Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery
Abstract
1. Introduction
2. Experiment
2.1. Chemicals and Materials
2.2. Synthesis of Partially Nitrided V2O5
2.3. Synthesis of Ca2+-Doped VNO
2.4. Material Characterization and Performance Evaluation
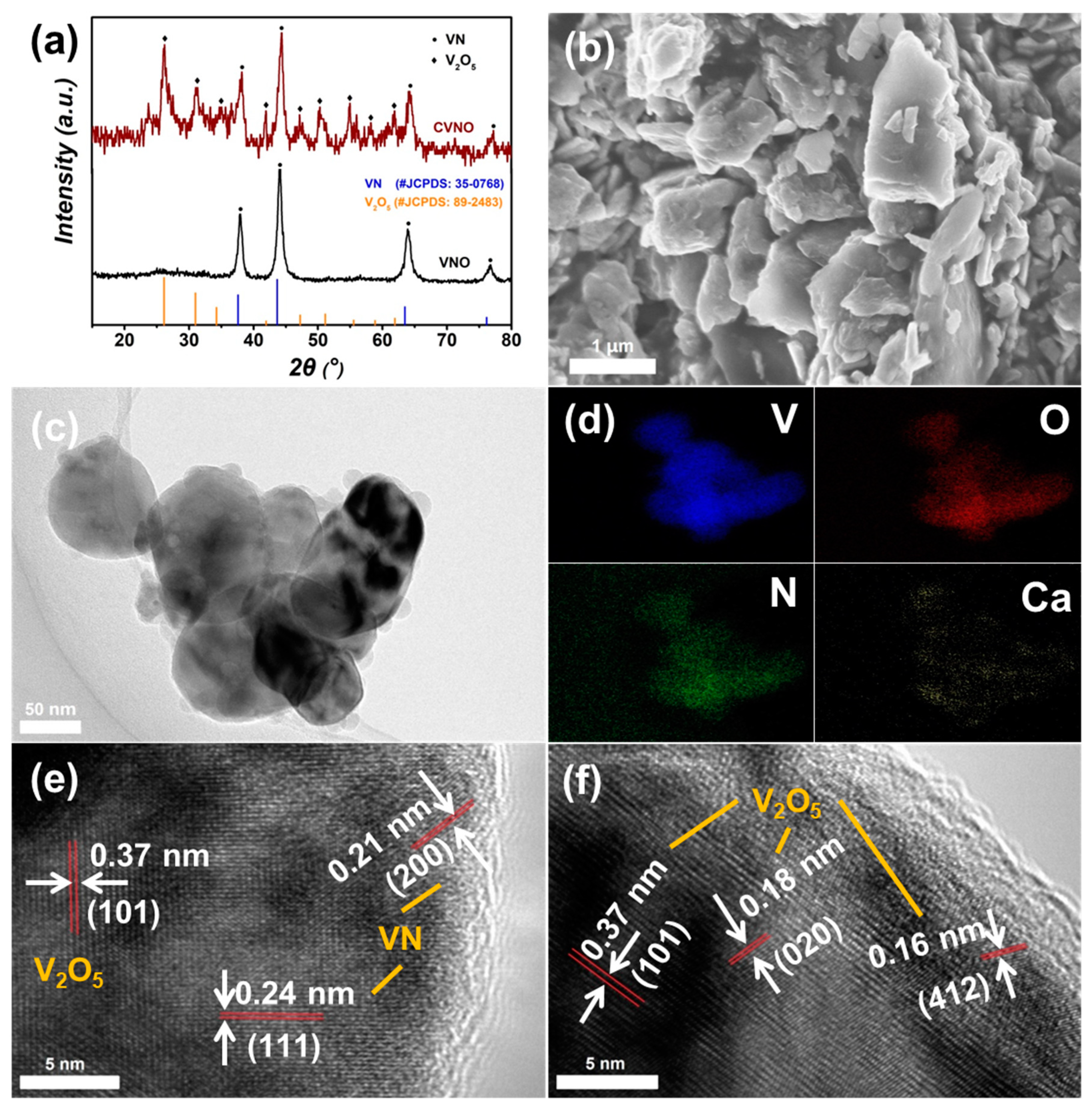
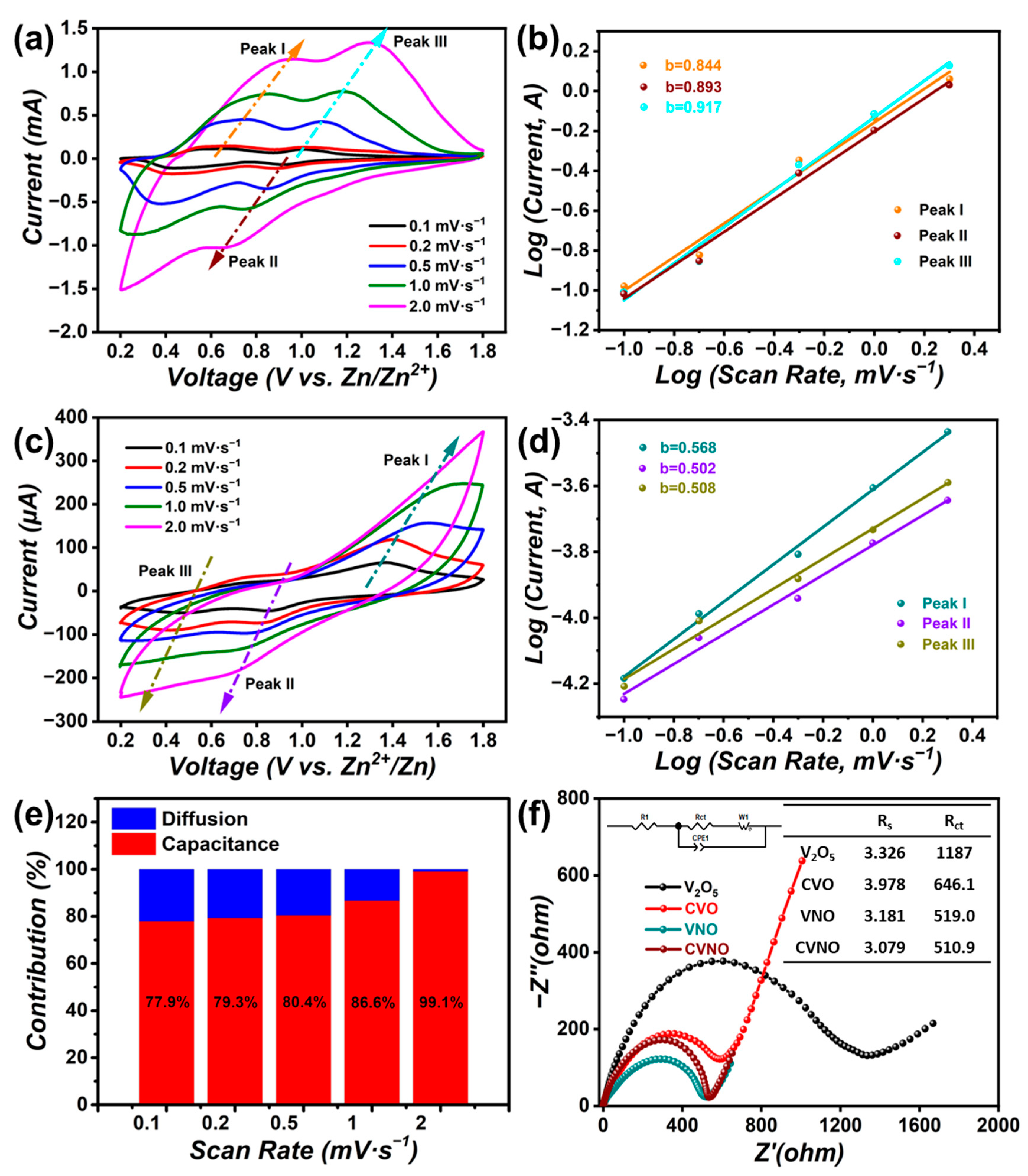
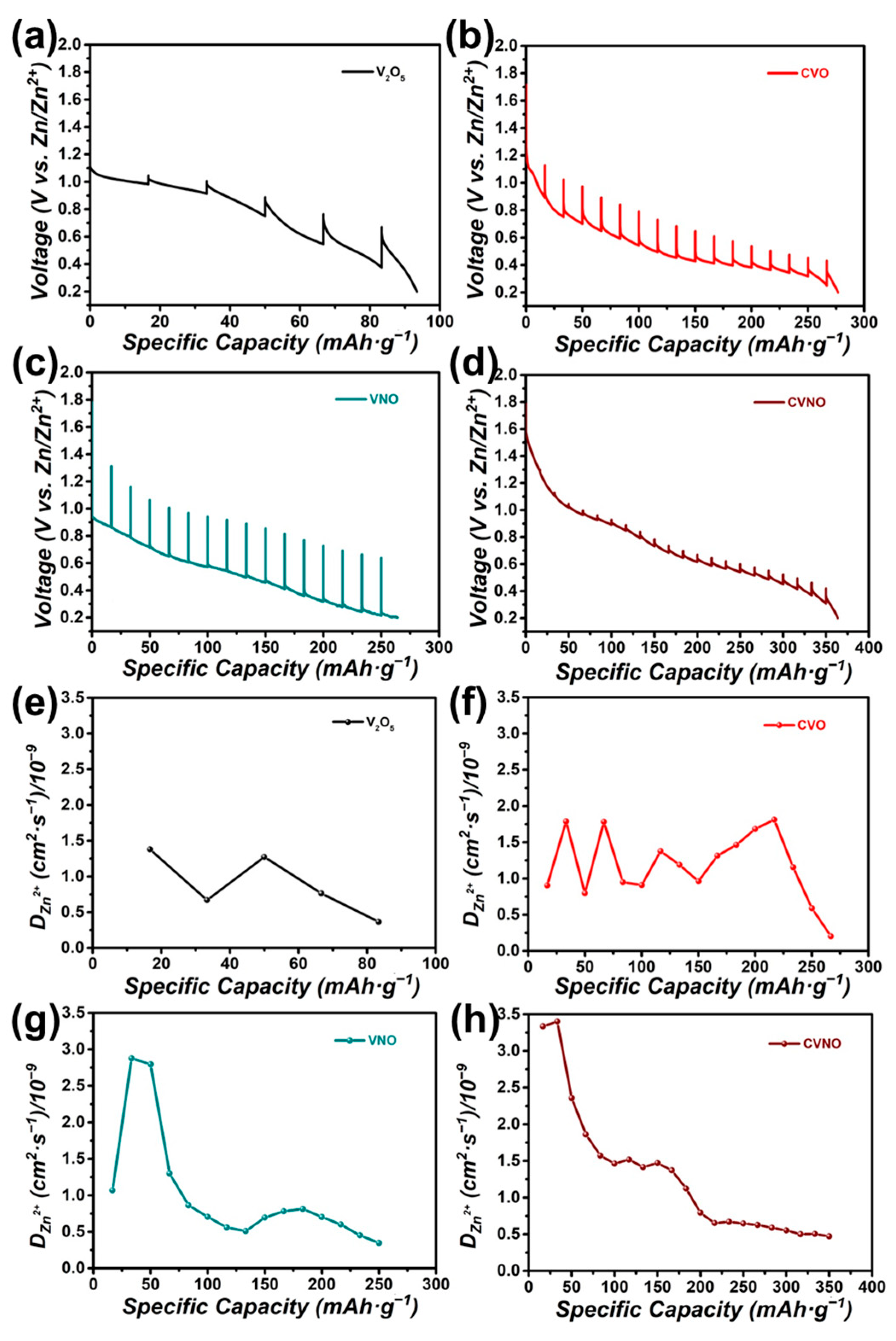
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaveevivitchai, W.; Manthiram, A. High-capacity zinc-ion storage in an open-tunnel oxide for aqueous and nonaqueous Zn-ion batteries. J. Mater. Chem. A 2016, 4, 18737–18741. [Google Scholar] [CrossRef]
- Yu, P.; Zeng, Y.X.; Zhang, H.Z.; Yu, M.H.; Tong, Y.X.; Lu, X. Flexible Zn-Ion Batteries: Recent Progresses and Challenges. Small 2019, 15, 1804760. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Dong, L.B.; Yang, W.; Yang, W.; Li, Y.; Wu, W.J.; Wang, G.X. Multivalent metal ion hybrid capacitors: A review with a focus on zinc-ion hybrid capacitors. J. Mater. Chem. A 2019, 7, 13810–13832. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Ma, J.X.; Cai, C.C.; Hu, G.W.; Wang, X.P.; Liu, Z.A.; Zhou, L.; Mai, L.Q. Porous V2O5 microspheres: A high-capacity cathode material for aqueous zinc-ion batteries. Chem. Commun. 2019, 55, 8486–8489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, L.; Zhang, P.; Wang, H.; Li, S.; Ji, S.; Wen, Z.; Sun, J. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl. Surf. Sci. 2020, 502, 144207. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Zheng, Q.; Li, X. Porous V2O5 yolk–shell microspheres for zinc ion battery cathodes: Activation responsible for enhanced capacity and rate performance. J. Mater. Chem. A 2020, 8, 5186–5193. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, Z.H.; Huang, Y.G. Monoclinic VO2(D) hollow nanospheres with super-long cycle life for aqueous zinc ion batteries. Nanoscale 2019, 11, 13032–13039. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, J.; Zhang, D.; Fang, Y.; Hu, F.; Zhu, K. VO2(B) nanobelts and reduced graphene oxides composites as cathode materials for low-cost rechargeable aqueous zinc ion batteries. Chem. Eng. J. 2020, 390, 124118. [Google Scholar] [CrossRef]
- Wang, L.L.; Huang, K.W.; Chen, J.T.; Zheng, J.R. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 2019, 5, eaax4279. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhao, Y.; Yang, Q.; Wang, D.; Huang, Z.; Liang, G.; Mo, F.; Liu, Z.; Zhi, C. Hydrated hybrid vanadium oxide nanowires as the superior cathode for aqueous Zn battery. Mater. Today Energy 2019, 14, 100361. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A Deep-Cycle Aqueous Zinc-Ion Battery Containing an Oxygen-Deficient Vanadium Oxide Cathode. Angew. Chem. 2019, 59, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Li, Q.; Yang, G.Z.; Wang, C.X. High-rate and durable aqueous zinc ion battery using dendritic V10O24· 12H2O cathode material with large interlamellar spacing. Electrochim. Acta 2018, 287, 60–67. [Google Scholar] [CrossRef]
- Li, Q.; Wei, T.; Ma, K.; Yang, G.; Wang, C. Boosting the Cyclic Stability of Aqueous Zinc-Ion Battery Based on Al-doped V10O24· 12H2O Cathode Materials. Acs Appl. Mater. Interfaces 2019, 11, 20888–20894. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Tang, Y.; Fang, G.Z.; Shan, L.T.; Guo, J.S.; Zhang, W.Y.; Wang, C.; Wang, L.B.; Zhou, J.; Liang, S.Q. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.Z.; Zhang, W.Y.; Zhou, J.; Shan, L.T.; Wang, L.B.; Wang, C.; Lin, T.Q.; Tang, Y.; Liang, S.Q. Mechanistic Insights of Zn2+ Storage in Sodium Vanadates. Adv. Energy Mater. 2018, 8, 1801819. [Google Scholar] [CrossRef]
- Tang, B.Y.; Fang, G.Z.; Zhou, J.; Wang, L.B.; Lei, Y.P.; Wang, C.; Lin, T.Q.; Tang, Y.; Liang, S.Q. Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 2018, 51, 579–587. [Google Scholar] [CrossRef]
- Tang, B.Y.; Zhou, J.; Fang, G.Z.; Guo, S.; Guo, X.; Shan, L.T.; Tang, Y.; Liang, S.Q. Structural Modification of V2O5 as High-Performance Aqueous Zinc-Ion Battery Cathode. J. Electrochem. Soc. 2019, 166, A480–A486. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Pan, Z.; Xu, L.; Zheng, J.; Gao, Z.; Hu, T.; Meng, C. Facile hydrothermal synthesis and electrochemical properties of (NH4)2V10O25·8H2O nanobelts for high-performance aqueous zinc ion batteries. Electrochim. Acta 2020, 332, 135506. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Pan, Z.; Xu, L.; Zheng, J.; Gao, Z.; Hu, T.; Meng, C.; Wang, J. NH4V3O8·0.5H2O nanobelts with intercalated water molecules as a high performance zinc ion battery cathode. Mater. Chem. Front. 2020, 4, 1434–1443. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Edit. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as Cathode Material for High-Performance Aqueous Zinc Ion Batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef]
- Li, J.; McColl, K.; Lu, X.; Sathasivam, S.; Dong, H.; Kang, L.; Li, Z.; Zhao, S.; Kafizas, A.G.; Wang, R.; et al. Multi-Scale Investigations of δ-Ni0.25V2O5·nH2O Cathode Materials in Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000058. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-Ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Bian, R.L.; Song, D.; Si, W.P.; Zhang, T.; Zhang, Y.X.; Lu, P.Y.; Feng, H.; Liang, J. Carbon nanotubes@nickel cobalt sulfide nanosheets for high-performance supercapacitors. Chem. Electro. Chem. 2020, 17, 3663–3669. [Google Scholar] [CrossRef]
- Fang, G.Z.; Hang, S.Q.; Chen, Z.X.; Cui, P.X.; Zheng, X.S.; Pan, A.Q.; Lu, B.G.; Lu, X.H.; Zhou, J. Simultaneous Cationic and Anionic Redox Reactions Mechanism Enabling High-Rate Long-Life Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2019, 29, 1905267. [Google Scholar] [CrossRef]
- Ding, J.W.; Du, Z.G.; Li, B.; Wang, L.Z.; Wang, S.W.; Gong, Y.J.; Yang, S.B. Unlocking the Potential of Disordered Rocksalts for Aqueous Zinc-Ion Batteries. Adv. Mater. 2019, 31, 1904369. [Google Scholar] [CrossRef]
- Huang, K.; Bi, K.; Liang, C.; Lin, S.; Zhang, R.; Wang, W.J.; Tang, H.L.; Lei, M. Novel VN/C nanocomposites as methanol-tolerant oxygen reduction electrocatalyst in alkaline electrolyte. Sci. Rep. 2015, 5, 11351. [Google Scholar] [CrossRef]
- Zhong, Y.; Chao, D.L.; Deng, S.J.; Zhan, J.Y.; Fang, R.Y.; Xia, Y.; Wang, Y.D.; Wang, X.L.; Xia, X.H.; Tu, J.P. Confining Sulfur in Integrated Composite Scaffold with Highly Porous Carbon Fibers/Vanadium Nitride Arrays for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1706391. [Google Scholar] [CrossRef]
- He, J.R.; Manthiram, A. Long-Life, High-Rate Lithium-Sulfur Cells with a Carbon-Free VN Host as an Efficient Polysulfide Adsorbent and Lithium Dendrite Inhibitor. Adv. Energy Mater. 2020, 10, 1903241. [Google Scholar] [CrossRef]
- Galesic, I.; Kolbesen, B.O. Formation of vanadium nitride by rapid thermal processing. Thin Solid Film. 1999, 349, 14–18. [Google Scholar] [CrossRef]
- Liu, W.; Dong, L.; Jiang, B.; Huang, Y.; Wang, X.; Xu, C.; Kang, Z.; Mou, J.; Kang, F. Layered vanadium oxides with proton and zinc ion insertion for zinc ion batteries. Electrochim. Acta 2019, 320, 134565. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, L.Q.; Zhang, B.; Feng, J.M.; Zhang, J.F.; Ou, X.; Hou, F.; Liang, J. A flexible carbon nanotube@V2O5 film as a high-capacity and durable cathode for zinc ion batteries. J. Energy Chem. 2021, 59, 126–133. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Xu, B.; Wang, Y.Y.; Qiu, M.D.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Cheng, F.Y. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.-E. Li+ Ion Insertion in TiO2 (Anatase). 2. Voltammetry on Nanoporous Films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhu, C.R.; Yang, P.H.; Xia, X.H.; Liu, J.L.; Wang, J.; Fan, X.F.; Savilov, S.V.; Lin, J.Y.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef]
- Liu, T.C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of molybdenum nitrides as materials for electrochemical capacitors —Comparison with ruthenium oxide. J. Electrochem. Soc. 1998, 145, 1882–1888. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, Y.J.; Liu, Y.H.; Wang, C.S. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.Y.; Liu, Y.C.; Zhao, Q.; Lei, K.X.; Chen, C.C.; Liu, X.S.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3) 2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, D.S.; Lee, H.J.; Jung, Y.; Choi, J.W. Hydrated Intercalation for High-Performance Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2019, 9, 1900083. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, L.; Zhang, B.; Wang, J.; Liu, J.; Tan, Q.; Wan, H.; Jiang, J. A durable VO2(M)/Zn battery with ultrahigh rate capability enabled by pseudocapacitive proton insertion. J. Mater. Chem. A 2020, 8, 1731–1740. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bian, R.; Sang, Z.; Tan, S.; Liang, J.; Wang, L.; Hou, F. Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery. Batteries 2023, 9, 352. https://doi.org/10.3390/batteries9070352
Zhang X, Bian R, Sang Z, Tan S, Liang J, Wang L, Hou F. Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery. Batteries. 2023; 9(7):352. https://doi.org/10.3390/batteries9070352
Chicago/Turabian StyleZhang, Xueqi, Ruilin Bian, Zhiyuan Sang, Shandong Tan, Ji Liang, Liqun Wang, and Feng Hou. 2023. "Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery" Batteries 9, no. 7: 352. https://doi.org/10.3390/batteries9070352
APA StyleZhang, X., Bian, R., Sang, Z., Tan, S., Liang, J., Wang, L., & Hou, F. (2023). Anion and Cation Co-Modified Vanadium Oxide for Cathode Material of Aqueous Zinc-Ion Battery. Batteries, 9(7), 352. https://doi.org/10.3390/batteries9070352







