Overcoming Challenges in Development of Manganese Oxide Supercapacitor Cathodes by Alkali-Free Hydrothermal Synthesis
Abstract
1. Introduction
2. Materials and Methods
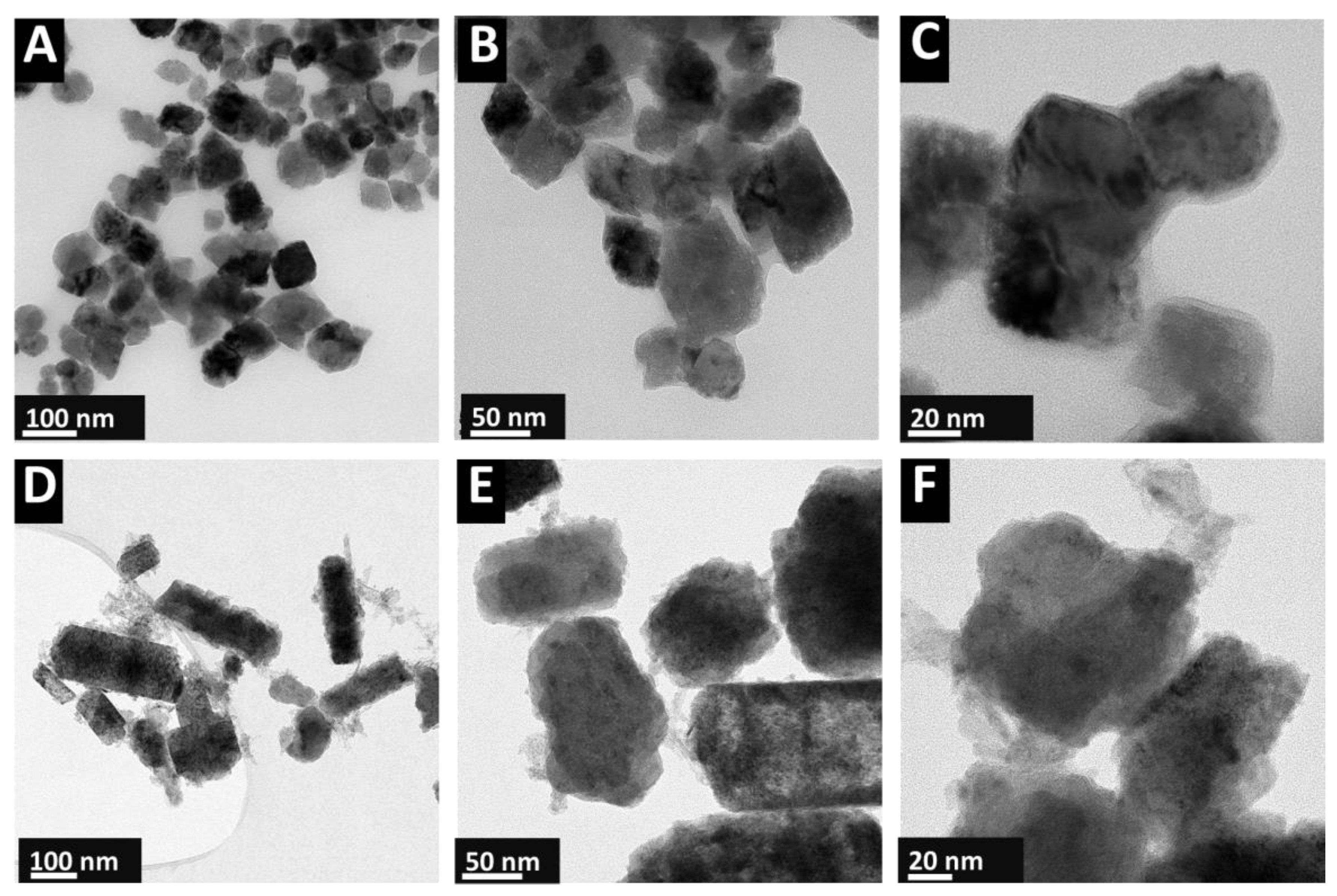
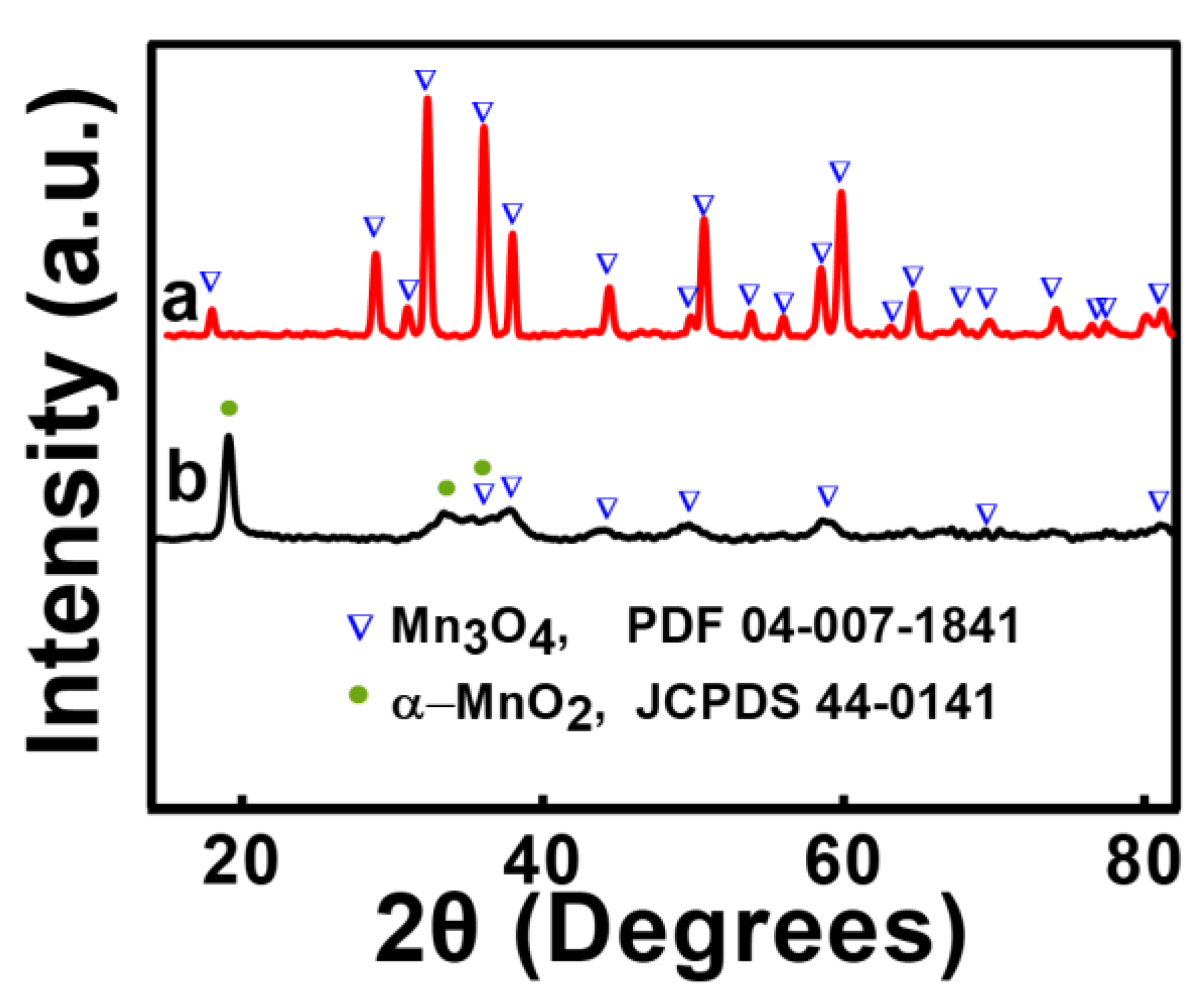
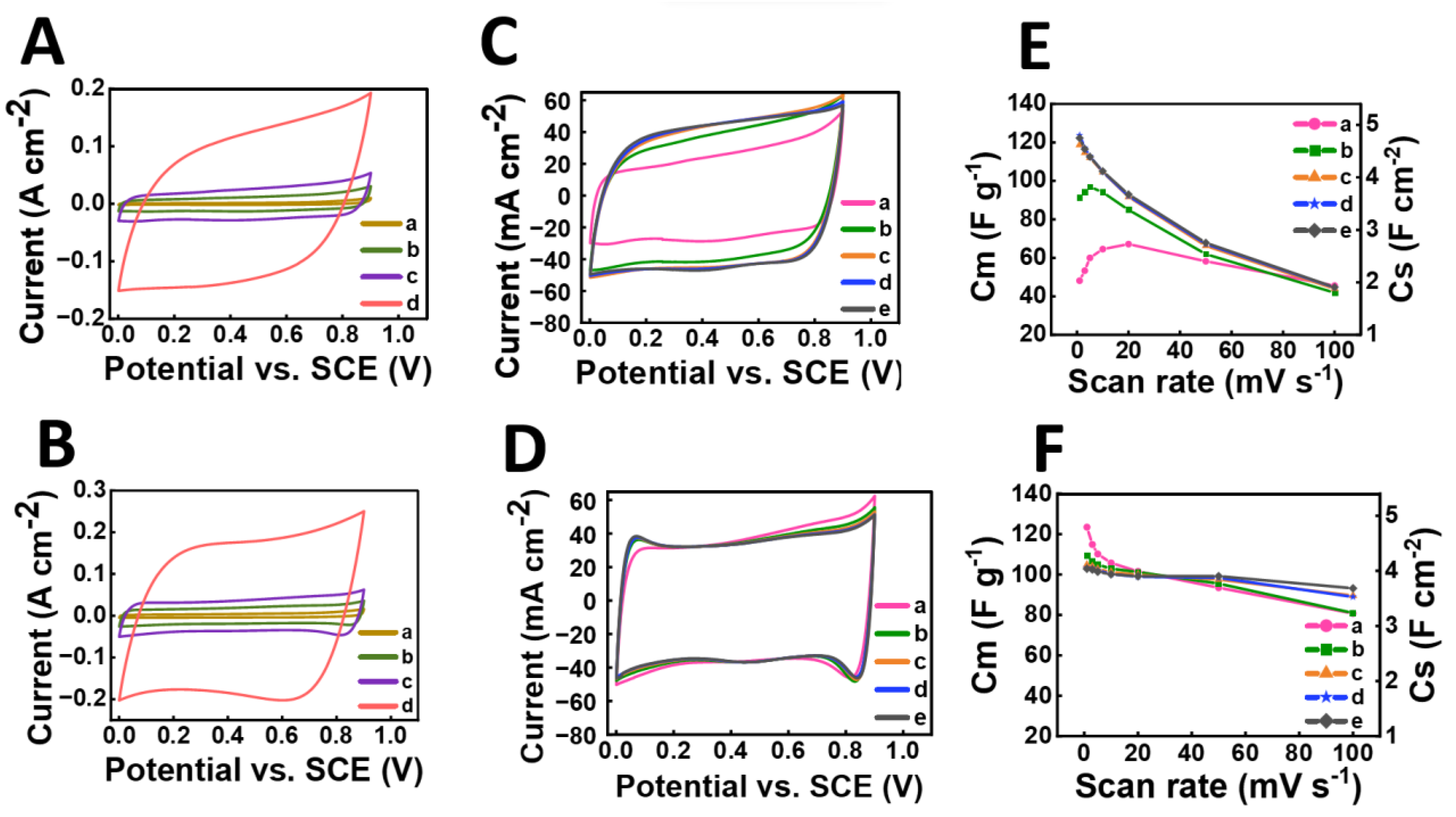
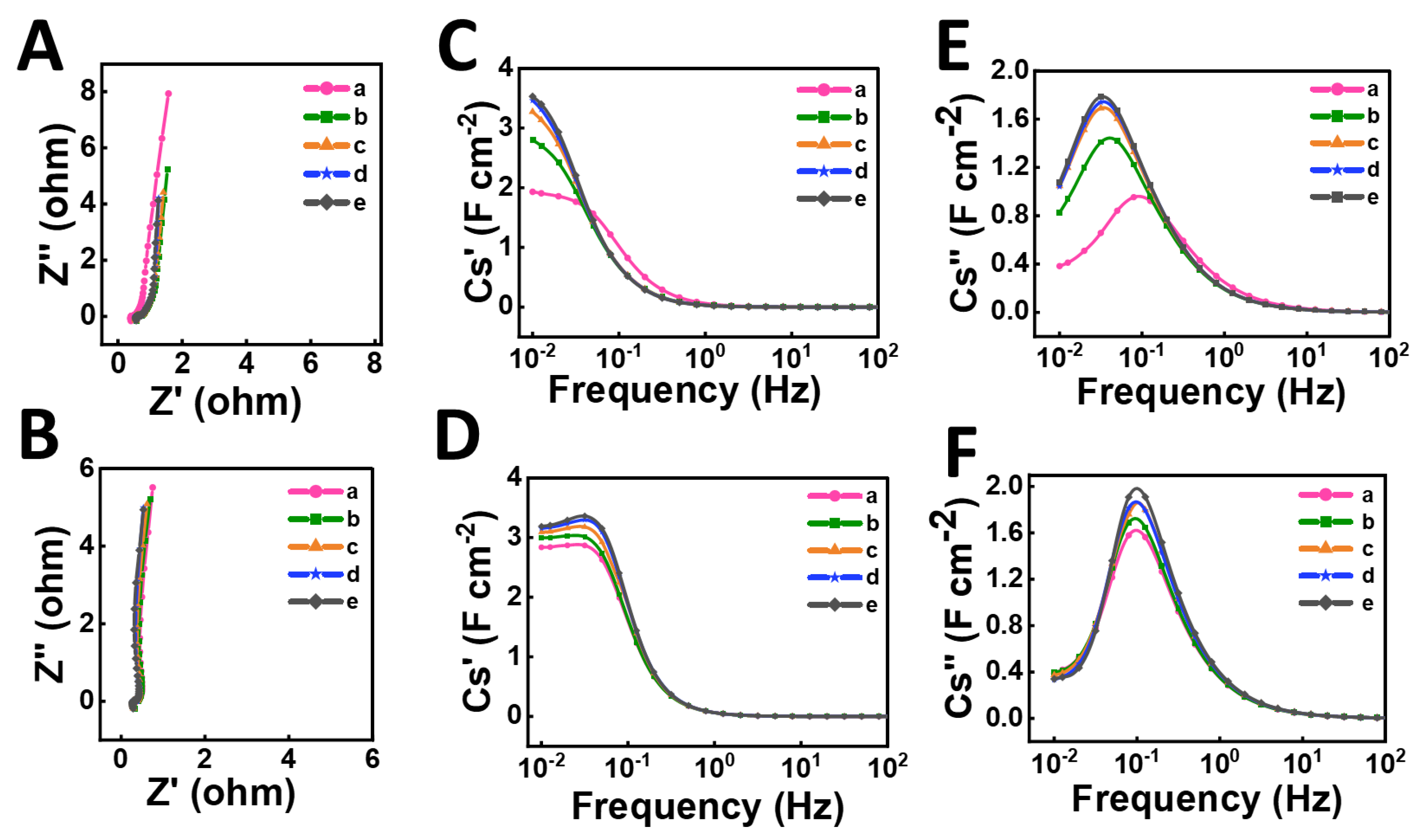
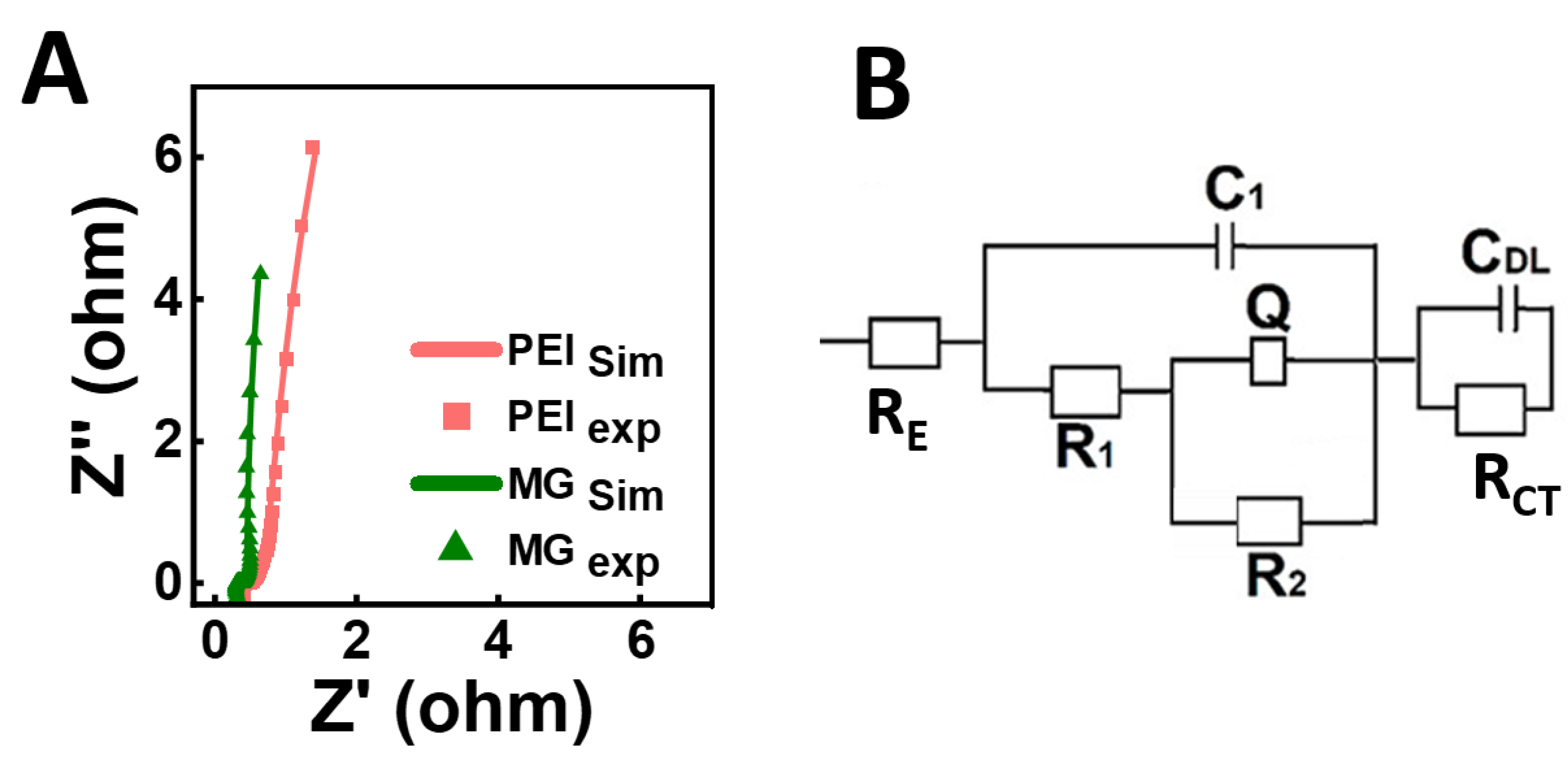
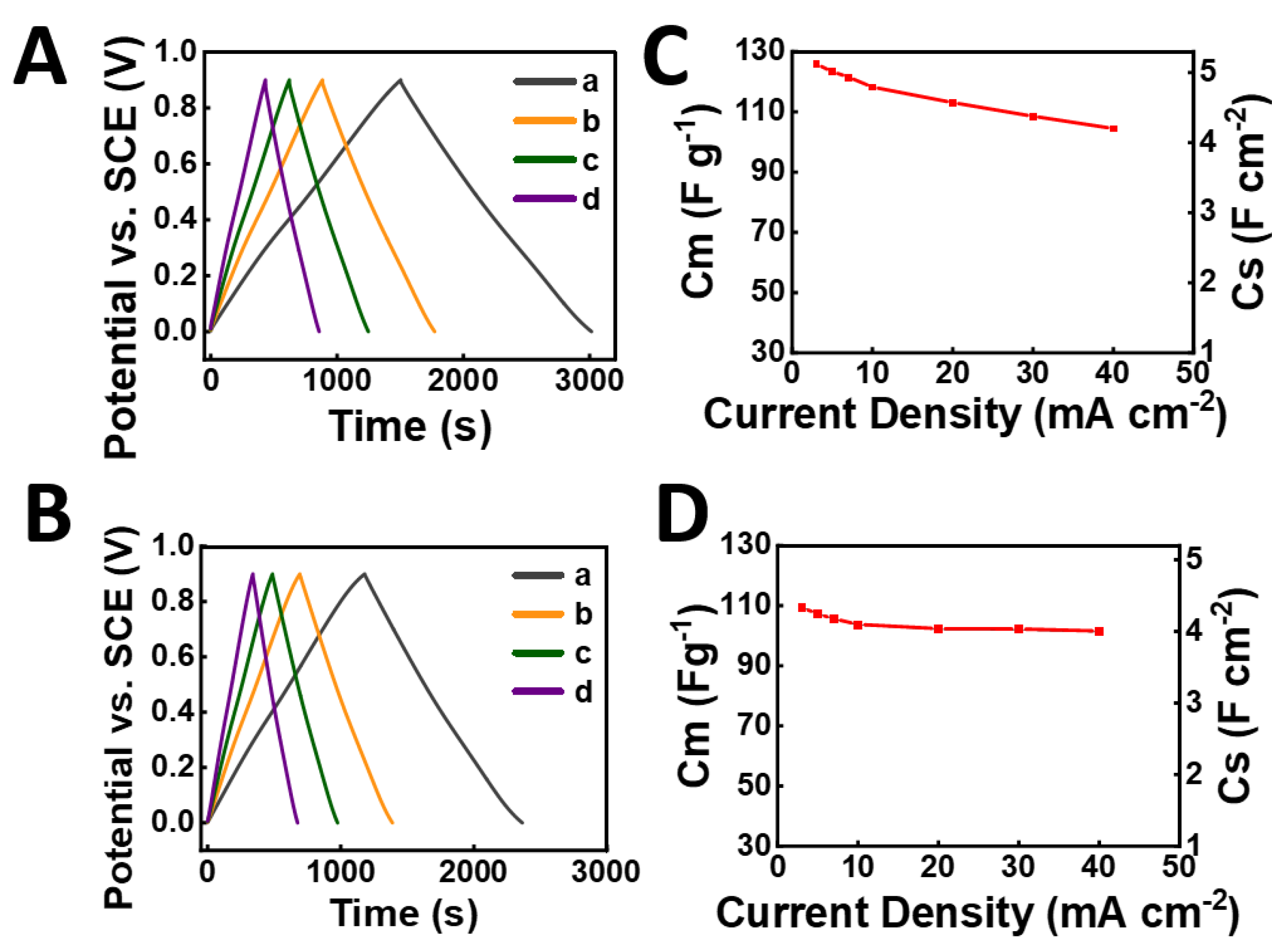
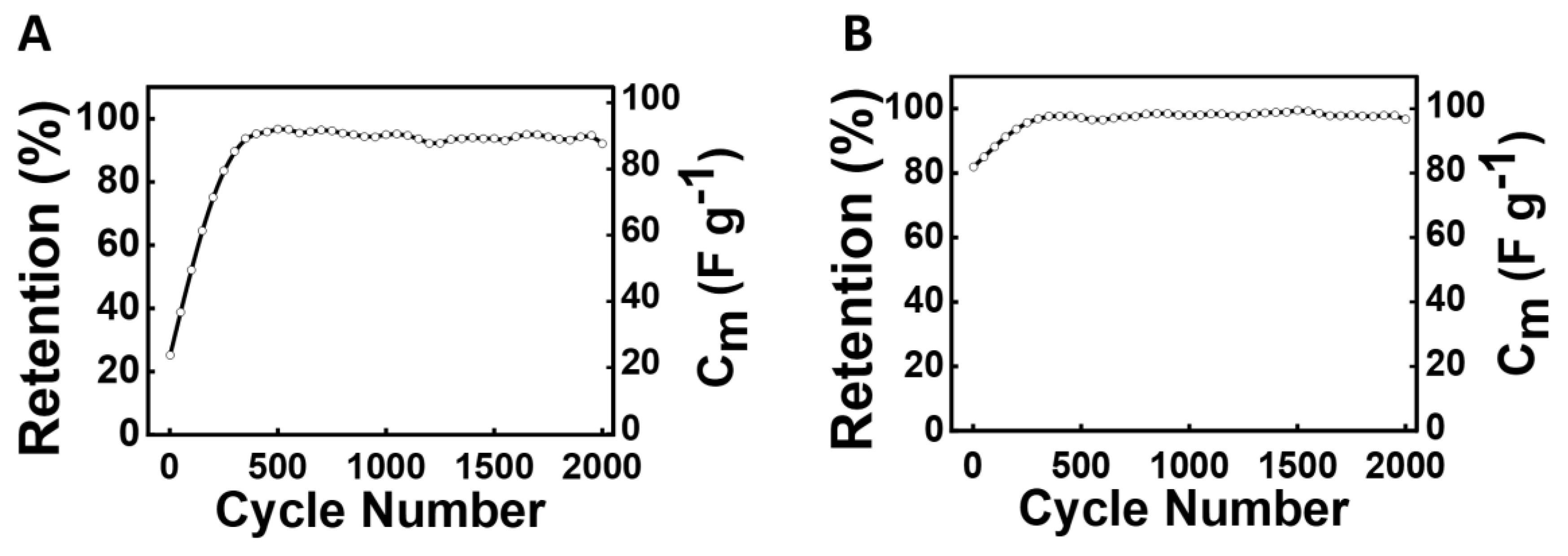
3. Results and Discussion
3(2 − δ)Na+(aq) + 3NaδMnOx·H2O + 3SO42-(aq) + (6x − 3δ − 8)e−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.; Yang, C.; Xu, Q.; Zhao, W.; Zhao, J.; Wang, K.; Chen, H.-C. Ag-Doping Effect on MnO2 Cathodes for Flexible Quasi-Solid-State Zinc-Ion Batteries. Batteries 2022, 8, 267. [Google Scholar] [CrossRef]
- Li, Z.; Ji, C.; Guo, F.; Mi, H.; Zhu, X.; Qiu, J. A multi-interface CoNi-SP/C heterostructure for quasi-solid-state hybrid supercapacitors with a graphene oxide-containing hydrogel electrolyte. J. Mater. Chem. A 2022, 10, 4671–4682. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Wu, X. Surface Selenization of NiCo-Layered Double Hydroxide Nanosheets for High-Performance Supercapacitors. Batteries 2023, 9, 49. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Yamani, Z.H.; Bozkurt, A. Sulfonated hollow silica spheres as electrolyte store/release agents: High-performance supercapacitor applications. Energy Technol. 2019, 7, 1900511. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, J.; Li, X.; Yue, L.; Liu, Q.; Lu, S.; Asiri, A.M.; Hu, J.; Luo, Y.; Sun, X. Recent advances in perovskite oxides as electrode materials for supercapacitors. Chem. Commun. 2021, 57, 2343–2355. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Yuan, B.; Li, Y.; Yu, L.; Zhao, W. Three-dimensional hollow nickel phosphate microspheres with controllable hoya-like structure for high-performance enzymeless glucose detection and supercapacitor. Appl. Surf. Sci. 2022, 588, 152928. [Google Scholar] [CrossRef]
- Alqarni, A.N.; Cevik, E.; Gondal, M.; Almessiere, M.; Baykal, A.; Bozkurt, A.; Slimani, Y.; Hassan, M.; Iqbal, A.; Alotaibi, S.A. Synthesis and design of vanadium intercalated spinal ferrite (Co0. 5Ni0. 5VxFe1. 6−xO4) electrodes for high current supercapacitor applications. J. Energy Storage 2022, 51, 104357. [Google Scholar] [CrossRef]
- Lin, S.; Yang, F.; Yang, Z.; Wang, J.; Xiang, L. Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution. Nanomaterials 2023, 13, 1179. [Google Scholar] [CrossRef]
- Mineo, G.; Scuderi, M.; Pezzotti Escobar, G.; Mirabella, S.; Bruno, E. Engineering of Nanostructured WO3 Powders for Asymmetric Supercapacitors. Nanomaterials 2022, 12, 4168. [Google Scholar] [CrossRef]
- Yang, W.; Eraky, H.; Zhang, C.; Hitchcock, A.P.; Zhitomirsky, I. Scanning transmission X-ray microscopy studies of electrochemical activation and capacitive behavior of Mn 3 O 4 supercapacitor electrodes. J. Mater. Chem. A 2022, 10, 18267–18277. [Google Scholar] [CrossRef]
- Gagrani, A.; Ding, B.; Wang, Y.; Tsuzuki, T. pH dependent catalytic redox properties of Mn3O4 nanoparticles. Mater. Chem. Phys. 2019, 231, 41–47. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Poon, R.; Zhitomirsky, I. High areal capacitance of Mn3O4-carbon nanotube electrodes. Mater. Lett. 2018, 215, 4–7. [Google Scholar] [CrossRef]
- Milne, J.; Zhitomirsky, I. Application of octanohydroxamic acid for liquid-liquid extraction of manganese oxides and fabrication of supercapacitor electrodes. J. Colloid Interface Sci. 2018, 515, 50–57. [Google Scholar] [CrossRef]
- Shar, S.S.; Cevik, E.; Bozkurt, A.; Yaman, C.; Almutari, Z.; Kayed, T.S. Molybdate incorporated poly (acrylic acid) electrolytes for use in quasi-solid state carbon based supercapacitors: Redox-active polychelates. Electrochim. Acta 2020, 354, 136770. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Bozkurt, A.; Amine, R.; Amine, K. Bio-inspired redox mediated electrolyte for high performance flexible supercapacitor applications over broad temperature domain. J. Power Sources 2020, 474, 228544. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Iqbal, A.; Akhtar, S.; Bozkurt, A. Synthesis of hierarchical multilayer N-doped Mo2C@ MoO3 nanostructure for high-performance supercapacitor application. J. Energy Storage 2022, 46, 103824. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Gutiérrez, G.L.; Dávila, O.O.; Aguilar, C.L.; Jiménez, M.D.; González, R.S.; Sirés, I.; Brillas, E.; Fabregat-Safont, D.; Navarro, A.R.; Arandes, J.B. Electrochemical oxidation of meglumine in a pharmaceutical formulation using a nanocomposite anode. Electrochim. Acta 2023, 437, 141457. [Google Scholar] [CrossRef]
- Fusina, A.; Degot, P.; Touraud, D.; Kunz, W.; Nardello-Rataj, V. Enhancement of water solubilization of quercetin by meglumine and application of the solubilization concept to a similar system. J. Mol. Liq. 2022, 368, 120756. [Google Scholar] [CrossRef]
- Hussien, M.A.; Essa, E.; El-Gizawy, S.A. Investigation of the effect of formulation additives on telmisartan dissolution rate: Development of oral disintegrating tablets. Eur. J. Biomed. Pharm. Sci 2019, 6, 12–20. [Google Scholar]
- Parikh, B.; Patel, D.; Patel, C.; Dave, J.; Gothi, G.; Patel, T. Formulation optimization and evaluation of immediate release tablet of telmisartan. J. Glob. Pharma Technol. 2010, 2, 79–84. [Google Scholar]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and improved dissolution of glycyrrhetinic acid solid dispersion by alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef]
- Manley, K.; Bravo-Nuevo, A.; Minton, A.R.; Sedano, S.; Marcy, A.; Reichman, M.; Tobia, A.; Artlett, C.M.; Gilmour, S.K.; Laury-Kleintop, L.D. Preclinical study of the long-range safety and anti-inflammatory effects of high-dose oral meglumine. J. Cell. Biochem. 2019, 120, 12051–12062. [Google Scholar] [CrossRef]
- Palchevska, T.; Saliy, O.; Baula, O.; Palchevskyi, K.; Onishchuk, O. The role of excipients of trometamolum and meglumine in the formation of biopharmaceutical properties of medicinal products of various pharmacites. Farmatsevtychnyi Zhurnal 2021, 4, 64–75. [Google Scholar] [CrossRef]
- Sawamura, T.; Okuyama, M.; Maeda, H.; Obata, A.; Kasuga, T. Preparation of calcium-phosphate cements with high compressive strength using meglumine as a water reducer. J. Ceram. Soc. Jpn. 2016, 124, 223–228. [Google Scholar] [CrossRef]
- Guo, R.-Y.; An, Z.-M.; Mo, L.-P.; Wang, R.-Z.; Liu, H.-X.; Wang, S.-X.; Zhang, Z.-H. Meglumine: A novel and efficient catalyst for one-pot, three-component combinatorial synthesis of functionalized 2-amino-4 H-pyrans. ACS Comb. Sci. 2013, 15, 557–563. [Google Scholar] [CrossRef]
- Sravya, G.; Suresh, G.; Zyryanov, G.V.; Balakrishna, A.; Madhu Kumar Reddy, K.; Suresh Reddy, C.; Venkataramaiah, C.; Rajendra, W.; Bakthavatchala Reddy, N. A meglumine catalyst–based synthesis, molecular docking, and antioxidant studies of dihydropyrano [3,2-b] chromenedione derivatives. J. Heterocycl. Chem. 2020, 57, 355–369. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Tang, F.; Uchikoshi, T.; Ozawa, K.; Sakka, Y. Effect of polyethylenimine on the dispersion and electrophoretic deposition of nano-sized titania aqueous suspensions. J. Eur. Ceram. Soc. 2006, 26, 1555–1560. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, F.; Suzuki, T.S.; Sakka, Y. Role of the initial degree of ionization of polyethylenimine in the dispersion of silicon carbide nanoparticles. J. Am. Ceram. Soc. 2003, 86, 189–191. [Google Scholar] [CrossRef]
- Dietrich, A.; Neubrand, A. Effects of particle size and molecular weight of polyethylenimine on properties of nanoparticulate silicon dispersions. J. Am. Ceram. Soc. 2001, 84, 806–812. [Google Scholar] [CrossRef]
- Laarz, E.; Bergström, L. Dispersing WC–Co powders in aqueous media with polyethylenimine. Int. J. Refract. Met. Hard Mater. 2000, 18, 281–286. [Google Scholar] [CrossRef]
- Rubianes, M.D.; Rivas, G.A. Dispersion of multi-wall carbon nanotubes in polyethylenimine: A new alternative for preparing electrochemical sensors. Electrochem. Commun. 2007, 9, 480–484. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Lu, Q.; Xiao, Z.; Ma, X.; Feng, Y. Preparation of Mn3O4 microspheres via glow discharge electrolysis plasma as a high-capacitance supercapacitor electrode material. J. Alloys Compd. 2022, 926, 166775. [Google Scholar] [CrossRef]
- Chen, J.; Chu, K.; Sun, S.; Chen, H.; Song, B.; Wang, J.; Liu, Z.; Zhu, L. Synthesis of magnetic core-shell Fe3O4-Mn3O4 composite for degradation of sulfadiazine via peroxymonosulfate activation: Characterization, mechanism and toxicity analysis. J. Environ. Chem. Eng. 2023, 11, 109230. [Google Scholar] [CrossRef]
- He, L.; Zhang, G.; Dong, Y.; Zhang, Z.; Xue, S.; Jiang, X. Polyetheramide templated synthesis of monodisperse Mn3O4 nanoparticles with controlled size and study of the electrochemical properties. Nano-Micro Lett. 2014, 6, 38–45. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol–gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Jeong, Y.; Manthiram, A. Nanocrystalline manganese oxides for electrochemical capacitors with neutral electrolytes. J. Electrochem. Soc. 2002, 149, A1419. [Google Scholar] [CrossRef]
- Dong, W.; Sakamoto, J.S.; Dunn, B. Electrochemical properties of vanadium oxide aerogels. Sci. Technol. Adv. Mater. 2003, 4, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface modification of MnO2 and carbon nanotubes using organic dyes for nanotechnology of electrochemical supercapacitors. J. Mater. Chem. A 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Barai, H.R.; Lopa, N.S.; Ahmed, F.; Khan, N.A.; Ansari, S.A.; Joo, S.W.; Rahman, M.M. Synthesis of Cu-Doped Mn3O4@Mn-Doped CuO Nanostructured Electrode Materials by a Solution Process for High-Performance Electrochemical Pseudocapacitors. ACS Omega 2020, 5, 22356–22366. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; Nawwar, M.; Zhitomirsky, I. Overcoming Challenges in Development of Manganese Oxide Supercapacitor Cathodes by Alkali-Free Hydrothermal Synthesis. Batteries 2023, 9, 365. https://doi.org/10.3390/batteries9070365
Awad M, Nawwar M, Zhitomirsky I. Overcoming Challenges in Development of Manganese Oxide Supercapacitor Cathodes by Alkali-Free Hydrothermal Synthesis. Batteries. 2023; 9(7):365. https://doi.org/10.3390/batteries9070365
Chicago/Turabian StyleAwad, Mahmoud, Mohamed Nawwar, and Igor Zhitomirsky. 2023. "Overcoming Challenges in Development of Manganese Oxide Supercapacitor Cathodes by Alkali-Free Hydrothermal Synthesis" Batteries 9, no. 7: 365. https://doi.org/10.3390/batteries9070365
APA StyleAwad, M., Nawwar, M., & Zhitomirsky, I. (2023). Overcoming Challenges in Development of Manganese Oxide Supercapacitor Cathodes by Alkali-Free Hydrothermal Synthesis. Batteries, 9(7), 365. https://doi.org/10.3390/batteries9070365









