Abstract
Aqueous sodium-ion batteries (ASIBs) represent a promising battery technology for stationary energy storage, due to their attractive merits of low cost, high abundance, and inherent safety. Recently, a variety of advanced cathode, anode, and electrolyte materials have been developed for ASIBs, which not only enhance our fundamental understanding of the Na insertion mechanism, but also facilitate the research and development of practical ASIB systems. Among these electrode materials, iron-based materials are of particular importance because of the high abundance, low price, and low toxicity of Fe elements. However, to our knowledge, there are no review papers that specifically discuss the properties of Fe-based materials for ASIBs yet. In this review, we present the recent research progress on Fe-based cathode/anode materials, which include polyanionic compounds, Prussian blue, oxides, carbides, and selenides. We also discuss the research efforts to build Fe-based ASIB full cells. Lastly, we share our perspectives on the key challenges that need to be addressed and suggest alternative directions for aqueous Na-ion batteries. We hope this review paper can promote more research efforts on the development of low-cost and low-toxicity materials for aqueous battery applications.
1. Introduction
The storage of renewable energy sources (solar and wind) requires the development of a low-cost, long-cycling, and high-safety battery system [1,2]. Currently, lithium-ion batteries (LIBs) demonstrate immense success in portable electronics and electric vehicles, and they have also been actively studied for stationary energy storage [3,4]. However, the intrinsically low lithium abundance (~20 ppm) in Earth’s crust and the uneven Li distribution concurrently contribute to a high battery cost [5,6], making them unaffordable for grid-scale energy storage. Additionally, the LIB electrolyte is based on the use of volatile and flammable carbonate solvents [7], which brings about safety concerns. Therefore, it is indispensable to develop an alternative battery system that can better satisfy the demands of grid-scale energy storage.
Since the 2010s, sodium-ion batteries (SIBs) have attracted considerable attention as an alternative to LIBs for large-scale energy storage, due to Na’s much higher elemental abundance (~23,000 ppm), ubiquitous distribution, and potentially lower cost [8,9,10,11,12]. Although the large Na+ ion radius (1.02 Å vs. 0.76 Å of Li+) caused some difficulties in early-stage SIB exploration [13,14,15,16], extensive investigations from worldwide researchers have successfully identified promising materials for near-future commercialization. Layered metal oxides [17,18,19], Prussian blue [20,21,22], and phosphates [23,24,25] are three leading cathode materials, and hard carbon is the most promising anode candidate [26,27,28,29]. There are several excellent review papers that discuss the prospects and challenges of the commercialization of SIBs [30,31,32,33], and readers can refer to these articles for more information. Despite the essential research progress, non-aqueous SIBs still rely on volatile and flammable carbonate electrolytes, which have similar safety concerns to LIBs [34]. Moreover, it remains questionable that non-aqueous SIBs offer a competitive levelized energy cost compared with lead-acid batteries [10], particularly when we consider the use of NaPF6 salts, carbonate solvents, and dry room assembly conditions for SIBs.
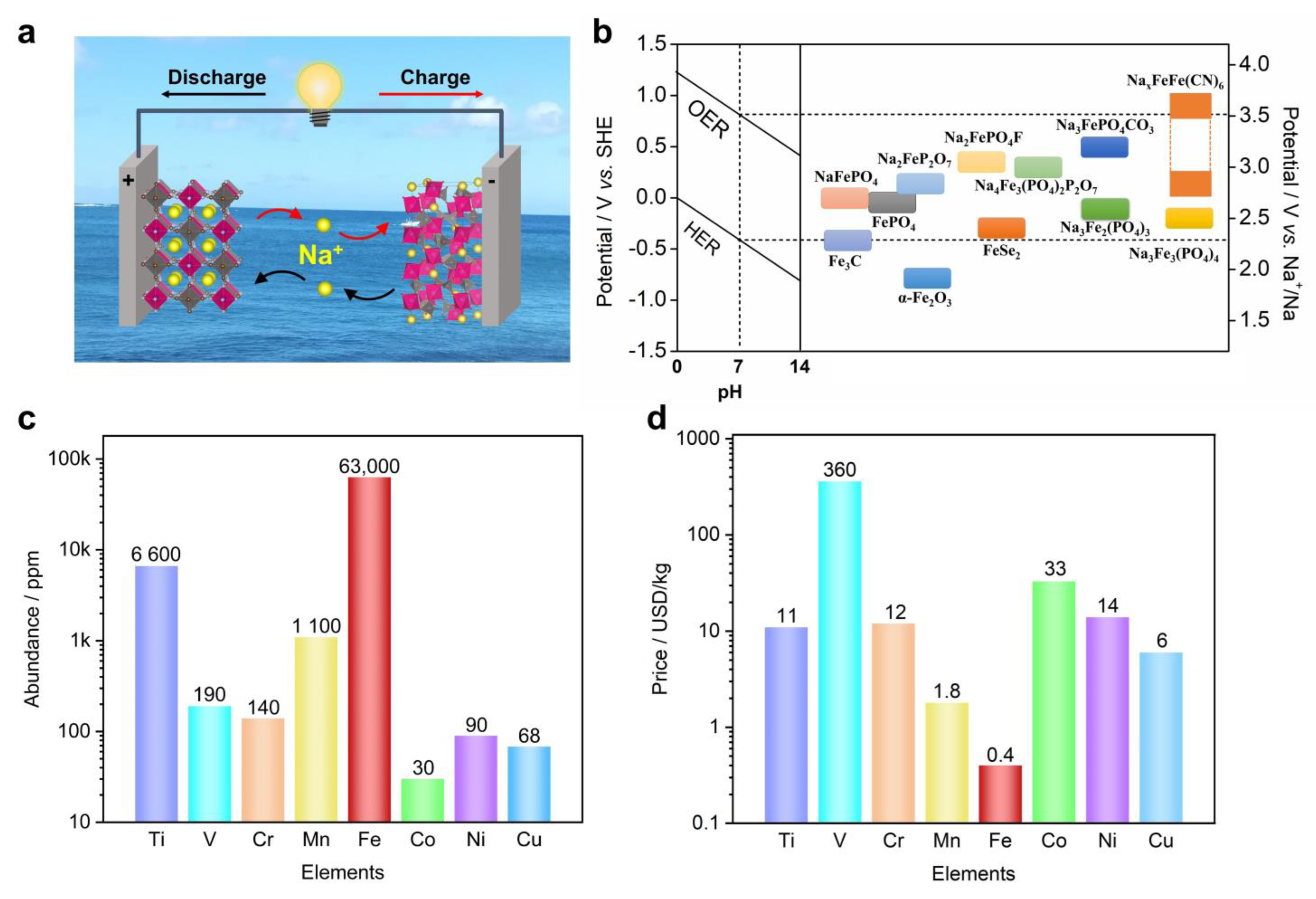
Due to these limitations of SIBs, there is a parallel interest in developing aqueous sodium-ion batteries (ASIBs), because of the cost-effective and non-flammable nature of aqueous electrolytes [35,36]. Furthermore, cheap and common salts could be used to make electrolytes, such as sodium sulfate, sodium nitrate, or even sodium chloride [22,37]. Additionally, ASIBs can be manufactured in ambient conditions, which eliminates the need to build or use dry rooms. Therefore, ASIBs exhibit a lower cost and higher safety than non-aqueous SIBs, which are more attractive for stationary energy storage. Figure 1a shows the “rocking-chair” working mechanism of ASIBs, where two insertion compounds serve as the cathode and anode in an aqueous Na-ion electrolyte. During the charge process, the cathode loses electrons and releases Na+ ions, whereas the anode receives electrons and hosts Na+ ions. The discharge process is the reverse of this.
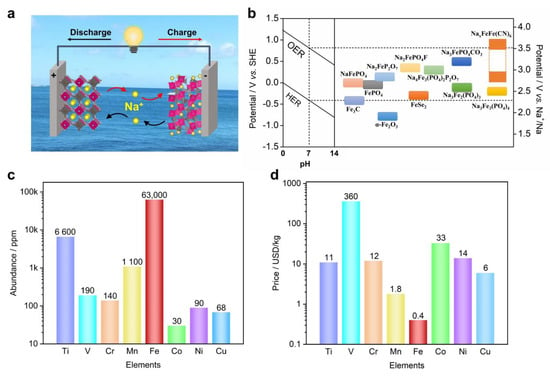
Figure 1.
(a) The “rocking−chair” working mechanism of aqueous sodium−ion batteries, where two insertion electrode materials serve as the cathode and anode, and the electrolyte is an aqueous Na−ion solution; (b) the reaction potentials of Fe−based materials in aqueous electrolytes, with a reference to non−aqueous systems; (c) the elemental abundance of transition metals in Earth’s crust; (d) the elemental price of transition metals. The data were retrieved from the Wikipedia webpage.
Compared with non-aqueous electrolytes, aqueous electrolytes generally have a much narrower electrochemical window, due to the oxidative and reductive decomposition of water molecules, i.e., oxygen evolution reactions (OERs) and hydrogen evolution reactions (HERs). [38] The thermodynamically stable electrochemical window of water is 1.23 V (Figure 1b), where OER and HER take place at a relative potential of 1.23–0.059 × pH and −0.059 × pH vs. standard hydrogen electrodes (SHEs), respectively. [39] For instance, ina neutral state (pH = 7), OERs and HERs tend to happen at +0.817 V and −0.413 V vs. SHEs, which corresponds to 3.53 V and 2.3 V vs. Na+/Na, respectively. Note that the Na+/Na redox couple exhibits a relative potential of −2.713 V vs. SHE. Therefore, researchers could screen suitable electrode materials from a non-aqueous SIB database and apply them to ASIBs. We need to point out that, in practical conditions, aqueous electrolytes support a wider window of 1.5–1.8 V, due to the overpotential contributions from OER and HER [40]. Recently, researchers have worked to increase the salt/solvent ratio and proposed the concept of concentrated or “water-in-salt” (WiS) electrolytes [41,42,43,44], which further enlarges the electrolyte window to 2–3.0 V. For instance, Hu et al. reported an inert-cation-assisted WiS electrolyte, which comprises tetraethylammonium (TEA+) inert cations and Na+ cations [42]. The very high ion concentration of 31 mol kg−1 enabled a broad window of 3.3 V, which supported the functioning of a new anode (NaTiOPO4). Moreover, the Na-ion full cell reached a 1.74 V voltage and high energy of 71 Wh kg−1. Very recently, Wang et al. demonstrated a NaClO4/NaOTF (17 + 2 mol kg−1) bi-salt WiS electrolyte, which can expand the electrochemical window to 4.4 V [45]. This electrolyte can effectively suppress the material dissolution of Na3V2(PO4)3, and the symmetrical Na3V2(PO4)3||Na3V2(PO4)3 full cell demonstrated a voltage of 1.75 V and an energy density of 70 Wh kg−1. These new electrolytes not only enable more electrode materials to work for ASIBs, but also effectively increase the full cell voltage and energy density.
Compared with other aqueous batteries, such as Ca2+, Mg2+, Al3+, K+, and NH4+ ions, [46,47,48,49,50] ASIBs have the advantages of abundant electrode material choices, which are facilitated by the moderate Na+ size and the monovalent cation charge. The bulk size of K+ and NH4+ limits the cation insertion to electrode structures, while the high charge density of Ca2+, Mg2+, and Al3+ restricts the cation diffusion process. Due to these advantages, the commercialization of ASIBs was attempted by Aquion Energy between 2008 and 2017 [51], which further highlights the attractive merits of ASIBs. To date, a variety of electrode materials have been investigated for ASIBs, including metal oxides [52,53], metal phosphates [54,55], metal hexacyanoferrates (Prussian blue analogues) [56,57], and other compounds [58]. In general, these materials possess one or more transition metal elements for redox reactions, such as titanium, vanadium, chromium, manganese, iron, cobalt, nickel, and copper. Among these materials, iron-based ones are of particular importance for ASIBs, because Fe is the most abundant element (50,000 ppm, Figure 1c) and the most cost-effective element (0.4 USD/kg, Figure 1d) [59,60]. Moreover, Fe-based materials are generally non-toxic or low-toxicity, and are thus vastly different from chromium, vanadium, or cobalt-based materials [61]. Additionally, the Fe element exhibits multiple valance states of +2, +3, and +4 [62], and could be utilized for both cathode and anode reactions, depending on the materials or crystal structures. Based on these discussions, it is appealing to demonstrate Fe-based ASIBs for sustainable energy storage. Nevertheless, there are no review papers on this topic yet.
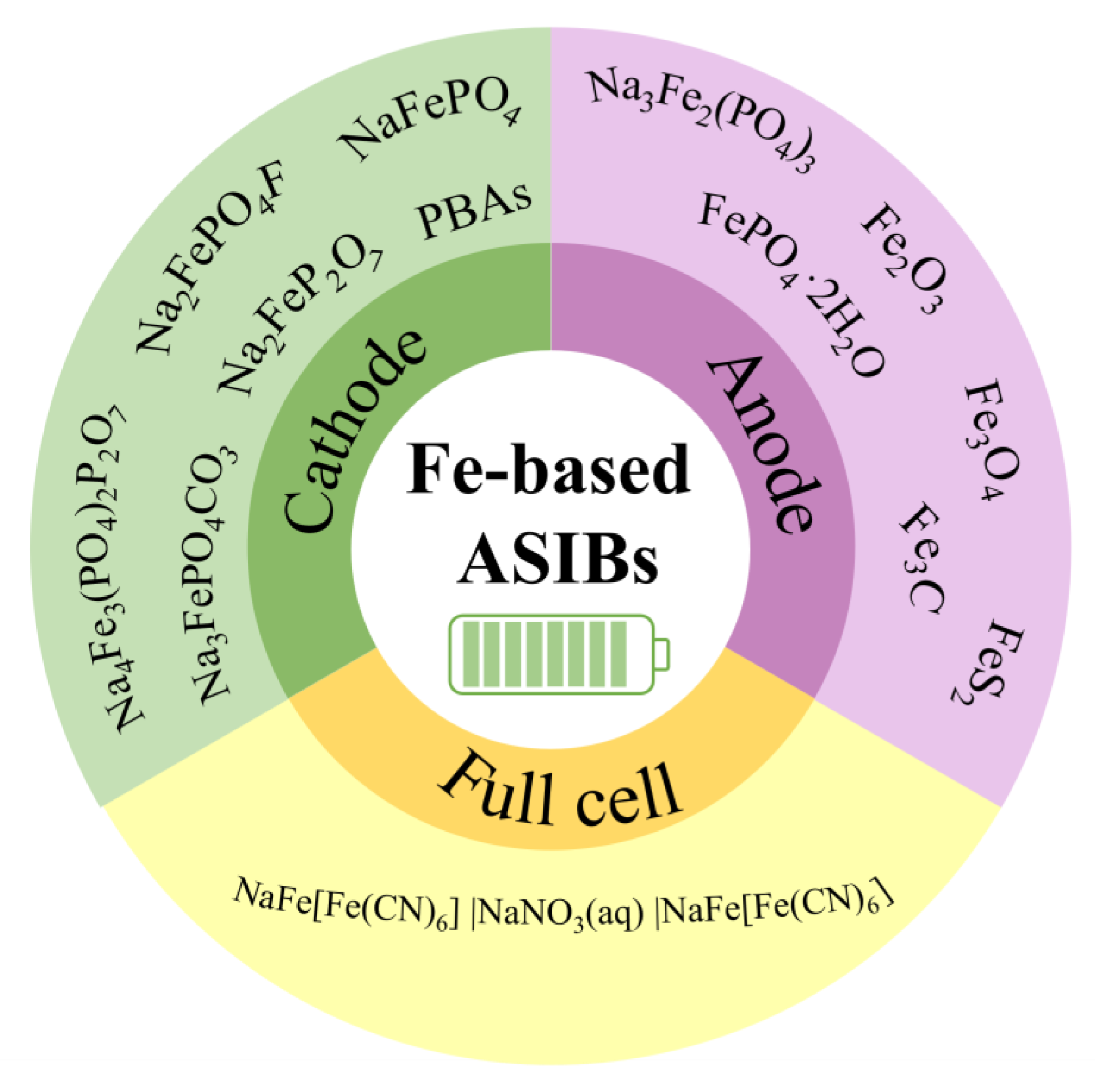
In this review, we summarize the research progress on Fe-based cathode and anode materials for ASIBs(Figure 2). We talk about the synthesis methods, crystal structures, reaction mechanisms, and electrochemical properties of these materials, and we point out some research limitations, as well. The recent efforts to assemble Fe-based aqueous Na-ion full cells are also discussed. Lastly, we share our perspectives on the key challenges in Fe-based materials and suggest some feasible solutions to overcome these challenges. This review paper will evoke more research interest in the use of Fe-based materials for aqueous batteries and sustainable energy storage.
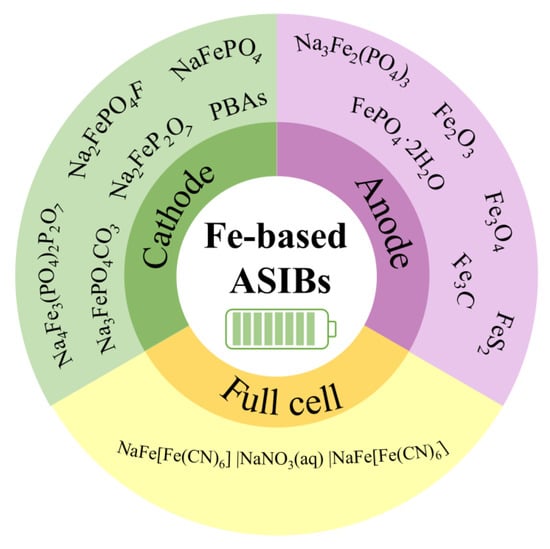
Figure 2.
Fe-based cathode, anode, and full cells for ASIBs.
2. Iron-Based Cathode Materials
Cathode materials generally play a decisive role in full cell energy density, and are expected to exhibit a high reaction potential and a high capacity. To date, Fe-based cathode materials primarily include polyanionic compounds (phosphates, pyrophosphates, and mixed anions) and Prussian blue analogues (PBA).
2.1. Polyanionic Compounds
Recently, polyanionic compounds have received extensive attention for ASIBs, due to their stable structures and tunable reaction potentials. In this paper, we will start with the NaFePO4 material, which is one of the earliest compounds in ASIB research. This material also attracted considerable interest at the beginning of ASIB research. Then, we will discuss other polyanionic compounds that show better performance than the NaFePO4 material.
2.1.1. NaFePO4 Cathode
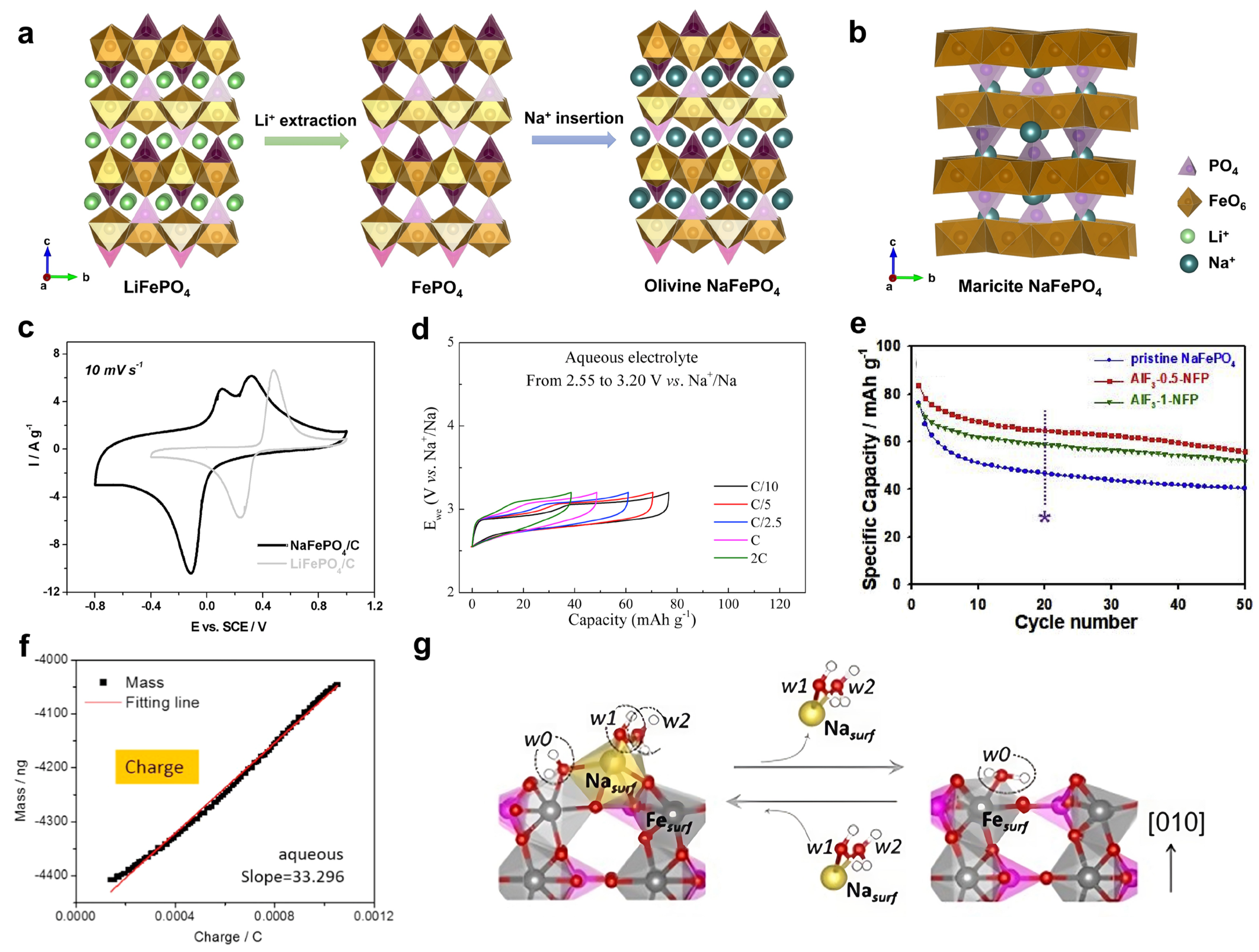
Phosphate compounds are promising electrode materials for battery applications, due to their versatile crystal structures, stable P-O bonds, and relatively high reaction potentials [63]. One of the most representative phosphate examples is the LiFePO4 cathode, which was first developed by John Goodenough in 1997 and is now used as a leading cathode in some electric vehicles [64,65]. The LiFePO4 material exhibits an olivine structure (space group: Pnma) and has a one-dimensional Li+ diffusion channel, which leads to a two-phase transition reaction between LiFePO4 and FePO4 (Figure 3a). As a result, the LiFePO4 features a flat reaction potential of +3.45 V vs. Li+/Li, a high theoretical capacity of ~170 mAh g−1, and superior cycling performance [64,65]. Due to these attractive properties of LiFePO4, its sodium version, NaFePO4, naturally receives immediate attention in early-stage ASIB studies.
However, the Li+/Na+ ion size difference is large in crystal structures, as are the differences in the reaction mechanisms in the AFePO4 framework (A = Li and Na). Unlike olivine LiFePO4, NaFePO4 crystalizes in two distinct crystal structures, i.e., maricite and olivine [66]. The maricite phase is thermodynamically more stable, but it does not exhibit a well-defined Na+ diffusion channel (Figure 3b) [67,68]. Consequently, the maricite phase is electrochemically inactive for Na+ insertion, and research efforts have focused on the olivine NaFePO4 phase. Based on the Fe2+/Fe3+ redox couple, the olivine NaFePO4 cathode exhibits a high theoretical capacity of 154 mAh g−1.
To prepare olivine NaFePO4, researchers generally use olivine LiFePO4 as the starting compound, extract Li+ ions from its structure, and then, re-insert Na+ ions to form an olivine NaFePO4 phase (Figure 3a). In 2013, Mentus et al. used the electrochemical ion-exchange method to prepare a NaFePO4 material [69], where LiFePO4 was subjected to successive cyclic voltammetry (CV) scanning in a saturated NaNO3 electrolyte. Compared with LiFePO4, NaFePO4 exhibits a 0.3 V lower reaction potential (Figure 3c). Moreover, NaFePO4 has one cathodic peak but demonstrates two anodic peaks, which are due to the intermediate phase of Na2/3FePO4 during the charging process. In this study, the authors used CV to explore electrochemical performance, but the galvanostatic charge/discharge (GCD) properties remain unknown.
In 2015, Cabanas et al. used a chemical method to prepare an olivine NaFePO4 material and systematically compared its Na insertion performance in aqueous and non-aqueous electrolytes [70]. They first used nitronium tetrafluoroborate (NO2BF4) to oxidize LiFePO4 into FePO4, and then, they utilized sodium iodide (NaI) to reduce FePO4 into NaFePO4. They found that NaFePO4 demonstrated much faster reaction kinetics and lower polarization in aqueous Na2SO4 electrolytes than non-aqueous NaClO4/EC-PC (EC: ethylene carbonate; PC: propylene carbonate) electrolytes. At a rate of 0.1 C, the polarization was 0.27 V in aqueous electrolytes (Figure 3d), lower than 0.44 V in non-aqueous electrolytes. When tested in the same potential range, aqueous electrolytes led to good capacity utilization of 50% at a rate of 2 C (Figure 3d), whereas non-aqueous ones showed a minimal reaction capacity. These results indicate that NaFePO4 could work as a higher-rate cathode in ASIBs. Then, the authors assembled an ASIB full cell of NaFePO4||NaTi2(PO4)3, which exhibited a cell voltage of ~0.6 V and stable cycling of 20 cycles. This work systematically investigated NaFePO4 battery performance in aqueous electrolytes, but the overall performance appears premature, which warrants further improvement. For instance, the NaFePO4 cathode only delivered a moderate capacity of ~75 mAh g−1 at room temperature, which corresponds to only 50% of the theoretical capacity. When the temperature increased to 55 °C, this cathode managed to give ~110 mAh g−1. Meanwhile, the NaFePO4 cycling performance was not satisfactory. The authors found that NaFePO4 suffered from fast capacity fading in a wider potential range of −0.2~0.6 V vs. SHE, and they excluded the material dissolution reason based on inductively coupled plasma (ICP) analysis. Thus, the capacity decay mechanism requires further investigation.
In 2019, Tron et al. investigated the capacity fading mechanism in NaFePO4 and proposed an artificial aluminum fluoride (AlF3) coating to enhance its cycling life [71]. They found that surface deterioration was primarily responsible for the poor cycling in bare NaFePO4, where the electrode–electrolyte side reactions led to the formation of iron oxides or iron hydroxides. To address this issue, they coated AlF3 on the NaFePO4 surface for electrode protection. Consequently, the coated electrode not only exhibited a higher initial Coulombic efficiency, but also demonstrated better cycling stability (Figure 3e). Unfortunately, even with surface coating, the NaFePO4 cathode was still limited to 50 cycles, which is much inferior to non-aqueous systems. For instance, Loh et al. reported that NaFePO4 showed outstanding capacity retention of 70% after 6000 cycles in non-aqueous electrolytes [72]. Therefore, there is a large performance gap between non-aqueous and aqueous electrolytes, and sophisticated characterization methods are needed to understand the capacity fading mechanism, which will help to demonstrate a long-cycling NaFePO4-based ASIB. For instance, electrochemical quartz crystal microbalance (EQCM) tests might provide alternative insights into the interfacial ion insertion process [73]. Pan et al. and Cakan et al. carried out in situ EQCM tests of the NaFePO4 electrode in aqueous electrolytes, and the reaction mass ratios were found to be 31–33 g mol−1 and 36–39 g mol−1, respectively [73,74]. Although these two studies have some discrepancies, it is evident that both Na+ ions and water molecules participate in surface redox reactions, because the molar mass of naked Na+ ions is 23 g mol−1. The participation of water molecules may explain the formation of FeO or Fe(OH)2 on the NaFePO4 surface, which leads to the capacity fading.
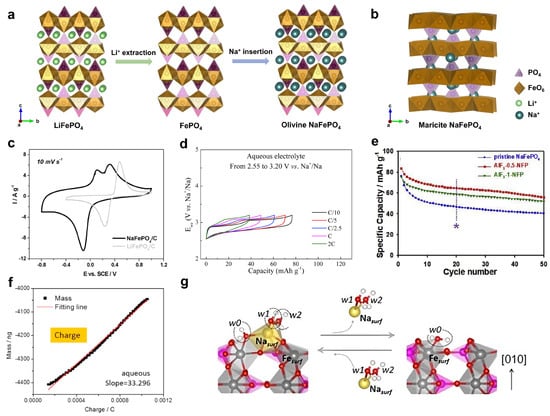
Figure 3.
(a) The structural transition between olivine LiFePO4, olivine FePO4, and olivine NaFePO4. (b) The crystal structure of maricite NaFePO4. (c) A CV curve comparison between the Li insertion in LiFePO4 and Na insertion in NaFePO4. Reprinted from reference [70], with permission from Elsevier. (d) The rate performance of NaFePO4 in aqueous electrolytes. Reprinted from reference [70], with permission from Elsevier. (e) A cycling performance comparison between bare NaFePO4 and AlF3−coated NaFePO4. The * in the Figure 3e indicates the cycling performance comparison at 20th cycle. Reprinted from reference [71], with permission from Elsevier. (f) EQCM analysis of the Na insertion process in NaFePO4. Reprinted from reference [73], with permission from Elsevier. (g) Theoretical simulations on Na insertion in NaFePO4 in aqueous electrolytes. Reprinted from reference [73], with permission from Elsevier.
Figure 3.
(a) The structural transition between olivine LiFePO4, olivine FePO4, and olivine NaFePO4. (b) The crystal structure of maricite NaFePO4. (c) A CV curve comparison between the Li insertion in LiFePO4 and Na insertion in NaFePO4. Reprinted from reference [70], with permission from Elsevier. (d) The rate performance of NaFePO4 in aqueous electrolytes. Reprinted from reference [70], with permission from Elsevier. (e) A cycling performance comparison between bare NaFePO4 and AlF3−coated NaFePO4. The * in the Figure 3e indicates the cycling performance comparison at 20th cycle. Reprinted from reference [71], with permission from Elsevier. (f) EQCM analysis of the Na insertion process in NaFePO4. Reprinted from reference [73], with permission from Elsevier. (g) Theoretical simulations on Na insertion in NaFePO4 in aqueous electrolytes. Reprinted from reference [73], with permission from Elsevier.
Another drawback related to the olivine NaFePO4 cathode is the complicated synthesis route, where LiFePO4 needs to serve as a sacrificial template, and it undergoes a two-step synthetic oxidation–reduction route. To solve this issue, Manjunatha et al. reported a low-temperature ionothermal method to prepare an olivine NaFePO4 material [75], where the reaction medium was a deep eutectic solvent, and the temperature was as low as 200 °C. When paired with a NaTi2(PO4)3 anode in 5 M NaNO3 electrolytes, the NaFePO4 cathode delivered a good capacity of ~97 mAh g−1 at a rate of 0.2 and reasonable capacity retention (78%) over 50 cycles.
2.1.2. Other Polyanionic Compounds
Besides the intensive studies on the NaFePO4 cathode, other Fe-based phosphate materials have also attracted certain attention for ASIBs. However, due to the limited number of publications, we will discuss these materials in one section and categorize them as other polyanionic compounds.
Compared with NaFePO4, sodium iron pyrophosphate (Na2FeP2O7) exhibits a relatively higher Na insertion potential, due to the stronger inductive effect of the [P2O7]4- groups [76,77]. More importantly, Na2FeP2O7 could be readily synthesized via a conventional solid-state method [76,77], which does not need to use lithium compounds as the starting precursor. This is beneficial for large-scale synthesis. Na2FeP2O7 has a triclinic crystal structure (P-1, No.2) and exhibits a theoretical capacity of ~97 mAh g−1 based on one-electron Fe2+/Fe3+ redox, which is lower than NaFePO4 but still acceptable as a Na-ion cathode.
Kim et al. used a simple solid-state method and prepared a carbon-coated Na2FeP2O7 material [78]. They found that aqueous electrolytes led to lower polarization and faster rate performance than non-aqueous electrolytes, which is akin to the NaFePO4 case. The Na2FeP2O7 electrode delivered a good capacity of ~87 mAh g−1 in a wide potential range of −0.26~0.94 V vs. SHE, with an average potential of ~0.25 V vs. SHE (Figure 4a). However, the capacity decreased to ~65 mAh g−1 in a narrow range of 0.04~0.94 V. This cathode showed an impressive rate performance of 50 C and excellent capacity retention of 86% after 300 cycles. Apparently, the Na2FeP2O7 cathode exhibited better cycling stability than NaFePO4. The authors also used advanced characterization tools to investigate the reaction mechanism. Fe-edge X-ray absorption near edge structure (XANES) analysis revealed that Fe element valance changes from +2 to +3 during the charge process, which indicates the oxidization of Na2FeP2O7. They also performed ex situ XRD analysis of the cycled electrode, which showed no peak change or intensity degradation, confirming the reaction reversibility. Later, Okada et al. further investigated electrolytes’ influence on Na2FeP2O7 performance, [79] where three different electrolytes were compared, i.e., Na2SO4 (2.0 M), NaNO3 (4.0 M), and NaClO4 (4.0 M). They found that these electrolytes led to similar cycling for Na2FeP2O7, but NaNO3 showed the worst cycling for the Na2FeP2O7||NaTi2(PO4)3 full cell, due to the nitrate decomposition and electrode corrosion reactions. Considering the strong oxidizing capabilities and potential explosiveness of sodium perchlorate, the authors concluded that 2.0 M Na2SO4 is the most promising electrolyte for aqueous Na2FeP2O7||NaTi2(PO4)3 full cells, which maintain ~89% capacity over 30 cycles.
Despite the easy synthesis of Na2FeP2O7, its moderate potential and relatively low capacity restrict the energy density of ASIB full cells. Hence, it is crucial to develop other Fe-based materials with higher potentials or capacities. Na4Fe3(PO4)2P2O7 is an interesting mixed polyanionic material, [80] which adopts an orthorhombic structure (space group: Pn21a) with large open channels. This material exhibits an even higher potential than NaFePO4 and Na2FeP2O7. The Fe ions exist in their 2+ state, and theoretically, all these Fe2+ ions could be oxidized to Fe3+. Consequently, this material can support the 3-Na insertion reaction, which corresponds to a theoretical capacity of ~129 mAh g−1. Compared with Na2FeP2O7, Na4Fe3(PO4)2P2O7 shows both a higher capacity and a higher reaction potential. Cabanas et al. studied Na4Fe3(PO4)2P2O7 performance in 1 M Na2SO4 electrolytes, [81] which delivered a specific capacity of ~84 mAh g−1 and an average potential of ~0.30 V vs. SHE (Figure 4b). However, the cycling stability of Na4Fe3(PO4)2P2O7 is less satisfactory, with 74% capacity retention in 50 cycles. To understand the capacity fading mechanism, the authors of [81] immersed Na4Fe3(PO4)2P2O7 in electrolytes and used ICP to detect the dissolved iron and phosphorus concentration, which were found to be 0.1% and 2.1%, respectively. Due to the much higher phosphorus content, it is likely that pyrophosphate anions undergo hydrolysis side reactions, which result in active mass loss and iron oxide precipitation. Therefore, surface coating could be necessary to reinforce cycling stability.
Na2FePO4F represents another promising ASIB cathode with a high reaction potential, due to the presence of electron-withdrawing fluoride anions [82]. Meanwhile, this cathode exhibits a theoretical capacity of 124 mAh g−1, comparable to Na4Fe3(PO4)2P2O7. This material crystalizes in an orthorhombic crystal structure with a space group of Pbcn. Barpanda et al. first studied Na2FePO4F performance in a concentrated electrolyte of 17 m NaClO4 (m: mol kg−1) [83], which may help to inhibit the material dissolution. As a result, Na2FePO4F delivers a capacity of ~84 mAh g−1 (Figure 4c), a high-rate capability of 5.0 mA cm−2, and stable cycling of 100 cycles. When coupled with a NaTi2(PO4)3 anode, the full cell shows a moderate cell voltage of ~0.7 V and decent cycling retention of 65% over 100 cycles. Relatively inferior cycling in full cells should result from the capacity fading on the anode side.
Besides [P2O7]4− and F− anions, carbonate anions have also been introduced to the Na-Fe-PO system to make new compounds. For instance, Na3FePO4CO3 is another promising cathode with a theoretical capacity of ~191 mAh g−1, due to its potential two-Na insertion via Fe3+/Fe2+ and Fe3+/Fe4+ redox couples. [84] This capacity even exceeds the NaFePO4 material. In 2020, Okada et al. briefly reported its Na insertion performance in a conference abstract, [79] which described an initial charge/discharge capacity of ~130/112 mAh g−1 in 17 m NaClO4 electrolytes. However, other information, such as electrochemical or structural characterization, is not available. In 2021, Manjunatha et al. systematically investigated Na3FePO4CO3’s battery performance in a 2.0 M Na2SO4 electrolyte [85]. In a typical CV test, the Na3FePO4CO3 electrode demonstrated a pair of oxidization/reduction peaks at 0.54/0.32 V vs. SCE (SCE: saturated calomel electrode), which converted to 0.78/0.56 V vs. SHE. On average, the Na insertion potential was 0.67 V vs. SHE, which is much higher than that of previous Fe-based materials. However, the potential gap of 0.22 V was not negligible, which indicates sluggish Na insertion kinetics. As a result, the GCD tests revealed that this cathode delivers a moderate capacity of ~80 mAh g−1 and considerable polarization of 0.5 V (Figure 4d), which leads to very low energy efficiency. When paired with the common NaTi2(PO4)3 anode, the full cell exhibited a reasonable rate capability of 2C and stable cycling of 100 cycles. Nevertheless, GCD curves were not shown for full cells, and the voltage hysteresis, energy density, and power density remain unknown.
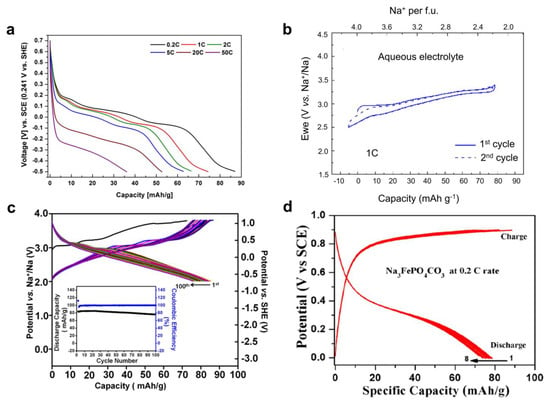
Figure 4.
Electrochemical performance of other Fe−based polyanionic compounds. (a) The discharge curves of the Na2FeP2O7 cathode. Reprinted from reference [78], with permission from authors. (b) GCD curves of the Na4Fe3(PO4)2P2O7 cathode. Reprinted with permission from reference [81]. Copyright 2018 American Chemical Society. (c) GCD curves of the Na2FePO4F cathode, where the inset is the cycling performance. Reprinted with permission from reference [84]. Copyright 2018 WILEY−VCH Verlag GmbH & Co. KGaA. (d) GCD curves of the Na3FePO4CO3 cathode. Reprinted from reference [85], with permission from IOP Publishing.
Figure 4.
Electrochemical performance of other Fe−based polyanionic compounds. (a) The discharge curves of the Na2FeP2O7 cathode. Reprinted from reference [78], with permission from authors. (b) GCD curves of the Na4Fe3(PO4)2P2O7 cathode. Reprinted with permission from reference [81]. Copyright 2018 American Chemical Society. (c) GCD curves of the Na2FePO4F cathode, where the inset is the cycling performance. Reprinted with permission from reference [84]. Copyright 2018 WILEY−VCH Verlag GmbH & Co. KGaA. (d) GCD curves of the Na3FePO4CO3 cathode. Reprinted from reference [85], with permission from IOP Publishing.
2.2. Prussian Blue Analogues
In addition to polyanionic compounds, Prussian blue analogues (PBAs) represent another class of Fe-based materials for ASIBs. PBAs exhibit a general chemical formula of AxM[Fe(CN)6]y·□1−y·zH2O (0 ≤ x ≤ 2, 0 ≤ y ≤1; z varies with the experimental conditions), where A, M, and □ stand for alkali metals, transition metals, and Fe(CN)6 vacancies, respectively [86]. PBAs usually possess a face-centered cubic structure, which is built up via the three-dimensional connection of Fe-CN-M bonds. The alkali metal cations and zeolitic water molecules occupy the center of nano-voids. Compared with iron-based polyanionic materials, PBAs hold greater promise for ASIBs. Firstly, PBAs theoretically undergo a 2-Na insertion reaction, which leads to a high theoretical capacity of ~170 mAh g−1 [87]. Secondly, the [Fe(CN)6]3−/[Fe(CN)6]4− redox couple exhibits a high reaction potential of +0.4~1.0 V vs. SHE, which is promising for cathode reactions [88]. Thirdly, the large open framework facilitates fast and reversible Na insertion reactions, which results in excellent rate and cycling performance [89]. Lastly, the synthesis of PBAs is based on an aqueous precipitation method [90], which is more cost-effective than the solid-state synthesis of iron phosphates. To date, there are several excellent review papers on PBA materials for non-aqueous and aqueous SIBs [20,91,92,93], and readers may refer to these publications for more information. Here, we limit our discussion to NaxFe[Fe(CN)6]y·□1−y·zH2O materials only, considering that the focus is on the use of Fe-based materials for ASIBs.
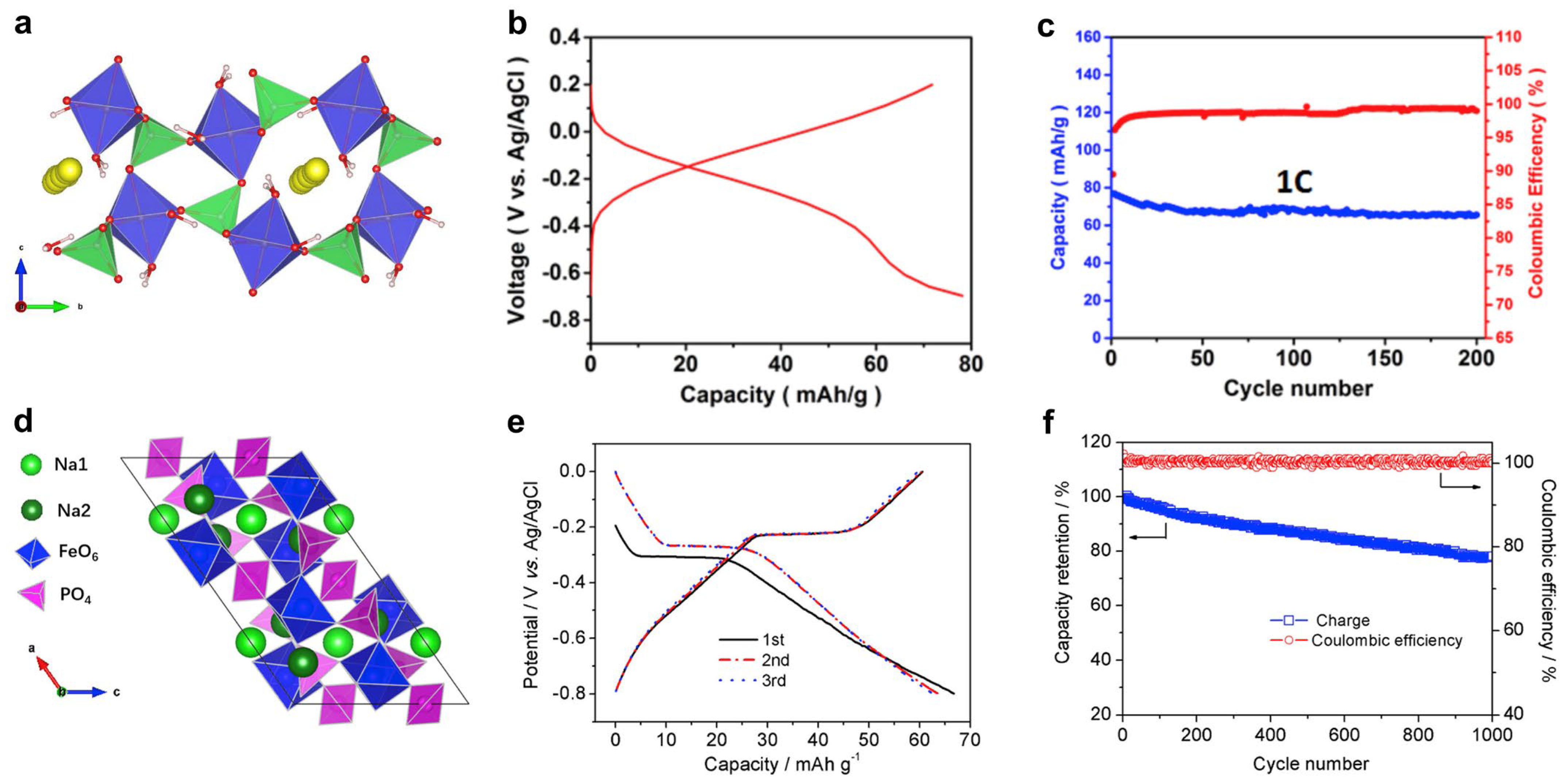
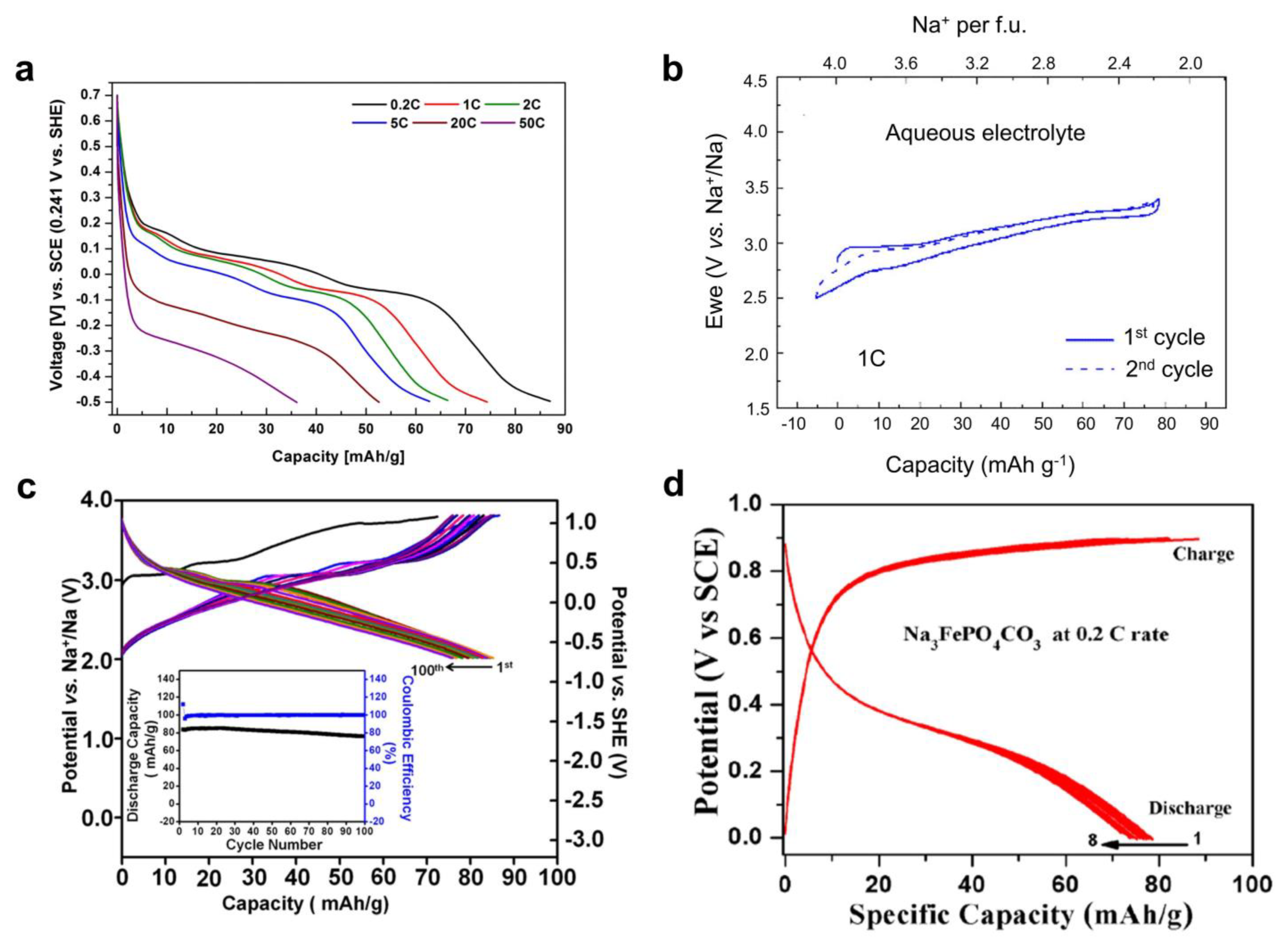
Based on the valence state of carbon-coordinated and nitrogen-coordinated iron ions, the NaxFe[Fe(CN)6]y·□1-y·zH2O material can be further divided into Prussian yellow (PY, +3 and +3), Prussian blue (PB, +2 and +3), and Prussian white (PW, +2 and +2) [88], as shown in Figure 5a. Note that in the PB structure, the carbon-coordinated and nitrogen-coordinated iron are in a +2 and +3 state, respectively. It is known that Fe(CN)6 vacancies degrade PBA crystal structures and lead to low capacities and capacity fading [89,90,94,95,96]. To suppress these vacancies, Yang et al. used a slow crystallization method and prepared a low-defect PY compound of Fe[Fe(CN)6]0.87·□0.13 [21], that contained only 13% Fe(CN)6 vacancies, much lower than the 25–30% in conventional PBAs. When tested for ASIBs, this PY cathode delivered a high capacity of ~125 mAh g−1 (Figure 5b) and stable cycling of 500 cycles with 83% retention. It also showed an encouraging rate performance of 20 C, which surpasses most iron phosphate materials. By contrast, the conventional PBA materials with 30% Fe(CN)6 vacancies suffered from severe capacity fading (Figure 5c). Li et al. further compared Li insertion and Na insertion in PY structures [97]. Interestingly, PY supported a reversible Na insertion reaction with a good capacity of ~120 mAh g−1, but it exhibited fast capacity fading for Li-ion insertion. The inferior Li-ion cycling was due to the very large size of hydrated Li+ ions, which cannot easily enter PBA channels. By contrast, Na+ ions can become de-solvated and readily enter PBA structures. This comparison further highlights the promise of PBAs for ASIB applications.
Regardless of the high capacity and stable cycling of PY materials, they do not have removable Na+ ions in their initial structures, which challenges full cell assembly. Yang et al. attempted to use sodium iodide (NaI) to reduce the PY compound, but the amount of introduced Na+ ions was quite limited [21]. Therefore, it is more favorable to directly prepare Na-rich PBA materials. In 2016, Cabanas et al. prepared a PB material of Na0.75Fe1.08[Fe(CN)6]·3.5H2O and studied its performance in 1 M Na2SO4 electrolytes [98]. When tested in a controlled voltage range, this cathode delivered a moderate capacity of ~61 mAh g−1 (Figure 5d) and stable cycling of 200 cycles with 84% retention. To further enhance the cycling performance, Huang et al. prepared a similar PB material of Na0.65Fe[Fe(CN)6]0.91·□0.09·2.7H2O and developed a graphene oxide (GO) suspension-based electrolyte [99]. GO’s introduction to 1 m NaClO4 electrolyte formed an ion-selective membrane on the separator, which helped to suppress the Fe3+ dissolution and crossover to the anode. Therefore, this new electrolyte led to superior long cycling of 17,000 cycles with 65.1% capacity retention, which would be the longest cycling among all the Fe-based ASIB materials. However, we need to note that the moderate Na+ concentration (0.65) in the structure will inevitably lead to a low initial charge capacity, which still complicates the full cell assembly.
In comparison with PY and PB, the PW material Na2FeFe(CN)6 is the most promising choice for full cell applications. However, this material is prone to oxidization because both Fe ions exist in the +2 state, so it requires delicate material synthesis and protection. Wu et al. used Na4Fe(CN)6 as the single iron source and added sodium chloride, hydrogen chloride, and poly-(vinylpyrrolidone) to synthesize the PW material [100]. Well-defined PW cubes (~2 μm) were obtained, and the chemical formula was found to be Na1.29Fe[Fe(CN)6]0.91·□0.09, which exhibited higher Na content than previous PB materials. This cathode showed a good capacity of ~107 mAh g−1 and minimal capacity fading after 1100 cycles. When paired with an activated carbon for a hybrid capacitor, the device exhibited an average voltage of ~0.8 V and energy density of ~30 Wh kg−1. Later, Zhang et al. used a citrate-assisted co-precipitation method to prepare PW compounds [101], where ascorbic acid was added to prevent Fe2+ oxidation. As a result, they achieved an even higher Na content and obtained a chemical formula of Na1.74Fe[Fe(CN)6]0.94·□0.06·3.3H2O. This PW material exhibited a high capacity of ~120 mAh g−1 (Figure 4e) and a high rate capability of 3000 mA g−1. However, the cycling stability was not satisfactory, with 32% capacity retention over 1000 cycles. The authors thus doped 24% nickel ions into the PW structure, which stabilized the crystal structure and led to 73% capacity retention over 1000 cycles (Figure 5f).
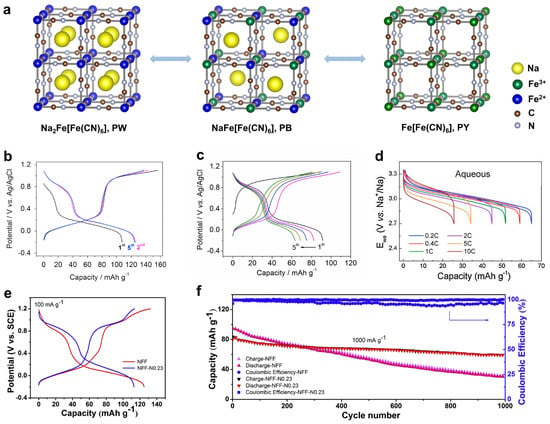
Figure 5.
Structural and electrochemical properties of PBA materials. (a) The structural transition between FeFe(CN)6, NaFeFe(CN)6, and Na2FeFe(CN)6 materials. (b) GCD curves of the low−vacancy FeFe(CN)6 material. Reprinted from reference [21], with permission from Elsevier. (c) GCD curves of the conventional NaFeFe(CN)6 material with high vacancies. Reprinted from reference [21], with permission from Elsevier. (d) GCD curves of the NaFeFe(CN)6 cathode. Reprinted from reference [98], with permission from Elsevier. (e) GCD curves of the Na2FeFe(CN)6 and nickel−doped Na2FeFe(CN)6 materials. Reprinted from reference [101], with permission from Elsevier. (f) A cycling performance comparison between pure Na2FeFe(CN)6 and nickel−doped Na2FeFe(CN)6 cathode. Reprinted from reference [101], with permission from Elsevier.
Figure 5.
Structural and electrochemical properties of PBA materials. (a) The structural transition between FeFe(CN)6, NaFeFe(CN)6, and Na2FeFe(CN)6 materials. (b) GCD curves of the low−vacancy FeFe(CN)6 material. Reprinted from reference [21], with permission from Elsevier. (c) GCD curves of the conventional NaFeFe(CN)6 material with high vacancies. Reprinted from reference [21], with permission from Elsevier. (d) GCD curves of the NaFeFe(CN)6 cathode. Reprinted from reference [98], with permission from Elsevier. (e) GCD curves of the Na2FeFe(CN)6 and nickel−doped Na2FeFe(CN)6 materials. Reprinted from reference [101], with permission from Elsevier. (f) A cycling performance comparison between pure Na2FeFe(CN)6 and nickel−doped Na2FeFe(CN)6 cathode. Reprinted from reference [101], with permission from Elsevier.
3. Iron-Based Anode Materials
To date, most ASIBs have utilized NaTi2(PO4)3 as the prominent anode material [102], due to its good capacity of 100–120 mAh g−1 and low reaction potential of −0.6 V vs. SHE. However, titanium-based materials are generally expensive, which increases the overall cost of ASIBs. Moreover, the Ti4+/Ti3+ redox potential is low enough to trigger noticeable HER side-reactions (−0.4 V vs. SHE) in conventional aqueous electrolytes [102]. In this regard, Fe-based anode materials represent an attractive direction, because of their much lower cost and slightly higher redox potentials. Currently, Fe-based anode materials include iron phosphates, oxides, carbides, and selenides.
3.1. Phosphate Materials
Iron phosphate materials are generally used as cathode materials in non-aqueous SIBs, and their average insertion potentials range from 2.3 to 3.0 V vs. Na+/Na [103]. If converted to aqueous electrolytes, these potentials are −0.4~0.3 V vs. SHE, suggesting that some phosphates can serve as anode candidates for aqueous SIBs.
In 2018, Zaghib et al. reported an amorphous iron phosphate hydrate (FePO4·2H2O) as a low-cost and cycle-stable anode for ASIBs [104]. This material is commercially available with a low price of ~300 USD/ton, which is much lower than NaTi2(PO4)3 (~10,000 USD/ton). Based on Fe3+/Fe2+ redox, this material can host 1 Na+ per formula and transforms to NaFePO4, corresponding to a high theoretical capacity of ~143 mAh g−1. Figure 6a shows the proposed Na+ diffusion pathway in its structure. When tested in 1 M Na2SO4, this anode delivered a moderate capacity of ~70 mAh g−1 (Figure 6b), which represents only 50% capacity utilization. The average insertion potential was ~0 V vs. SHE, which is much higher than NaTi2(PO4)3, and thus, effectively avoids HER reactions. However, the high potential in the anode led to low voltage in the full cell system. When paired with a Na0.44MnO2 cathode, the full cell only showed a low voltage of ~0.5 V, which is not suitable for practical use. Moreover, the half-cell and full-cell cycling performances were limited to 200 (Figure 6c) and 300 cycles, respectively.
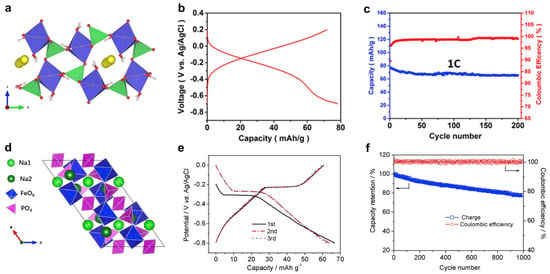
Figure 6.
Structural and electrochemical properties of iron phosphate anode materials. (a) The crystal structure and Na–diffusion manner in the hydrated FePO4·2H2O material. The yellow circles represent the Na+ ions. The green tetrahedron is the [PO4] group, while the blue octahedron is the [FeO6] group. (b) GCD curves of the FePO4·2H2O anode. (c) The cycling performance of the FePO4·2H2O anode. (a–c) were reprinted from reference [103], with permission from Elsevier. (d) Crystal structures of the NASICON Na3Fe2(PO4)3. (e) GCD curves of the Na3Fe2(PO4)3 anode. (f) Cycling performance of the Na3Fe2(PO4)3 anode. (d–f) were reprinted from reference [104,105], with permission from Elsevier.
To further improve anode performance, Feng et al. investigated other iron phosphate materials with different stoichiometries and crystal structures [105,106]. In 2019, they presented a NASICON-type Na3Fe2(PO4)3 (Figure 6d) as a low-cost, high-rate, and long-cycling anode material in 17 m NaClO4 electrolytes. [105] By utilizing the Fe3+/Fe2+ redox couple, this anode could host 1 Na+ and exhibited a reasonable capacity of ~60 mAh g−1 (Figure 6e). Its reaction potential of ~0 V vs. SHE is close to the previously reported value for FePO4·2H2O, but it exhibited a much better rate capability of 100 C and excellent cycling of 1000 cycles (Figure 6f), which likely resulted from the stable NASICON crystal structures and well-defined Na insertion channels. Ex situ XRD analysis revealed the formation of a Na4Fe2(PO4)3 phase at the end of discharge, which accounts for the reaction plateau at ~0 V. Additionally, X-ray photoelectron spectroscopy (XPS) showed that the Na/Fe ratio increased from 1.55 to 1.94 when the electrode was fully discharged, which further confirms the formation of Na4Fe2(PO4)3.
In 2021, Feng et al. demonstrated another layer-structured Na3Fe3(PO4)4 material as a high-performance anode [106]. This material accommodates two Na+ ions, and thus, exhibited a higher capacity of ~80 mAh g−1, which surpasses FePO4·2H2O and Na3Fe2(PO4)3. Additionally, the reaction potential was found to be −0.2 V vs. SHE, lower than NaTi2(PO4)3 (−0.6 V) but higher than HER (−0.4 V), which enabled the maintenance of a good balance between full cell voltages and water decomposition reactions. Furthermore, its desirable layered structure with roomy spaces facilitated a fast and reversible Na insertion process, which translated to a predominantly high rate of 200 C and extremely long cycling of 6000 cycles with 72% retention. Such electrode performance has set a record in Fe-based anode materials. The authors used in operando synchrotron XRD and Fe K-edge XANES spectra to study the reaction mechanism. During the discharge, there was no extra XRD peak, and the (200), (110), and (022) peaks progressively shifted to lower positions. This means that the Na3Fe2(PO4)3 anode works on a solid-solution Na insertion reaction, where the lattice structure expands when the Na+ insertion takes place. The binding energy of the Fe element also moved to a lower energy value, which indicates an Fe3+/Fe2+ redox reaction. On average, the Fe valance state lowered from +3 to +2.3, which corresponds to a two-Na insertion reaction. Therefore, the Na insertion reaction is a reversible transition between the Na3Fe3(PO4)4 and Na5Fe3(PO4)4 materials.
3.2. Oxides, Carbides, and Selenides
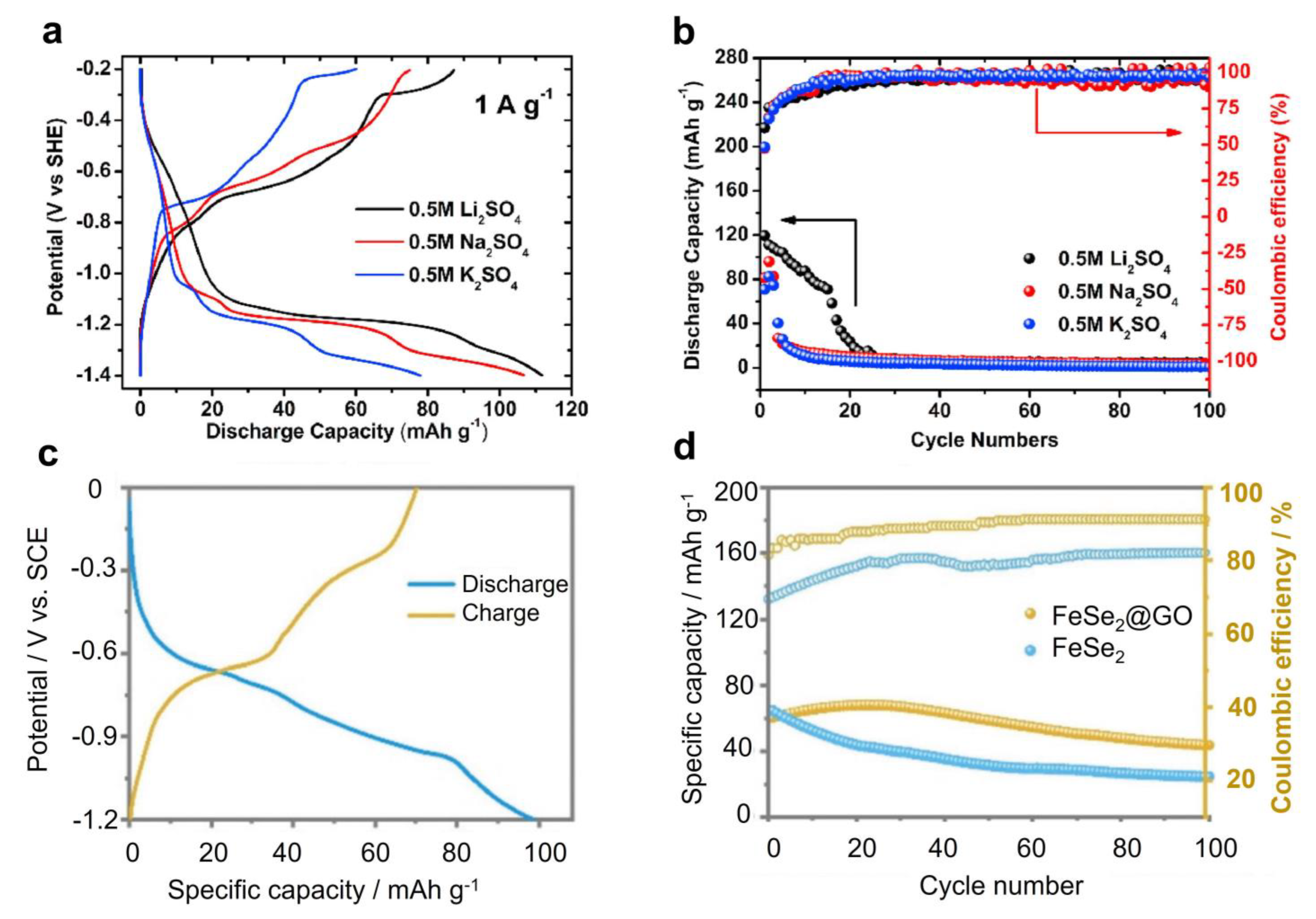
Iron oxides (Fe2O3 or Fe3O4) are highly abundant and cheap materials, and also receive some attention for ASIB anode applications. Lokhande et al. deposited Fe2O3 thin film on stainless steel and evaluated its performance in 1 M Na2SO4, which showed a capacity of 78.6 mAh g−1 at 5 mV s−1 in a voltage range of 1.0 V [107]. This capacity is reasonable, but thin films have low mass loading and are not suitable for practical applications. Nwanya et al. synthesized nano-sized α-Fe2O3 spheres and tested their performance in 0.5 M Na2SO4 [108]. When scanned in a wide electrochemical window of −0.8 to 1.0 V vs. Ag/AgCl, this material delivered a very low capacity of 65 C g−1, which corresponds to ~18 mAh g−1 only. The low capacity could have resulted from the capacitive reaction mechanism and higher mass loading. To pursue a higher capacity, Cheng et al. lowered the discharge cut-off potential to −1.4 vs. Ag/AgCl, which forced Fe2O3 to partially undergo conversion reactions [109]. As a result, it showed an enhanced capacity of ~80 mAh g−1 in 0.5 M Na2SO4 (Figure 7a). However, due to Fe ion dissolution, the capacity quickly faded to 0 mAh g−1 within 10 cycles (Figure 7b). Based on these results, it appears that Fe2O3 cannot maintain a high capacity and long cycling at the same time. Hence, researchers studied Fe3O4 as an alternative material. Ma et al. prepared an Fe3O4@rGO composite (rGO: reduced graphene oxide) and examined its Na insertion properties in 0.5 M Na2SO4 [110]. At 1 mA cm−2, this anode exhibited a moderate capacity of ~64 mAh g−1 in a 1.0 V potential range. At 8 mA cm−2, it also retained ~90% capacity over 1000 cycles, indicating a reversible Na+ (de)absorption process.
Iron carbides (Fe3C) are interesting materials due to their good chemical stability and thermal stability [111]. However, pure Fe3C nanoparticles exhibit low electronic conductivity, which constrains their electrochemical performance. Wang et al. prepared porous Fe3C@rGO composites and tested their performance in 6 M KOH [112], where they demonstrated a capacity of ~95.3 mAh g−1. They also assembled a full cell based on this anode and a Na0.5MnO2 cathode in 1 M Na2SO4, which supported a high charging voltage of ~2.4 V. However, the pure Na-storage performance of this anode was not shown. Moreover, the reaction mechanism remains elusive.
Iron selenides (FeSe2) are another type of material that exhibits a high capacity in non-aqueous SIBs. However, they suffer from the material dissolution issue in aqueous electrolytes, due to the solubility og Se-based species [113]. To overcome this shortcoming, Xing et al. used GO to encapsulate FeSe2 to form a composite electrode, which exhibited a moderate capacity of ~60 mAh g−1 and an average reaction potential of −0.35 V vs. SHE (Figure 6c) [113]. Compared with the pristine FeSe2 electrode, the rGO coated one exhibited improved cycling performance over 100 cycles (Figure 7d). Ex situ XRD results revealed that the reaction mechanism is based on the conversion between FeSe2 and NaxFe2Se4/Na2Se. The authors further assembled an FeSe2@rGO-Na3V2(PO4)2F3 full battery, which showed a high voltage (~1.7 V), good energy density (53.4 W h kg−1), and excellent rate performance. However, the cycling performance was limited to 50 cycles only.
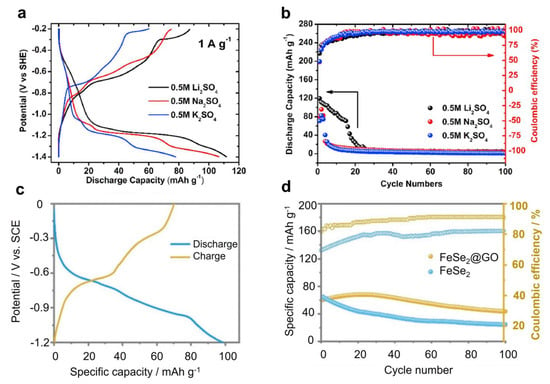
Figure 7.
Electrochemical performance of Fe2O3 and FeSe2. (a) GCD curves of the Fe2O3 anode for different cation storage. (b) The cycling performance of the Fe2O3 anode. Figure 6a,b were reprinted from reference [109], with permission from Elsevier. (c) GCD curves of the FeSe2 anode. (d) A cycling performance comparison between pure FeSe2 and FeSe2@GO. Figure 6c,d were reprinted from ref− erence [113], with permission from the Royal Society of Chemistry.
Figure 7.
Electrochemical performance of Fe2O3 and FeSe2. (a) GCD curves of the Fe2O3 anode for different cation storage. (b) The cycling performance of the Fe2O3 anode. Figure 6a,b were reprinted from reference [109], with permission from Elsevier. (c) GCD curves of the FeSe2 anode. (d) A cycling performance comparison between pure FeSe2 and FeSe2@GO. Figure 6c,d were reprinted from ref− erence [113], with permission from the Royal Society of Chemistry.
4. Iron-Based ASIB Full Cells
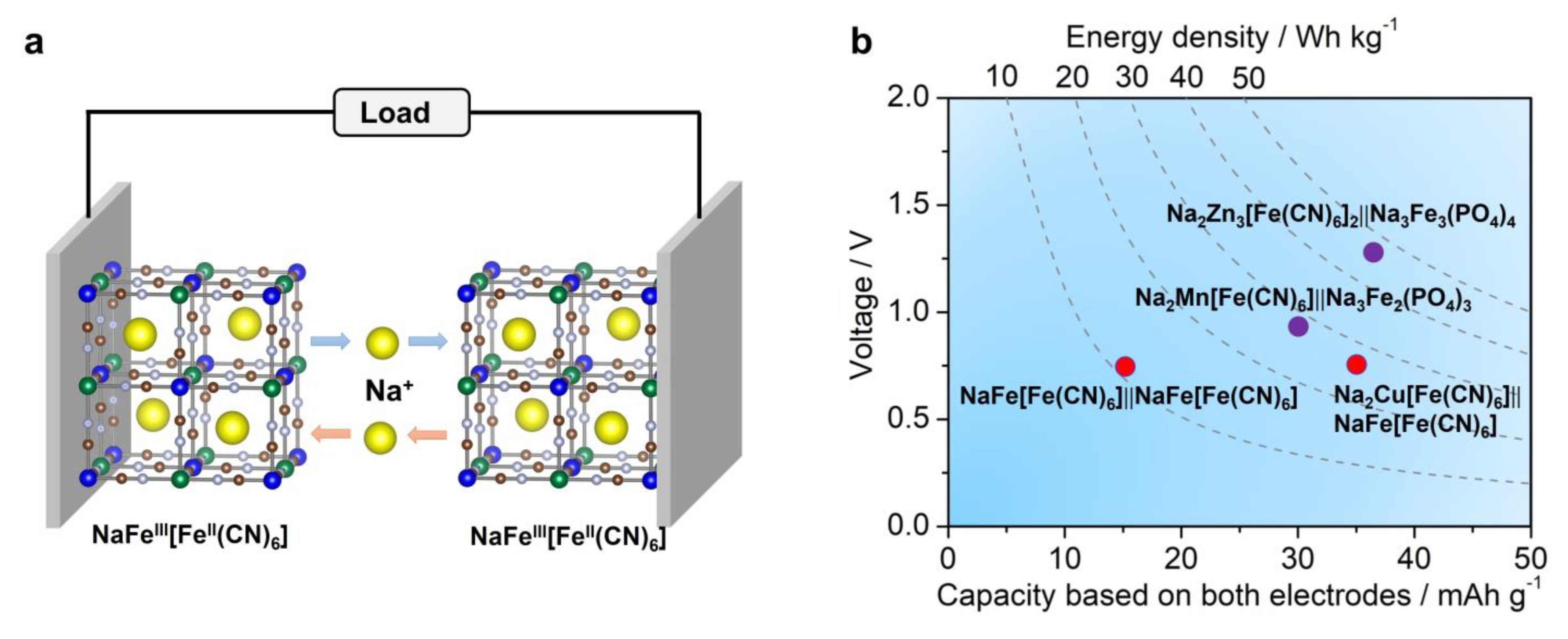
Although many iron-based cathode and anode materials have been developed for half-cell studies, ASIB full cells, assembled from all-Fe-based materials, are under-explored. To our knowledge, there is only one full cell system that solely utilizes an Fe-based cathode and anode. In 2017, Yang et al. prepared an Fe[Fe(CN)6] (PY) material and used it in bipolar electrodes in ASIB full cells [114]. As discussed in the PBA section, two pairs of redox center exist in this material, which are nitrogen-coordinated and carbon-coordinated Fe3+/Fe2+ couples, respectively. Their reaction potentials differ by ~0.70 V, which is reasonable for full cell operation. The authors pre-activated the PY electrode in a 1 M NaNO3 electrolyte to prepare a Na-containing NaFeFe(CN)6 material, which was used for full cell assembly (Figure 8a). The full cell reaction mechanism is expressed as follows:
NaFeIII[FeII(CN)6] + NaFeIII[FeII(CN)6] ↔ FeIII[FeIII(CN)6] + Na2FeII[FeII(CN)6]
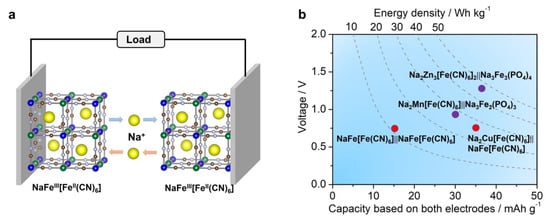
Figure 8.
(a) A scheme of the all−PBA full cell system, where the cathode and anode are composed of the same NaFe[Fe(CN)6] material; (b) a capacity, voltage, and energy density comparison of different Fe−based ASIB full cells. The above figures were plotted by the authors.
This full cell delivered an average voltage of ~0.7 V, a high rate performance of 20 C, and stable cycling for 200 cycles. However, we need to point out that the average capacity was only ~14 mAh g−1 based on the total mass of the cathode and anode, which gave rise to low energy density of ~10 Wh kg−1. This energy is too low to be practical.
Aside from Yang’s work, there are several studies that incorporate other transition metals to increase the cell voltage and energy density of full cells, where the majority of the capacity still comes from Fe3+/Fe2+ redox. For discussion purposes, we also include these works here. In 2019, Wang et al. used two PBA electrodes to fabricate an all-PBA-based ASIB [115]. The cathode was Na2Cu[Fe(CN)6], while the anode was NaFe[Fe(CN)6]. The full cell reaction is written as follows:
Na2Cu[FeII(CN)6] + NaFeIII[FeII(CN)6] ↔ NaCu[FeIII(CN)6] + Na2FeII[FeII(CN)6]
Compared with FeFe(CN)6 full cells, this ASIB system exhibited a similar cell voltage of ~0.70 V but higher energy density of ~27 Wh kg−1 (Figure 8b). It also delivered a good rate capability of 20 C and stable cycling of 250 cycles.
Feng et al. used another approach to develop Fe-based ASIB full cells, where the cathode was a PBA material, but the anode was an iron phosphate material [105,106]. In 2019, they assembled a full cell based on a Na2MnFe(CN)6 cathode, a Na3Fe2(PO4)3 anode, and concentrated electrolytes [99]. The full cell reaction can be written as follows:
Na2Mn[FeII(CN)6] + Na3FeIII2(PO4)3 ↔ NaMn[FeIII(CN)6] + Na4FeIIIFeII(PO4)3.
This full cell demonstrated an average cell voltage of ~0.9 V and energy density of ~27 Wh kg−1 (Figure 8b). It also supported a high rate of 40 C and stable cycling of 700 cycles with 70% capacity retention. Akin to Fe, the manganese element is also Earth-abundant and low-cost, which is attractive for ASIB applications. Later, Feng et al. constructed another ASIB full cell based on a Na2Zn3[Fe(CN)6] cathode, a layer-structured Na3Fe3(PO4)4 anode, and concentrated electrolytes [100]. The full cell reaction can be expressed as:
Na2Zn3[FeII(CN)6]2 + Na3FeIII3(PO4)4 ↔ Zn3[FeIII(CN)6]2 + Na5FeIIIFeII2(PO4)4.
This full cell gave a promising voltage of ~1.2 V (Figure 8b), a high energy density of ~46 Wh kg−1, an ultra-high rate of 200 C, and long cycling of 3000 cycles. Such a performance greatly exceeded previously reported Fe-based ASIBs, indicating the promise of combining a PBA cathode and a phosphate anode.
5. Summary and Outlook
Fe-based ASIBs are appealing for stationary energy storage, due to the desirable combination of Na/Fe elements and aqueous electrolytes. Therefore, low cost, high sustainability, and high safety can be expected. Here, we suggest some directions to further improve ASIB performance (Table 1).

Table 1.
Electrochemical properties of typical iron-based cathode and anode materials.
Regarding cathodes, we believe Na4Fe3(PO4)2P2O7 and Na2Fe[Fe(CN)]6 materials are competitive candidates, due to their high potentials, high capacities, and easy synthesis. Currently, their cycling performance is not particularly long, but it could be further improved by using concentrated electrolytes or surface coating, which can effectively suppress electrode–electrolyte side reactions and material dissolution. Regarding anodes, sodium iron phosphates are more promising than iron oxides, carbides, or selenides. Currently, Na3Fe2(PO4)3 and Na3Fe3(PO4)4 materials have shown encouraging performance in concentrated electrolytes, which may be further boosted if surface coating is used, or artificial solid-electrolyte interphase (SEI) can be formed. Moreover, we emphasize that there are many other iron phosphate materials in non-aqueous SIBs, which need extensive examination in aqueous electrolytes.
On the full cell level, more research efforts are required to demonstrate the efficacy of all-iron-based ASIB full cells. Currently, there are limited publications in this direction, and the performance of these cells is sub-optimal. For potential commercialization, aqueous Na-ion full cells should exhibit competitive properties (energy, cycling, price, etc.) compared with existing aqueous batteries, especially lead-acid batteries [116]. Lead-acid batteries exhibit an energy density of ~30 Wh kg−1, which is based on the entire mass of the battery system. However, the current ASIB studies only consider the active mass for academic research purposes. Moreover, most studies use a high current rate to demonstrate long cycling, which should also be realized at a low current rate. To further decrease the battery price, low-cost and anti-corrosive current collectors and electrolytes should be developed.
In summary, the development of advanced Fe-based ASIBs warrants the holistic design of cathode materials, anode materials, electrolytes, and full cells. Different approaches should be considered and compared, such as the carbon coating, surface modification, electrolyte design, and pouch cell assembly, to demonstrate more practical Fe-based Na-ion full cells. We hope this review can provide an overall picture of the research status of aqueous Na-ion batteries, and that it will motivate researchers to develop more effective strategies to expediate ASIB research and development. If successful, ASIBs will play an important role in stationary energy storage and contribute to the use of renewable energy sources.
Author Contributions
Conceptualization, Z.F. and X.W.; investigation, S.C. and S.Q; data curation, S.C., S.Q., S.K. and J.F.F.G.; writing—original draft preparation, S.C., S.Q., S.K. and J.F.F.G.; writing—review and editing, Z.F. and X.W.; supervision, Z.F. and X.W.; funding acquisition, Z.F. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
Z.F. (Zhenxing Feng) acknowledges financial support from the U.S. National Science Foundation (award numbers: CBET-2016192 and CBET-1949870) and the Oregon Metal Initiative. X.W. acknowledges support from NSF Center for the Advancement of Wearable Technologies (No. 1849243).
Data Availability Statement
This paper contains no supporting data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Yang, J.; Wang, W.; Shao, Y.; Liu, P.; Whittingham, M.S. The TWh challenge: Next generation batteries for energy storage and electric vehicles. Next Energy 2023, 1, 100015. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A perspective on the sustainability of cathode materials used in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Von Wald Cresce, A.; Xu, K. Aqueous lithium-ion batteries. Carbon Energy 2021, 3, 721–751. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-ion battery materials and electrochemical properties reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 7070–7072. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem. 2013, 125, 4731–4734. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Komaba, S.; Itabashi, T.; Watanabe, M.; Groult, H.; Kumagai, N. Electrochemistry of graphite in Li and Na salt codissolving electrolyte for rechargeable batteries. J. Electrochem. Soc. 2007, 154, A322. [Google Scholar] [CrossRef]
- Kubota, K.; Yabuuchi, N.; Yoshida, H.; Dahbi, M.; Komaba, S. Layered oxides as positive electrode materials for Na-ion batteries. MRS Bull. 2014, 39, 416–422. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Xu, S.; Bai, J.; Xiao, R.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L.; Huang, X. A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries. Nat. Commun. 2013, 4, 2365. [Google Scholar] [CrossRef]
- Mu, L.-Q.; Hu, Y.-S.; Chen, L.-Q. New layered metal oxides as positive electrode materials for room-temperature sodium-ion batteries. Chin. Phys. B 2015, 24, 038202. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Sun, M.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Low-defect Prussian blue nanocubes as high capacity and long life cathodes for aqueous Na-ion batteries. Nano Energy 2015, 13, 117–123. [Google Scholar] [CrossRef]
- Wu, X.; Sun, M.; Guo, S.; Qian, J.; Liu, Y.; Cao, Y.; Ai, X.; Yang, H. Vacancy-free Prussian blue nanocrystals with high capacity and superior cyclability for aqueous sodium-ion batteries. ChemNanoMat 2015, 1, 188–193. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Xiao, L.; Ai, X.; Cao, Y.; Yang, H. Phosphate framework electrode materials for sodium ion batteries. Adv. Sci. 2017, 4, 1600392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Jiang, X.; Li, R.; Yuan, D.; Ai, X.; Yang, H.; Cao, Y. A safer sodium-ion battery based on nonflammable organic phosphate electrolyte. Adv. Sci. 2016, 3, 1600066. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. High-safety symmetric sodium-ion batteries based on nonflammable phosphate electrolyte and double Na3V2(PO4)3 electrodes. ACS Appl. Mater. Interfaces 2019, 11, 27833–27838. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Henderson, W.A.; Sushko, M.L.; Shao, Y.; Xiao, J.; Wang, W.; Engelhard, M.H.; Nie, Z.; Liu, J. Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 2016, 19, 279–288. [Google Scholar] [CrossRef]
- Li, Z.; Jian, Z.; Wang, X.; Rodríguez-Pérez, I.A.; Bommier, C.; Ji, X. Hard carbon anodes of sodium-ion batteries: Undervalued rate capability. Chem. Commun. 2017, 53, 2610–2613. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New mechanistic insights on Na-ion storage in nongraphitizable carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef]
- Deng, J.; Luo, W.B.; Chou, S.L.; Liu, H.K.; Dou, S.X. Sodium-ion batteries: From academic research to practical commercialization. Adv. Energy Mater. 2018, 8, 1701428. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. Boost sodium-ion batteries to commercialization: Strategies to enhance initial Coulombic efficiency of hard carbon anode. Nano Energy 2021, 82, 105738. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus Prussian blue analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The scale-up and commercialization of nonaqueous Na-ion battery technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 22–42. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.M. Towards high power high energy aqueous sodium-ion batteries: The NaTi2(PO4)3/Na0. 44MnO2 system. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Wu, X.; Cao, Y.; Ai, X.; Qian, J.; Yang, H. A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3–Na2NiFe(CN)6 intercalation chemistry. Electrochem. Commun. 2013, 31, 145–148. [Google Scholar] [CrossRef]
- Bin, D.; Wang, F.; Tamirat, A.G.; Suo, L.; Wang, Y.; Wang, C.; Xia, Y. Progress in aqueous rechargeable sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703008. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Xia, Y. Recent progress in aqueous lithium-ion batteries. Adv. Energy Mater. 2012, 2, 830–840. [Google Scholar] [CrossRef]
- Sui, Y.; Ji, X. Anticatalytic strategies to suppress water electrolysis in aqueous batteries. Chem. Rev. 2021, 121, 6654–6695. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, L.; Yue, J.; Zhang, Q.; Zhou, A.; Borodin, O.; Suo, L.; Li, H.; Chen, L.; Xu, K.; et al. High-voltage aqueous Na-ion battery enabled by inert-cation-assisted water-in-salt electrolyte. Adv. Mater. 2020, 32, 1904427. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, Y.; Zhang, C.; Leonard, D.P.; Markir, A.; Lu, J.; Ji, X. Reverse dual-ion battery via a ZnCl2 water-in-salt electrolyte. J. Am. Chem. Soc. 2019, 141, 6338–6344. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Wang, Y.; Rong, X.; Sun, W.; Fan, X.; Xu, S.; Schroeder, M.A.; Cresce, A.V.; Wang, F. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv. Energy Mater. 2017, 7, 1701189. [Google Scholar] [CrossRef]
- Jin, T.; Ji, X.; Wang, P.F.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S.; et al. High-Energy Aqueous Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 11943–11948. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Wu, F.; Jing, Y.; Dong, H.; Rao, K.K.; Chi, X.; Fang, F.; Yao, Y. An aqueous Ca-ion battery. Adv. Sci. 2017, 4, 1700465. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.L.; Dong, X.; Truhlar, D.G.; Wang, Y.; Wang, C.; Xia, Y. Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett. 2017, 2, 1115–1121. [Google Scholar] [CrossRef]
- Yan, C.; Lv, C.; Wang, L.; Cui, W.; Zhang, L.; Dinh, K.N.; Tan, H.; Wu, C.; Wu, T.; Ren, Y. Architecting a stable high-energy aqueous Al-ion battery. J. Am. Chem. Soc. 2020, 142, 15295–15304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, W.; Zhou, Z.; Li, C.; Zhang, M.; Yuan, X.; Hu, J.; Chen, H.; Li, R. An ultra-long life aqueous full K-ion battery. J. Mater. Chem. A 2021, 9, 2822–2829. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Aquion_Energy (accessed on 26 April 2023).
- Pang, G.; Nie, P.; Yuan, C.; Shen, L.; Zhang, X.; Zhu, J.; Ding, B. Enhanced Performance of Aqueous Sodium-Ion Batteries Using Electrodes Based on the NaTi2(PO4)3/MWNTs–Na0.44MnO2 System. Energy Technol. 2014, 2, 705–712. [Google Scholar] [CrossRef]
- Kim, D.J.; Ponraj, R.; Kannan, A.G.; Lee, H.-W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0. 44MnO2 in aqueous and non-aqueous electrolytes. J. Power Source 2013, 244, 758–763. [Google Scholar] [CrossRef]
- Shen, X.; Han, M.; Li, X.; Zhang, P.; Yang, C.; Liu, H.; Hu, Y.-S.; Zhao, J. Regulated Synthesis of α-NaVOPO4 with an Enhanced Conductive Network as a High-Performance Cathode for Aqueous Na-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-F.; Lan, W.-H.; Yeh, Y.-W.; Chang, W.-S.; Yang, C.-C.; Lin, J.-C. Hydrothermal synthesis of sodium titanium phosphate nanoparticles as efficient anode materials for aqueous sodium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 7074–7079. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Deng, Z.; Li, X.; Wang, B.; Chen, X.; Ong, S.P. Water contributes to higher energy density and cycling stability of Prussian blue analogue cathodes for aqueous sodium-ion batteries. Chem. Mater. 2019, 31, 5933–5942. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef]
- Deng, W.; Shen, Y.; Qian, J.; Yang, H. A polyimide anode with high capacity and superior cyclability for aqueous Na-ion batteries. Chem. Commun. 2015, 51, 5097–5099. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Abundance_of_the_chemical_elements (accessed on 9 June 2023).
- Available online: https://en.wikipedia.org/wiki/Prices_of_chemical_elements (accessed on 16 May 2023).
- Fang, Y.; Chen, Z.; Xiao, L.; Ai, X.; Cao, Y.; Yang, H. Recent progress in iron-based electrode materials for grid-scale sodium-ion batteries. Small 2018, 14, 1703116. [Google Scholar] [CrossRef]
- Okada, S.; Yamaki, J.-I. Iron-based cathodes/anodes for Li-ion and post Li-ion batteries. J. Ind. Eng. Chem. 2004, 10, 1104–1113. [Google Scholar]
- Liu, Y.; Li, W.; Xia, Y. Recent progress in polyanionic anode materials for Li(Na)-ion batteries. Electrochem. Energy Rev. 2021, 4, 447–472. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. High-performance olivine NaFePO4 microsphere cathode synthesized by aqueous electrochemical displacement method for sodium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 17977–17984. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, M.; Mohamed, Z.; Ling, C.D.; Lu, J.; Tamaru, M.; Yamada, A.; Barpanda, P. Magnetic structures of NaFePO4 maricite and triphylite polymorphs for sodium-ion batteries. Inorg. Chem. 2013, 52, 8685–8693. [Google Scholar] [CrossRef]
- Kim, J.; Seo, D.-H.; Kim, H.; Park, I.; Yoo, J.-K.; Jung, S.-K.; Park, Y.-U.; Goddard Iii, W.A.; Kang, K. Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. Energy Environ. Sci. 2015, 8, 540–545. [Google Scholar] [CrossRef]
- Vujković, M.; Mentus, S. Fast sodiation/desodiation reactions of electrochemically delithiated olivine LiFePO4 in aerated aqueous NaNO3 solution. J. Power Source 2014, 247, 184–188. [Google Scholar] [CrossRef]
- Fernández-Ropero, A.J.; Saurel, D.; Acebedo, B.; Rojo, T.; Casas-Cabanas, M. Electrochemical characterization of NaFePO4 as positive electrode in aqueous sodium-ion batteries. J. Power Source 2015, 291, 40–45. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.H.; Park, Y.D.; Lee, C.Y.; Mun, J.; Tron, A. Artificially coated NaFePO4 for aqueous rechargeable sodium-ion batteries. J. Alloys Compd. 2019, 784, 720–726. [Google Scholar] [CrossRef]
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F. High-performance NaFePO4 formed by aqueous ion-exchange and its mechanism for advanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 4882–4892. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Amine, J.; Duan, Y.; Zheng, J.; Lin, Y.; Pan, F. In-situ mass-electrochemical study of surface redox potential and interfacial chemical reactions of Li (Na) FePO4 nanocrystals for Li (Na)-ion batteries. Nano Energy 2017, 37, 90–97. [Google Scholar] [CrossRef]
- Sevinc, S.; Tekin, B.; Ata, A.; Morcrette, M.; Perrot, H.; Sel, O.; Demir-Cakan, R. In-situ tracking of NaFePO4 formation in aqueous electrolytes and its electrochemical performances in Na-ion/polysulfide batteries. J. Power Source 2019, 412, 55–62. [Google Scholar] [CrossRef]
- Shiprath, K.; Manjunatha, H.; Ratnamala, A.; Ramesh, S.; Naidu, K.C.B. Electrochemical Study of NaFePO4 Cathode Material in Aqueous Sodium-ion Electrolyte. Biointerface Res. Appl. Chem. 2021, 13, 186. [Google Scholar]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A safe cathode for rechargeable sodium-ion batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Kim, H.; Shakoor, R.A.; Park, C.; Lim, S.Y.; Kim, J.S.; Jo, Y.N.; Cho, W.; Miyasaka, K.; Kahraman, R.; Jung, Y. Na2FeP2O7 as a promising iron-based pyrophosphate cathode for sodium rechargeable batteries: A combined experimental and theoretical study. Adv. Funct. Mater. 2013, 23, 1147–1155. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lim, C.H.; Kim, J.-H.; Kim, D.K. Na2FeP2O7 as a positive electrode material for rechargeable aqueous sodium-ion batteries. RSC Adv. 2014, 4, 9799–9802. [Google Scholar] [CrossRef]
- Nakamoto, K.; Kano, Y.; Kitajou, A.; Okada, S. Electrolyte dependence of the performance of a Na2FeP2O7//NaTi2(PO4)3 rechargeable aqueous sodium-ion battery. J. Power Source 2016, 327, 327–332. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Yuan, T.; Cao, S.; Liu, S.; Xu, L.; Yang, H.; Ai, X.; Chen, Z.; Cao, Y. Na4Fe3(PO4) 2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Storage Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Fernández-Ropero, A.J.; Zarrabeitia, M.; Reynaud, M.; Rojo, T.; Casas-Cabanas, M. Toward safe and sustainable batteries: Na4Fe3(PO4)2P2O7 as a low-cost cathode for rechargeable aqueous Na-ion batteries. J. Phys. Chem. C 2018, 122, 133–142. [Google Scholar] [CrossRef]
- Kawabe, Y.; Yabuuchi, N.; Kajiyama, M.; Fukuhara, N.; Inamasu, T.; Okuyama, R.; Nakai, I.; Komaba, S. Synthesis and electrode performance of carbon coated Na2FePO4F for rechargeable Na batteries. Electrochem. Commun. 2011, 13, 1225–1228. [Google Scholar] [CrossRef]
- Sharma, L.; Nakamoto, K.; Sakamoto, R.; Okada, S.; Barpanda, P. Na2FePO4F Fluorophosphate as Positive Insertion Material for Aqueous Sodium-Ion Batteries. ChemElectroChem 2019, 6, 444–449. [Google Scholar] [CrossRef]
- Xie, B.; Sakamoto, R.; Kitajou, A.; Nakamoto, K.; Zhao, L.; Okada, S.; Fujita, Y.; Oka, N.; Nishida, T.; Kobayashi, W. Cathode properties of Na3FePO4CO3 prepared by the mechanical ball milling method for Na-ion batteries. Sci. Rep. 2020, 10, 3278. [Google Scholar] [CrossRef] [PubMed]
- Shiprath, K.; Manjunatha, H.; Ratnam, K.V.; Janardan, S. Synthesis of Flower-Like, Hyperbranched Na3FePO4CO3 Nanocrystals and Their Electrochemical Performance as Cathodes in Aqueous Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 080523. [Google Scholar] [CrossRef]
- Herren, F.; Fischer, P.; Ludi, A.; Hälg, W. Neutron diffraction study of Prussian Blue, Fe4[Fe(CN)6]3. xH2O. Location of water molecules and long-range magnetic order. Inorg. Chem. 1980, 19, 956–959. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; McDowell, M.T.; Huggins, R.A.; Cui, Y. The effect of insertion species on nanostructured open framework hexacyanoferrate battery electrodes. J. Electrochem. Soc. 2011, 159, A98. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y. Prussian blue analogs cathodes for aqueous zinc ion batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Du, G.; Pang, H. Recent advancements in Prussian blue analogues: Preparation and application in batteries. Energy Storage Mater. 2021, 36, 387–408. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian blue analogs as battery materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Wu, X.; Ji, X. Prussian blue analogues as electrodes for aqueous monovalent ion batteries. Electrochem. Energy Rev. 2022, 5, 242–262. [Google Scholar] [CrossRef]
- Wu, X.; Shao, M.; Wu, C.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Low defect FeFe(CN)6 framework as stable host material for high performance Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 23706–23712. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Deng, W.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Single-crystal FeFe(CN)6 nanoparticles: A high capacity and high rate cathode for Na-ion batteries. J. Mater. Chem. A 2013, 1, 10130–10134. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Wei, C.; Hu, L.; Qian, J.; Cao, Y.; Ai, X.; Wang, J.; Yang, H. Highly crystallized Na2CoFe(CN)6 with suppressed lattice defects as superior cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, W.; Li, H.; Zhang, J.; Kang, F.; Li, B. Investigation of iron hexacyanoferrate as a high rate cathode for aqueous batteries: Sodium-ion batteries and lithium-ion batteries. Electrochim. Acta 2018, 270, 96–103. [Google Scholar] [CrossRef]
- Fernández-Ropero, A.J.; Piernas-Muñoz, M.J.; Castillo-Martínez, E.; Rojo, T.; Casas-Cabanas, M. Electrochemical characterization of NaFe2(CN)6 Prussian blue as positive electrode for aqueous sodium-ion batteries. Electrochim. Acta 2016, 210, 352–357. [Google Scholar] [CrossRef]
- Bi, H.; Luo, Y.; Zhao, C.; Ma, L.; Huang, H. Graphene oxide suspension-based electrolyte promotes the cycling performance of aqueous sodium-ion batteries through the interaction between metal ions, free water molecules and functional groups. J. Power Source 2023, 555, 232380. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Z.; Li, C.; Chen, B.; Wang, Y.; Fu, L.; Zhu, Y.; Liu, X.; Wu, Y. Prussian blue as positive electrode material for aqueous sodium-ion capacitor with excellent performance. RSC Adv. 2016, 6, 109340–109345. [Google Scholar] [CrossRef]
- Wang, J.; Mi, C.; Nie, P.; Dong, S.; Tang, S.; Zhang, X. Sodium-rich iron hexacyanoferrate with nickel doping as a high performance cathode for aqueous sodium ion batteries. J. Electroanal. Chem. 2018, 818, 10–18. [Google Scholar] [CrossRef]
- Park, S.I.; Gocheva, I.; Okada, S.; Yamaki, J.-I. Electrochemical properties of NaTi2(PO4)3 anode for rechargeable aqueous sodium-ion batteries. J. Electrochem. Soc. 2011, 158, A1067. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Ge, X.; Li, S.; Wang, X.; Wang, S.; Zhang, L.; Zhang, Z. Iron-Phosphate-Based Cathode Materials for Cost-Effective Sodium-Ion Batteries: Development, Challenges, and Prospects. Adv. Mater. Interfaces 2022, 9, 2200515. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Laul, D.; Zhu, W.; Provencher, M.; Trudeau, M.L.; Guerfi, A.; Zaghib, K. Ultra-low cost and highly stable hydrated FePO4 anodes for aqueous sodium-ion battery. J. Power Source 2018, 374, 211–216. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, X.; Wang, M.; Lucero, M.; Wang, Y.; Wang, J.; Yang, Z.; Xu, W.; Wang, Q.; Gu, M.; et al. NASICON-type Na3Fe2(PO4)3 as a low-cost and high-rate anode material for aqueous sodium-ion batteries. Nano Energy 2019, 64, 103941. [Google Scholar] [CrossRef]
- Qiu, S.; Lucero, M.; Wu, X.; Wang, Q.; Wang, M.; Wang, Y.; Samarakoon, W.S.; Bolding, M.R.; Yang, Z.; Huang, Y.; et al. Revealing the Fast and Durable Na+ Insertion Reactions in a Layered Na3Fe3(PO4)4 Anode for Aqueous Na-Ion Batteries. ACS Mater. Au 2021, 2, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gund, G.S.; Dubal, D.P.; Chodankar, N.R.; Cho, J.Y.; Gomez-Romero, P.; Park, C.; Lokhande, C.D. Low-cost flexible supercapacitors with high-energy density based on nanostructured MnO2 and Fe2O3 thin films directly fabricated onto stainless steel. Sci. Rep. 2015, 5, 12454. [Google Scholar] [CrossRef] [PubMed]
- Nwanya, A.C.; Ndipingwi, M.M.; Ikpo, C.O.; Ezema, F.I.; Iwuoha, E.I.; Maaza, M. Biomass mediated multi layered NaNixCo1− xO2 (x= 0.4) and α-Fe2O3 nanoparticles for aqueous sodium ion battery. J. Electroanal. Chem. 2020, 858, 113809. [Google Scholar] [CrossRef]
- Lan, P.; Liu, T.; Ji, X.; Cheng, S. Charge storage behavior and reaction mechanism of α-Fe2O3 as anodes for aqueous batteries. J. Alloys Compd. 2021, 859, 157789. [Google Scholar] [CrossRef]
- Lu, K.; Li, D.; Gao, X.; Dai, H.; Wang, N.; Ma, H. An advanced aqueous sodium-ion supercapacitor with a manganous hexacyanoferrate cathode and a Fe3O4/rGO anode. J. Mater. Chem. A 2015, 3, 16013–16019. [Google Scholar] [CrossRef]
- Li, N.N.; Sheng, Z.M.; Huang, H.; Chang, C.K.; Jia, R.P.; Han, S. Fe2O3 nanoparticles encapsulated with N-doped porous graphitic shells approached by oxidizing Fe3C@C precursor for high-performance sodium-ion batteries. J. Alloys Compd. 2019, 792, 25–31. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, X.; Wan, H.; Zhang, B.; Liu, X.; Li, L.; Wang, C.; Gan, Y.; Liang, P.; Wang, Y. Metal–organic framework-derived high conductivity Fe3C with porous carbon on graphene as advanced anode materials for aqueous battery-supercapacitor hybrid devices. J. Power Source 2020, 448, 227403. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Kong, D.; Fan, H.; Gao, X.; Cui, Y.; Jiang, T.; Ren, Y.; Cai, T.; Xing, W. Realizing an aqueous sodium-ion battery with a super-high discharge voltage based on a novel FeSe2@rGO anode. Inorg. Chem. Front. 2022, 9, 1622–1629. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Niu, F.; Li, X.; Wang, C.; Yang, J. FeFe(CN)6 Nanocubes as a Bipolar Electrode Material in Aqueous Symmetric Sodium-Ion Batteries. ChemPlusChem 2017, 82, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.; Liang, C.; Yan, M.; Jiang, Y. An All-Prussian-Blue-Based Aqueous Sodium-Ion Battery. ChemElectroChem 2019, 6, 4848–4853. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.-G.; Yang, J.; Xue, S.-C.; Gao, H.-L.; Yan, X.-H.; Huo, Q.-Y.; Wang, S.-W.; Cao, Y.; Yan, J. Advances and challenges in improvement of the electrochemical performance for lead-acid batteries: A comprehensive review. J. Power Source 2022, 520, 230800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).