Toward Scalable Liquid-Phase Synthesis of Sulfide Solid Electrolytes for All-Solid-State Batteries
Abstract
1. Introduction
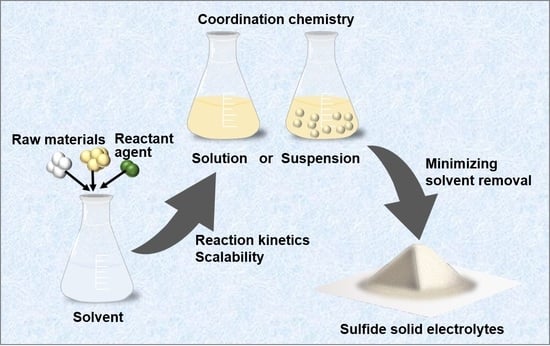
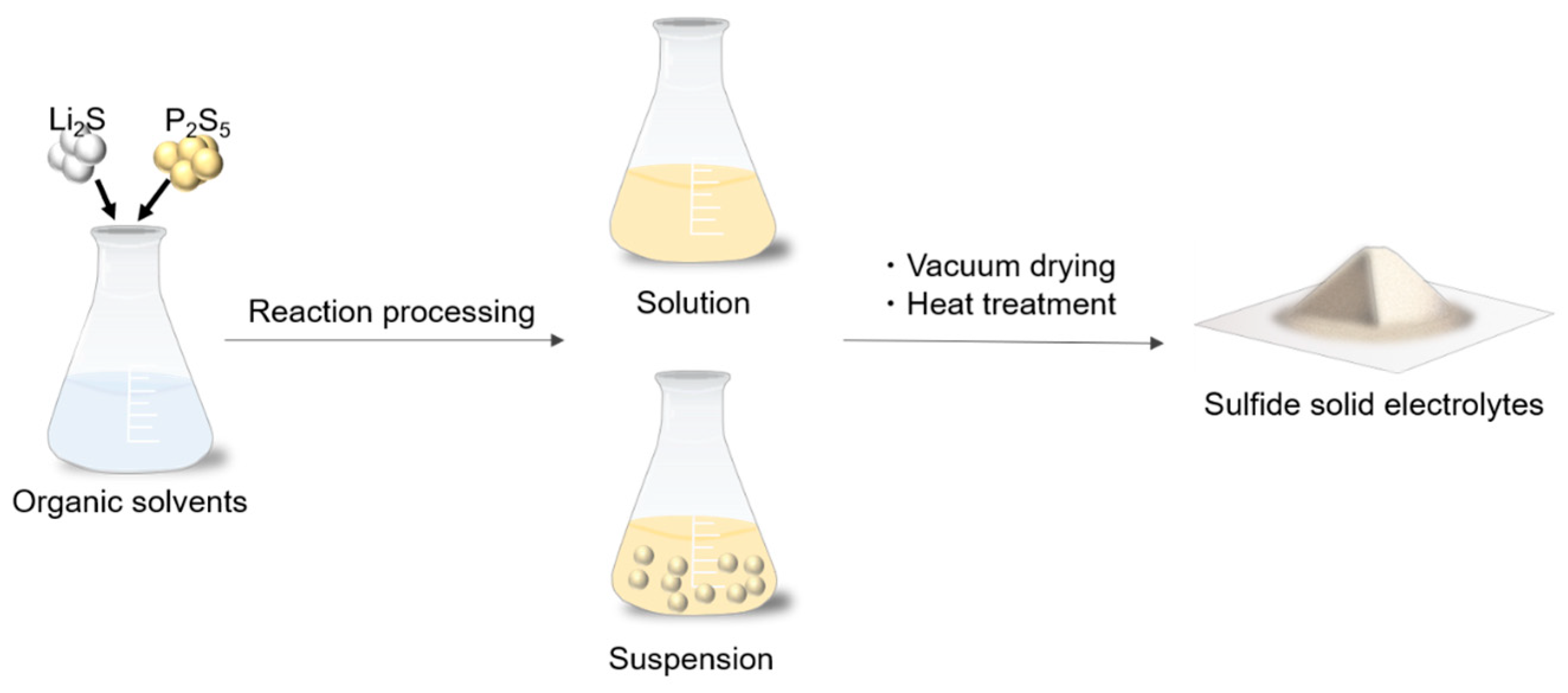
2. The Liquid-Phase Synthesis Method
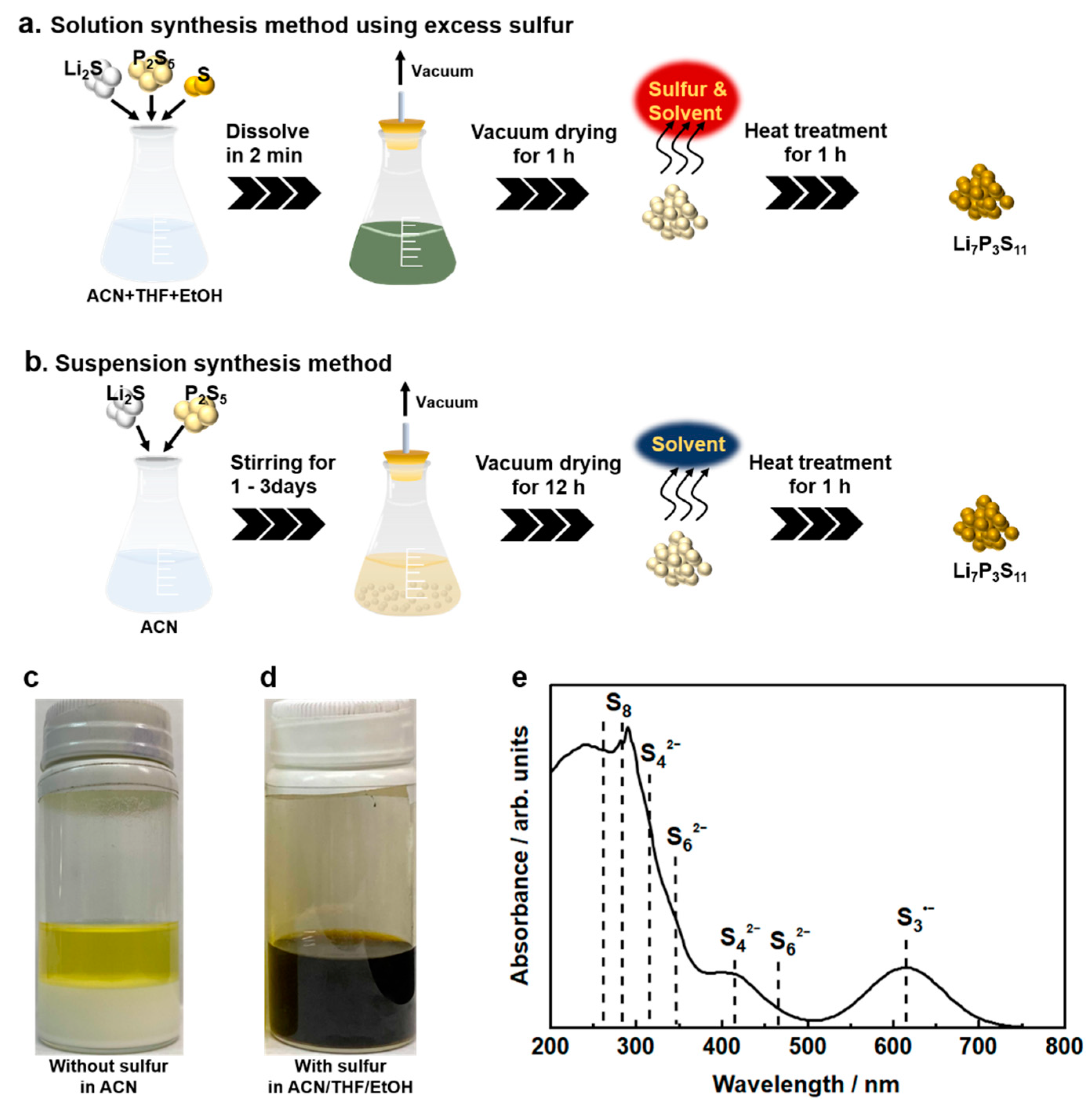
2.1. Suspension Synthesis
2.2. Solution Synthesis
3. Solvent Selection
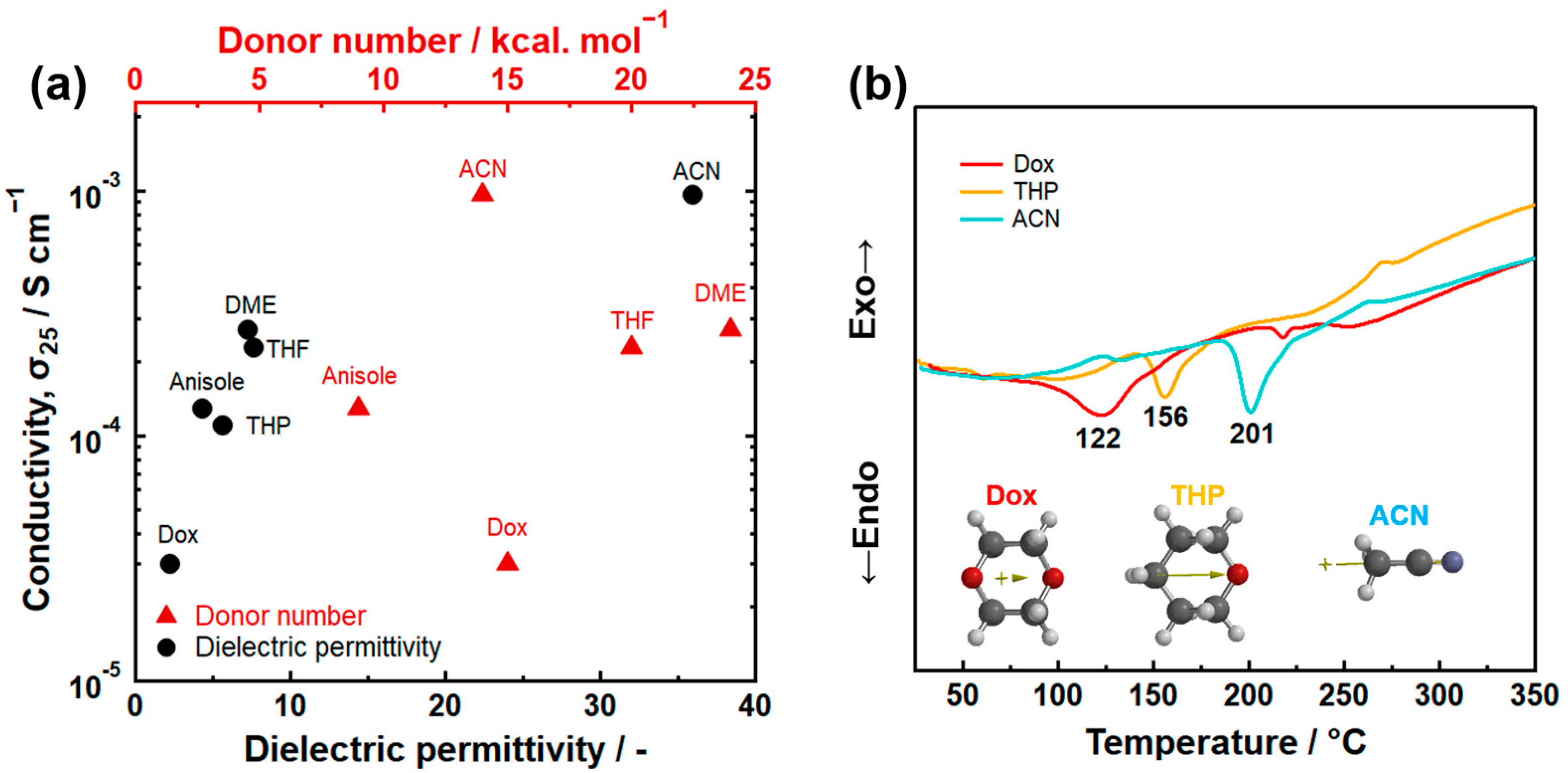
3.1. Donor Number
3.2. Dielectric Permittivity
3.3. Solubility
4. Strategies for Rapid Synthesis
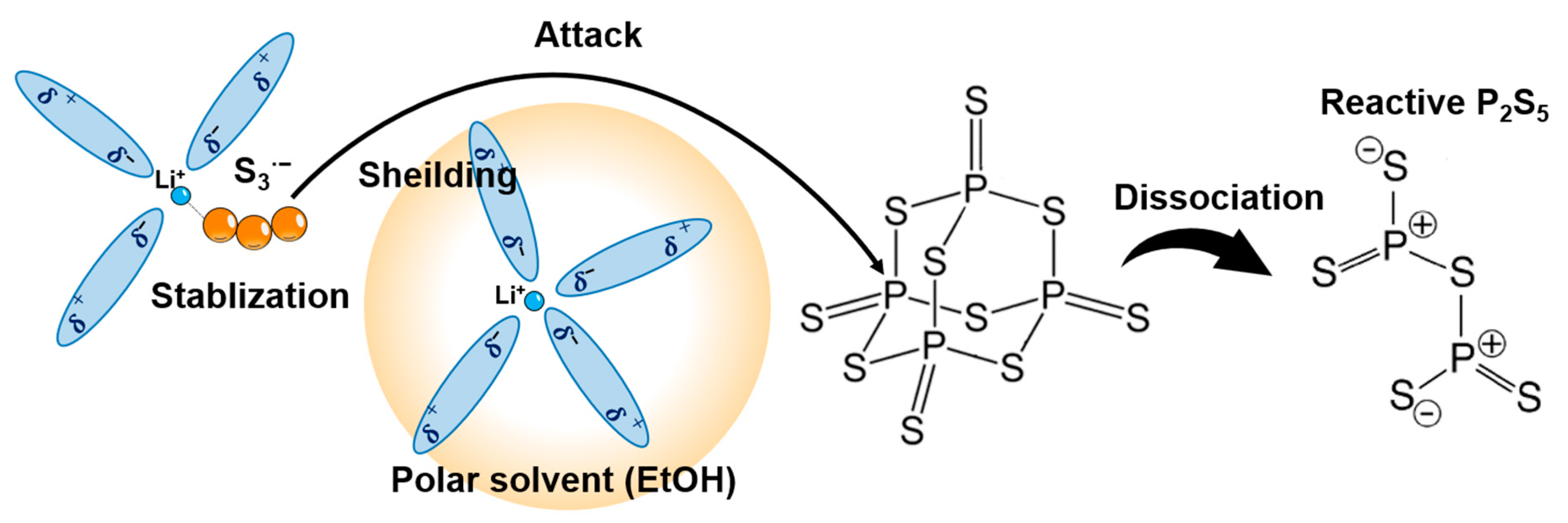
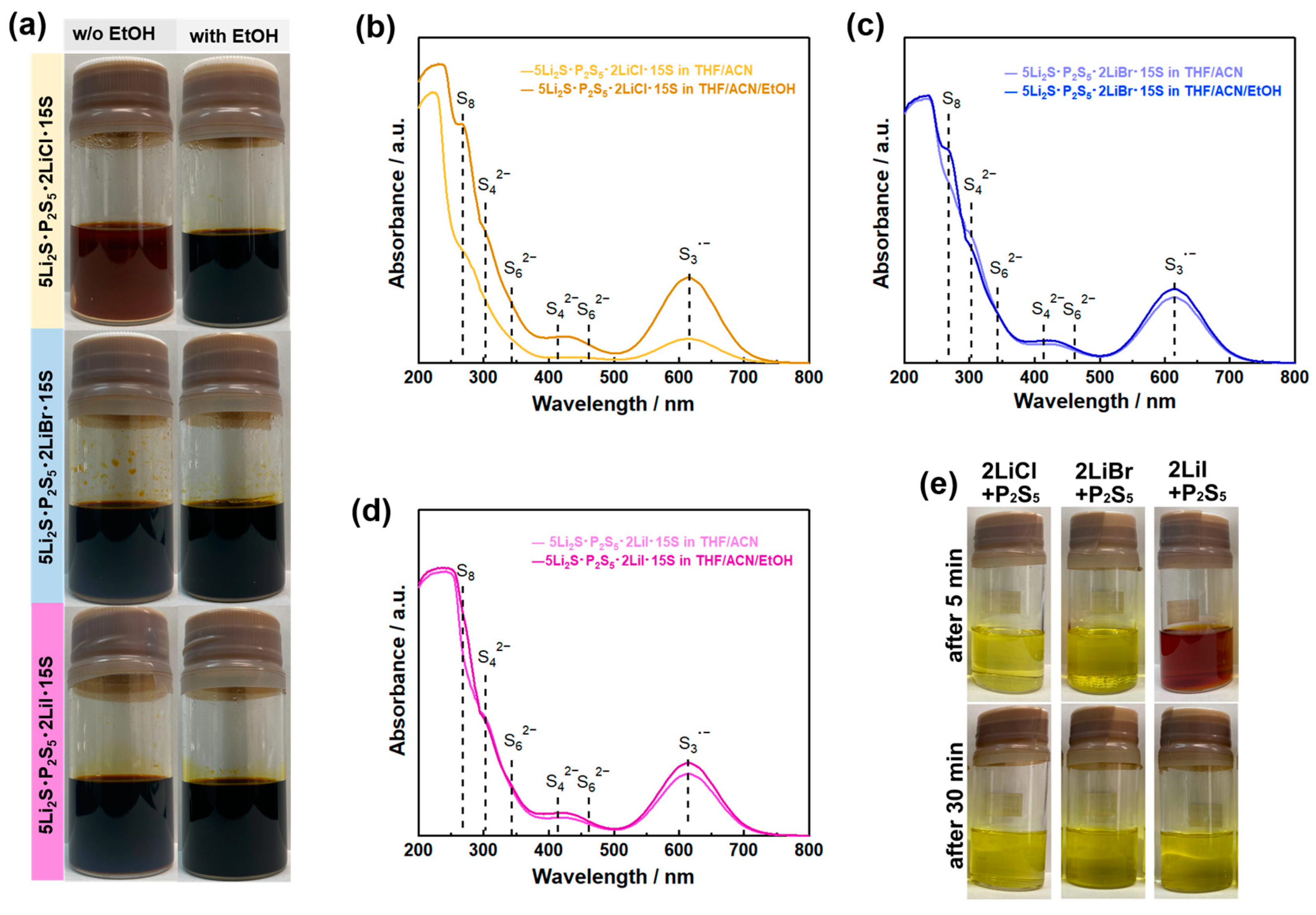
4.1. Solubilizer
4.2. Reactant Agent
5. Low-Cost Synthesis
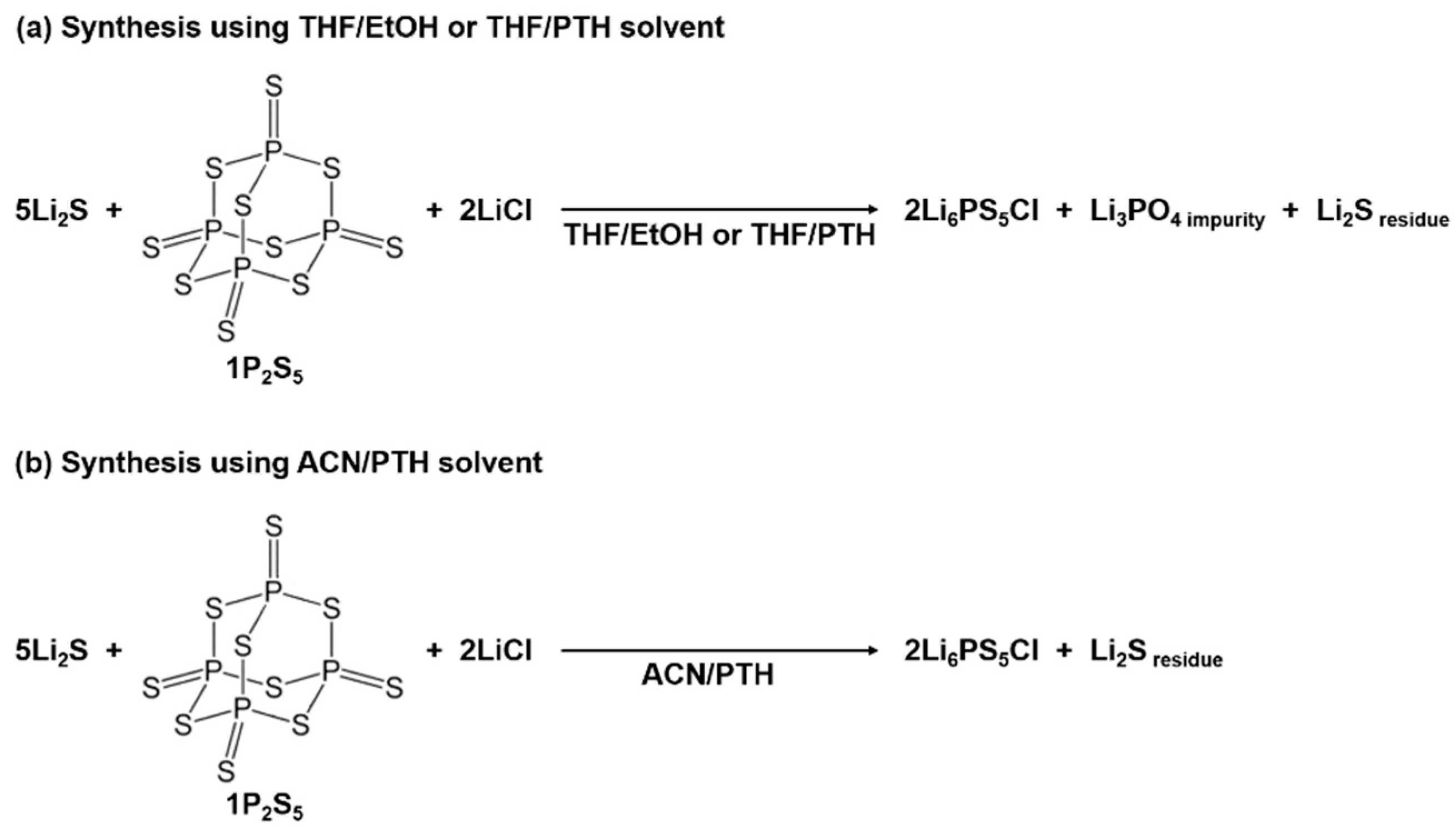
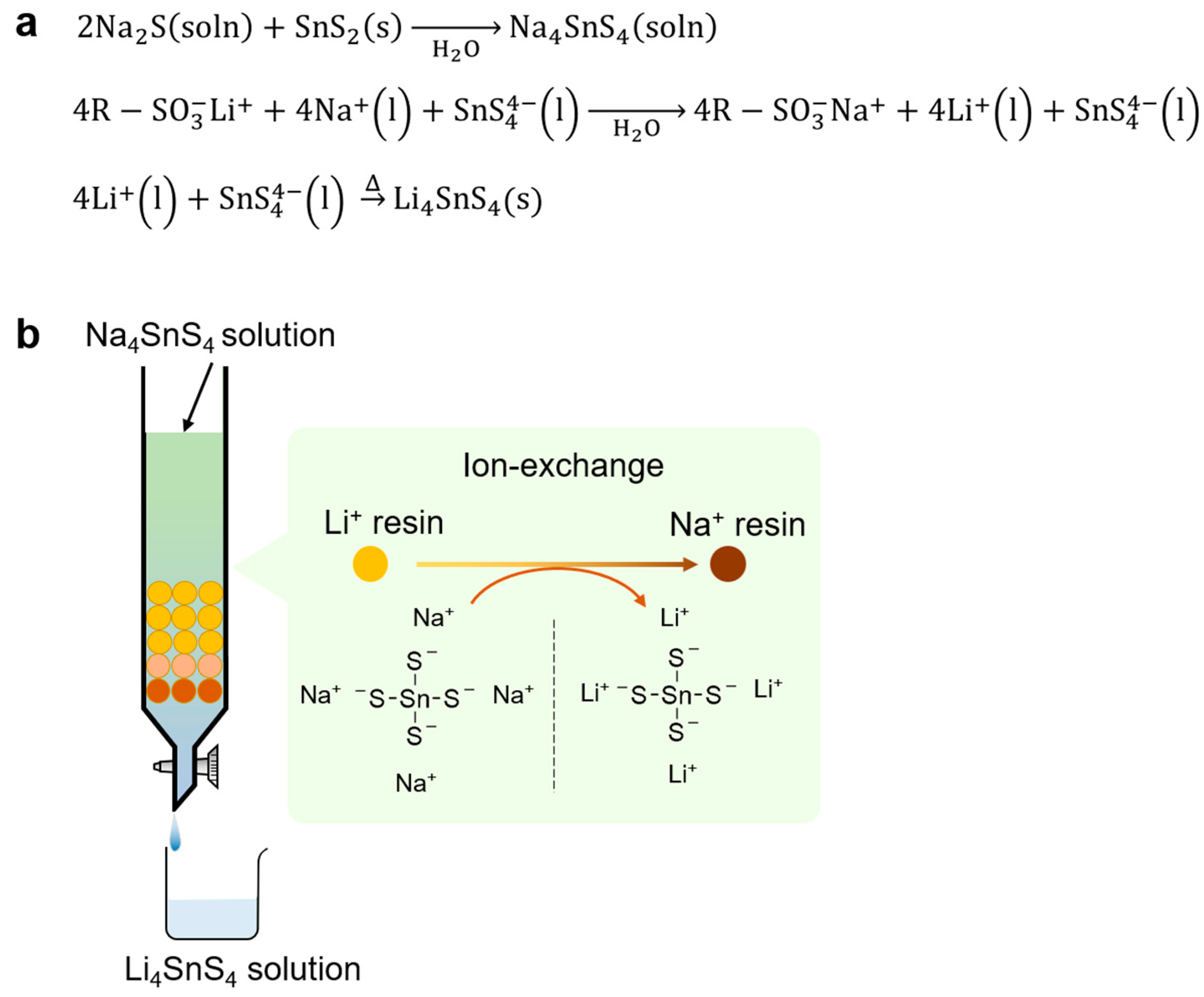
5.1. Ion-Exchange Method
5.2. Sulfurizing Agent
6. Environmental Aspects
7. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Lim, H.-D.; Park, J.-H.; Shin, H.-J.; Jeong, J.; Kim, J.T.; Nam, K.-W.; Jung, H.-G.; Chung, K.Y. A review of challenges and issues concerning interfaces for all-solid-state batteries. Energy Storage Mater. 2020, 25, 224–250. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131–1901172. [Google Scholar] [CrossRef]
- Yamane, H.; Shibata, M.; Shimane, Y.; Junke, T.; Seino, Y.; Adams, S.; Minami, K.; Hayashi, A.; Tatsumisago, M. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ion. 2007, 178, 1163–1167. [Google Scholar] [CrossRef]
- Kamaya, N.; Hommna, K.; Yamakawa, Y.; Hirayama, M.; Kannno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103–16118. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Char, K.; Kim, K.J.; Choi, W.J. Issues and Advances in Scaling up Sulfide-Based All-Solid-State Batteries. Acc. Chem. Res. 2021, 54, 3390–3402. [Google Scholar] [CrossRef]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Gamo, H. Development of All-Solid-State Batteries with Sulfide Solid Electrolytes. Ph.D. Thesis, Toyohashi University of Technology, Toyohashi, Japan, 2023. [Google Scholar]
- Ghidiu, M.; Ruhl, J.; Culver, S.P.; Zeier, W.G. Solution-based synthesis of lithium thiophosphate superionic conductors for solid-state batteries: A chemistry perspective. J. Mater. Chem. A 2019, 7, 17735–17753. [Google Scholar] [CrossRef]
- Gutmann, V. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 1976, 18, 225–255. [Google Scholar] [CrossRef]
- Calpa, M.; Nakajima, H.; Mori, S.; Goto, Y.; Mizuguchi, Y.; Moriyoshi, C.; Kuroiwa, Y.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Formation Mechanism of β-Li3PS4 through Decomposition of Complexes. Inorg. Chem. 2021, 60, 6964–6970. [Google Scholar] [CrossRef]
- Gamo, H.; Nagai, A.; Matsuda, A. The effect of solvent on reactivity of the Li2S–P2S5 system in liquid-phase synthesis of Li7P3S11 solid electrolyte. Sci. Rep. 2021, 11, 21097–21104. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.C.; Xia, X.H.; Yao, Z.J.; Wang, X.L.; Gu, C.D.; Tu, J.P. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution-evaporation method for all-solid-state lithium ion batteries. Electrochim. Acta 2016, 219, 235–240. [Google Scholar] [CrossRef]
- Wang, X.; Ye, L.; Nan, C.W.; Li, X. Effect of Solvents on a Li10GeP2S12-Based Composite Electrolyte via Solution Method for Solid-State Battery Applications. ACS Appl. Mater. Interfaces 2022, 14, 46627–46634. [Google Scholar] [CrossRef]
- Yamamoto, M.; Terauchi, Y.; Sakuda, A.; Takahashi, M. Binder-free sheet-type all-solid-state batteries with enhanced rate capabilities and high energy densities. Sci. Rep. 2018, 8, 1212–1221. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Ito, S.; Nakakita, M.; Aihara, Y.; Uehara, T.; Machida, N. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent. J. Power Sources 2014, 271, 342–345. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Yu, Z.; Bowden, M.; Miller, Q.R.S.; Chen, P.; Scharf, H.T.; Mueller, K.T.; Lu, D.; Xiao, J.; et al. Wet-chemical synthesis of Li7P3S11 with tailored particle size for solid state electrolytes. Chem. Eng. J. 2022, 429, 132334. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yang, S.; Takahashi, M.; Ohara, K.; Uchiyama, T.; Watanabe, T.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; Muto, H.; et al. High Ionic Conductivity of Liquid-Phase-Synthesized Li3PS4 Solid Electrolyte, Comparable to That Obtained via Ball Milling. ACS Appl. Energy Mater. 2021, 4, 2275–2281. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Hirahara, E.; Morikawa, K.; Muto, H.; Matsuda, A. One-pot liquid phase synthesis of (100−x)Li3PS4–xLiI solid electrolytes. J. Power Sources 2017, 365, 7–11. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Morikawa, K.; Totani, M.; Muto, H.; Matsuda, A. Chemical synthesis of Li3PS4 precursor suspension by liquid-phase shaking. Solid State Ion. 2016, 285, 2–5. [Google Scholar] [CrossRef]
- Rajagopal, R.; Subramanian, Y.; Jung, Y.J.; Kang, S.; Ryu, K.-S. Rapid Synthesis of Highly Conductive Li6PS5Cl Argyrodite-Type Solid Electrolytes Using Pyridine Solvent. ACS Appl. Energy Mater. 2022, 5, 9266–9272. [Google Scholar] [CrossRef]
- Ghidiu, M.; Schlem, R.; Zeier, W.G. Pyridine Complexes as Tailored Precursors for Rapid Synthesis of Thiophosphate Superionic Conductors. Batteries Supercaps 2021, 4, 607–611. [Google Scholar] [CrossRef]
- Maniwa, R.; Calpa, M.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Synthesis of sulfide solid electrolytes from Li2S and P2S5 in anisole. J. Mater. Chem. A 2021, 9, 400–405. [Google Scholar] [CrossRef]
- Matsuda, A.; Muto, H.; Phuc, N.H.H. Preparation of Li3PS4 Solid Electrolyte by Liquid-Phase Shaking Using Organic Solvents with Carbonyl Group as Complex Forming Medium. J. Jpn. Soc. Powder Powder Metall. 2016, 63, 976–980. [Google Scholar] [CrossRef]
- Shi, J.; Liu, G.; Weng, W.; Cai, L.; Zhang, Q.; Wu, J.; Xu, X.; Yao, X. Co3S4@Li7P3S11 Hexagonal Platelets as Cathodes with Superior Interfacial Contact for All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 14079–14086. [Google Scholar] [CrossRef]
- Calpa, M.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process. RSC Adv. 2017, 7, 46499–46504. [Google Scholar] [CrossRef]
- Rangasamy, E.; Liu, Z.; Gobet, M.; Pilar, K.; Sahu, G.; Zhou, W.; Wu, H.; Greenbaum, S.; Liang, C. An Iodide-Based Li7P2S8I Superionic Conductor. J. Am. Chem. Soc. 2015, 137, 1384–1387. [Google Scholar] [CrossRef]
- Hikima, K.; Yamamoto, T.; Phuc, N.H.H.; Matsuda, R.; Muto, H.; Matsuda, A. Improved ionic conductivity of Li2S-P2S5-LiI solid electrolytes synthesized by liquid-phase synthesis. Solid State Ion. 2020, 354, 115403–115408. [Google Scholar] [CrossRef]
- Zhou, L.; Park, K.-H.; Sun, X.; Lalere, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019, 4, 265–270. [Google Scholar] [CrossRef]
- Indrawan, R.F.; Gamo, H.; Nagai, A.; Matsuda, A. Chemically Understanding the Liquid-Phase Synthesis of Argyrodite Solid Electrolyte Li6PS5Cl with the Highest Ionic Conductivity for All-Solid-State Batteries. Chem. Mater. 2023, 35, 2549–2558. [Google Scholar] [CrossRef]
- Calpa, M.; Rosero-Navarro, N.C.; Miura, A.; Terai, K.; Utsuno, F.; Tadanaga, K. Formation mechanism of thiophosphate anions in the liquid-phase synthesis of sulfide solid electrolytes using polar aprotic solvents. Chem. Mater. 2020, 32, 9627–9632. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, D.; Bowden, M.; Khoury, P.Z.E.; Han, K.S.; Deng, Z.D.; Xiao, J.; Zhang, J.-G.; Liu, J. Mechanism of Formation of Li7P3S11 Solid Electrolytes through Liquid Phase Synthesis. Chem. Mater. 2018, 30, 990–997. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wu, J.; Jiang, Y.; Hung, S.; Zhao, B.; Chen, Z.; Zhang, J. Reaction mechanism of Li2S-P2S5 system in acetonitrile based on wet chemical synthesis of Li7P3S11 solid electrolyte. Chem. Eng. J. 2020, 393, 124706–124714. [Google Scholar] [CrossRef]
- Tsukasaki, H.; Mori, S.; Shiotani, S.; Yamamura, H. Ionic conductivity and crystallization process in the Li2S–P2S5 glass electrolyte. Solid State Ion. 2018, 317, 122–126. [Google Scholar] [CrossRef]
- Yubuchi, S.; Uematsu, M.; Hotehama, C.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. An argyrodite sulfide-based superionic conductor synthesized by a liquid-phase technique with tetrahydrofuran and ethanol. J. Mater. Chem. A 2019, 7, 558–566. [Google Scholar] [CrossRef]
- Ruhl, J.; Riegger, L.M.; Ghidiu, M.; Zeier, W.G. Impact of Solvent Treatment of the Superionic Argyrodite Li6PS5Cl on Solid-State Battery Performance. Adv. Energy Sustain. Res. 2021, 2, 2000077–2000086. [Google Scholar] [CrossRef]
- Song, Y.B.; Kim, D.H.; Kwak, H.; Han, D.; Kang, S.; Lee, J.H.; Bak, S.-M.; Nam, K.-W.; Lee, H.-W.; Jung, Y.S. Tailoring Solution-Processable Li Argyrodites Li6+xP1-xMxS5I (M = Ge, Sn) and Their Microstructural Evolution Revealed by Cryo-TEM for All-Solid-State Batteries. Nano Lett. 2020, 20, 4337–4345. [Google Scholar] [CrossRef]
- Yubuchi, S.; Uematsu, M.; Deguchi, M.; Hayashi, A.; Tatsumisago, M. Lithium-Ion-Conducting Argyrodite-Type Li6PS5X (X = Cl, Br, I) Solid Electrolytes Prepared by a Liquid-Phase Technique Using Ethanol as a Solvent. ACS Appl. Energy Mater. 2018, 1, 3622–3629. [Google Scholar] [CrossRef]
- Heo, Y.J.; Seo, S.D.; Hwang, S.H.; Choi, S.H.; Kim, D.W. One-pot aprotic solvent-enabled synthesis of superionic Li-argyrodite solid electrolyte. Int. J. Energy Res. 2022, 46, 17644–17653. [Google Scholar] [CrossRef]
- Ito, A.; Kimura, T.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Liquid-phase synthesis of Li3PS4 solid electrolyte using ethylenediamine. J. Sol-Gel Sci. Technol. 2021, 101, 2–7. [Google Scholar] [CrossRef]
- Kimura, T.; Ito, A.; Nakano, T.; Hotehama, C.; Kowada, H.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Crystalline precursor derived from Li3PS4 and ethylenediamine for ionic conductors. J. Sol-Gel Sci. Technol. 2022, 104, 627–634. [Google Scholar] [CrossRef]
- Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries. J. Mater. Chem. A 2014, 2, 5095–5099. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Deng, Z.; Wu, E.A.; Nguyen, H.; Douz, J.-M.; Wang, X.; Cheng, J.-h.; Ong, S.P.; Meng, Y.S.; et al. Enabling Thin and Flexible Solid-State Composite Electrolytes by the Scalable Solution Process. ACS Appl. Energy Mater. 2019, 2, 6542–6550. [Google Scholar] [CrossRef]
- Woo, J.; Song, Y.B.; Kwak, H.; Jun, S.; Jang, B.Y.; Park, J.; Kim, K.T.; Park, C.; Lee, C.; Park, K.-H.; et al. Liquid-Phase Synthesis of Highly Deformable and Air-Stable Sn-Substituted Li3PS4 for All-Solid-State Batteries Fabricated and Operated under Low Pressures. Adv. Energy Mater. 2023, 13, 2203292. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, K.-H.; Kim, J.C.; Wi, T.-U.; Ha, A.R.; Song, Y.B.; Oh, D.Y.; Woo, J.; Kweon, S.H.; Yeom, S.J.; et al. Universal Solution Synthesis of Sulfide Solid Electrolytes Using Alkahest for All-Solid-State Batteries. Adv. Mater. 2022, 34, 2200083–2200093. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. A berzelius reagent, phosphorus decasulfide (P4S10), in organic syntheses. Chem. Rev. 2010, 110, 3419–3478. [Google Scholar] [CrossRef]
- Gutmann, V. Empirical parameters for donor and accepter properties of solvents. Electrochim. Acta 1976, 21, 661–670. [Google Scholar] [CrossRef]
- Fan, B.; Xu, Y.; Ma, R.; Luo, Z.; Wang, F.; Zhang, X.; Ma, H.; Fan, P.; Xue, B.; Han, W. Will Sulfide Electrolytes be Suitable Candidates for Constructing a Stable Solid/Liquid Electrolyte Interface? ACS Appl. Mater. Interfaces 2020, 12, 52845–52856. [Google Scholar] [CrossRef] [PubMed]
- Gamo, H.; Nishida, J.; Nagai, A.; Hikima, K.; Matsuda, A. Solution Processing via Dynamic Sulfide Radical Anions for Sulfide Solid Electrolytes. Adv. Energy Sustain. Res. 2022, 3, 2200019–2200027. [Google Scholar] [CrossRef]
- Yuan, M.; Mitzi, D.B. Solvent properties of hydrazine in the preparation of metal chalcogenide bulk materials and films. Dalton Trans. 2009, 31, 6078–6088. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.L.; Brutchey, R.L. Solution processing of chalcogenide materials using thiol–amine ‘alkahest’ solvent systems. Chem. Commun. 2017, 53, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Antunez, P.D.; Torelli, D.A.; Yang, F.; Rabuffetti, F.A.; Lewis, N.S.; Brutchey, R.L. Low Temperature Solution-Phase Deposition of SnS Thin Films. Chem. Mater. 2014, 26, 5444–5446. [Google Scholar] [CrossRef]
- Webber, D.H.; Brutchey, R.L. Alkahest for V2VI3 Chalcogenides: Dissolution of Nine Bulk Semiconductors in a Diamine-Dithiol Solvent Mixture. J. Am. Chem. Soc. 2013, 135, 15722–15725. [Google Scholar] [CrossRef]
- Ito, T.; Hori, S.; Hirayama, M.; Kanno, R. Liquid-phase synthesis of the Li10GeP2S12-type phase in the Li-Si-P-S-Cl system. J. Mater. Chem. A 2022, 10, 14392–14398. [Google Scholar] [CrossRef]
- Lim, H.; Lim, H.-K.; Xing, X.; Lee, B.-S.; Liu, H.; Coaty, C.; Kim, H.; Liu, P. Solid Electrolyte Layers by Solution Deposition. Adv. Mater. Interfaces 2018, 5, 1701328–1701336. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhou, H.; Xing, X.; Banerjee, A.; Holoubek, J.; Liu, H.; Meng, Y.S.; Liu, P. Thin Solid Electrolyte Layers Enabled by Nanoscopic Polymer Binding. ACS Energy Lett. 2020, 5, 955–961. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, I.-H.; Jo, S.C.; Kim, B.G.; Ha, Y.-C.; Lee, S.-M.; Kang, S.; Baeg, K.-J.; Park, J.-W. A Novel Strategy to Overcome the Hurdle for Commercial All-Solid-State Batteries via Low-Cost Synthesis of Sulfide Solid Electrolytes. Small Methods 2021, 5, 2100793–2100804. [Google Scholar] [CrossRef]
- Bieker, G.; Wellmann, J.; Kolek, M.; Jalkanen, K.; Winter, M.; Bieker, P. Influence of cations in lithium and magnesium polysulphide solutions: Dependence of the solvent chemistry. Phys. Chem. Chem. Phys. 2017, 19, 11152–11162. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Lei, C.; Meng, R.; Li, J.; He, X.; Liang, X. Electrolyte Solvation Chemistry for the Solution of High-Donor-Number Solvent for Stable Li–S Batteries. Small 2022, 18, 2200046–2200054. [Google Scholar] [CrossRef] [PubMed]
- Gamo, H.; Kusaba, I.; Hikima, K.; Matsuda, A. Rapid Solution Synthesis of Argyrodite-Type Li6PS5X (X = Cl, Br, and I) Solid Electrolytes Using Excess Sulfur. Inorg. Chem. 2023, 62, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Hikima, K.; Ogawa, K.; Gamo, H.; Matsuda, A. Li10GeP2S12 solid electrolytes synthesised via liquid-phase methods. Chem. Commun. 2023, 43, 6564–6567. [Google Scholar] [CrossRef]
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or Not Radical: Revisiting Lithium-Sulfur Electrochemistry in Nonaqueous Electrolytes. Adv. Energy Mater. 2015, 5, 1401801–1401806. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.-E.; Adams, B.D.; Garsuch, A.; Balasubramanian, M.; Nazar, L.F. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ. Sci. 2014, 7, 2697–2705. [Google Scholar] [CrossRef]
- Lim, H.-D.; Yue, X.; Xing, X.; Petrova, V.; Gonzalez, M.; Liu, H.; Liu, P. Designing solution chemistries for the low-temperature synthesis of sulfide-based solid electrolytes. J. Mater. Chem. A 2018, 6, 7370–7374. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Yamamoto, T.; Muto, H.; Matsuda, A. Fast synthesis of Li2S-P2S5-LiI solid electrolyte precursors. Inorg. Chem. Front. 2017, 4, 1660–1664. [Google Scholar] [CrossRef]
- Matsuda, R.; Kokubo, T.; Phuc, N.H.H.; Muto, H.; Matsuda, A. Preparation of ambient air-stable electrolyte Li4SnS4 by aqueous ion-exchange process. Solid State Ion. 2020, 345, 115190. [Google Scholar] [CrossRef]
- Han, A.; Tian, R.; Fang, L.; Wan, F.; Hu, X.; Zhao, Z.; Tu, F.; Song, D.; Zhang, X.; Yang, Y. A Low-Cost Liquid-Phase Method of Synthesizing High-Performance Li6PS5Cl Solid-Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 30824–30838. [Google Scholar] [CrossRef]
- Smith, W.H.; Vaselabadi, S.A.; Wolden, C.A. Argyrodite Superionic Conductors Fabricated from Metathesis-Derived Li2S. ACS Appl. Energy Mater. 2022, 5, 4029–4035. [Google Scholar] [CrossRef]
- Smith, W.H.; Vaselabadi, S.A.; Wolden, C.A. Synthesis of high-purity Li2S nanocrystals via metathesis for solid-state electrolyte applications. J. Mater. Chem. A 2023, 11, 7652–7661. [Google Scholar] [CrossRef]
- Smith, W.H.; Vaselabadi, S.A.; Wolden, C.A. Sustainable synthesis of SiS2 for solid-state electrolytes by cascaded metathesis. Mater. Today Commun. 2023, 35, 105574. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion. 2011, 182, 116–119. [Google Scholar] [CrossRef]
- Otoyama, M.; Kuratani, K.; Kobayashi, H. A systematic study on structure, ionic conductivity, and air-stability of xLi4SnS4·(1−x)Li3PS4 solid electrolytes. Ceram. Int. 2021, 47, 28377–28383. [Google Scholar] [CrossRef]
- Kimura, T.; Kato, A.; Hotehama, C.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes. Solid State Ion. 2019, 333, 45–49. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, D.Y.; Choi, Y.E.; Nam, Y.J.; Han, L.; Kim, J.-Y.; Xin, H.; Lin, F.; Oh, S.M.; Jung, Y.S. Solution-Processable Glass LiI-Li4SnS4 Superionic Conductors for All-Solid-State Li-Ion Batteries. Adv. Mater. 2016, 28, 1874–1883. [Google Scholar] [CrossRef]
- Zhao, F.; Liang, J.; Yu, C.; Sun, Q.; Li, X.; Adair, K.; Wang, C.; Zhao, Y.; Zhang, S.; Li, W.; et al. A Versatile Sn-Substituted Argyrodite Sulfide Electrolyte for All-Solid-State Li Metal Batteries. Adv. Energy Mater. 2020, 10, 1903422–1903431. [Google Scholar] [CrossRef]
- Otoyama, M.; Kuratani, K.; Kobayashi, H. Mechanochemical synthesis of air-stable hexagonal Li4SnS4-based solid electrolytes containing LiI and Li3PS4. RSC Adv. 2021, 11, 38880–38888. [Google Scholar] [CrossRef]
- Kimura, T.; Nakano, T.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Hydration and Dehydration Behavior of Li4SnS4 for Applications as a Moisture-Resistant All-Solid-State Battery Electrolyte. J. Phys. Chem. C 2023, 127, 1303–1309. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, Q.; Han, A.; Zhao, Z.; Hu, X.; Wan, F.; Yang, H.; Song, D.; Zhang, X.; Yang, Y. Green synthesis of the battery material lithium sulfide via metathetic reactions. Chem. Commun. 2022, 58, 5498–5501. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-S.; Hong, J.-W.; Choi, I.-H.; Sung, J.; Park, J.-H.; Park, H.; Kim, D.; Kim, B.G.; Ha, Y.-C.; Seo, J.; et al. Engineering green and sustainable solvents for scalable wet synthesis of sulfide electrolytes in high-energy-density all-solid-state batteries. Green Chem. 2023, 25, 1473–1487. [Google Scholar] [CrossRef]
| Product | Treatment | Solvent | Reaction Time (h) | Ionic Conductivity (mS cm−1) | Reference |
|---|---|---|---|---|---|
| Li3PS4 | Stirring | THF | 12 | 0.074 | [19] |
| Li3PS4 | Stirring | ACN | 48 | 0.12 | [14] |
| Li3PS4 | Shaking | EP | 6 | 0.20 | [33] |
| Li7P3S11 | Stirring | DME | 72 | 0.27 | [20] |
| Li7P3S11 | Stirring | ACN | 24 | 1.5 | [35] |
| Li7P3S11 | Sonication | ACN | 0.5 | 1.0 | [32] |
| Li7P3S11 | Microwave | anisole | 0.5 | 0.13 | [27] |
| Li7P2S8I | Stirring | ACN | 36 | 0.63 | [31] |
| Li7P2S8I | Shaking | EP | 6 | 0.34 | [23] |
| Li7P2S8I | Shaking | EP | 1 | 1.0 | [36] |
| Li6PS5Cl | Stirring | THF+EtOH | 36 | 2.4 | [37] |
| Li6PS5Cl | Stirring | ACN+PTH | 24 | 2.8 | [38] |
| Product | Treatment | Solvent | Reaction Time (h) | Ionic Conductivity (mS cm−1) | Reference |
|---|---|---|---|---|---|
| Li3PS4 | Stirring | EDA | 3 | 2.3 × 10−3 | [44] |
| Li7P3S11 | Stirring | EA | 2 | 0.12 | [21] |
| Li6−xPS5−xCl1+x (0 ≤ x ≤ 0.5) | Stirring | EDA | ― | 2.9 | [25] |
| Li10Ge2PS12 | Stirring | EDA+ET | 3 | 1.2 | [49] |
| Solvent | Donor Number [kcal·mol−1] | Dielectric Permittivity | Boiling Point [°C] |
|---|---|---|---|
| 1,4-dioxan | 15 | 2.2 | 101 |
| Carbon sulfide | 2 | 2.63 | 46 |
| Anisole | 9 | 4 | 154 |
| Tetrahydropyran | 22 | 5.5 | 88 |
| Ethyl acetate | 17 | 6 | 77 |
| 1,2-Dimethoxyethane | 24 | 7.2 | 83 |
| Tetrahydrofuran | 20 | 7.6 | 66 |
| Pyridine | 33 | 12 | 115 |
| Ethylene diamine | 55 | 14 | 116 |
| Ethanol | 32 | 19 | 78 |
| Dimethylformamide | 27 | 36 | 153 |
| Acetonitrile | 14 | 38 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamo, H.; Nagai, A.; Matsuda, A. Toward Scalable Liquid-Phase Synthesis of Sulfide Solid Electrolytes for All-Solid-State Batteries. Batteries 2023, 9, 355. https://doi.org/10.3390/batteries9070355
Gamo H, Nagai A, Matsuda A. Toward Scalable Liquid-Phase Synthesis of Sulfide Solid Electrolytes for All-Solid-State Batteries. Batteries. 2023; 9(7):355. https://doi.org/10.3390/batteries9070355
Chicago/Turabian StyleGamo, Hirotada, Atsushi Nagai, and Atsunori Matsuda. 2023. "Toward Scalable Liquid-Phase Synthesis of Sulfide Solid Electrolytes for All-Solid-State Batteries" Batteries 9, no. 7: 355. https://doi.org/10.3390/batteries9070355
APA StyleGamo, H., Nagai, A., & Matsuda, A. (2023). Toward Scalable Liquid-Phase Synthesis of Sulfide Solid Electrolytes for All-Solid-State Batteries. Batteries, 9(7), 355. https://doi.org/10.3390/batteries9070355