Solid Polymer Electrolytes for Zinc-Ion Batteries
Abstract
1. Introduction
2. Classification of SPEs
3. Homogenous Polymer Matrix SPEs
4. Hybrid Polymer Matrix SPEs
5. Nanocomposite Polymer Electrolytes
6. Summary and Perspectives
- In terms of SPEs, one of the major challenges is the low ionic conductivity at room temperature, which limits the performance of zinc-ion batteries. Normally, a high ionic conductivity will improve the efficiency of ion transportation that can help achieve great electrochemical performance and alleviate the reduction of zinc metal on the surface of the anode. To address this challenge, several strategies can be employed, including the incorporation of high-conductivity salts, functional polymers, and the use of nanocomposites.
- Another challenge is the mechanical stability of SPEs. Most polymers used as SPEs are soft and easily deformable, which can result in the formation of dendrites and short-circuits in the battery. To address this challenge, several strategies can be employed, including the incorporation of rigid segments into the polymer backbone, the use of crosslinked polymers, and the use of ceramic fillers. According to the methods used for lithium metal batteries, the addition of inorganic materials can improve the Young’s modulus of SPEs and efficiently suppress the growth of metal dendrites. Therefore, in terms of ZIBs, the principle can also be utilised to address the safety issue induced by zinc dendrites. To date, the related studies are very limited, so this strategy might be a promising method to improve the electrochemical property and safety of ZIBs in a future study.
- Furthermore, the long-term stability of SPEs needs to be further improved. Many SPEs undergo degradation over time due to the presence of the electrochemical active species, which can lead to the deterioration of ionic conductivity and capacity. Crosslinking and in situ polymerisation methods would be reasonable methods to improve the chemical and thermal stability of the SPEs. Additionally, the incorporation of inorganic nanomaterials would also help enhance the stability of SPEs attributed to the stability of the crystalline structures.
- Lastly, biomass-based SPEs show promising potential in improving the ionic conductivity, biocompatibility, and stability of the ZIBs. Biomass polymers or macromolecules usually contain plenty of functional groups, which can provide lots of active sites that can interact with zinc salts and electrolytes. Therefore, biomass-based SPEs can show great compatibility with zinc salts and electrolytes and thus have very stable crosslinking structures and ion channels. These advantages enable biomass-based SPEs as a research hotpot for high-performance and safe ZIBs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Schroeder, M.A.; Pollard, T.P.; Borodin, O.; Ding, M.S.; Sun, R.; Cao, L.; Ho, J.; Baker, D.R.; Wang, C. Critical factors dictating reversibility of the zinc metal anode. Energy Environ. Mater. 2020, 3, 516–521. [Google Scholar] [CrossRef]
- Brige, A.; Olsson, M.; Xiong, S.; Matic, A. A comparative study of hydroxyethylcellulose-based solid polymer electrolytes for solid state Zn batteries. Nano Sel. 2022, 4, 102–111. [Google Scholar] [CrossRef]
- Chuai, M.; Yang, J.; Tan, R.; Liu, Z.; Yuan, Y.; Xu, Y.; Sun, J.; Wang, M.; Zheng, X.; Chen, N. Theory-Driven Design of a Cationic Accelerator for High-Performance Electrolytic MnO2–Zn Batteries. Adv. Mater. 2022, 34, 2203249. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kohn, B.; Scheler, U.; Wang, F.; Oswald, S.; Löffler, M.; Tan, D.; Zhang, P.; Zhang, J.; Feng, X. A High-Voltage, Dendrite-Free, and Durable Zn–Graphite Battery. Adv. Mater. 2020, 32, 1905681. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, K.; Pei, P.; Wei, M.; Liu, X.; Xiao, Y.; Zhang, P. Zinc dendrite growth and inhibition strategies. Mater. Today Energy 2021, 20, 100692. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B.; Zhi, C. Dendrites in Zn-based batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of zinc dendrite growth in zinc-based batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Xu, D.; Dong, P.; Chee, M.O.L.; Li, X.; Zhu, K.; Ye, M.; Shen, J. Eliminating Zn dendrites by commercial cyanoacrylate adhesive for zinc ion battery. Energy Storage Mater. 2021, 36, 132–138. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, C.; Li, W.; Wu, T.; Zhang, Q.; Tang, Y.; Shao, M.; Wang, H. Revealing the Two-Dimensional Surface Diffusion Mechanism for Zinc Dendrite Formation on Zinc Anode. Small 2022, 18, 2104148. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design strategies for high-energy-density aqueous zinc batteries. Angew. Chem. 2022, 134, e202200598. [Google Scholar] [CrossRef]
- Dang, J.; Yin, M.; Pan, D.; Tian, Z.; Chen, G.; Zou, J.; Miao, H.; Wang, Q.; Yuan, J. Four-functional iron/copper sulfide heterostructure for alkaline hybrid zinc batteries and water splitting. Chem. Eng. J. 2023, 457, 141357. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Cai, Y.; Pam, M.E.; Yang, Y.; Zhang, D.; Wang, Y.; Huang, S. Designing Advanced Aqueous Zinc-Ion Batteries: Principles, Strategies, and Perspectives. Energy Environ. Mater. 2022, 5, 823–851. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Wang, L.-P.; Wang, P.-F.; Wang, T.-S.; Yin, Y.-X.; Guo, Y.-G.; Wang, C.-R. Prussian blue nanocubes as cathode materials for aqueous Na-Zn hybrid batteries. J. Power Sources 2017, 355, 18–22. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, Y.; Yang, S. Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 2018, 30, 1800762. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1. 1V3O7. 9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem. 2019, 131, 16508–16517. [Google Scholar] [CrossRef]
- Bósquez-Cáceres, M.F.; Hidalgo-Bonilla, S.; Morera Córdova, V.; Michell, R.M.; Tafur, J.P. Nanocomposite Polymer Electrolytes for Zinc and Magnesium Batteries: From Synthetic to Biopolymers. Polymers 2021, 13, 4284. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent advances in polymer electrolytes for zinc ion batteries: Mechanisms, properties, and perspectives. Adv. Energy Mater. 2020, 10, 1903977. [Google Scholar] [CrossRef]
- Mo, F.; Guo, B.; Ling, W.; Wei, J.; Chen, L.; Yu, S.; Liang, G. Recent Progress and Challenges of Flexible Zn-Based Batteries with Polymer Electrolyte. Batteries 2022, 8, 59. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Luo, L.; Yang, W.; Liu, Y.; Shen, Z.; Fan, X.-H. Self-healing solid polymer electrolyte with high ion conductivity and super stretchability for all-solid zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 36320–36329. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; MacKenzie, J.D. Fully printed, high energy density flexible zinc-air batteries based on solid polymer electrolytes and a hierarchical catalyst current collector. Flex. Pr. Electron. 2019, 4, 015010. [Google Scholar] [CrossRef]
- Lu, J.; Jaumaux, P.; Wang, T.; Wang, C.; Wang, G. Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J. Mater. Chem. A 2021, 9, 24175–24194. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, X.; Chen, A.; Dong, B.; Zhi, C. Liquid-Free All-Solid-State Zinc Batteries and Encapsulation-Free Flexible Batteries Enabled by In Situ Constructed Polymer Electrolyte. Angew. Chem. 2020, 132, 24044–24052. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, M.; Huang, S.; Tian, J.; Niu, Z. Thermal-Gated Polymer Electrolytes for Smart Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 16480–16484. [Google Scholar] [CrossRef]
- Fu, J.; Lee, D.U.; Hassan, F.M.; Yang, L.; Bai, Z.; Park, M.G.; Chen, Z. Flexible high-energy polymer-electrolyte-based rechargeable zinc–air batteries. Adv. Mater. 2015, 27, 5617–5622. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Ghosh, M.; Kurian, M.; Torris, A.; Dilwale, S.; Badiger, M.V.; Winter, M.; Nair, J.R.; Kurungot, S. An In Situ Cross-Linked Nonaqueous Polymer Electrolyte for Zinc-Metal Polymer Batteries and Hybrid Supercapacitors. Small 2020, 16, 2002528. [Google Scholar] [CrossRef]
- Lin, C.; Shinde, S.S.; Li, X.; Kim, D.H.; Li, N.; Sun, Y.; Song, X.; Zhang, H.; Lee, C.H.; Lee, S.U. Solid-State Rechargeable Zinc–Air Battery with Long Shelf Life Based on Nanoengineered Polymer Electrolyte. ChemSusChem 2018, 11, 3215–3224. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Muchakayala, R.; Song, S. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn (Tf) 2 complex system. J. Mater. Sci. Mater. Electron. 2020, 31, 6160–6173. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Deng, D.; Bai, Y.; Liu, M.; Zhao, X.; Xiong, X.; Lei, Y. Low-temperature resistant gel polymer electrolytes for zinc–air batteries. J. Mater. Chem. A 2022, 10, 19304–19319. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Huang, F.; Cai, Y.; Li, Y.; Ke, H.; Lv, P.; Wei, Q. “Water-in-Salt” Nonalkaline Gel Polymer Electrolytes Enable Flexible Zinc-Air Batteries with Ultra-Long Operating Time. Adv. Funct. Mater. 2022, 32, 2203204. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Liu, X.; Liu, J.; Zhao, N.; Zhong, C.; Hu, W.; Lu, J. Functionalized Nanocomposite Gel Polymer Electrolyte with Strong Alkaline-Tolerance and High Zinc Anode Stability for Ultralong-Life Flexible Zinc–Air Batteries. Adv. Mater. 2023, 35, 2209290. [Google Scholar] [CrossRef]
- Hong, Z.; Ahmad, Z.; Viswanathan, V. Design principles for dendrite suppression with porous polymer/aqueous solution hybrid electrolyte for Zn metal anodes. ACS Energy Lett. 2020, 5, 2466–2474. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yang, F.; Liu, S.; Zhang, S.; Mao, J.; Guo, Z. Electrolyte Engineering Enables High Performance Zinc-Ion Batteries. Small 2022, 18, 2107033. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.J.; Liu, J. Materials and structure design for solid-state zinc-ion batteries: A mini-review. Front. Energy Res. 2021, 8, 616665. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Hiralal, P.; Imaizumi, S.; Unalan, H.E.; Matsumoto, H.; Minagawa, M.; Rouvala, M.; Tanioka, A.; Amaratunga, G.A.J. Nanomaterial-enhanced all-solid flexible zinc−carbon batteries. ACS Nano 2010, 4, 2730–2734. [Google Scholar] [CrossRef]
- Tafur, J.P.; Abad, J.; Román, E.; Romero, A.J.F.J.E.C. Charge storage mechanism of MnO2 cathodes in Zn/MnO2 batteries using ionic liquid-based gel polymer electrolytes. ACS Nano 2015, 60, 190–194. [Google Scholar]
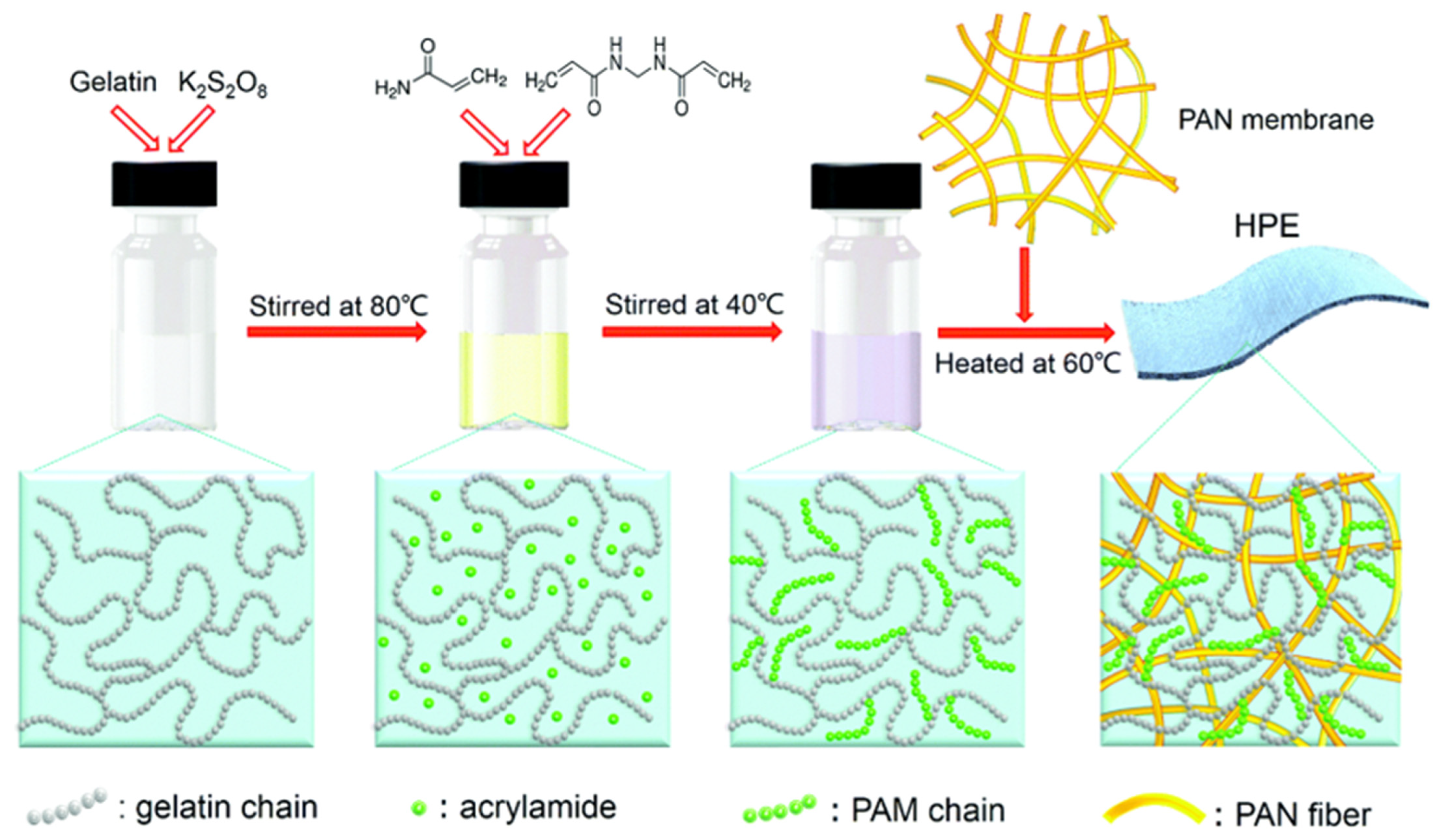
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.J.A.n. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018, 12, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Kettlgruber, G.; Siket, C.; Schwödiauer, R.; Bauer, S.J. Arrays of ultracompliant electrochemical dry gel cells for stretchable electronics. Adv. Mater. 2010, 22, 2065–2067. [Google Scholar] [CrossRef]
- Yi, J.; Guo, S.; He, P.; Zhou, H.J.E. Status and prospects of polymer electrolytes for solid-state Li–O 2 (air) batteries. Energy Environ. Sci. 2017, 10, 860–884. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Lv, Z.; Wang, Q.; Zhang, Y.; Lu, G.; Zhao, J.; Cui, G. In-situ formed all-amorphous poly (ethylene oxide)-based electrolytes enabling solid-state Zn electrochemistry. Chem. Eng. J. 2021, 417, 128096. [Google Scholar] [CrossRef]
- Kumar, G. Electrochemical characterization of poly(vinylidenefluoride)-zinc triflate gel polymer electrolyte and its application in solid-state zinc batteries. Solid State Ion. 2003, 160, 289–300. [Google Scholar] [CrossRef]
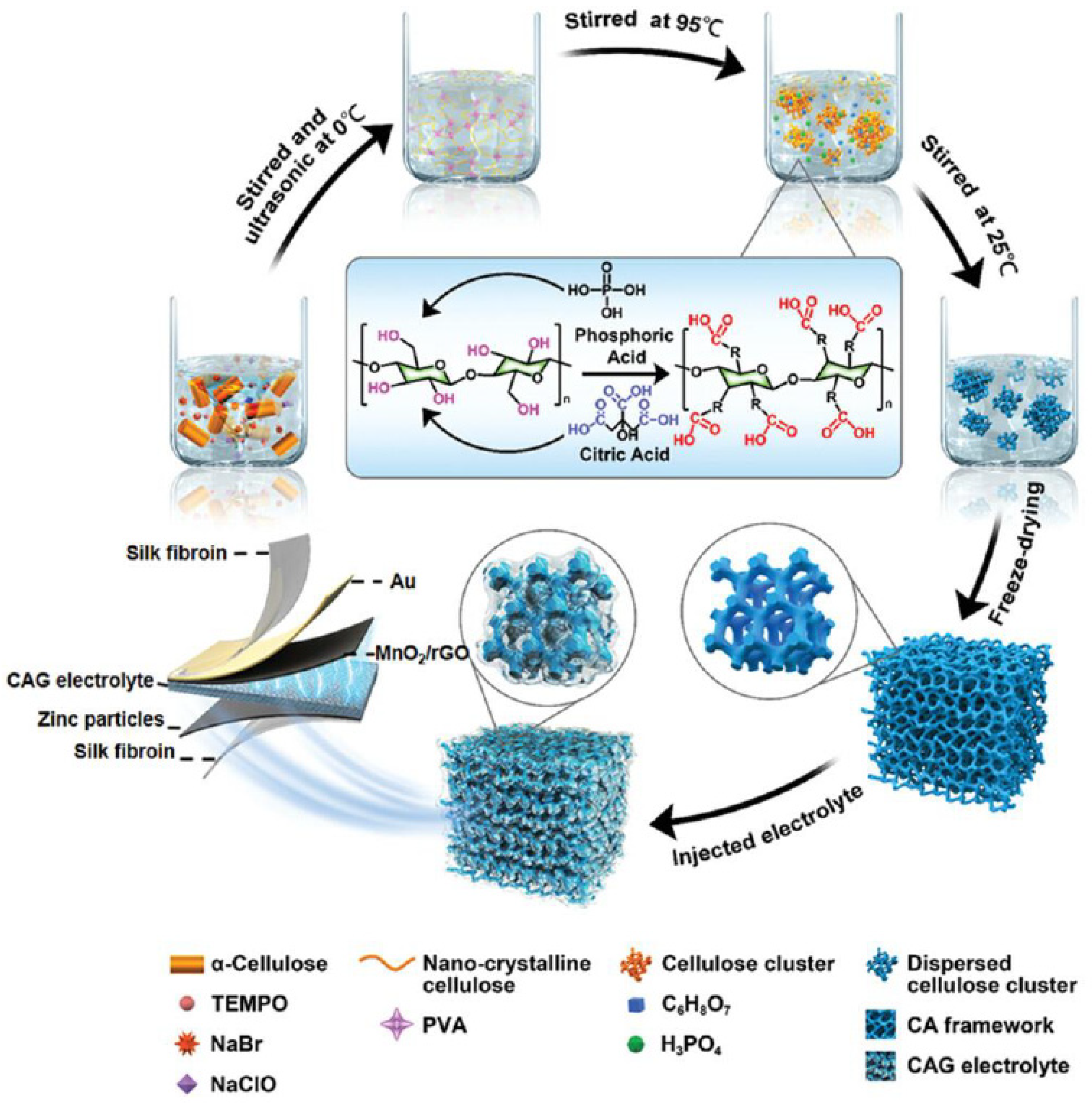
- Huang, Y.; Liu, J.; Zhang, J.; Jin, S.; Jiang, Y.; Zhang, S.; Li, Z.; Zhi, C.; Du, G.; Zhou, H. Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte. RSC Adv. 2019, 9, 16313–16319. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Liu, J.; Li, Z.; Jin, S.; Li, Z.; Zhang, S.; Zhou, H. Flexible and stable quasi-solid-state zinc ion battery with conductive guar gum electrolyte. Mater. Today Energy 2019, 14, 100349. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Xu, L.; Yang, C.; Hong, M.; Cui, M.; Clifford, B.C.; He, S.; Jing, S.; Yao, Y. A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Ye, H.; Xu, J.J. Zinc ion conducting polymer electrolytes based on oligomeric polyether/PVDF-HFP blends. J. Power Sources 2007, 165, 500–508. [Google Scholar] [CrossRef]
- Rathika, R.; Padmaraj, O.; Suthanthiraraj, S.A. Electrical conductivity and dielectric relaxation behaviour of PEO/PVdF-based solid polymer blend electrolytes for zinc battery applications. Ionics 2017, 24, 243–255. [Google Scholar] [CrossRef]
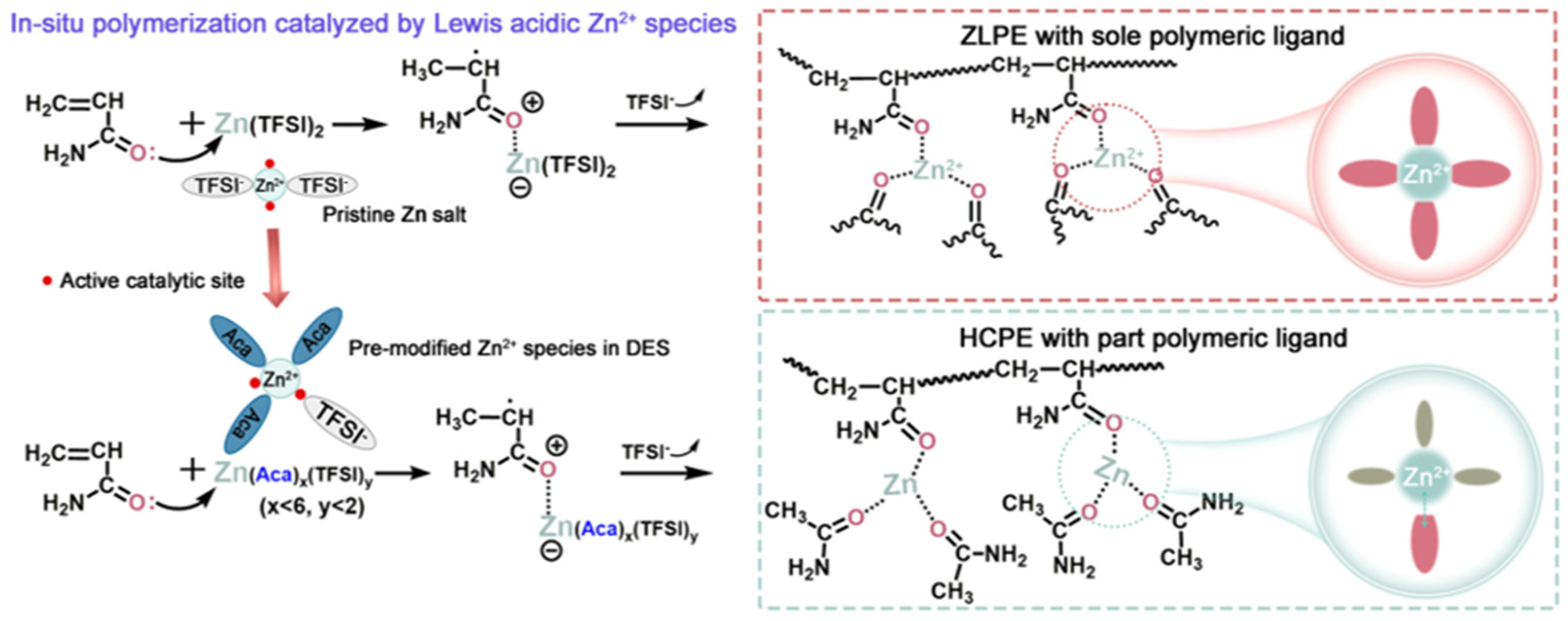
- Lu, G.; Qiu, H.; Du, X.; Sonigara, K.K.; Wang, J.; Zhang, Y.; Chen, Z.; Chen, L.; Ren, Y.; Zhao, Z.; et al. Heteroleptic Coordination Polymer Electrolytes Initiated by Lewis-Acidic Eutectics for Solid Zinc–Metal Batteries. Chem. Mater. 2022, 34, 8975–8986. [Google Scholar] [CrossRef]
- Li, H.; Han, C.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Wang, Z.; Liu, Z.; Tang, Z.; et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ. Sci. 2018, 11, 941–951. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, H.; Tawiah, B.; Jia, H.; Fu, S. Zwitterionic triple-network hydrogel electrolyte for advanced flexible zinc ion batteries. Compos. Commun. 2021, 28, 100942. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery. Sci. Rep. 2020, 10, 12587. [Google Scholar] [CrossRef] [PubMed]
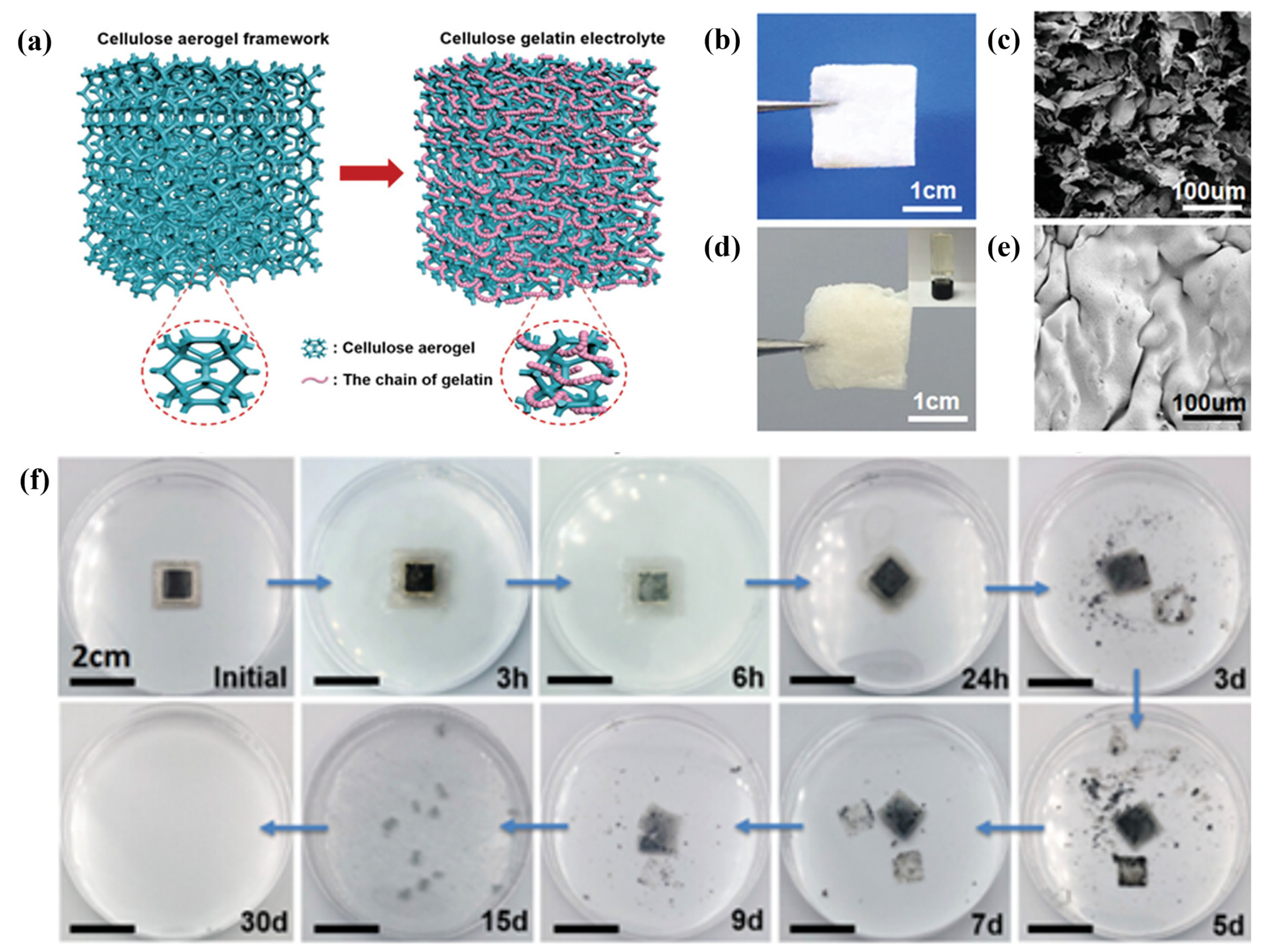
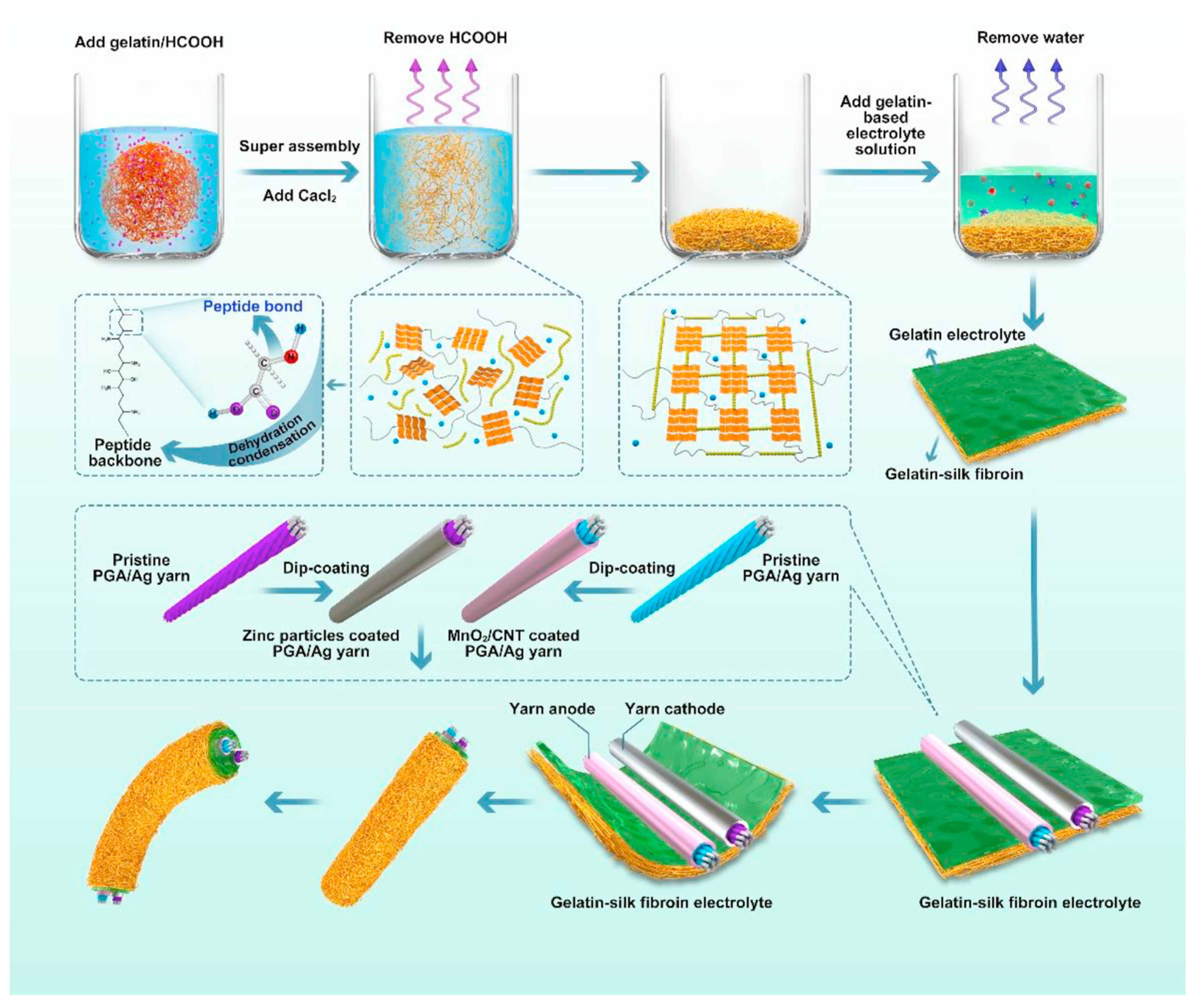
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Xie, L.; Xu, R.; Zhang, R.; Gao, M.; Tian, W.; Li, D.; Qiao, L.; Wang, T.; et al. Humidity-sensitive, shape-controllable, and transient zinc-ion batteries based on plasticizing gelatin-silk protein electrolytes. Mater. Today Energy 2021, 21, 100712. [Google Scholar] [CrossRef]
- Wang, W.; Liao, C.; Liew, K.M.; Chen, Z.; Song, L.; Kan, Y.; Hu, Y.J.J.o.M.C.A. A 3D flexible and robust HAPs/PVA separator prepared by a freezing-drying method for safe lithium metal batteries. J. Mater. Chem. A 2019, 7, 6859–6868. [Google Scholar] [CrossRef]
- Wang, W.; Yuen, A.C.Y.; Yuan, Y.; Liao, C.; Li, A.; Kabir, I.I.; Kan, Y.; Hu, Y.; Yeoh, G.H.J.C.E.J. Nano architectured halloysite nanotubes enable advanced composite separator for safe lithium metal batteries. Chem. Eng. J. 2023, 451, 138496. [Google Scholar] [CrossRef]
- Yuan, D.; Manalastas, W., Jr.; Zhang, L.; Chan, J.J.; Meng, S.; Chen, Y.; Srinivasan, M. Lignin@Nafion Membranes Forming Zn Solid-Electrolyte Interfaces Enhance the Cycle Life for Rechargeable Zinc-Ion Batteries. ChemSusChem 2019, 12, 4889–4900. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 2302098. [Google Scholar] [CrossRef]
- Sownthari, K.; Suthanthiraraj, S.A. Preparation and properties of biodegradable polymer-layered silicate nanocomposite electrolytes for zinc based batteries. Electrochim. Acta 2015, 174, 885–892. [Google Scholar] [CrossRef]
- Sai Prasanna, C.M.; Austin Suthanthiraraj, S. PVC/PEMA-based blended nanocomposite gel polymer electrolytes plasticized with room temperature ionic liquid and dispersed with nano-ZrO2 for zinc ion batteries. Polym. Compos. 2019, 40, 3402–3411. [Google Scholar] [CrossRef]
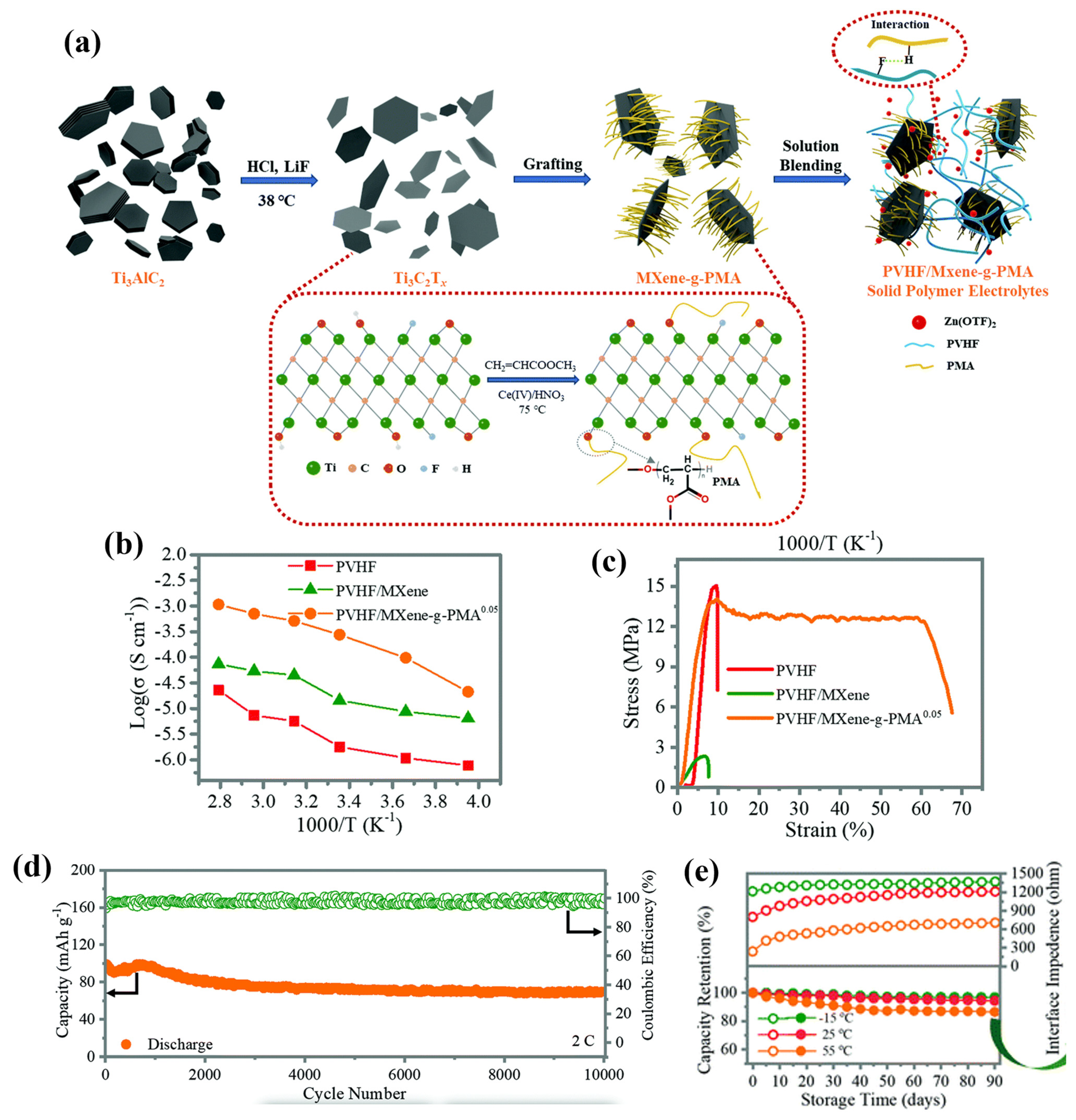
- Chen, Z.; Li, X.; Wang, D.; Yang, Q.; Ma, L.; Huang, Z.; Liang, G.; Chen, A.; Guo, Y.; Dong, B.; et al. Grafted MXene/polymer electrolyte for high performance solid zinc batteries with enhanced shelf life at low/high temperatures. Energy Environ. Sci. 2021, 14, 3492–3501. [Google Scholar] [CrossRef]
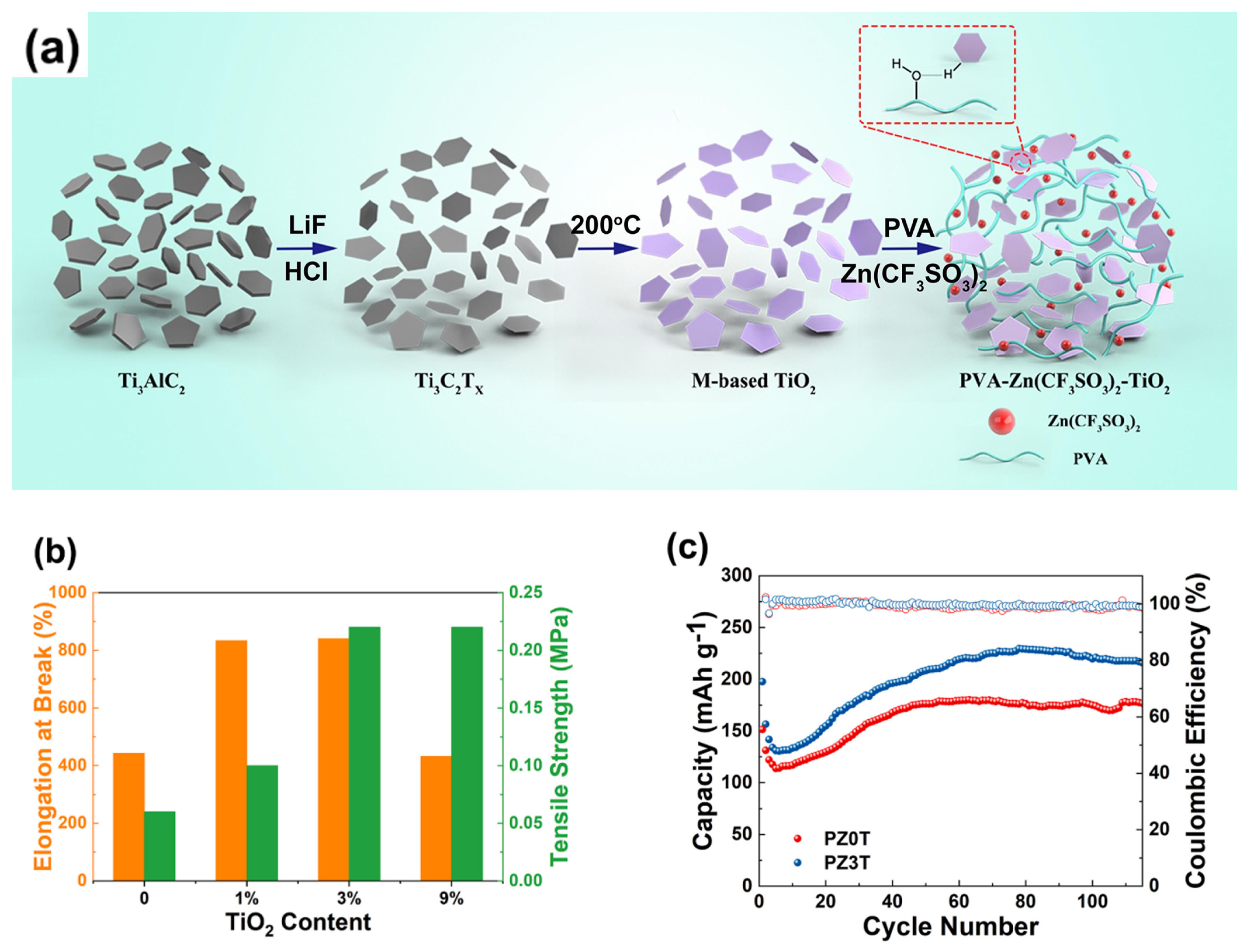
- Liu, C.; Tian, Y.; An, Y.; Yang, Q.; Xiong, S.; Feng, J.; Qian, Y. Robust and flexible polymer/MXene-derived two dimensional TiO2 hybrid gel electrolyte for dendrite-free solid-state zinc-ion batteries. Chem. Eng. J. 2022, 430, 132748. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.-C.; Liu, K.; Cui, Y. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly (ethylene oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef]
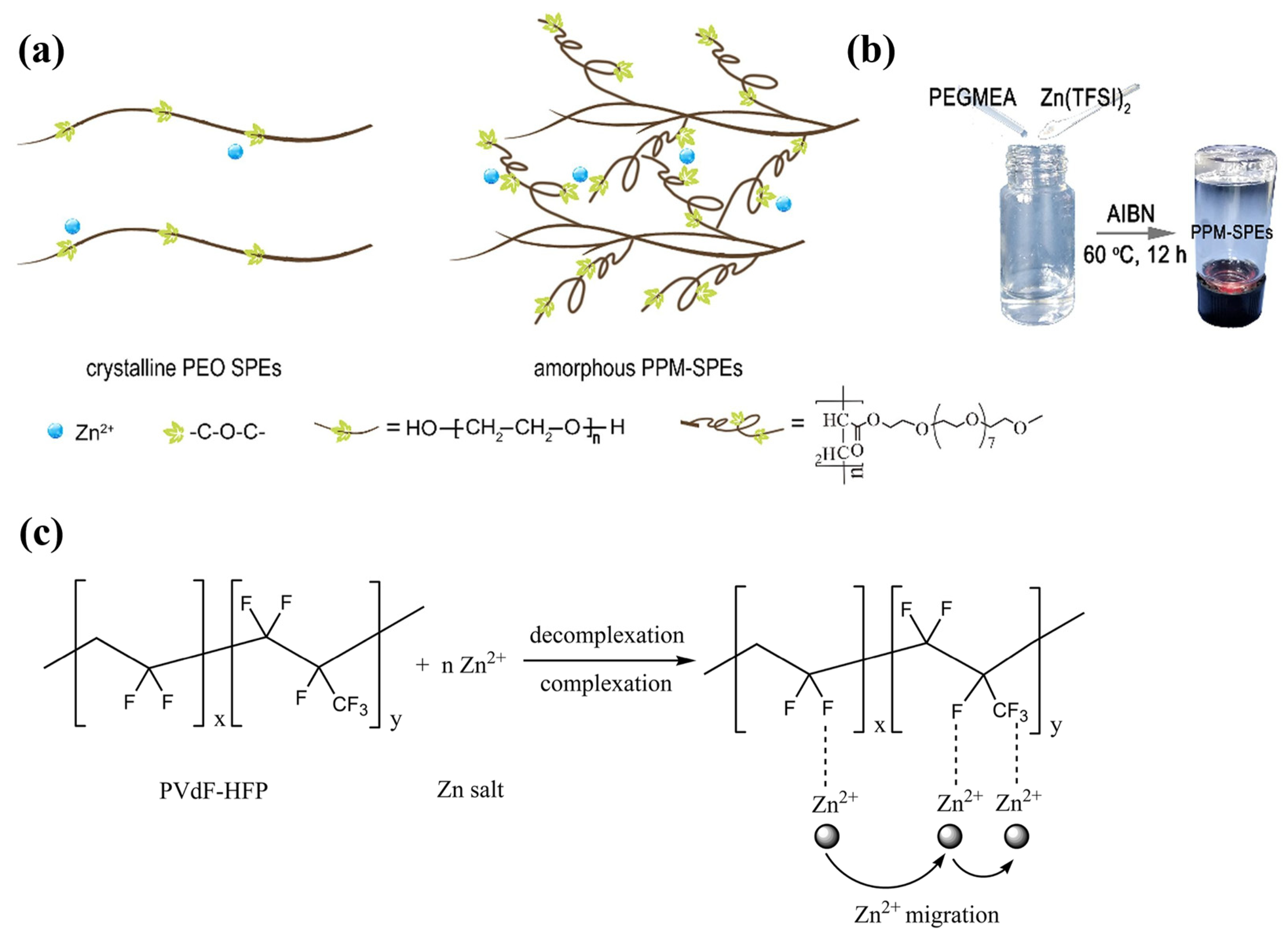
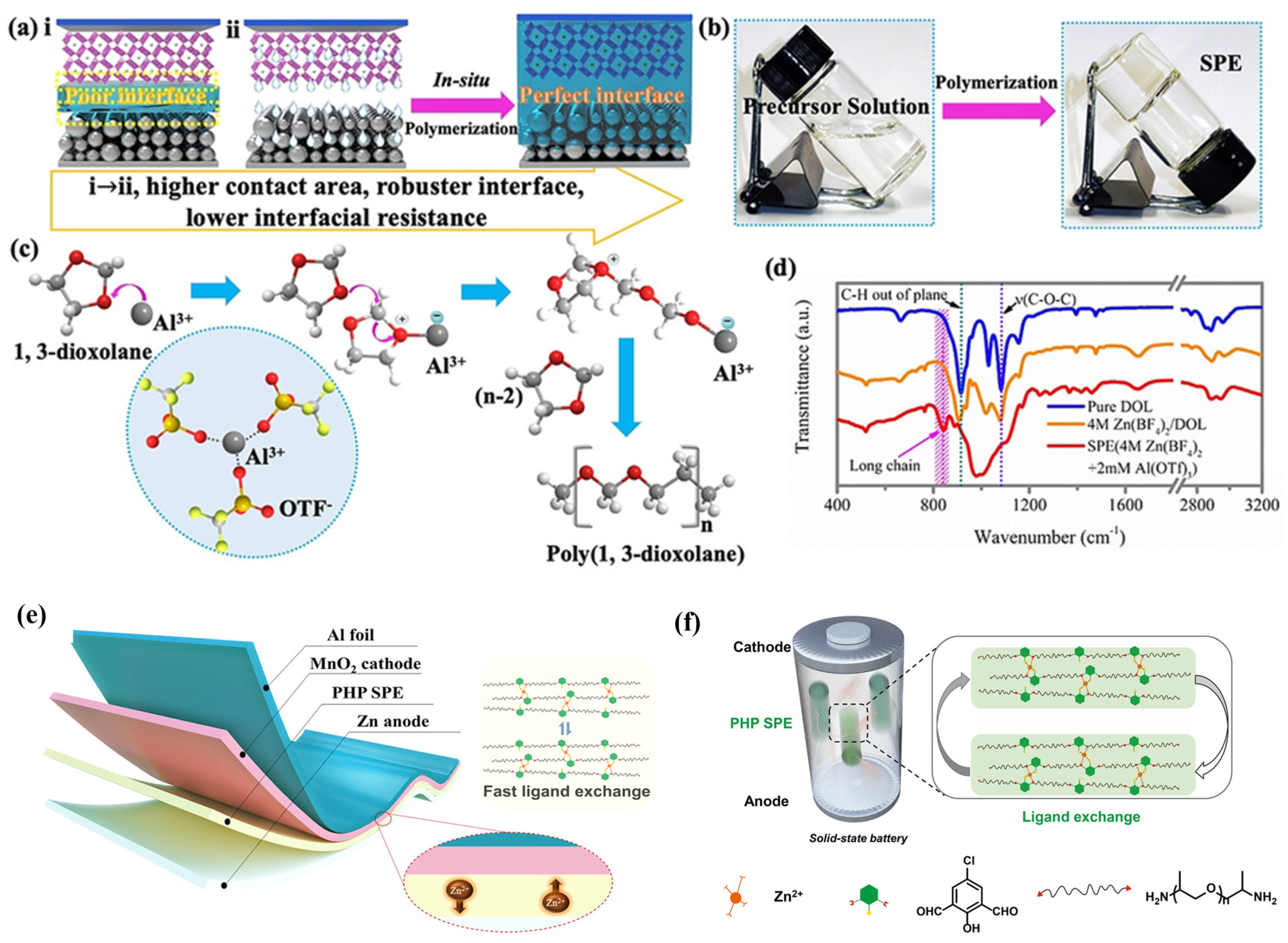
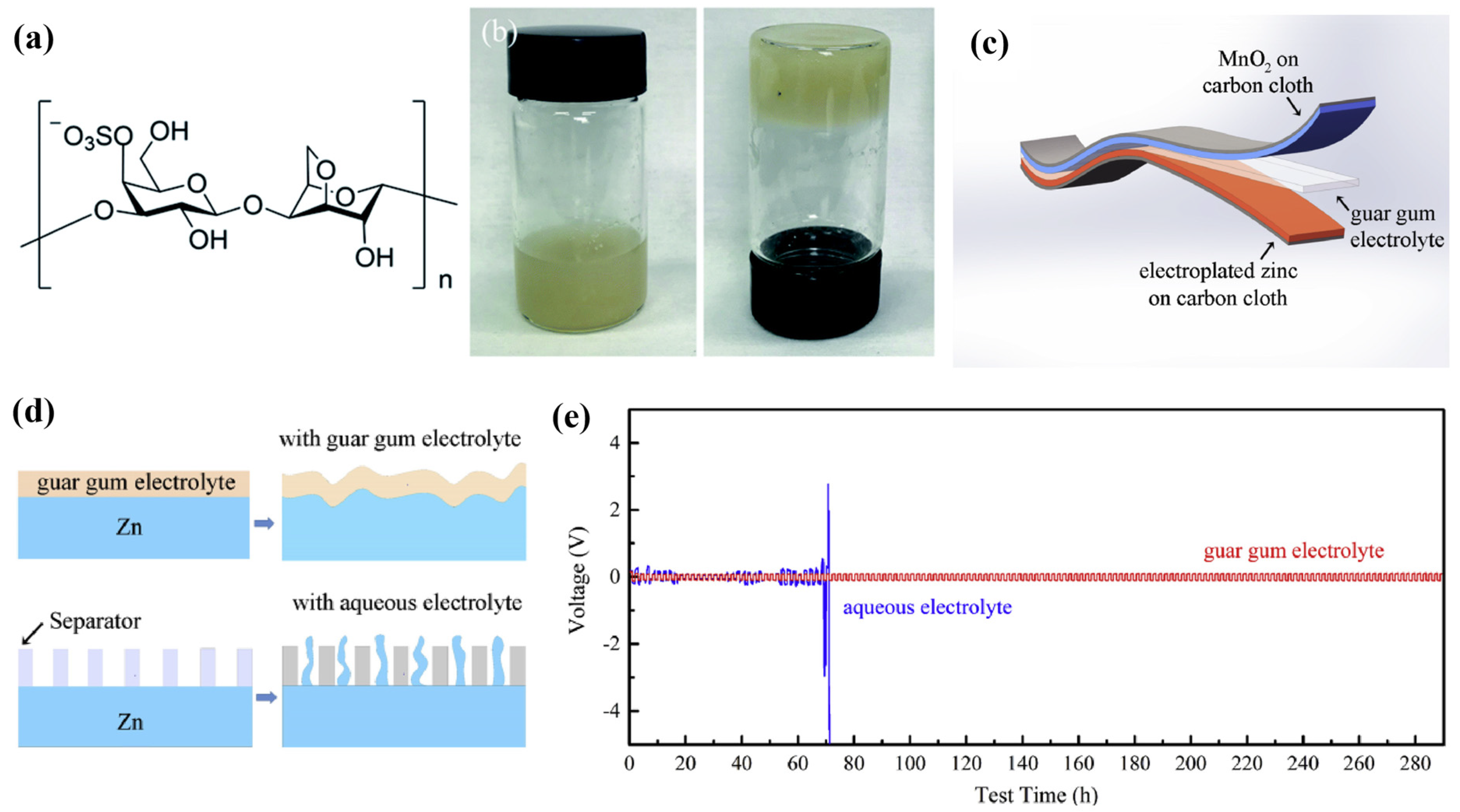
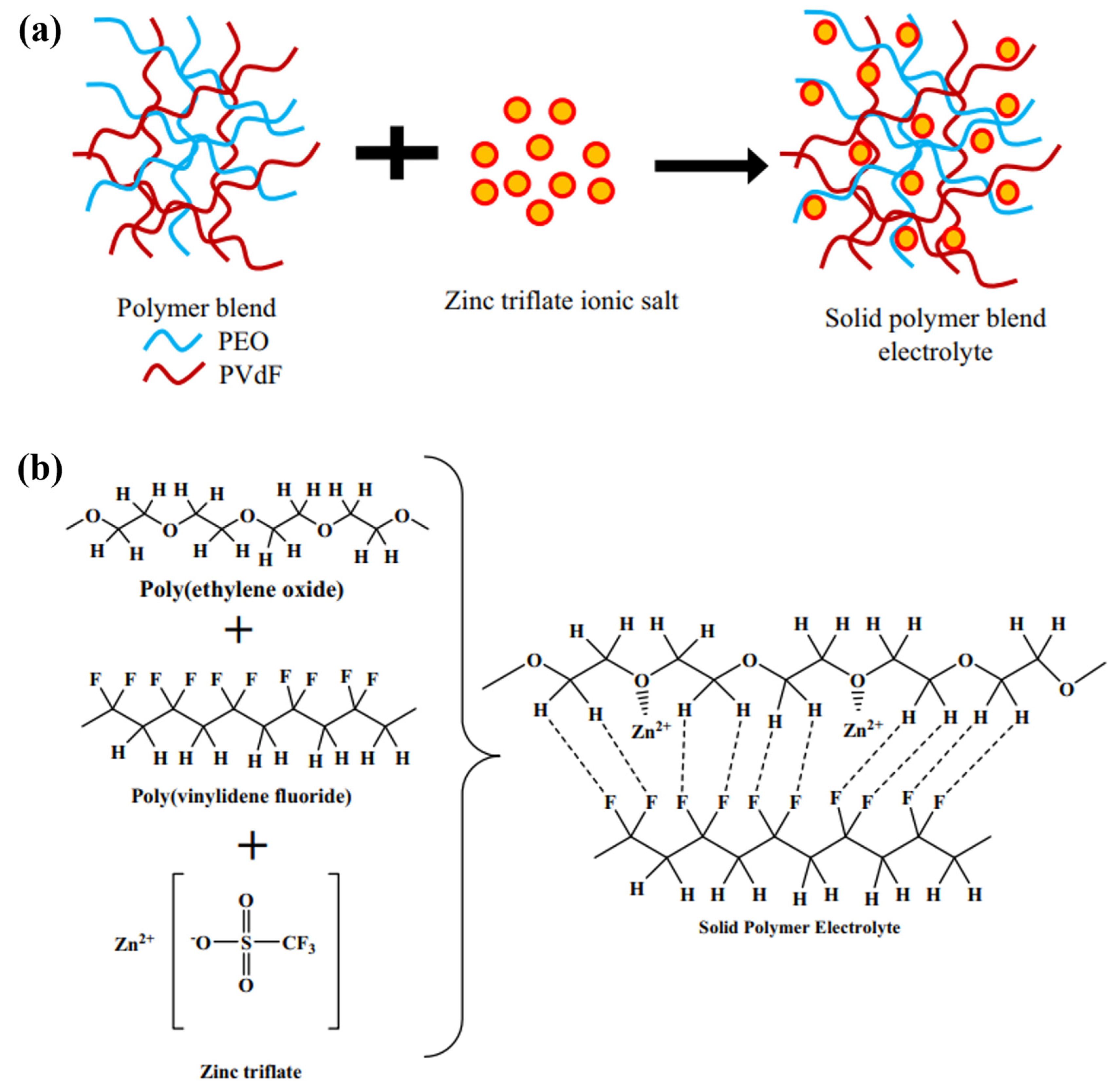

| Name | Molecule Structure | Ionic Conductivity (S cm−1) | Features | Year, Ref |
|---|---|---|---|---|
| PEO-PPM | 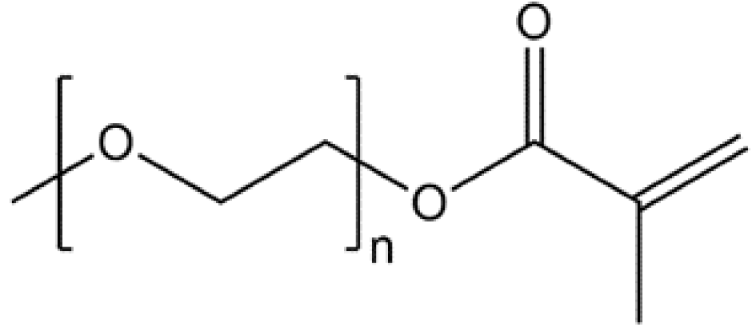 | 2.87 × 10−5 |
| 2021, [45] |
| PVdF-HFP | 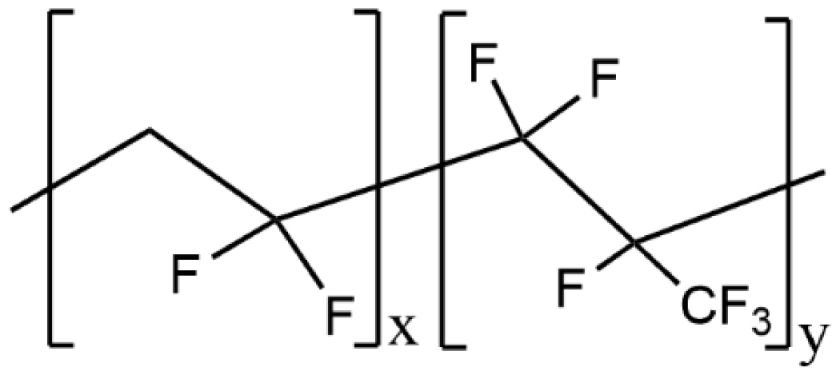 | 2.44 × 10−5 |
| 2020, [31] |
| Poly(1,3-dioxolane) | 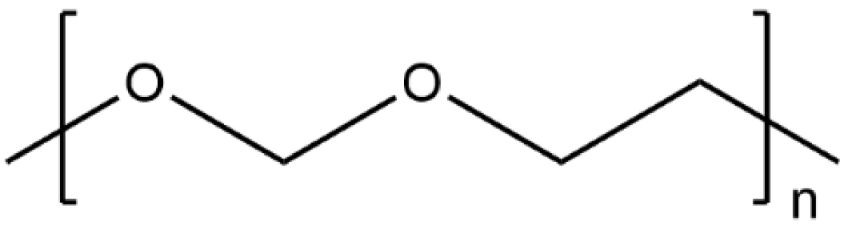 | 1.96 × 10−2 |
| 2020, [26] |
| PHP |  | 6 × 10−6 |
| 2021, [23] |
| HEC |  | 1 × 10−6 |
| 2022, [2] |
| Kappa-Carrageenan | 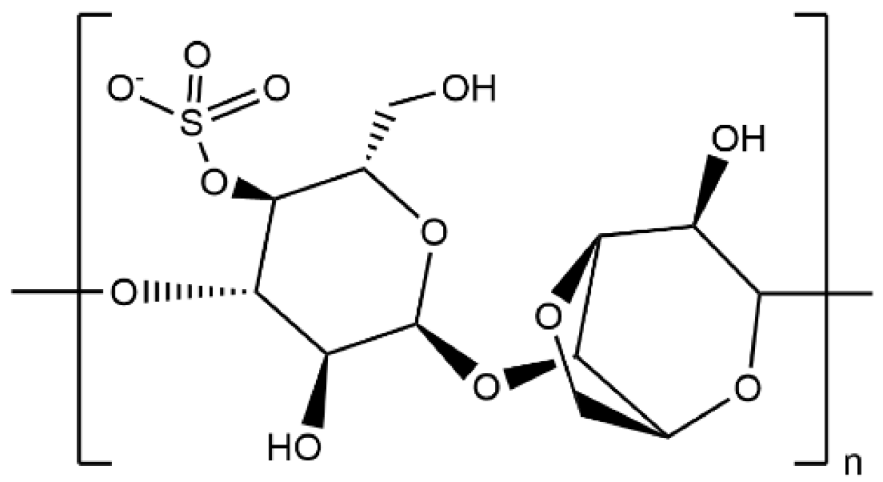 | 3.32 × 10−2 |
| 2019, [47] |
| Guar Gum | 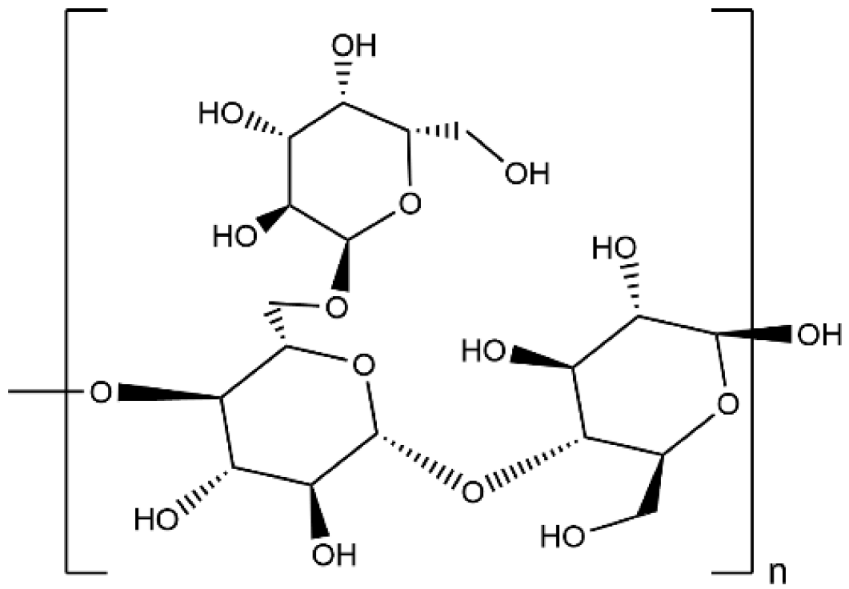 | 1.07 × 10−2 |
| 2019, [48] |
| Chitosan | 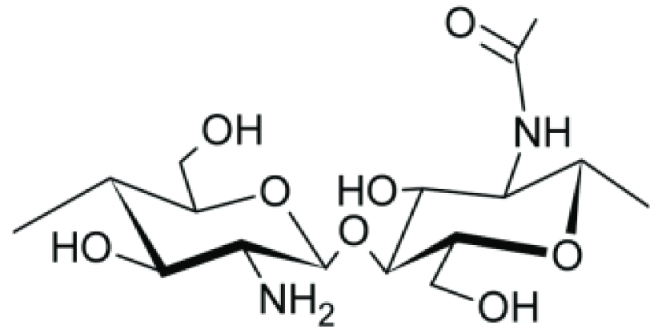 | 7.2 × 10−2 |
| 2022, [49] |
| Name | Molecule Structure | Ionic Conductivity (S cm−1) | Features | Year, Ref |
|---|---|---|---|---|
| PEO/PVDF |  | 2.5 × 10−5 |
| 2017, [51] |
| PAAM/Acetamide | 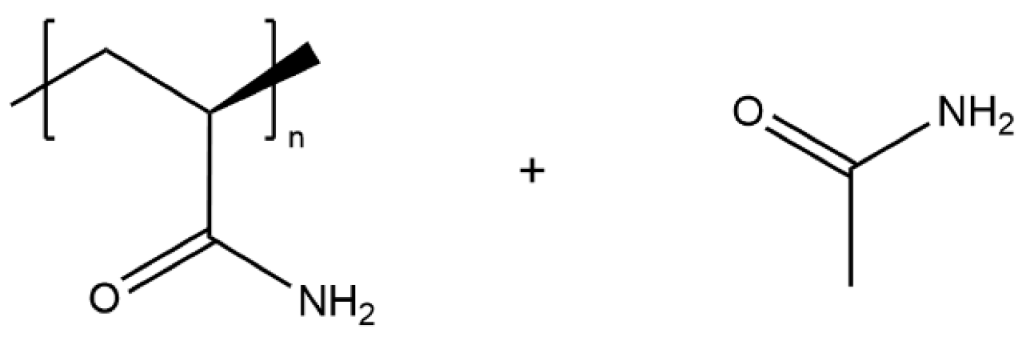 | 4.7 × 10−3 |
| 2022, [52] |
| Gelatin/PAM | 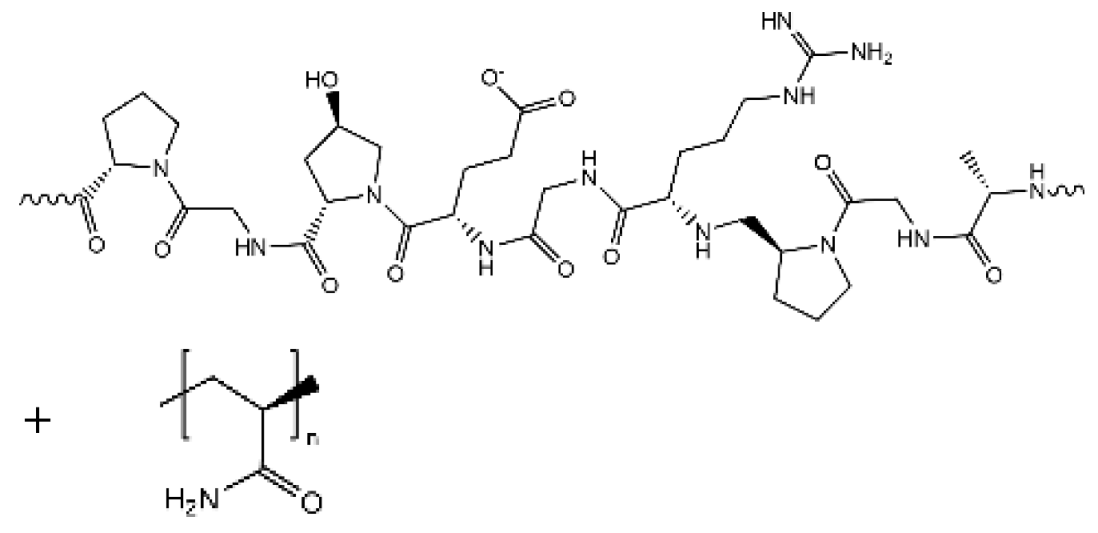 | 1.76 × 10−2 |
| 2018, [53] |
| PAM/DMAPS/Gelatin | 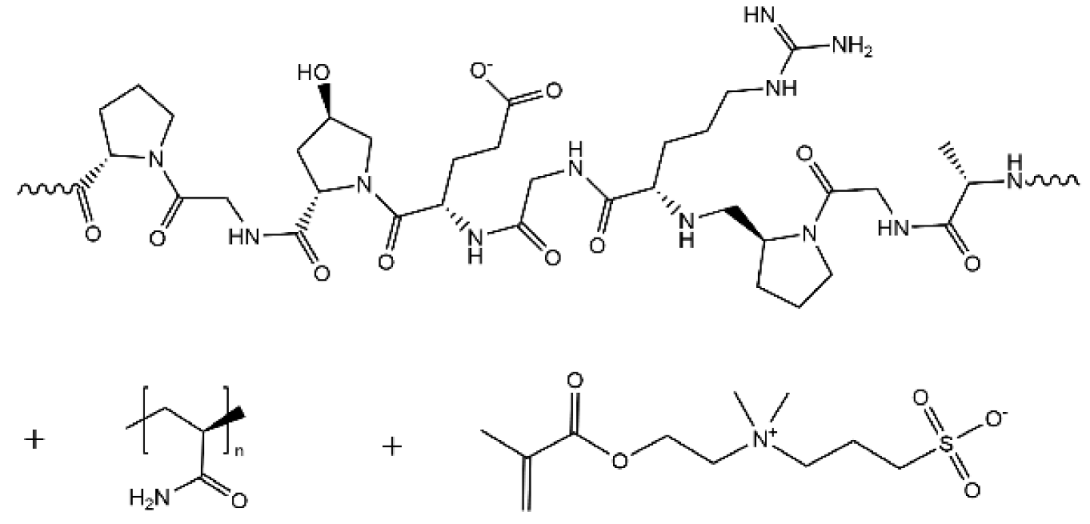 | 3.51× 10−2 |
| 2022, [54] |
| CMC/PNiPAM | 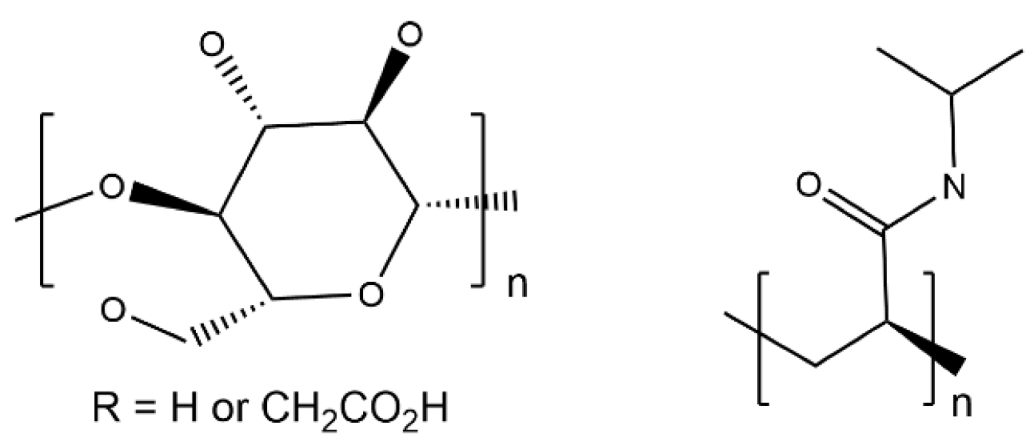 | 1.68 × 10−4 |
| 2020, [55] |
| Cellulose/Gelatin | 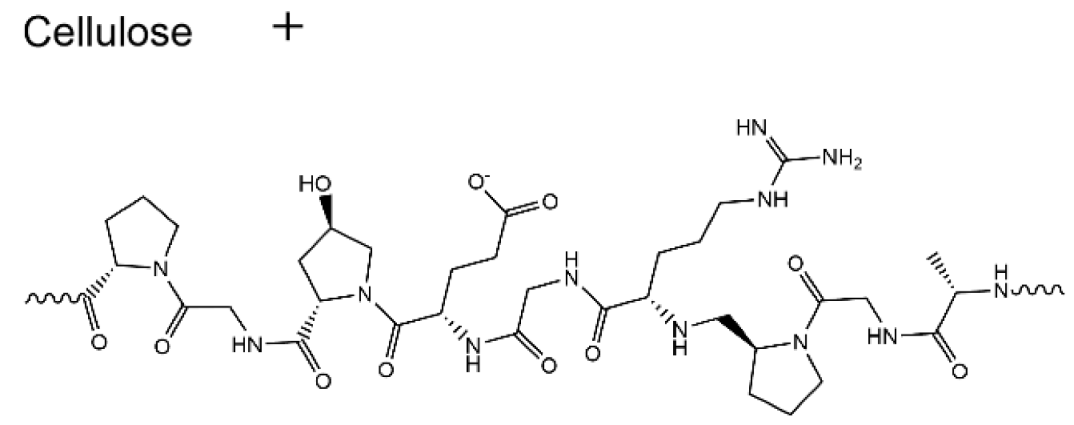 | 1.23 × 10−2 |
| 2022, [56] |
| Gelatin-silk fibroin |  | 5.68 × 10−3 |
| 2022, [57] |
| Lignin/Nafion | 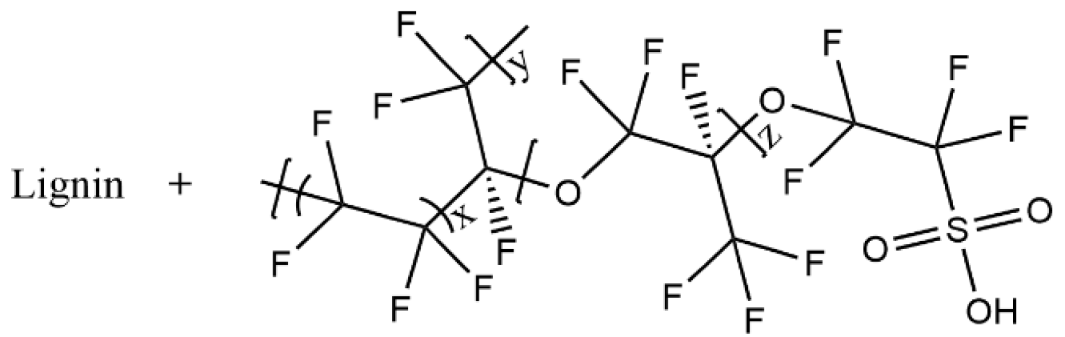 | 9.1 × 10−3 |
| 2019, [60] |
| Cellulose/CMC | 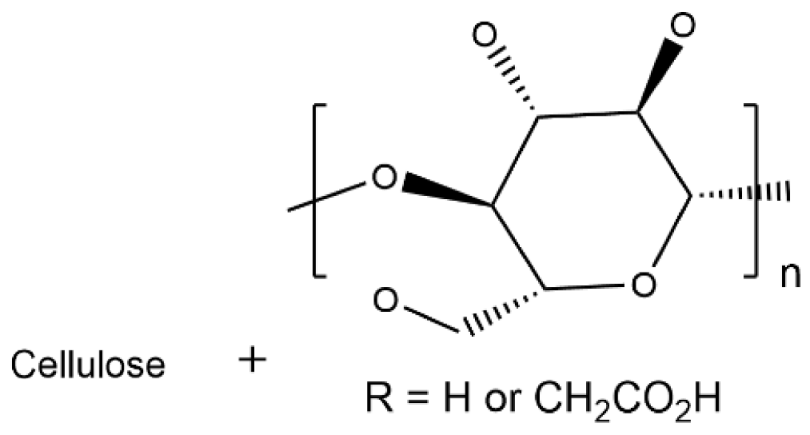 | 2.6 × 10−2 |
| 2023, [61] |
| Name | Molecule Structure | Ionic Conductivity (S cm−1) | Feature | Year, Ref |
|---|---|---|---|---|
| MMT/PCL | 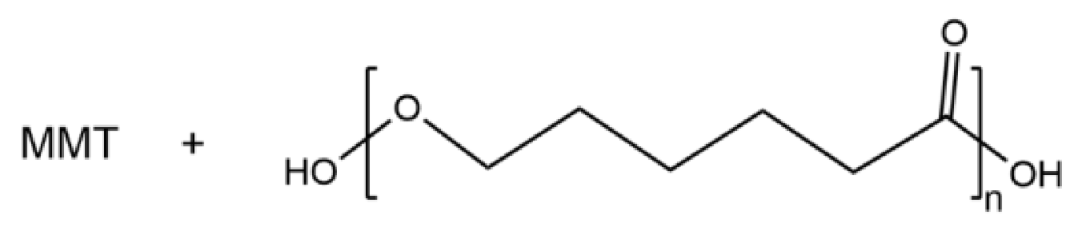 | 9.5 × 10−5 |
| 2015, [62] |
| PVC/PEMA/ZrO2 | 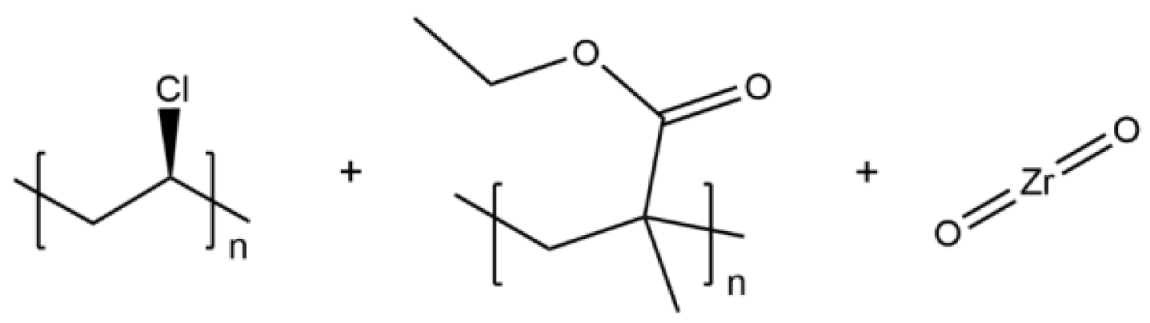 | 3.63 × 10−4 |
| 2019, [63] |
| PVHF/Mxene/PMA | 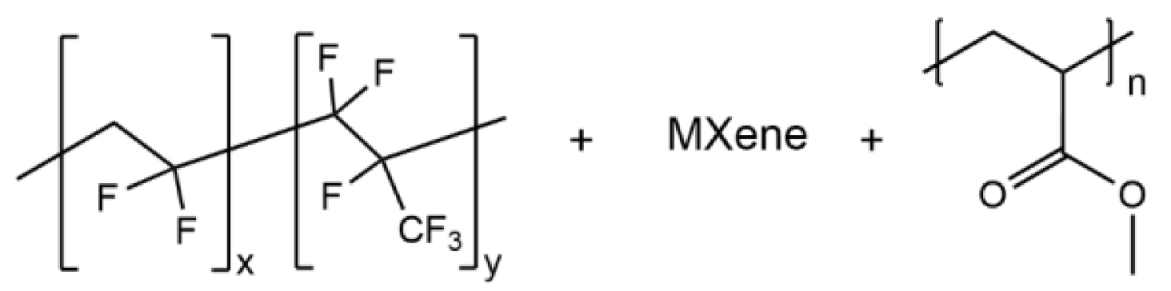 | 2.69 × 10−4 |
| 2021, [64] |
| PVA/TiO2 | 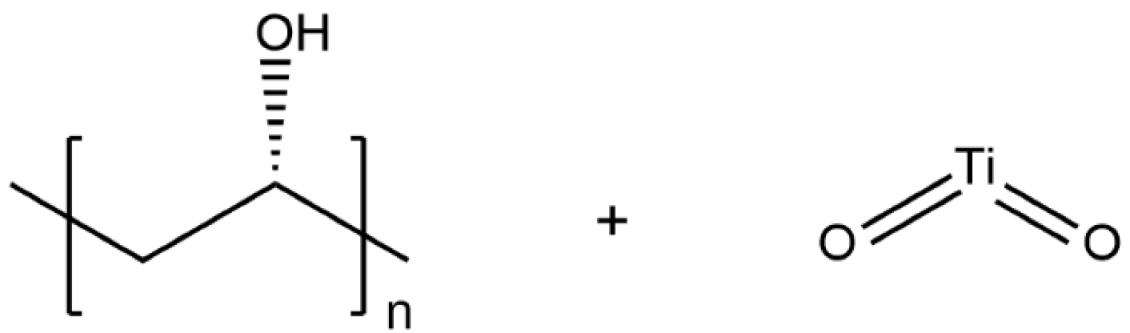 | 1.243 × 10−5 |
| 2022, [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cachinho Cordeiro, I.M.; Li, A.; Lin, B.; Ma, D.X.; Xu, L.; Eh, A.L.-S.; Wang, W. Solid Polymer Electrolytes for Zinc-Ion Batteries. Batteries 2023, 9, 343. https://doi.org/10.3390/batteries9070343
De Cachinho Cordeiro IM, Li A, Lin B, Ma DX, Xu L, Eh AL-S, Wang W. Solid Polymer Electrolytes for Zinc-Ion Batteries. Batteries. 2023; 9(7):343. https://doi.org/10.3390/batteries9070343
Chicago/Turabian StyleDe Cachinho Cordeiro, Ivan Miguel, Ao Li, Bo Lin, Daphne Xiuyun Ma, Lulu Xu, Alice Lee-Sie Eh, and Wei Wang. 2023. "Solid Polymer Electrolytes for Zinc-Ion Batteries" Batteries 9, no. 7: 343. https://doi.org/10.3390/batteries9070343
APA StyleDe Cachinho Cordeiro, I. M., Li, A., Lin, B., Ma, D. X., Xu, L., Eh, A. L.-S., & Wang, W. (2023). Solid Polymer Electrolytes for Zinc-Ion Batteries. Batteries, 9(7), 343. https://doi.org/10.3390/batteries9070343







