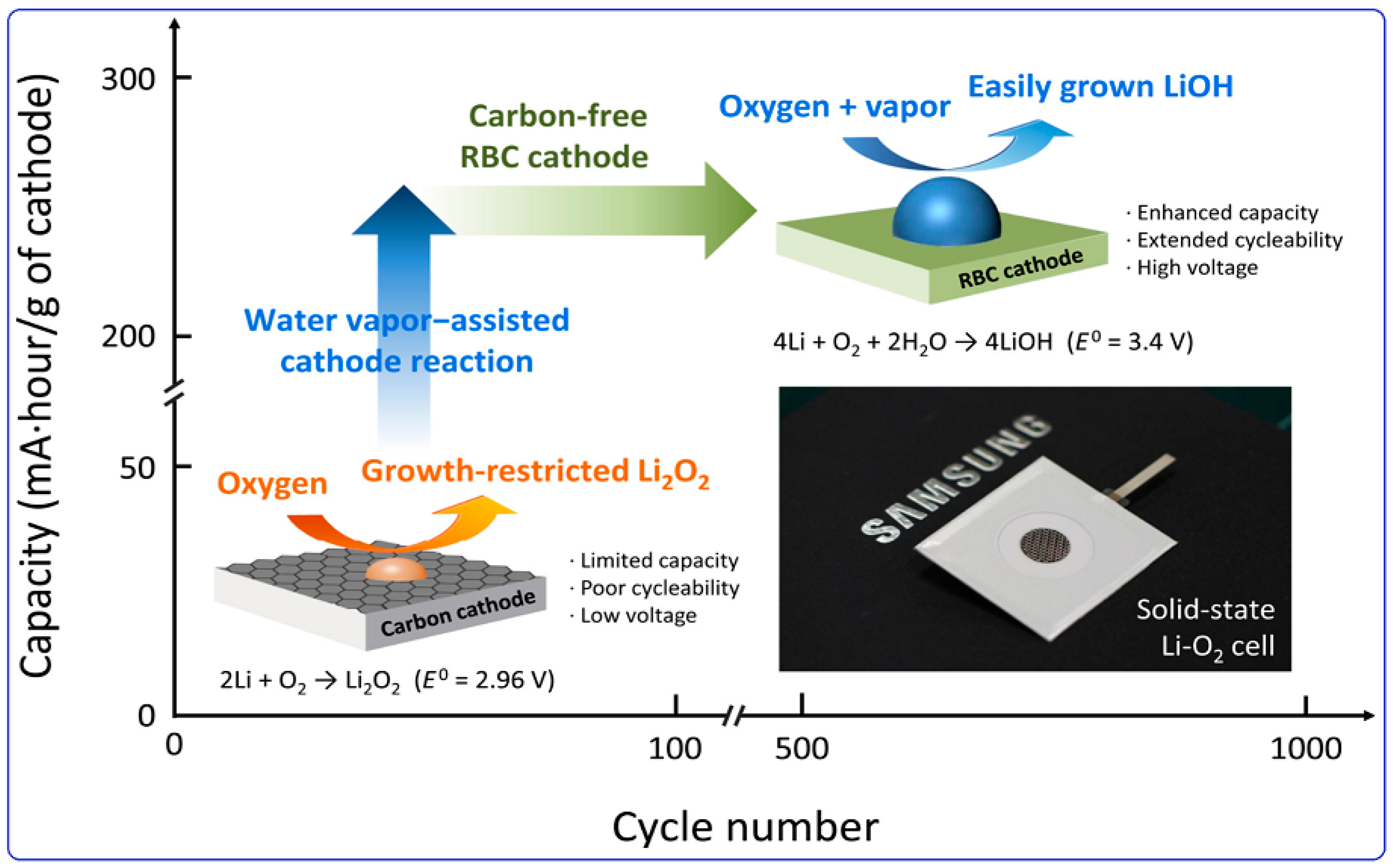
2.3. Cathode Architecture for Solid-State Lithium–Oxygen Batteries
3. Machine Learning of All-Solid-State Lithium Batteries
4. Recycling of All-Solid-State Lithium Batteries
- Material composition. The recyclability of SE materials depends on their chemical composition. Ideally, the materials should be composed of elements that are readily recyclable or can be extracted and reused efficiently.
- Purity level. The presence of impurities in SE materials can affect their recyclability. Contaminants or unwanted elements may need to be removed or separated during the recycling process.
- Manufacturing processes. The method used to produce SE materials can impact their recyclability. If the manufacturing process involves techniques that are difficult to reverse or require extensive energy input, it can hinder the material’s recyclability.
- Recycling technologies. Currently, there are various recycling technologies available for different types of materials. The recyclability of SE materials depends on the availability of appropriate recycling technologies that can efficiently recover the material components.
- Economic feasibility. The economic feasibility of recycling SE materials is an essential consideration. The cost of recycling should be reasonable relative to the cost of producing new materials.
- Research and development. Continuous research and development efforts are necessary to explore innovative recycling methods for SE materials. This can include the development of new recycling technologies or the improvement of existing ones.
5. Outlook and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wunderlich, P.; Küpper, J.; Simon, U. Optimizing discharge capacity of graphite nanosheet electrodes for lithium–oxygen batteries. Batteries 2020, 6, 36. [Google Scholar] [CrossRef]
- Ogasawara, T.; Débart, A.; Holzapfel, M.; Novák, P.; Bruce, P.G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef]
- Zheng, H.; Xiao, D.; Li, X.; Liu, Y.; Wu, Y.; Wang, J.; Jiang, K.; Chen, C.; Gu, L.; Wei, X.; et al. New insight in understanding oxygen reduction and evolution in solid-state lithium-oxygen batteries using an in situ environmental scanning electron microscope. Nano Lett. 2014, 14, 4245–4249. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, S.; Wang, X.; Xia, Y.; Xia, X.; Wu, J.; Gu, C.; Tu, J. Recent Developments of All-Solid-State Lithium Secondary Batteries with Sulfide Inorganic Electrolytes. Chem.—Eur. J. 2017, 24, 6007–6018. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-State Li–Metal Batteries: Challenges and Horizons of Oxide and Sulfide Solid Electrolytes and Their Interfaces. Adv. Energy Mater. 2020, 11, 2002689. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.; Yuan, H.; Cheng, X.; Huang, J.; Zhang, Q. Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies. Adv. Funct. Mater. 2021, 31, 2009925. [Google Scholar] [CrossRef]
- Sarkar, S.; Thangadurai, V. Critical Current Densities for High-Performance All-Solid-State Li-Metal Batteries: Fundamentals, Mechanisms, Interfaces, Materials, and Applications. ACS Energy Lett. 2022, 7, 1492–1527. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Tong, B.; Guo, F.; Wang, J.; Shi, Y.; Wen, R.; Zhou, Z.; Guo, L.; et al. Taming Interfacial Instability in Lithium–Oxygen Batteries: A Polymeric Ionic Liquid Electrolyte Solution. Adv. Energy Mater. 2019, 9, 1901967. [Google Scholar] [CrossRef]
- Wang, X.X.; Chi, X.W.; Li, M.L.; Guan, D.H.; Miao, C.L.; Xu, J.J. An integrated solid-state lithium-oxygen battery with highly stable anionic covalent organic frameworks electrolyte. Chem 2022, 9, 394–410. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Miao, X.; Wang, C.; Wang, C.; Zhang, Z.; Wang, R.; Yin, L. Designing Lithium Argyrodite Solid-State Electrolytes for High-Performance All-Solid-State Lithium Batteries. Batter. Supercaps 2021, 5, e202100288. [Google Scholar] [CrossRef]
- Vishnugopi, B.S.; Kazyak, E.; Lewis, J.A.; Nanda, J.; McDowell, M.T.; Dasgupta, N.P.; Mukherjee, P.P. Challenges and Opportunities for Fast Charging of Solid-State Lithium Metal Batteries. ACS Energy Lett. 2021, 6, 3734–3749. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Sun, X. Challenges and opportunities of nanostructured materials for aprotic rechargeable lithium–air batteries. Nano Energy 2013, 2, 443–467. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 452–471. [Google Scholar] [CrossRef]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable Artificial Solid Electrolyte Interphases for Lithium Batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Roberts, M.; Edström, K. The SEI layer formed on lithium metal in the presence of oxygen: A seldom considered component in the development of the Li–O2 battery. J. Power Sources 2013, 225, 40–45. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Wang, C.; Adair, K.; Sun, X. All-Solid-State Lithium Metal Batteries with Sulfide Electrolytes: Understanding Interfacial Ion and Electron Transport. Accounts Mater. Res. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Kitz, P.G.; Lacey, M.J.; Novák, P.; Berg, E.J. Operando investigation of the solid electrolyte interphase mechanical and transport properties formed from vinylene carbonate and fluoroethylene carbonate. J. Power Sources 2020, 477, 228567. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Park, J.-B.; Lee, S.H.; Jung, H.-G.; Aurbach, D.; Sun, Y.-K. Redox Mediators for Li-O2Batteries: Status and Perspectives. Adv. Mater. 2017, 30, 1704162. [Google Scholar] [CrossRef]
- Jung, J.W.; Cho, S.H.; Nam, J.S.; Kim, I.D. Current and future cathode materials for non-aqueous Li-air (O2) battery technology—A focused review. Energy Storage Mater. 2019, 24, 512–528. [Google Scholar] [CrossRef]
- Zahoor, A.; Ghouri, Z.K.; Hashmi, S.; Raza, F.; Ishtiaque, S.; Nadeem, S.; Ullah, I.; Nahm, K.S. Electrocatalysts for Lithium–Air Batteries: Current Status and Challenges. ACS Sustain. Chem. Eng. 2019, 7, 14288–14320. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zhang, W.; Huang, Y.; Liu, Y.; Wu, D.; Wang, L.; Chou, S. Toward high-performance lithium-oxygen batteries with cobalt-based transition metal oxide catalysts: Advanced strategies and mechanical insights. InfoMat 2021, 4, e12260. [Google Scholar] [CrossRef]
- Chalasani, D.; Lucht, B.L. Reactivity of electrolytes for lithium-oxygen batteries with Li2O2. ECS Electrochem. Lett. 2012, 1, A38–A42. [Google Scholar] [CrossRef]
- Yang, T.; Shu, C.; Zheng, R.; Li, M.; Hou, Z.; Hei, P.; Zhang, Q.; Mei, D.; Long, J. Dendrite-Free Solid-State Li-O2 Batteries Enabled by Organic-Inorganic Interaction Reinforced Gel Polymer Electrolyte. ACS Sustain. Chem. Eng. 2019, 7, 17362–17371. [Google Scholar] [CrossRef]
- Cao, D.; Sun, X.; Li, Q.; Natan, A.; Xiang, P.; Zhu, H. Lithium Dendrite in All-Solid-State Batteries: Growth Mechanisms, Suppression Strategies, and Characterizations. Matter 2020, 3, 57–94. [Google Scholar] [CrossRef]
- Sastre, J.; Futscher, M.H.; Pompizi, L.; Aribia, A.; Priebe, A.; Overbeck, J.; Stiefel, M.; Tiwari, A.N.; Romanyuk, Y.E. Blocking lithium dendrite growth in solid-state batteries with an ultrathin amorphous Li-La-Zr-O solid electrolyte. Commun. Mater. 2021, 2, 76. [Google Scholar] [CrossRef]
- Xin, X.; Ito, K.; Dutta, A.; Kubo, Y. Dendrite-Free Epitaxial Growth of Lithium Metal during Charging in Li–O2 Batteries. Angew. Chem. Int. Ed. 2018, 57, 13206–13210. [Google Scholar] [CrossRef]
- Hou, G.; Ma, X.; Sun, Q.; Ai, Q.; Xu, X.; Chen, L.; Li, D.; Chen, J.; Zhong, H.; Li, Y.; et al. Lithium Dendrite Suppression and Enhanced Interfacial Compatibility Enabled by an Ex Situ SEI on Li Anode for LAGP-Based All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18610–18618. [Google Scholar] [CrossRef]
- Kang, J.-H.; Park, J.; Na, M.; Choi, R.H.; Byon, H.R. Low-Temperature CO2-Assisted Lithium–Oxygen Batteries for Improved Stability of Peroxodicarbonate and Excellent Cyclability. ACS Energy Lett. 2022, 7, 4248–4257. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Yan, C.; Li, Z.; Zhao, C.; Xiang, R.; Yuan, H.; Huang, J.; Kuzmina, E.; Karaseva, E.; et al. A perspective on energy chemistry of low-temperature lithium metal batteries. iEnergy 2022, 1, 72–81. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar] [CrossRef]
- Hu, A.; Li, F.; Chen, W.; Lei, T.; Li, Y.; Fan, Y.; He, M.; Wang, F.; Zhou, M.; Hu, Y.; et al. Ion Transport Kinetics in Low-Temperature Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2202432. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, H.; Song, X.; Zhu, T.; Ye, Y.; Chen, J.; Yu, L.; Xu, J.; Chen, K. An extra-wide temperature all-solid-state lithium-metal battery operating from −73 °C to 120 °C. Energy Storage Mater. 2021, 39, 139–145. [Google Scholar] [CrossRef]
- Kitaura, H.; Zhou, H. All-solid-state lithium-oxygen battery with high safety in wide ambient temperature range. Sci. Rep. 2015, 5, 13271. [Google Scholar] [CrossRef]
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A highly stable and flexible zeolite electrolyte solid-state Li–air battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, X.-X.; Li, F.; Xu, J.-J. Fundamental Understanding and Construction of Solid-State Li−Air Batteries. Small Sci. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Yang, C.-S.; Gao, K.-N.; Zhang, X.-P.; Sun, Z.; Zhang, T. Rechargeable solid-state Li-air batteries: A status report. Rare Met. 2018, 37, 459–472. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Lu, W.; Sun, L.; Lin, L.; Zheng, M.; Sun, X.; Xie, H. PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries. Nano Energy 2021, 88, 106205. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2021, 64, 62–84. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, Q.; Du, X.; Zhang, J.; Zhang, H.; Zou, Z.; Zhou, X.; Cui, G. Poly(maleic anhydride) copolymers-based polymer electrolytes enlighten highly safe and high-energy-density lithium metal batteries: Advances and prospects. Nano Sel. 2020, 1, 59–78. [Google Scholar] [CrossRef]
- Yi, J.; Guo, S.; He, P.; Zhou, H. Status and prospects of polymer electrolytes for solid-state Li–O2 (air) batteries. Energy Environ. Sci. 2017, 10, 860–884. [Google Scholar] [CrossRef]
- Buannic, L.; Naviroj, M.; Miller, S.M.; Zagorski, J.; Faber, K.T.; Llordés, A. Dense freeze-cast Li 7 La 3 Zr 2 O 12 solid electrolytes with oriented open porosity and contiguous ceramic scaffold. J. Am. Ceram. Soc. 2018, 102, 1021–1029. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Qiu, M.; Li, X.; Li, C.; Li, R.; He, J.; Lin, G.; Qian, Q.; Wen, Z.; et al. Research progress in electrospinning engineering for all-solid-state electrolytes of lithium metal batteries. J. Energy Chem. 2021, 61, 253–268. [Google Scholar] [CrossRef]
- McOwen, D.W.; Xu, S.; Gong, Y.; Wen, Y.; Godbey, G.L.; Gritton, J.E.; Hamann, T.R.; Dai, J.; Hitz, G.T.; Hu, L.; et al. 3D-Printing Electrolytes for Solid-State Batteries. Adv. Mater. 2018, 30, e1707132. [Google Scholar] [CrossRef]
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing Strategies for Solid Electrolyte in Batteries. Front. Energy Res. 2020, 8, 571440. [Google Scholar] [CrossRef]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, K.; Chen, R.; Liu, T.; Zhang, Y.; Nan, C.W. Oxide Electrolytes for Lithium Batteries. J. Am. Ceram. Soc. 2015, 98, 3603–3623. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Q.; Tsai, C.L.; Tietz, F.; Guillon, O. A Novel Sol-Gel Method for Large-Scale Production of Nanopowders: Preparation of Li1.5Al0.5Ti1.5(PO4)3 as an Example. J. Am. Ceram. Soc. 2015, 99, 410–414. [Google Scholar] [CrossRef]
- Kang, I.; Kang, J. Low-cost iron-based electrocatalysts for high-performance Li–O2 batteries. Results Mater. 2023, 17, 100351. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, L.; Li, Y.; Wang, J.; Luo, Z.; Chen, W.; Liu, Z.; He, H. Aluminum silicate fiber membrane: A cost-effective substitute for fiber glass separator in Li–O2 battery. Mater. Today Energy 2020, 17, 100485. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, Y.; Qi, H.; Xiao, Z.; Lin, H.; Liu, J.; Guo, Z.; Wang, L.; Feng, S. A dendrite-free and stable anode for high-performance Li-O2 batteries by prestoring Li in reduced graphene oxide coated three-dimensional nickel foam. Chem. Commun. 2020, 56, 7645–7648. [Google Scholar] [CrossRef]
- Huang, G.; Wang, J.; Zhang, X. Electrode Protection in High-Efficiency Li-O2 Batteries. ACS Central Sci. 2020, 6, 2136–2148. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Xie, J. Building Better Li Metal Anodes in Liquid Electrolyte: Challenges and Progress. ACS Appl. Mater. Interfaces 2020, 13, 18–33. [Google Scholar] [CrossRef]
- Li, J.; Kong, Z.; Liu, X.; Zheng, B.; Fan, Q.H.; Garratt, E.; Schuelke, T.; Wang, K.; Xu, H.; Jin, H. Strategies to anode protection in lithium metal battery: A review. InfoMat 2021, 3, 1333–1363. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Xia, Y.; Chen, C.; Wang, F.; Tamirat, A.G.; Wang, Y.; Xia, Y.; Wang, L.; Feng, S. A flexible polymer-based Li-air battery using a reduced graphene oxide/Li composite anode. J. Mater. Chem. A 2018, 6, 6022–6032. [Google Scholar] [CrossRef]
- Deng, H.; Qiu, F.; Li, X.; Qin, H.; Zhao, S.; He, P.; Zhou, H. A Li-ion oxygen battery with Li-Si alloy anode prepared by a mechanical method. Electrochem. Commun. 2017, 78, 11–15. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, X.; Wang, Y.; Xia, Y. Correction: A lithium air battery with a lithiated Al–carbon anode. Chem. Commun. 2021, 57, 3724. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, M.; Zheng, L.; Guan, D.; Huang, X.; Xu, J.; Yu, J. Porous Materials Applied in Nonaqueous Li–O2 Batteries: Status and Perspectives. Adv. Mater. 2020, 32, 2002559. [Google Scholar] [CrossRef]
- Jeong, M.G.; Kwak, W.J.; Kim, J.Y.; Lee, J.K.; Sun, Y.K.; Jung, H.G. Uniformly distributed reaction by 3D host-lithium composite anode for high rate capability and reversibility of Li-O2 batteries. Chem. Eng. J. 2021, 427, 130914. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, L.; Gu, Y.; Hu, J.; Chen, Y.; Qi, P.; Zhao, X.; Peng, Y.; Deng, Z.; Liu, Z. High-Performance Li–O2 Batteries Based on All-Graphene Backbone. Adv. Funct. Mater. 2020, 30, 2007218. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, G.; Yin, L.; Li, F.; Bin Xu, B.; Dala, L.; Liu, X.; Luo, K. A Facile Surface Preservation Strategy for the Lithium Anode for High-Performance Li-O2 Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27316–27326. [Google Scholar] [CrossRef]
- Xia, Q.; Zan, F.; Zhang, Q.; Liu, W.; Li, Q.; He, Y.; Hua, J.; Liu, J.; Xu, J.; Wang, J.; et al. All-Solid-State Thin Film Lithium/Lithium-Ion Microbatteries for Powering the Internet of Things. Adv. Mater. 2022, 35, 2200538. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Jiang, H.; Xiong, S.; Feng, J.; Qian, Y. Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 2020, 78, 105344. [Google Scholar] [CrossRef]
- Hsueh, T.H.; Wang, M.-C.; Liu, S.-E.; Wu, B.-H.; Li, Y.-C.; Tsai, D.-G.; Chang, S.-M.; Shiue, A.; Chin, K.-Y. Sputtered silver on the current collector for anode-less NMC111 gel polymer electrolyte lithium batteries. Electrochem. Commun. 2023, 150, 107478. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Su, W.-N.; Shitaw, K.N.; Jiang, S.-K.; Abrha, L.H.; Weret, M.A.; Merso, S.K.; Hagos, T.M.; Huang, C.-J.; Lakshmanan, K.; et al. Multifunctional Electrospun PVDF-HFP Gel Polymer Electrolyte Membrane Suppresses Dendrite Growth in Anode-Free Li Metal Battery. Energy Storage Mater. 2023, 61, 102861. [Google Scholar] [CrossRef]
- Hasegawa, S.; Imanishi, N.; Zhang, T.; Xie, J.; Hirano, A.; Takeda, Y.; Yamamoto, O. Study on lithium/air secondary batteries-Stability of NASICON-type lithium ion conducting glass–ceramics with water. J. Power Sources 2009, 189, 371–377. [Google Scholar] [CrossRef]
- Bai, F.; Kakimoto, K.; Shang, X.; Mori, D.; Taminato, S.; Matsumoto, M.; Takeda, Y.; Yamamoto, O.; Izumi, H.; Minami, H.; et al. Water-Stable High Lithium-Ion Conducting Solid Electrolyte of Li1.4Al0.4Ge0.2Ti1.4(PO4)3–LiCl for Aqueous Lithium-Air Batteries. Front. Energy Res. 2020, 8, 187. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-Type Materials in Contact with Lithium Metal: Formation of Mixed Conducting Interphases (MCI) on Solid Electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Lewis, J.A.; Cortes, F.J.Q.; Boebinger, M.G.; Tippens, J.; Marchese, T.S.; Kondekar, N.P.; Liu, X.; Chi, M.; McDowell, M.T. Interphase Morphology between a Solid-State Electrolyte and Lithium Controls Cell Failure. ACS Energy Lett. 2019, 4, 591–599. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H. Composite Solid Electrolytes with NASICON-Type LATP and PVdF–HFP for Solid-State Lithium Batteries. Ind. Eng. Chem. Res. 2021, 60, 1494–1500. [Google Scholar] [CrossRef]
- Yow, Z.F.; Oh, Y.L.; Gu, W.; Rao, R.P.; Adams, S. Effect of Li+/H+ exchange in water treated Ta-doped Li7La3Zr2O12. Solid State Ion. 2016, 292, 122–129. [Google Scholar] [CrossRef]
- Zeng, X.; Martinolich, A.J.; See, K.A.; Faber, K.T. Dense garnet-type electrolyte with coarse grains for improved air stability and ionic conductivity. J. Energy Storage 2019, 27, 101128. [Google Scholar] [CrossRef]
- Huang, B.; Xu, B.; Zhang, J.; Li, Z.; Huang, Z.; Li, Y.; Wang, C.-A. Li-ion conductivity and stability of hot-pressed LiTa2PO8 solid electrolyte for all-solid-state batteries. J. Mater. Sci. 2020, 56, 2425–2434. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Ding, L.; Liu, Y.-Z.; Xiong, L.-P.; Chen, J.; Luo, X.-L. Room-temperature ionic conductivity of Ba, Y, Al co-doped Li7La3Zr2O12 solid electrolyte after sintering. Rare Met. 2020, 40, 2301–2306. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Inada, R.; Kimura, K.; Kusakabe, K.; Tojo, T.; Sakurai, Y. Synthesis and lithium-ion conductivity for perovskite-type Li3/8Sr7/16Ta3/4Zr1/4O3 solid electrolyte by powder-bed sintering. Solid State Ion. 2014, 261, 95–99. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Chien, P.; Wu, N.; Xin, S.; Xue, L.; Park, K.; Hu, Y.; Goodenough, J.B. A Perovskite Electrolyte That Is Stable in Moist Air for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 8587–8591. [Google Scholar] [CrossRef]
- Ma, S.B.; Kwon, H.J.; Kim, M.; Bak, S.; Lee, H.; Ehrlich, S.N.; Cho, J.; Im, D.; Seo, D. Mixed Ionic–Electronic Conductor of Perovskite LixLayMO3−δ toward Carbon-Free Cathode for Reversible Lithium–Air Batteries. Adv. Energy Mater. 2020, 10, 2001767. [Google Scholar] [CrossRef]
- Lim, C.; Kim, C.; Gwon, O.; Jeong, H.Y.; Song, H.-K.; Ju, Y.-W.; Shin, J.; Kim, G. Nano-perovskite oxide prepared via inverse microemulsion mediated synthesis for catalyst of lithium-air batteries. Electrochim. Acta 2018, 275, 248–255. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, Q.; Li, Y.; Tao, L.; Tan, H.; Yin, K.; Zhou, J.; Guo, S. Cesium Lead Bromide Perovskite-Based Lithium-Oxygen Batteries. Nano Lett. 2021, 21, 4861–4867. [Google Scholar] [CrossRef]
- Sahu, G.; Lin, Z.; Li, J.; Liu, Z.; Dudney, N.; Liang, C. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li 4 SnS 4. Energy Environ. Sci. 2013, 7, 1053–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, X.; Zheng, C.; Liu, X.; Chen, Z.; Yang, W.; Lin, J.; Huang, F. Chemistry Design Towards a Stable Sulfide-Based Superionic Conductor Li4Cu8Ge3S12. Angew. Chem. Int. Ed. 2019, 58, 7673–7677. [Google Scholar] [CrossRef]
- Ohtomo, T.; Hayashi, A.; Tatsumisago, M.; Kawamoto, K. Characteristics of the Li2O-Li2S-P2S 5 glasses synthesized by the two-step mechanical milling. J. Non-Cryst. Solids 2013, 364, 57–61. [Google Scholar] [CrossRef]
- Otoyama, M.; Kuratani, K.; Kobayashi, H. A systematic study on structure, ionic conductivity, and air-stability of xLi4SnS4·(1−x)Li3PS4 solid electrolytes. Ceram. Int. 2021, 47, 28377–28383. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, e1901131. [Google Scholar] [CrossRef] [PubMed]
- Hanghofer, I.; Redhammer, G.; Rohde, S.; Hanzu, I.; Senyshyn, A.; Wilkening, H.M.R.; Rettenwander, D. Untangling the Structure and Dynamics of Lithium-Rich Anti-Perovskites Envisaged as Solid Electrolytes for Batteries. Chem. Mater. 2018, 30, 8134–8144. [Google Scholar] [CrossRef]
- Emly, A.; Kioupakis, E.; Van der Ven, A. Phase Stability and Transport Mechanisms in Antiperovskite Li 3 OCl and Li 3 OBr Superionic Conductors. Chem. Mater. 2013, 25, 4663–4670. [Google Scholar] [CrossRef]
- Mohamed, M.A.A.; Gorbunov, M.V.; Valldor, M.; Hampel, S.; Gräßler, N.; Mikhailova, D. Tuning the electrochemical properties by anionic substitution of Li-rich antiperovskite (Li 2 Fe)S 1− x Se x O cathodes for Li-ion batteries. J. Mater. Chem. A 2021, 9, 23095–23105. [Google Scholar] [CrossRef]
- Li, S.; Zhu, J.; Wang, Y.; Howard, J.W.; Lü, X.; Li, Y.; Kumar, R.S.; Wang, L.; Daemen, L.L.; Zhao, Y. Reaction mechanism studies towards effective fabrication of lithium-rich anti-perovskites Li3OX (X = Cl, Br). Solid State Ion. 2016, 284, 14–19. [Google Scholar] [CrossRef]
- Senevirathne, K.; Day, C.S.; Gross, M.D.; Lachgar, A.; Holzwarth, N. A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure. Solid State Ion. 2013, 233, 95–101. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Kalubarme, R.S.; Jadhav, A.H.; Gil Seo, J. Highly stable bilayer of LiPON and B2O3 added Li1.5Al0.5Ge1.5(PO4) solid electrolytes for non-aqueous rechargeable Li-O2 batteries. Electrochim. Acta 2016, 199, 126–132. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.-P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef]
- Steinle, D.; Wu, F.; Kim, G.-T.; Passerini, S.; Bresser, D. PEO-based Interlayers for LAGP-type Solid-State Lithium-Metal Batteries. In Electrochemical Society Meeting Abstracts 242; No. 4; The Electrochemical Society, Inc.: Atlanta, GA, USA, 2022. [Google Scholar] [CrossRef]
- Dussart, T.; Stevens, P.; Toussaint, G.; Laberty-Robert, C. Study of Solid State Lithium Batteries with a Ceramic Electrolyte. In Electrochemical Society Meeting Abstracts 237; No. 2; The Electrochemical Society, Inc.: Montréal, QC, Canada, 2022. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li 5 La 3 M 2 O 12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La 3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Qin, S.; Zhu, X.; Jiang, Y.; Ling, M.; Hu, Z.; Zhu, J. Growth of self-textured Ga3+-substituted Li7La3Zr2O12 ceramics by solid state reaction and their significant enhancement in ionic conductivity. Appl. Phys. Lett. 2018, 112, 113901. [Google Scholar] [CrossRef]
- Meesala, Y.; Jena, A.; Chang, H.; Liu, R.-S. Recent Advancements in Li-Ion Conductors for All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2017, 2, 2734–2751. [Google Scholar] [CrossRef]
- Kobi, S.; Amardeep; Vyas, A.; Bhargava, P.; Mukhopadhyay, A. Al and Mg Co-Doping Towards Development of Air-Stable and Li-Ion Conducting Li-La-Zirconate Based Solid Electrolyte Exhibiting Low Electrode/Electrolyte Interfacial Resistance. J. Electrochem. Soc. 2020, 167, 120519. [Google Scholar] [CrossRef]
- Abrha, L.H.; Hagos, T.T.; Nikodimos, Y.; Bezabh, H.K.; Berhe, G.B.; Hagos, T.M.; Huang, C.-J.; Tegegne, W.A.; Jiang, S.-K.; Weldeyohannes, H.H.; et al. Dual-Doped Cubic Garnet Solid Electrolytes with Superior Air Stability. ACS Appl. Mater. Interfaces 2020, 12, 25709–25717. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Li7La3Zr2O12 electrolyte stability in air and fabrication of a Li/Li7La3Zr 2O12/Cu0.1V2O5 solid-state battery. J. Power Sources 2013, 239, 326–331. [Google Scholar] [CrossRef]
- Jia, M.; Bi, Z.; Shi, C.; Zhao, N.; Guo, X. Air-stable dopamine-treated garnet ceramic particles for high-performance composite electrolytes. J. Power Sources 2020, 486, 229363. [Google Scholar] [CrossRef]
- Duan, H.; Chen, W.; Fan, M.; Wang, W.; Yu, L.; Tan, S.; Chen, X.; Zhang, Q.; Xin, S.; Wan, L.; et al. Building an Air Stable and Lithium Deposition Regulable Garnet Interface from Moderate-Temperature Conversion Chemistry. Angew. Chem. Int. Ed. 2020, 59, 12069–12075. [Google Scholar] [CrossRef]
- Li, R.; Liao, K.; Zhou, W.; Li, X.; Meng, D.; Cai, R.; Shao, Z. Realizing fourfold enhancement in conductivity of perovskite Li0.33La0.557TiO3 electrolyte membrane via a Sr and Ta co-doping strategy. J. Membr. Sci. 2019, 582, 194–202. [Google Scholar] [CrossRef]
- Le, H.T.T.; Ngo, D.T.; Didwal, P.N.; Fisher, J.G.; Park, C.-N.; Kim, I.-D.; Park, C.-J. Highly efficient and stable solid-state Li–O2 batteries using a perovskite solid electrolyte. J. Mater. Chem. A 2019, 7, 3150–3160. [Google Scholar] [CrossRef]
- Xu, H.; Chien, P.-H.; Shi, J.; Li, Y.; Wu, N.; Liu, Y.; Hu, Y.-Y.; Goodenough, J.B. High-performance all-solid-state batteries enabled by salt bonding to perovskite in poly(ethylene oxide). Proc. Natl. Acad. Sci. USA 2019, 116, 18815–18821. [Google Scholar] [CrossRef]
- Dawson, J.A.; Famprikis, T.; Johnston, K.E. Anti-perovskites for solid-state batteries: Recent developments, current challenges and future prospects. J. Mater. Chem. A 2021, 9, 18746–18772. [Google Scholar] [CrossRef]
- Dixit, M.; Muralidharan, N.; Bisht, A.; Jafta, C.J.; Nelson, C.T.; Amin, R.; Essehli, R.; Balasubramanian, M.; Belharouak, I. Tailoring of the Anti-Perovskite Solid Electrolytes at the Grain-Scale. ACS Energy Lett. 2023, 8, 2356–2364. [Google Scholar] [CrossRef]
- Ye, Y.; Deng, Z.; Gao, L.; Niu, K.; Zhao, R.; Bian, J.; Li, S.; Lin, H.; Zhu, J.; Zhao, Y. Lithium-Rich Anti-perovskite Li 2 OHBr-Based Polymer Electrolytes Enabling an Improved Interfacial Stability with a Three-Dimensional-Structured Lithium Metal Anode in All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2021, 13, 28108–28117. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, Y.; Zhao, F.; Adair, K.; Zhao, R.; Li, S.; Zou, R.; Zhao, Y.; Sun, X. Antiperovskite Electrolytes for Solid-State Batteries. Chem. Rev. 2022, 122, 3763–3819. [Google Scholar] [CrossRef]
- Deng, Z.; Ni, D.; Chen, D.; Bian, Y.; Li, S.; Wang, Z.; Zhao, Y. Anti-perovskite materials for energy storage batteries. InfoMat 2021, 4, e12252. [Google Scholar] [CrossRef]
- Yu, P.; Ye, Y.; Zhu, J.; Xia, W.; Zhao, Y. Optimized Interfaces in Anti-Perovskite Electrolyte-Based Solid-State Lithium Metal Batteries for Enhanced Performance. Front. Chem. 2021, 9, 786956. [Google Scholar] [CrossRef]
- Fang, H.; Jena, P. Li-rich antiperovskite superionic conductors based on cluster ions. Proc. Natl. Acad. Sci. USA 2017, 114, 11046–11051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Yu, J. Zeolite-Based Electrolytes: A Promising Choice for Solid-State Batteries. PRX Energy 2022, 1, 031001. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Q.; Liu, Y.; Yao, P.; Liu, C.; Liu, X.; Wu, J.; Lavorgna, M. Integrate multifunctional ionic sieve lithiated X zeolite-ionic liquid electrolyte for solid-state lithium metal batteries with ultralong lifespan. Chem. Eng. J. 2021, 433, 133522. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Alfutimie, A. Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column. Energies 2022, 15, 347. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Q.; Zhang, Q.; Yao, P.; Liu, X.; Wu, J. A flexible solid polymer electrolyte enabled with lithiated zeolite for high performance lithium battery. Nano Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Z.; Chu, F.; Xue, Z.; Wu, F.; Yu, Y. Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: Challenges, strategies, and prospects. InfoMat 2021, 4, e12248. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Bermudez, V.d.Z.; Fidalgo-Marijuan, A.; Zhang, Q.; Lanceros-Méndez, S. Metal-organic frameworks and zeolite materials as active fillers for lithium-ion battery solid polymer electrolytes. Mater. Adv. 2021, 2, 3790–3805. [Google Scholar] [CrossRef]
- Le, H.T.T.; Ngo, D.T.; Kim, Y.J.; Park, C.N.; Park, C.J. A perovskite-structured aluminium-substituted lithium lanthanum titanate as a potential artificial solid-electrolyte interface for aqueous rechargeable lithium-metal-based batteries. Electrochim. Acta 2017, 248, 232–242. [Google Scholar] [CrossRef]
- Khan, T.T.; Park, C.-J. Solid-State Li-O2 Battery Using a Perovskite Type Solid Electrolyte with an Improved Interfacial Property. In Electrochemical Society Meeting Abstracts 235; No. 2; The Electrochemical Society, Inc.: Dallas, TX, USA, 2019. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Zhang, X.; He, P.; Zhou, H. Hybrid polymer electrolyte for Li–O2 batteries. Green Energy Environ. 2018, 4, 3–19. [Google Scholar] [CrossRef]
- Yi, J.; Zhou, H. A Unique Hybrid Quasi-Solid-State Electrolyte for Li-O2 Batteries with Improved Cycle Life and Safety. Chemsuschem 2016, 9, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Sashmitha, K.; Rani, M.U. A comprehensive review of polymer electrolyte for lithium-ion battery. Polym. Bull. 2022, 80, 89–135. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Matsuo, A.; Takase, K.; Doi, S.; Hisanaga, A.; Onimura, K.; Oishi, T. Conductivity enhancement of polyacrylonitrile-based electrolytes by addition of cascade nitrile compounds. J. Power Sources 2000, 90, 33–38. [Google Scholar] [CrossRef]
- Li, S.; Ren, W.; Huang, Y.; Zhou, Q.; Luo, C.; Li, Z.; Li, X.; Wang, M.; Cao, H. Building more secure LMBs with gel polymer electrolytes based on dual matrices of PAN and HPMC by improving compatibility with anode and tuning lithium ion transference. Electrochim. Acta 2021, 391, 138950. [Google Scholar] [CrossRef]
- Huq, R.; Koksbang, R.; Tonder, P.; Farrington, G.C. Effect of plasticizers on the properties of new ambient temperature polymer electrolyte. Electrochim. Acta 1992, 37, 1681–1684. [Google Scholar] [CrossRef]
- Abraham, K.M.; Alamgir, M. Li+-Conductive Solid Polymer Electrolytes with Liquid-Like Conductivity. J. Electrochem. Soc. 1990, 137, 1657–1658. [Google Scholar] [CrossRef]
- Tran, H.K.; Wu, Y.-S.; Chien, W.-C.; Wu, S.-H.; Jose, R.; Lue, S.J.; Yang, C.-C. Composite polymer electrolytes based on PVA/PAN for all-solid-state lithium metal batteries operated at room temperature. ACS Appl. Energy Mater. 2020, 3, 11024–11035. [Google Scholar] [CrossRef]
- Chen, W.P.; Duan, H.; Shi, J.-L.; Qian, Y.; Wan, J.; Zhang, X.-D.; Sheng, H.; Guan, B.; Wen, R.; Yin, Y.-X.; et al. Bridging Interparticle Li+ Conduction in a Soft Ceramic Oxide Electrolyte. J. Am. Chem. Soc. 2021, 143, 5717–5726. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, S.J. Alkaline composite PEO-PVA-glass-fibre-mat polymer electrolyte for Zn-air battery. J. Power Sources 2002, 112, 497–503. [Google Scholar] [CrossRef]
- Wen, Z.; Itoh, T.; Uno, T.; Kubo, M.; Yamamoto, O. Thermal, electrical, and mechanical properties of composite polymer electrolytes based on cross-linked poly(ethylene oxide-co-propylene oxide) and ceramic filler. Solid State Ion. 2003, 160, 141–148. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Hou, G.; Cheng, H.; Li, F. Homogeneous and Fast Ion Conduction of PEO-Based Solid-State Electrolyte at Low Temperature. Adv. Funct. Mater. 2020, 30, 2007172. [Google Scholar] [CrossRef]
- Fauteux, D.; Massucco, A.; McLin, M.; Van Buren, M.; Shi, J. Lithium polymer electrolyte rechargeable battery. Electrochim. Acta 1995, 40, 2185–2190. [Google Scholar] [CrossRef]
- Weston, J.; Steele, B. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wu, X.; Wang, J.; Li, Q.; Pan, K.; He, J. Research Progress and Application of PEO-Based Solid State Polymer Composite Electrolytes. Front. Energy Res. 2021, 9, 726738. [Google Scholar] [CrossRef]
- Li, C.; Xue, P.; Chen, L.; Liu, J.; Wang, Z. Reducing the crystallinity of PEO-based composite electrolyte for high performance lithium batteries. Compos. Part B Eng. 2022, 234, 109729. [Google Scholar] [CrossRef]
- He, K.; Cheng, S.H.; Hu, J.; Zhang, Y.; Yang, H.; Liu, Y.; Liao, W.; Chen, D.; Liao, C.; Cheng, X.; et al. In-Situ Intermolecular Interaction in Composite Polymer Electrolyte for Ultralong Life Quasi-Solid-State Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 12116–12123. [Google Scholar] [CrossRef]
- Ramesh, S.; Wen, L.C. Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionics 2009, 16, 255–262. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Li, Y.J.; Fan, C.Y.; Zhang, J.P.; Wu, X.L. A promising PMHS/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Dalton Trans. 2018, 47, 14932–14937. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, X.; Rashid, A.; Hu, Z.; Sun, P.; Zhang, Q.; Zhang, L. Organic polymeric filler-amorphized poly(ethylene oxide) electrolyte enables all-solid-state lithium–metal batteries operating at 35 °C. J. Mater. Chem. A 2020, 8, 13351–13363. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Harding, J.R.; Shao-Horn, Y.; Hammond, P.T. Understanding the chemical stability of polymers for lithium-air batteries. Chem. Mater. 2015, 27, 550–561. [Google Scholar] [CrossRef]
- Uludağ, A.A.; Tokur, M.; Algul, H.; Cetinkaya, T.; Uysal, M.; Akbulut, H. High stable Li-air battery cells by using PEO and PVDF additives in the TEGDME/LiPF6 electrolytes. Int. J. Hydrogen Energy 2016, 41, 6954–6964. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Santhosh, P.; Manesh, K.M.; Nho, J.H.; Kim, S.H.; Hwang, C.-G.; Lee, K.-P. Development of electrospun PVdF-PAN membrane-based polymer electrolytes for lithium batteries. J. Membr. Sci. 2008, 325, 683–690. [Google Scholar] [CrossRef]
- Ramesh, S.; Lu, S.-C. Enhancement of ionic conductivity and structural properties by 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid in poly(vinylidene fluoride-hexafluoropropylene)-based polymer electrolytes. J. Appl. Polym. Sci. 2012, 126, E484–E492. [Google Scholar] [CrossRef]
- Kim, K.M.; Ryu, K.S.; Kang, S.-G.; Chang, S.H.; Chung, I.J. The Effect of Silica Addition on the Properties of Poly((vinylidene fluoride)-co-hexafluoropropylene)-Based Polymer Electrolytes. Macromol. Chem. Phys. 2001, 202, 866–872. [Google Scholar] [CrossRef]
- Saikia, D.; Chen-Yang, Y.; Chen, Y.; Li, Y.; Lin, S. Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P(VDF-HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 2008, 234, 24–32. [Google Scholar] [CrossRef]
- Liu, T.; Chang, Z.; Yin, Y.; Chen, K.; Zhang, Y.; Zhang, X. The PVDF-HFP gel polymer electrolyte for Li-O2 battery. Solid State Ion. 2018, 318, 88–94. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Zhang, P.; Li, H.; Mi, H. PVDF-HFP based polymer electrolytes with high Li+ transference number enhancing the cycling performance and rate capability of lithium metal batteries. Appl. Surf. Sci. 2021, 574, 151593. [Google Scholar] [CrossRef]
- Celik, M.; Kızılaslan, A.; Can, M.; Cetinkaya, T.; Akbulut, H. Electrochemical investigation of PVDF: HFP gel polymer electrolytes for quasi-solid-state Li-O2 batteries: Effect of lithium salt type and concentration. Electrochim. Acta 2021, 371, 137824. [Google Scholar] [CrossRef]
- Rajendran, S.; Mahendran, O.; Kannan, R. Ionic conductivity studies in composite solid polymer electrolytes based on methylmethacrylate. J. Phys. Chem. Solids 2002, 63, 303–307. [Google Scholar] [CrossRef]
- Liew, C.; Durairaj, R.; Ramesh, S. Rheological studies of PMMA-PVC based polymer blend electrolytes with LiTFSI as doping salt. PLoS ONE 2014, 9, e102815. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Sedlaříková, M.; Vondrák, J.; Pařízek, L. PMMA-Based Electrolytes for Li-Ion Batteries. ECS Trans. 2016, 74, 159–164. [Google Scholar] [CrossRef]
- Flora, X.H.; Ulaganathan, M.; Babu, R.S.; Rajendran, S. Evaluation of lithium ion conduction in PAN/PMMA-based polymer blend electrolytes for Li-ion battery applications. Ionics 2012, 18, 731–736. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Gui, X.; Lin, S.; Tu, Y. A Promising PMMA/m-MgO All-Solid-State Electrolyte for Lithium-Oxygen Batteries. J. Electrochem. Soc. 2021, 168, 020514. [Google Scholar] [CrossRef]
- Liu, X.; Xin, X.; Shen, L.; Gu, Z.; Wu, J.; Yao, X. Poly(methyl methacrylate)-Based Gel Polymer Electrolyte for High-Performance Solid State Li-O2 Battery with Enhanced Cycling Stability. ACS Appl. Energy Mater. 2021, 4, 3975–3982. [Google Scholar] [CrossRef]
- Kim, Y.D.; Jo, Y.K.; Jo, N.J. Electrochemical performance of poly(vinyl alcohol)-based solid polymer electrolyte for lithium polymer batteries. J. Nanosci. Nanotechnol. 2012, 12, 3529–3533. [Google Scholar] [CrossRef]
- Yang, C.C.; Wu, G. Study of microporous PVA/PVC composite polymer membrane and it application to MnO2 capacitors. Mater. Chem. Phys. 2009, 114, 948–955. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, M.; Subadevi, R. Effect of salt concentration in poly(vinyl alcohol)-based solid polymer electrolytes. J. Power Sources 2003, 124, 225–230. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, H.Z.; Yang, C.C. Modification and characterization of semi-crystalline poly(vinyl alcohol) with interpenetrating poly(acrylic acid) by UV radiation method for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2008, 322, 74–80. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Zhou, S.; Hu, H. Mechanical integrity degradation and control of all-solid-state lithium battery with physical aging poly (vinyl alcohol)-based electrolyte. Polymers 2020, 12, 1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, J.; Zhao, Y.; Zheng, M.; Li, X.; Sun, X. All-solid-state lithium batteries enabled by sulfide electrolytes: From fundamental research to practical engineering design. Energy Environ. Sci. 2021, 14, 2577–2619. [Google Scholar] [CrossRef]
- Song, S.; Qin, X.; Ruan, Y.; Li, W.; Xu, Y.; Zhang, D.; Thokchom, J. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte. J. Power Sources 2020, 461, 228146. [Google Scholar] [CrossRef]
- Castillo, J.; Santiago, A.; Judez, X.; Garbayo, I.; Clemente, J.A.C.; Morant-Miñana, M.C.; Villaverde, A.; González-Marcos, J.A.; Zhang, H.; Armand, M.; et al. Safe, Flexible, and High-Performing Gel-Polymer Electrolyte for Rechargeable Lithium Metal Batteries. Chem. Mater. 2021, 33, 8812–8821. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2018, 136, 27–46. [Google Scholar] [CrossRef]
- Wang, J.; Huang, G.; Yan, J.-M.; Ma, J.-L.; Liu, T.; Shi, M.-M.; Yu, Y.; Zhang, M.-M.; Tang, J.-L.; Zhang, X.-B. Hybrid solid electrolyte enabled dendrite-free Li anodes for high-performance quasi-solid-state lithium-oxygen batteries. Natl. Sci. Rev. 2020, 8, nwaa150. [Google Scholar] [CrossRef]
- Ouyang, H.; Min, S.; Yi, J.; Liu, X.; Ning, F.; Qin, J.; Jiang, Y.; Zhao, B.; Zhang, J. Tuning composite solid-state electrolyte interface to improve the electrochemical performance of lithium-oxygen battery. Green Energy Environ. 2022, 8, 1195–1204. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Liu, J.; Song, H.; Liu, Y.; Wang, P.; He, P.; Xu, J.; Zhou, H. Ultra-fine surface solid-state electrolytes for long cycle life all-solid-state lithium-air batteries. J. Mater. Chem. A 2018, 6, 21248–21254. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Iv, W.J.E.; Deng, D.Z.; Yang, J.; Xiao, J. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A 2016, 4, 15266–15280. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, J.; Li, X.; Holmes, N.; Wang, C.; Wang, J.; Zhao, F.; Li, S.; Sun, Q.; Yang, X.; et al. Halide-based solid-state electrolyte as an interfacial modifier for high performance solid-state Li–O2 batteries. Nano Energy 2020, 75, 105036. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Q.; Luo, J.; Liang, J.; Liu, Y.; Zhang, L.; Wang, J.; Deng, S.; Lin, X.; Yang, X.; et al. 3D Porous Garnet/Gel Polymer Hybrid Electrolyte for Safe Solid-State Li-O2 Batteries with Long Lifetimes. Chem. Mater. 2020, 32, 10113–10119. [Google Scholar] [CrossRef]
- Chamaani, A.; Chawla, N.; Safa, M.; El-Zahab, B. One-Dimensional Glass Micro-Fillers in Gel Polymer Electrolytes for Li-O2 Battery Applications. Electrochim. Acta 2017, 235, 56–63. [Google Scholar] [CrossRef]
- Luo, K.; Zhu, G.; Zhao, Y.; Luo, Z.; Liu, X.; Zhang, K.; Li, Y.; Scott, K. Enhanced cycling stability of Li-O2 batteries by using a polyurethane/SiO2/glass fiber nanocomposite separator. J. Mater. Chem. A 2018, 6, 7770–7776. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Xu, M.; Xia, Q.; Liu, J.; Zhao, S.; Chen, L.; Pan, A.; Ivey, D.G.; Wei, W. Cross-linked branching nanohybrid polymer electrolyte with monodispersed TiO2 nanoparticles for high performance lithium-ion batteries. J. Power Sources 2016, 317, 103–111. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhao, T.S.; Wei, Z.H.; Tan, P.; Zhao, G. A novel solid-state Li–O2 battery with an integrated electrolyte and cathode structure. Energy Environ. Sci. 2015, 8, 2782–2790. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhao, T.S.; Wei, Z.H.; Tan, P.; An, L. A high-rate and long cycle life solid-state lithium-air battery. Energy Environ. Sci. 2015, 8, 3745–3754. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, T.; Tan, P.; Wei, Z.; Wu, M. A high-performance solid-state lithium-oxygen battery with a ceramic-carbon nanostructured electrode. Nano Energy 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, H.; Zhao, H.; Zhou, Z.; Wei, Z. A simple and effective method to prepare dense Li1.3Al0.3Ti1.7(PO4)3 solid–state electrolyte for lithium-oxygen batteries. Ionics 2020, 26, 6049–6056. [Google Scholar] [CrossRef]
- Gong, H.; Xue, H.; Lu, X.; Gao, B.; Wang, T.; He, J.; Ma, R. All solid-state lithium-oxygen batteries with MOF-derived nickel cobaltate nanoflake arrays as high-performance oxygen cathodes. Chem. Commun. 2019, 55, 10689–10692. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Y.; Sun, Q.; Wang, C.; Luo, J.; Lin, X.; Yang, X.; Zhao, Y.; Li, R.; Zhao, S.; et al. Transition of the Reaction from Three-Phase to Two-Phase by Using a Hybrid Conductor for High-Energy-Density High-Rate Solid-State Li-O2 Batteries. Angew. Chem. Int. Ed. 2021, 60, 5821–5826. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, N.; Li, Y.; Guo, X.; Feng, X.; Liu, X.; Liu, Z.; Cui, G.; Zheng, H.; Gu, L.; et al. A rechargeable Li-air fuel cell battery based on garnet solid electrolytes. Sci. Rep. 2017, 7, srep41217. [Google Scholar] [CrossRef]
- Jiang, F.; Ma, L.; Sun, J.; Guo, L.; Peng, Z.; Cui, Z.; Li, Y.; Guo, X.; Zhang, T. Deciphering the Enigma of Li 2 CO 3 Oxidation Using a Solid-State Li–Air Battery Configuration. ACS Appl. Mater. Interfaces 2021, 13, 14321–14326. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y.; Liu, T.; Yang, X.; Chang, Z.; Zhang, X. Hybrid electrolyte with robust garnet-ceramic electrolyte for lithium anode protection in lithium-oxygen batteries. Nano Res. 2018, 11, 3434–3441. [Google Scholar] [CrossRef]
- Kufian, M.; Ramesh, S.; Arof, A. PMMA-LiTFSI based gel polymer electrolyte for lithium-oxygen cell application. Opt. Mater. 2021, 120, 111418. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Gu, Z.; Zhao, X.; Guo, D.; Yao, X. Polyimide-Based Solid-State Gel Polymer Electrolyte for Lithium-Oxygen Batteries with a Long-Cycling Life. ACS Appl. Mater. Interfaces 2023, 15, 7014–7022. [Google Scholar] [CrossRef]
- Wang, J.; Huang, G.; Chen, K.; Zhang, X. An Adjustable-Porosity Plastic Crystal Electrolyte Enables High-Performance All-Solid-State Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2020, 59, 9382–9387. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, J. Cathodes for Solid-State Lithium–Oxygen Cells: Roles of Nasicon Glass-Ceramics. J. Electrochem. Soc. 2010, 157, A611–A616. [Google Scholar] [CrossRef]
- Balaish, M.; Peled, E.; Golodnitsky, D.; Ein-Eli, Y. Liquid-Free Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2014, 54, 436–440. [Google Scholar] [CrossRef]
- Yu, W.; Xue, C.; Hu, B.; Xu, B.; Li, L.; Nan, C.-W. Oxygen- and dendrite-resistant ultra-dry polymer electrolytes for solid-state Li–O2 batteries. Energy Storage Mater. 2020, 27, 244–251. [Google Scholar] [CrossRef]
- Yi, J.; Liu, Y.; Qiao, Y.; He, P.; Zhou, H. Boosting the Cycle Life of Li-O2 Batteries at Elevated Temperature by Employing a Hybrid Polymer-Ceramic Solid Electrolyte. ACS Energy Lett. 2017, 2, 1378–1384. [Google Scholar] [CrossRef]
- Yang, T.; Shu, C.; Zheng, R.; Hu, A.; Hou, Z.; Li, M.; Ran, Z.; Hei, P.; Long, J. Excellent electrolyte-electrode interface stability enabled by inhibition of anion mobility in hybrid gel polymer electrolyte based Li–O2 batteries. J. Membr. Sci. 2020, 604, 118051. [Google Scholar] [CrossRef]
- Wu, S.; Yi, J.; Zhu, K.; Bai, S.; Liu, Y.; Qiao, Y.; Ishida, M.; Zhou, H. A Super-Hydrophobic Quasi-Solid Electrolyte for Li-O2 Battery with Improved Safety and Cycle Life in Humid Atmosphere. Adv. Energy Mater. 2016, 7, 1601759. [Google Scholar] [CrossRef]
- Shu, C.; Long, J.; Dou, S.; Wang, J. Component-Interaction Reinforced Quasi-Solid Electrolyte with Multifunctionality for Flexible Li–O 2 Battery with Superior Safety under Extreme Conditions. Small 2019, 15, e1804701. [Google Scholar] [CrossRef]
- Chawla, N.; Chamaani, A.; Safa, M.; Herndon, M.; El-Zahab, B. Mechanism of ionic impedance growth for palladium-containing cnt electrodes in lithium-oxygen battery electrodes and its contribution to battery failure. Batteries 2019, 5, 15. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.; Kwon, H.J.; Bak, S.-M.; Jaye, C.; Fischer, D.A.; Yoon, G.; Park, J.O.; Seo, D.-H.; Ma, S.B.; et al. Carbon-free high-performance cathode for solid-state Li-O2 battery. Sci. Adv. 2022, 8, abm8584. [Google Scholar] [CrossRef]
- Pakseresht, S.; Al-Ogaili, A.W.M.; Cetinkaya, T.; Celik, M.; Akbulut, H. Prevention of side reactions with a unique carbon-free catalyst biosynthesized by a virus template for non-aqueous and quasi-solid-state Li–O2 batteries. J. Power Sources 2021, 509, 230374. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, B.; Zhang, F.; Cheng, Z.; He, P.; Zhou, H. Integrated solid electrolyte with porous cathode by facilely one-step sintering for an all-solid-state Li-O2 battery. Nanotechnology 2019, 30, 364003. [Google Scholar] [CrossRef]
- Li, C.; Huang, G.; Yu, Y.; Xiong, Q.; Yan, J.; Zhang, X. Three Birds with One Stone: An Integrated Cathode–Electrolyte Structure for High-Performance Solid-State Lithium–Oxygen Batteries. Small 2022, 18, 2107833. [Google Scholar] [CrossRef] [PubMed]
- Muthukkumaran, A.; Ravichandran, A.; Shanbhag, S.; Arjun, R.; Rengaswamy, R. Lithium-air battery electrocatalyst identification using Machine Learning and SciBERT word embeddings. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022; Volume 51, pp. 1429–1434. [Google Scholar] [CrossRef]
- Sharma, P.; Bora, B.J. A Review of Modern Machine Learning Techniques in the Prediction of Remaining Useful Life of Lithium-Ion Batteries. Batteries 2022, 9, 13. [Google Scholar] [CrossRef]
- Wang, A.; Zou, Z.; Wang, D.; Liu, Y.; Li, Y.; Wu, J.; Avdeev, M.; Shi, S. Identifying Chemical Factors Affecting Reaction Kinetics in Li-air Battery via ab initio Calculations and Machine Learning. Energy Storage Mater. 2020, 35, 595–601. [Google Scholar] [CrossRef]
- Mishra, A.K.; Rajput, S.; Karamta, M.; Mukhopadhyay, I. Exploring the Possibility of Machine Learning for Predicting Ionic Conductivity of Solid-State Electrolytes. ACS Omega 2023, 8, 16419–16427. [Google Scholar] [CrossRef]
- Waidha, A.I.; Salihovic, A.; Jacob, M.; Vanita, V.; Aktekin, B.; Brix, K.; Wissel, K.; Kautenburger, R.; Janek, J.; Ensinger, W.; et al. Recycling of All-Solid-State Li-ion Batteries: A Case Study of the Separation of Individual Components Within a System Composed of LTO, LLZTO and NMC. Chemsuschem 2023, 16, e202202361. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef] [PubMed]
- Schwich, L.; Küpers, M.; Finsterbusch, M.; Schreiber, A.; Fattakhova-Rohlfing, D.; Guillon, O.; Friedrich, B. Recycling strategies for ceramic all-solid-state batteries—Part i: Study on possible treatments in contrast to li-ion battery recycling. Metals 2020, 10, 1523. [Google Scholar] [CrossRef]
- Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
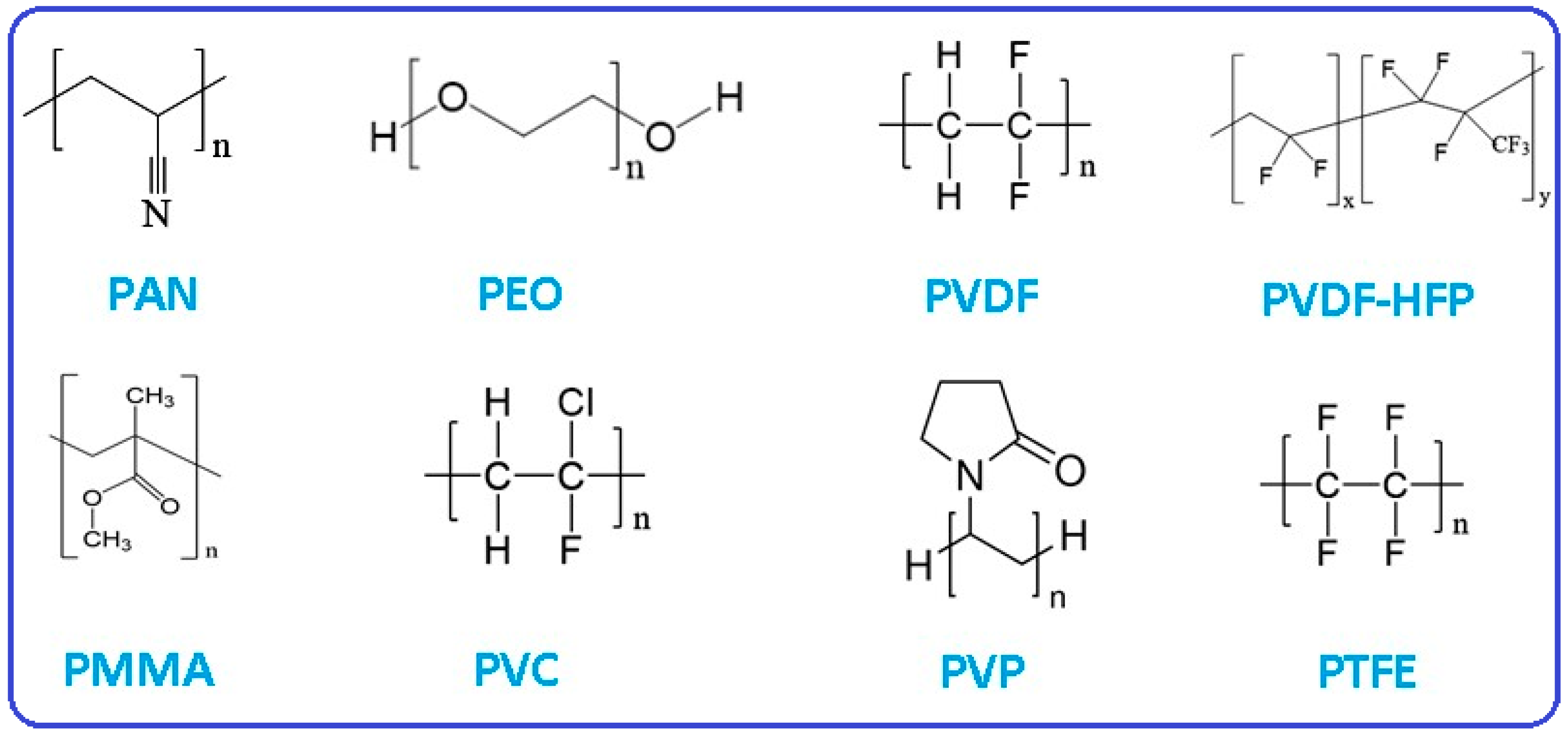
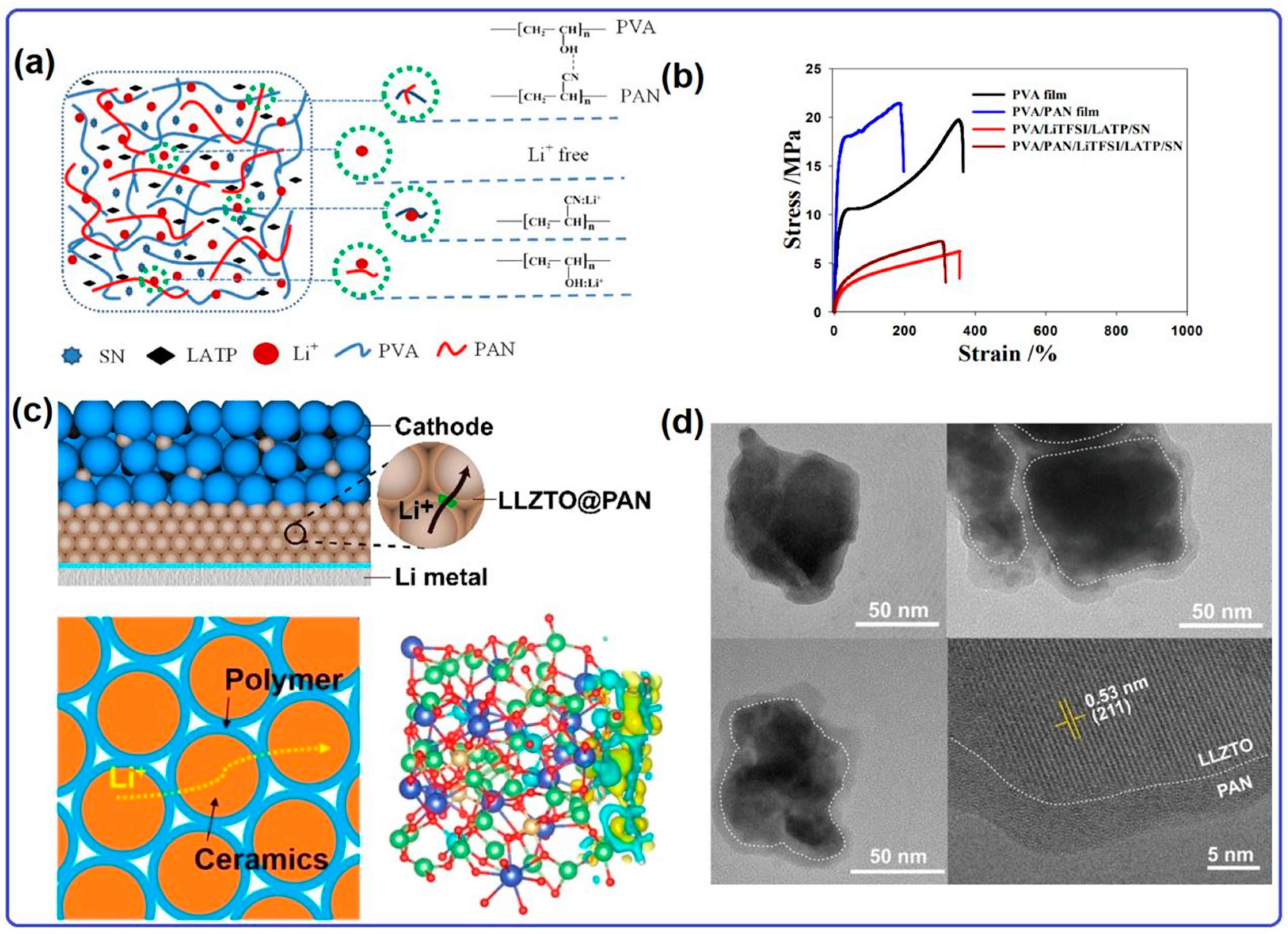
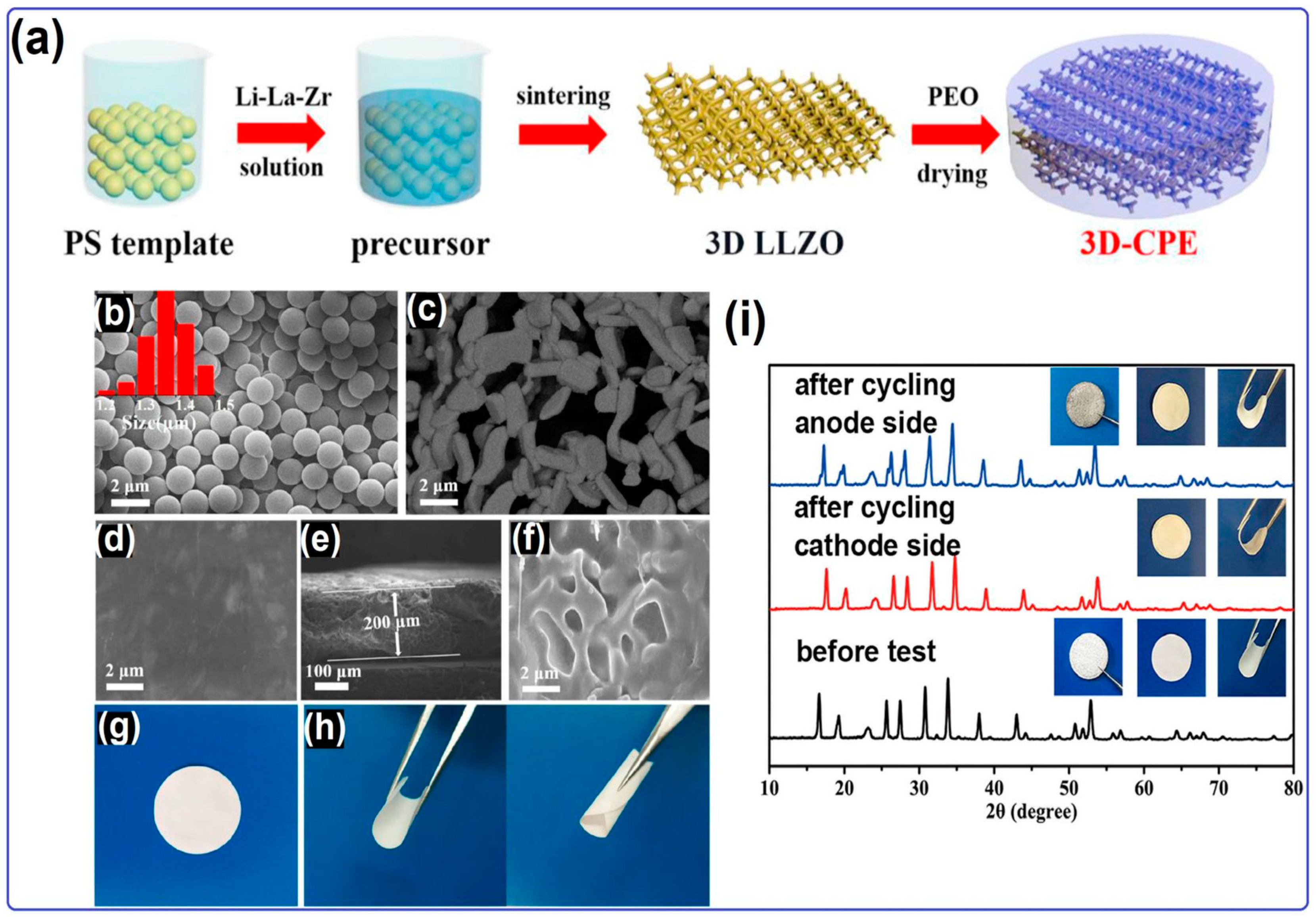
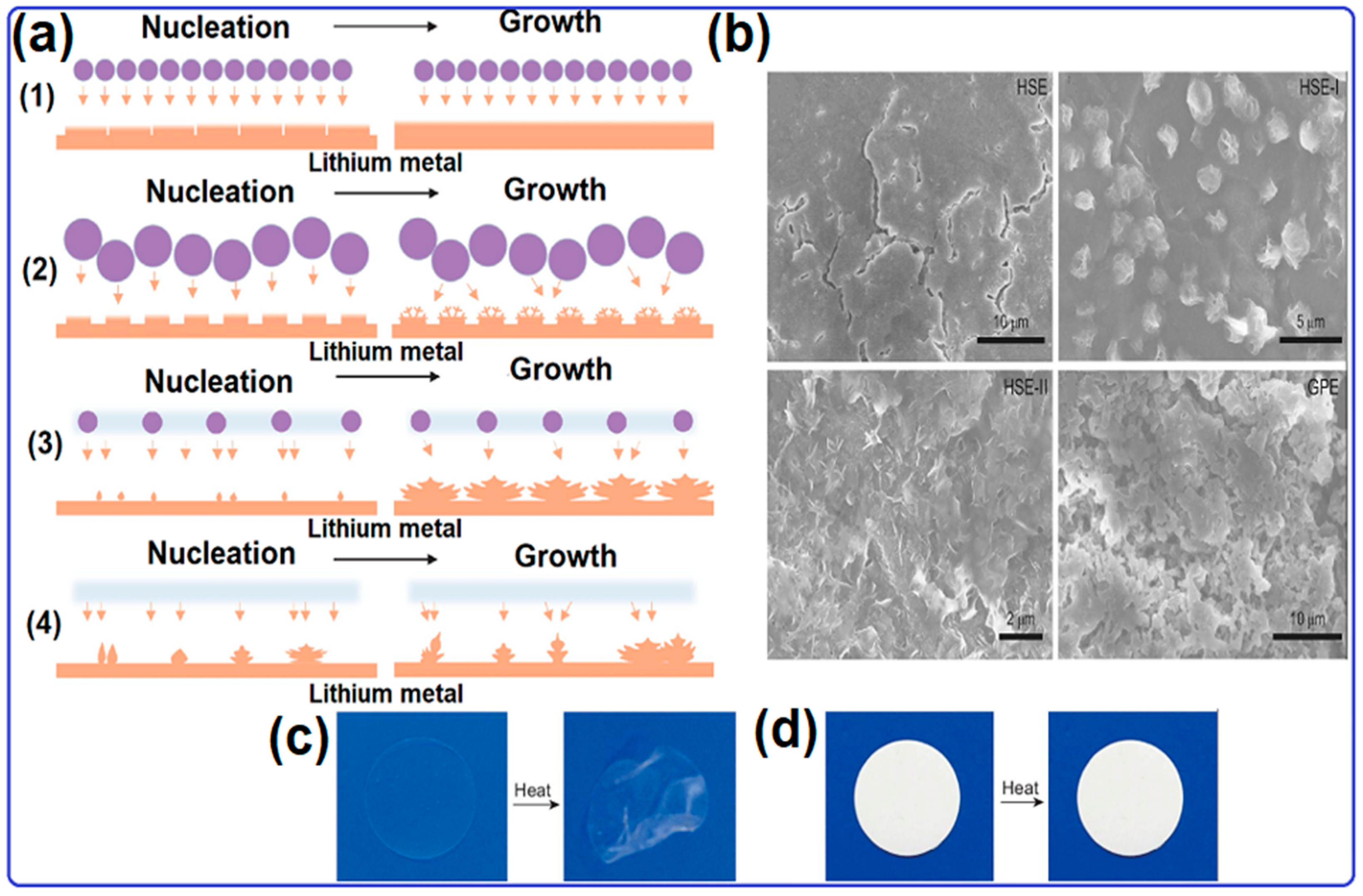
| SSEs | Typ. | Advantages | Challenges | σLi (S cm−1) | Refs. |
|---|---|---|---|---|---|
| Oxide-based | NASICON and LISICON | Air stability and mechanical strength Substitution, composite material, and protective layer | Li anode instability | 10−5–10−3 | [74,75,76,77,78] |
| Garnet | Stability with Li metal and mechanical strength Substitution, protective layer, changing the ratio of Li+, and introducing additives | Sensitive to CO2 and humidity | 10−5–10−3 | [79,80,81,82,83] | |
| Perovskite | Air stability, low cost, and mechanical strength Substitution and composite material | Li anode instability | 10−5–10−3 | [84,85,86,87,88] | |
| Sulfide-based | Thio-LISICON and Li2S-MxSy | High σLi Substitution, composite material, and ion exchange | Sensitive to O2 and humidity | 10−4–10−2 | [89,90,91,92,93] |
| Other type | Anti-perovskite | Stability with Li metal and light weight substitution | Poor cycling and structural durabilities | 10−4–10−2 | [94,95,96,97] |
| LiPON | Li metal stability and mechanical rigidity Properties of bond and functional group | Low σLi and expensive | 10−6 | [98,99] |
| Solid-State Electrolytes | Ionic Conductivity (S cm−1) | Li Transfer Number | Li Salt | Cycle Number | Ref. |
|---|---|---|---|---|---|
| LATP | 7 × 10−4 | - | - | 100 | [189] |
| LATP | 0.71 | - | - | 50 | [190] |
| LATP | - | - | - | 1174 | [191] |
| LATP | 5.23 × 10−4 | - | - | 200 | [192] |
| LAGP | 3.9 × 10−4 | - | - | 27 | [182] |
| LAGP | 2 × 10−4 | - | - | 20 | [40] |
| LAGP | 4.5 × 10−4 | - | - | 80 | [193] |
| LAGP/LiTaO3 | - | - | - | 59 | [194] |
| LAGP/Li3InCl6 | 13 × 10−4 | - | - | 33 | [184] |
| Al-doped LLTO/PVDF-HFP | 3.17 × 10−4 | - | LiTFSI | 132 | [115] |
| LLZTO | 16 × 10−4 | - | PPC:LiTFSI | 50 | [195] |
| LLZTO | 16 × 10−4 | - | - | 5 | [196] |
| LLZT-xAl2O3 | - | - | - | 43 | [197] |
| LiXZM | 2.67 × 10−4 | - | - | 149 | [41] |
| Poly (methyl methacrylate) | 2.5 × 10−2 | 0.47 | LiTFSI | - | [198] |
| Polyimide | 0.44 | 0.596 | LiTFSI | 156 | [199] |
| SN/LiTFSI/P(VDF-HFP)/BHT | 3.87 × 10−4 | - | LiTFSI | 130 | [200] |
| PEO/LiBETI/Li2O/BN/LAGP | - | - | LiBETI/Li2O | 40 | [201] |
| PEO/LiTf | - | - | LiTf | 40 | [202] |
| P(VDF-HFP)/LiFSI | 0.79 × 10−4 | - | LiFSI | 60 | [203] |
| LLZTO/SN | 2.73 × 10−4 | 0.48 | LiTFSI | 60 | [181] |
| LLZO/PS | 9.2 × 10−5 | - | LiTFSI | 50 | [177] |
| LAGP/PMS | 3.2 × 10−4 | 0.75 | LiTFSI | 160 | [204] |
| PVDF-HFP/Ti3AlC2 | 5.45 × 10−4 | 0.47 | LiTFSI | 200 | [205] |
| SiO2/PIB | 9.1 × 10−4 | - | LiTFSI | 150 | [206] |
| PVDF-HFP/SiO2 | 9.3 × 10−4 | - | LiTFSI | 89 | [207] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakseresht, S.; Celik, M.; Guler, A.; Al-Ogaili, A.W.M.; Kallio, T. Recent Advances in All-Solid-State Lithium–Oxygen Batteries: Challenges, Strategies, Future. Batteries 2023, 9, 380. https://doi.org/10.3390/batteries9070380
Pakseresht S, Celik M, Guler A, Al-Ogaili AWM, Kallio T. Recent Advances in All-Solid-State Lithium–Oxygen Batteries: Challenges, Strategies, Future. Batteries. 2023; 9(7):380. https://doi.org/10.3390/batteries9070380
Chicago/Turabian StylePakseresht, Sara, Mustafa Celik, Aslihan Guler, Ahmed Waleed Majeed Al-Ogaili, and Tanja Kallio. 2023. "Recent Advances in All-Solid-State Lithium–Oxygen Batteries: Challenges, Strategies, Future" Batteries 9, no. 7: 380. https://doi.org/10.3390/batteries9070380
APA StylePakseresht, S., Celik, M., Guler, A., Al-Ogaili, A. W. M., & Kallio, T. (2023). Recent Advances in All-Solid-State Lithium–Oxygen Batteries: Challenges, Strategies, Future. Batteries, 9(7), 380. https://doi.org/10.3390/batteries9070380







