Exploring the Performance and Mass-Transfer Characteristics of Porous Zinc Anodes for Membraneless Hybrid-Flow Batteries
Abstract
1. Introduction
2. Experimental Details
2.1. Experimental Materials
2.2. Preparation of Zinc Anodes
2.3. Physicochemical Characterizations
2.4. Half- and Full-Cell Charge–Discharge Cycling
2.5. COMSOL Multiphysics Numerical Modelling
- (1)
- The distance between the initial electrode and the deposited metal zinc is negligible;
- (2)
- The electrodes are uniform in texture, and the structure of electrodes will not be changed by zinc deposition;
- (3)
- The electrolyte has uniform viscosity and is an incompressible fluid, and its side reactions are negligible;
- (4)
- Regardless of the gravity effect of the battery system, the battery operates in a stable state;
- (5)
- The side reactions occurring on the electrode, such as the self-discharge reaction caused by direct contact between the metal zinc in the negative electrode and the electrolyte, are neglected.
3. Results and Discussions
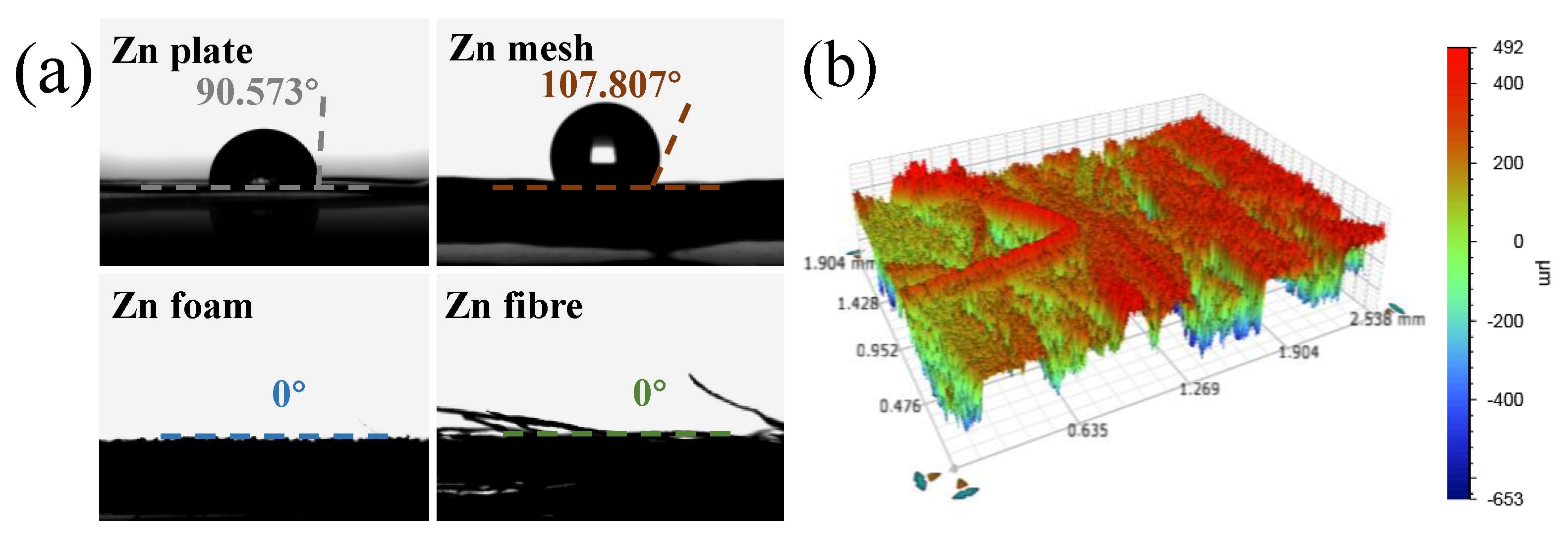
3.1. Characterizations of Two- and Three-Dimensional Zinc Anodes
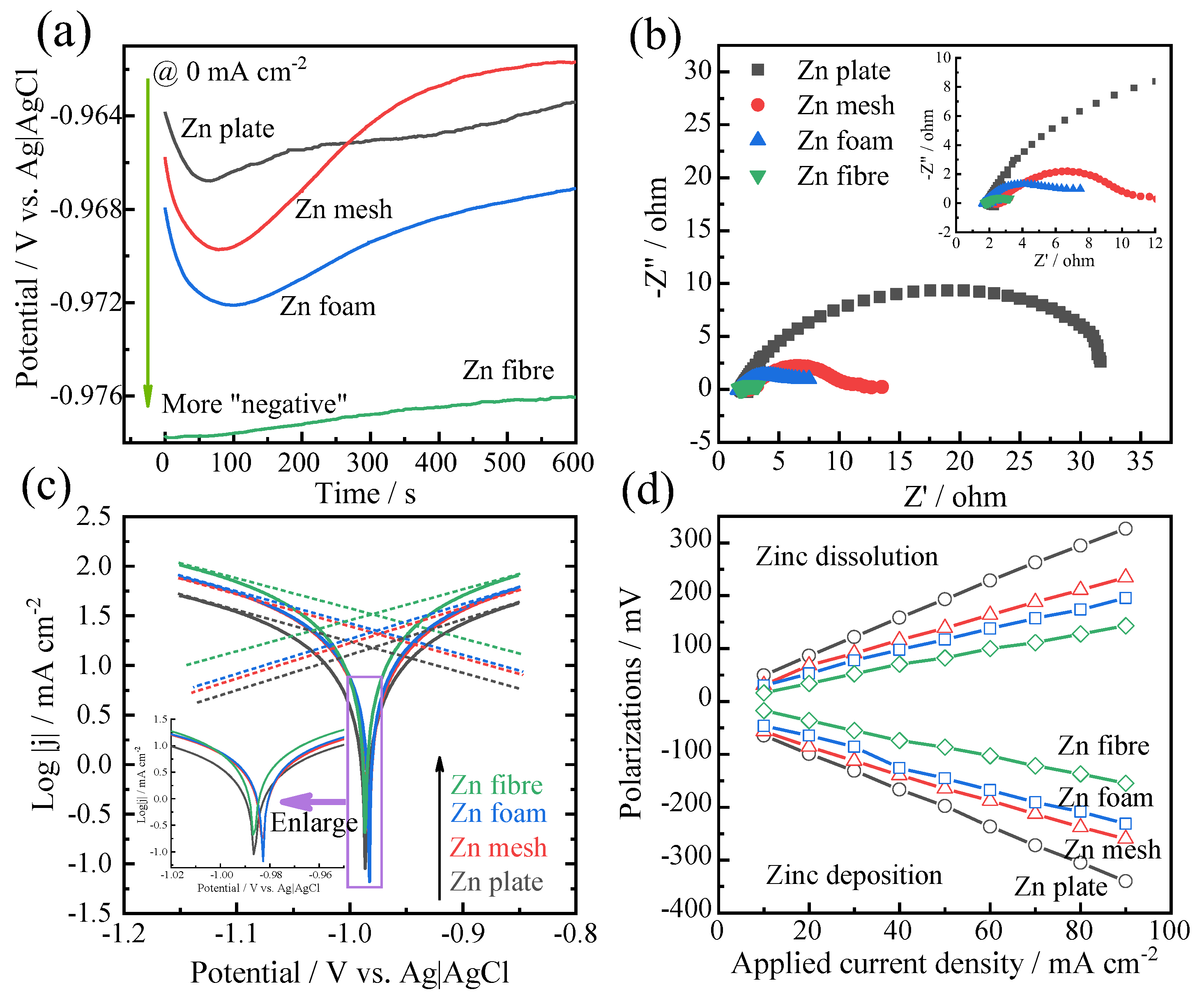
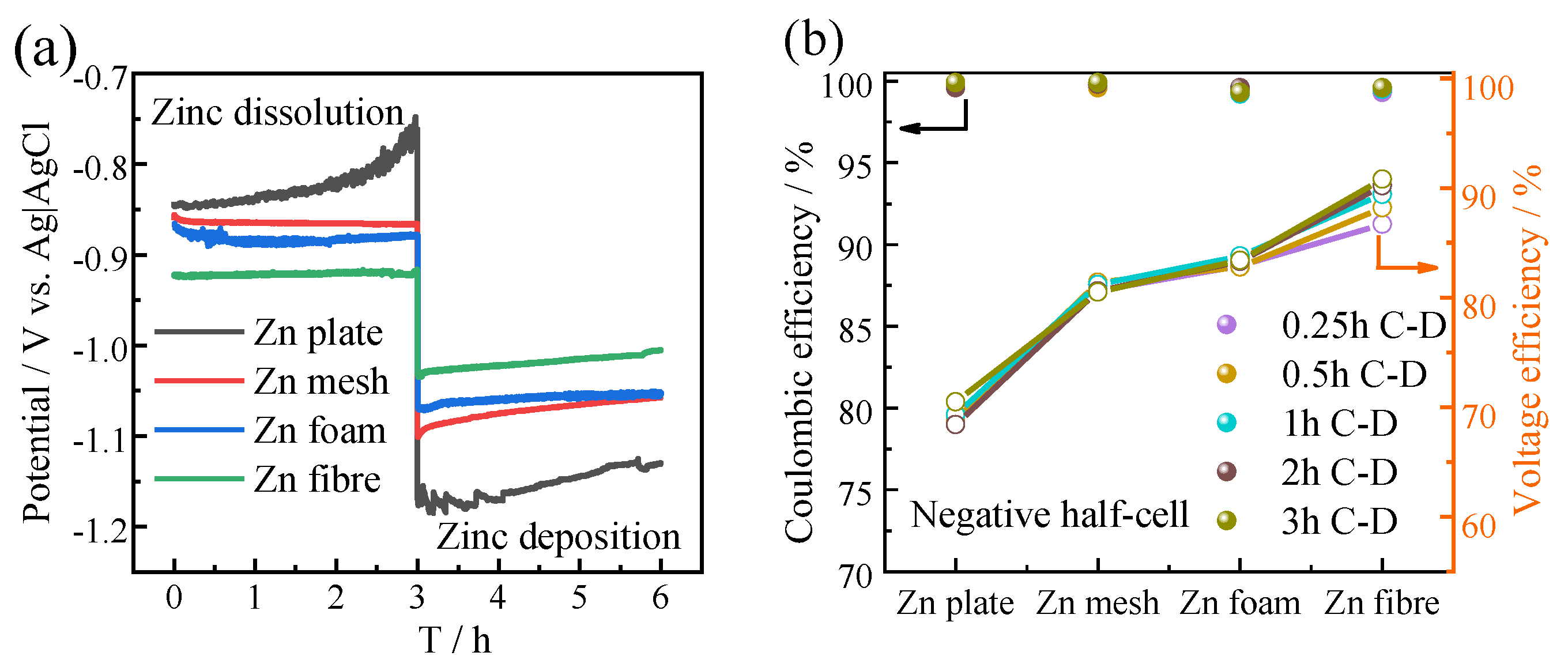
3.2. Half-Cell Study of Zinc Anodes
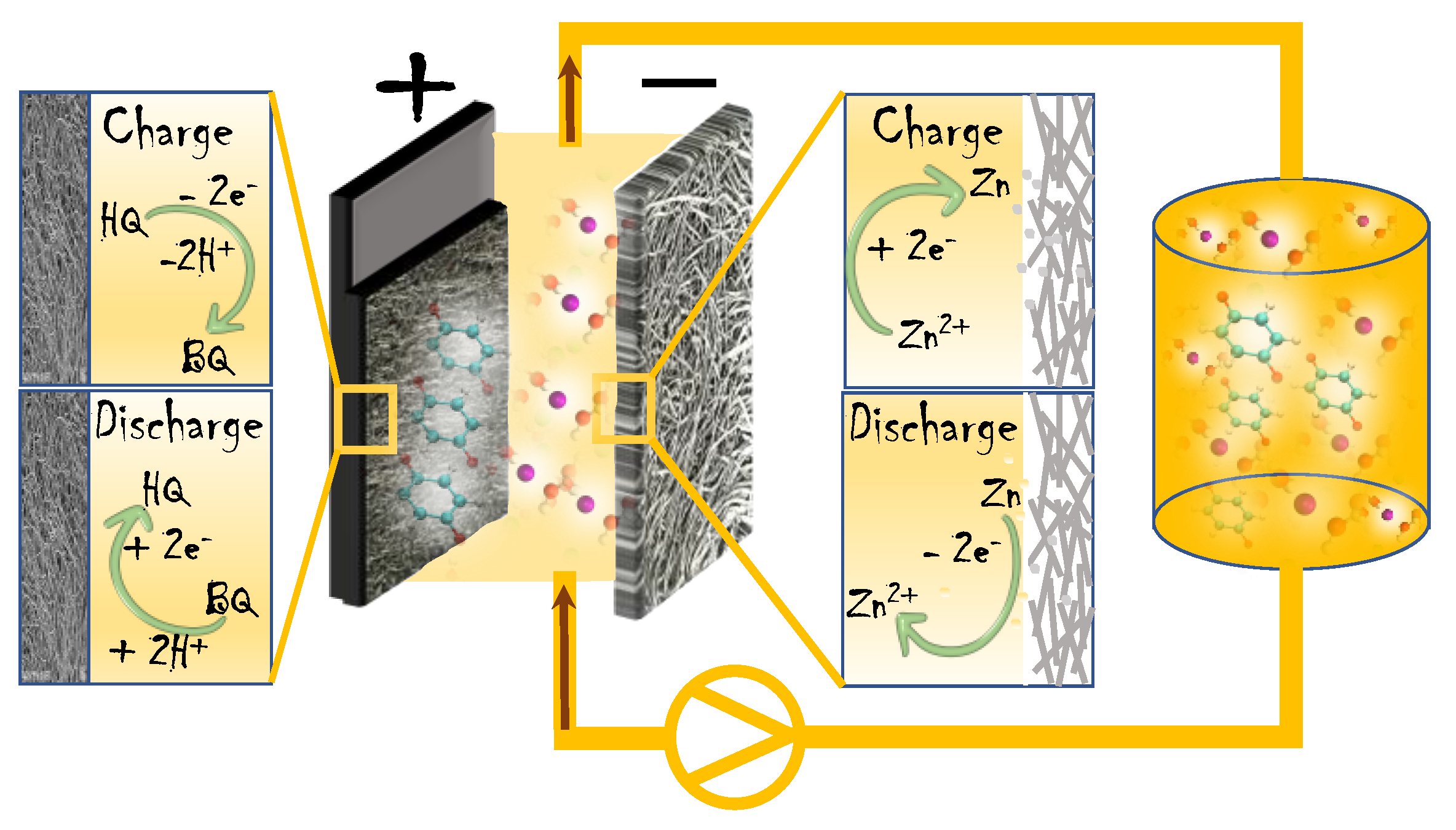
3.3. Full-Cell Study of a Membraneless Hybrid-Flow Battery
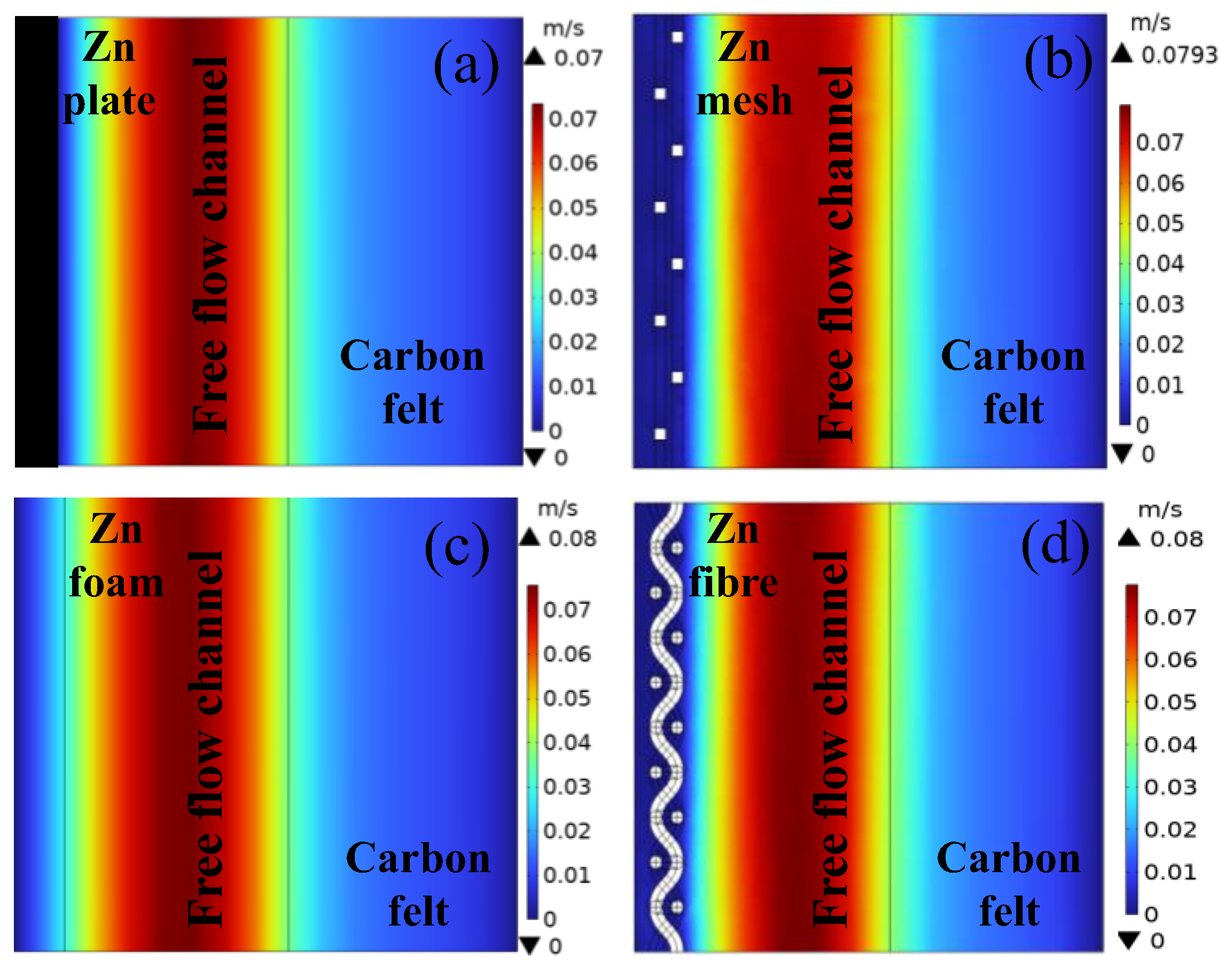
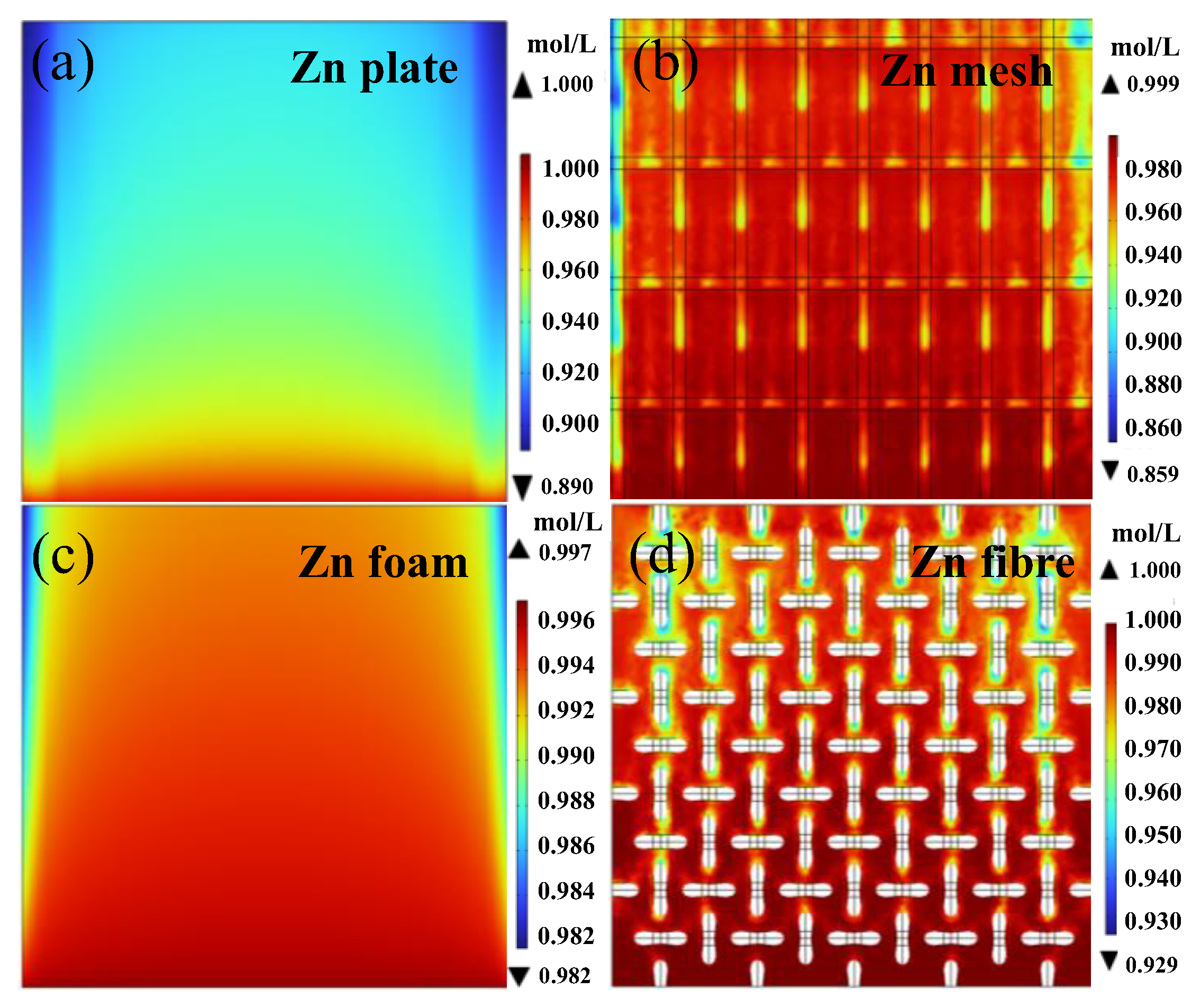
3.3.1. Mass-Transport Analysis Based on Multiphysics Models
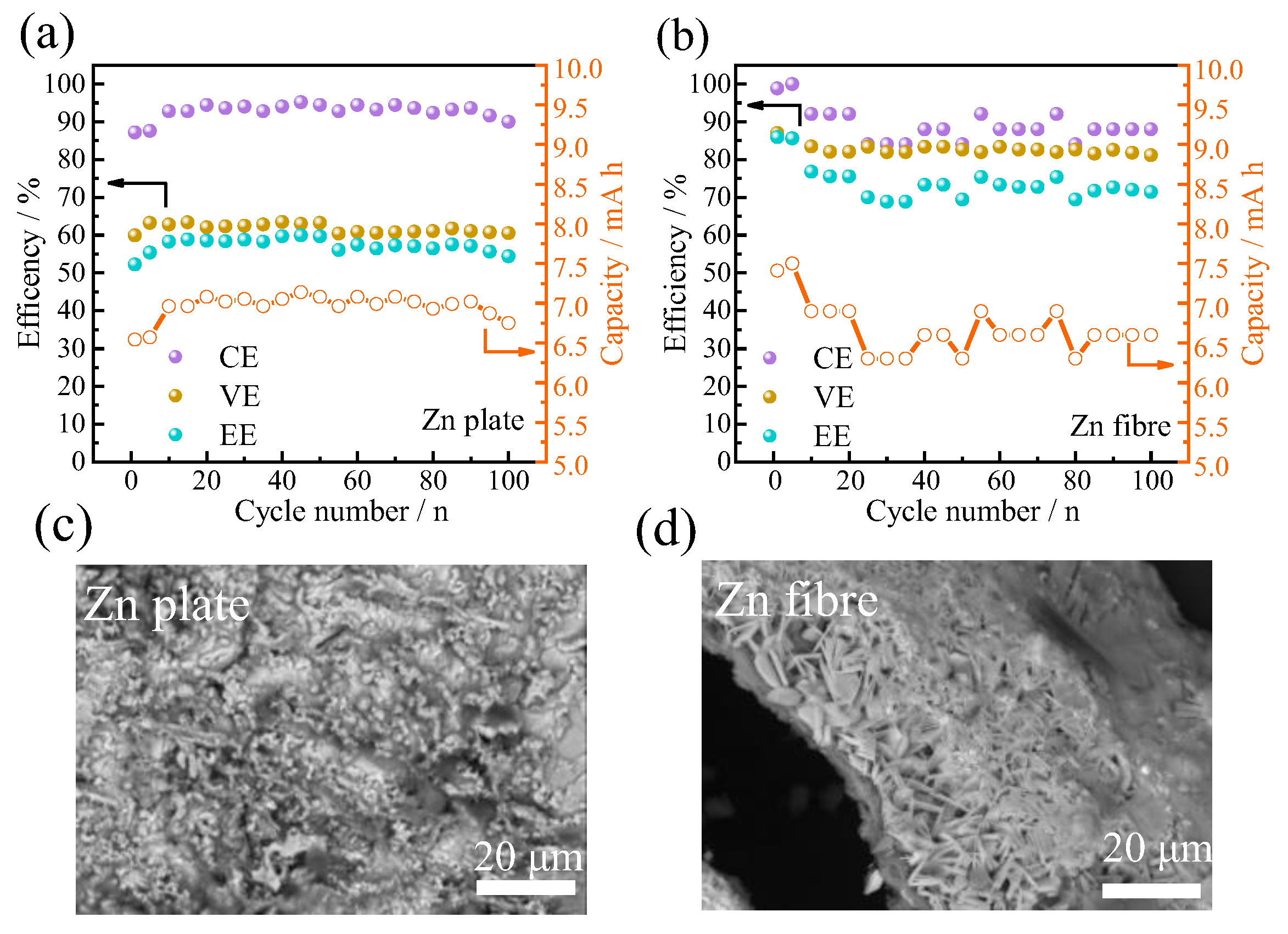
3.3.2. Battery Performance Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Hao, R.; Liu, X.; Zhang, N.; Guo, W.; Zhang, Z.; Liu, C.; Liu, Y.; Duan, C.; Qin, J. Thermodynamic performance analysis of an improved coal-fired power generation system coupled with geothermal energy based on organic Rankine cycle. Renew. Energy 2022, 201, 273–290. [Google Scholar] [CrossRef]
- Song, Y.L.K.; Zhang, X.; Jiang, J.; Sun, C.; Lan, H. A Review on Interoperability of Wireless Charging Systems for Electric Vehicles. Energies 2023, 16, 1653. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kim, J.H.; Hossain, S. Recent advances of energy storage technologies for grid: A comprehensive review. Energy Storage 2022, 4, e322. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Emmett, R.K.; Roberts, M.E. Recent developments in alternative aqueous redox flow batteries for grid-scale energy storage. J. Power Sources 2021, 506, 230087. [Google Scholar] [CrossRef]
- Schubert, C.; Hassen, W.F.; Poisl, B.; Seitz, S.; Schubert, J.; Usabiaga, E.O.; Gaudo, P.M.; Pettinger, K.-H. Hybrid Energy Storage Systems Based on Redox-Flow Batteries: Recent Developments, Challenges, and Future Perspectives. Batteries 2023, 9, 211. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Xu, Q.; Mohamed, M.R.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A. Future perspective on redox flow batteries: Aqueous versus nonaqueous electrolytes. Curr. Opin. Chem. Eng. 2022, 37, 100833. [Google Scholar] [CrossRef]
- Mongird, V.V.K.; Alam, J.; Vartanian, C.; Sprenkle, V.; Baxter, R. Grid Energy Storage Technology Cost and Performance Assessment. Energy 2020, 5, 6–15. [Google Scholar]
- Brushett, F.R.; Aziz, M.J.; Rodby, K.E. On Lifetime and Cost of Redox-Active Organics for Aqueous Flow Batteries. ACS Energy Lett. 2020, 5, 879–884. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Sun, H.Z. C-Y. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Mohamed, M.R.; Xu, Q.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A.; Liao, Q. Capital cost evaluation of conventional and emerging redox flow batteries for grid storage applications. Electrochim. Acta 2023, 437, 141460. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Leung, P.; Velasco, P.Q.; Pahlevani, M.; Walsh, F.C.; Roberts, E.P.L.; de Le, C.P. A nonaqueous organic redox flow battery using multi-electron quinone molecules. J. Power Sources 2021, 500, 229942. [Google Scholar] [CrossRef]
- Yadav, G.G.; Weiner, M.; Upreti, A.; Huang, J.; Lambert, T.N.; Arnot, D.J.; Schorr, N.B.; Bell, N.S.; Turney, D.; Hawkins, B.; et al. The advent of membrane-less zinc-anode aqueous batteries with lithium battery-like voltage. Mater Horiz 2022, 9, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, Y.; Lek, D.G.; Liu, T.; Lin, F.; Luo, W.; Huang, S.; Gao, M.; Wang, X.; Zhi, Y.; et al. Membrane-free redox flow cell based on thermally regenerative electrochemical cycle for concurrent electricity storage, cooling and waste heat harnessing of perovskite solar cells. J. Power Sources 2022, 548, 232081. [Google Scholar] [CrossRef]
- Ma, L.; Zhi, C. Zn electrode/electrolyte interfaces of Zn batteries: A mini review. Electrochem. Commun. 2021, 122, 106898. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Zhang, H.; Li, X. Anode for Zinc-Based Batteries: Challenges, Strategies, and Prospects. ACS Energy Lett. 2021, 6, 2765–2785. [Google Scholar] [CrossRef]
- Yuan, Z.; Yin, Y.; Xie, C.; Zhang, H.; Yao, Y.; Li, X. Advanced Materials for Zinc-Based Flow Battery: Development and Challenge. Adv. Mater. 2019, 31, e1902025. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Yang, Y.-S.; Wen, Y.-H.; Cao, G.-P.; Wang, X.-D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Leung, P.; de Le, C.P.; Walsh, F.C. An undivided zinc–cerium redox flow battery operating at room temperature (295 K). Electrochem. Commun. 2011, 13, 770–773. [Google Scholar] [CrossRef]
- Kong, T.; Liu, J.; Zhou, X.; Xu, J.; Xie, Y.; Chen, J.; Li, X.; Wang, Y. Stable Operation of Aqueous Organic Redox Flow Batteries in Air Atmosphere. Angew. Chem. Int. Ed. 2023, 62, e202214819. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Hu, B.; Li, H.; Ravivarma, M.; Feng, Y.; Song, J. Conjugate-Driven Electron Density Delocalization of Piperidine Nitroxyl Radical for Stable Aqueous Zinc Hybrid Flow Batteries. Angew. Chem. Int. Ed. 2022, 61, e202115908. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Martin, T.; Shah, A.A.; Mohamed, M.R.; Anderson, M.A.; Palma, J. Membrane-less hybrid flow battery based on low-cost elements. J. Power Sources 2017, 341, 36–45. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Zhang, S.; Bo, G.; Wang, C.; Guo, Z. Designing Dendrite-Free Zinc Anodes for Advanced Aqueous Zinc Batteries. Adv. Funct. Mater. 2020, 30, 2001263. [Google Scholar] [CrossRef]
- Han, D.; Wu, S.; Zhang, S.; Deng, Y.; Cui, C.; Zhang, L.; Long, Y.; Li, H.; Tao, Y.; Weng, Z.; et al. A Corrosion-Resistant and Dendrite-Free Zinc Metal Anode in Aqueous Systems. Small 2020, 16, e2001736. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B.; Zhi, C. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, e2001854. [Google Scholar] [CrossRef]
- Chamoun, M.; Hertzberg, B.J.; Gupta, T.; Davies, D.; Bhadra, S.; Van Tassell, B.; Erdonmez, C.; Steingart, D.A. Hyper-dendritic nanoporous zinc foam anodes. NPG Asia Mater. 2015, 7, e178. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Sun, Y. Anode corrosion in aqueous Zn metal batteries. eScience 2023, 3, 100093. [Google Scholar] [CrossRef]
- Borchers, N.; Clark, S.; Horstmann, B.; Jayasayee, K.; Juel, M.; Stevens, P. Innovative zinc-based batteries. J. Power Sources 2021, 484, 229309. [Google Scholar] [CrossRef]
- Guo, N.; Huo, W.; Dong, X.; Sun, Z.; Lu, Y.; Wu, X.; Dai, L.; Wang, L.; Lin, H.; Liu, H.; et al. A Review on 3D Zinc Anodes for Zinc Ion Batteries. Small Methods 2022, 6, e2200597. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Sassin, M.B.; Chervin, C.N.; DeSario, P.A.; Parker, J.F.; Long, J.W.; Rolison, D.R. Fabricating architected zinc electrodes with unprecedented volumetric capacity in rechargeable alkaline cells. Energy Storage Mater. 2020, 27, 370–376. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Wang, Y.; Cong, J.; Wu, X.; Song, X.; Ma, Y.; Xiang, H.; Huang, Y. Regulating Zn(002) Deposition toward Long Cycle Life for Zn Metal Batteries. ACS Energy Lett. 2022, 8, 372–380. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Elezzabi, A.Y. Transparent Zinc-Mesh Electrodes for Solar-Charging Electrochromic Windows. Adv. Mater. 2020, 32, e2003574. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance—Dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117–1124. [Google Scholar] [CrossRef]
- Parker, J.F.; Nelson, E.S.; Wattendorf, M.D.; Chervin, C.N.; Long, J.W.; Rolison, D.R. Retaining the 3D framework of zinc sponge anodes upon deep discharge in Zn-air cells. ACS Appl. Mater. Interfaces 2014, 6, 19471–19476. [Google Scholar] [CrossRef]
- Xue, R.; Kong, J.; Wu, Y.; Wang, Y.; Kong, X.; Gong, M.; Zhang, L.; Lin, X.; Wang, D. Highly reversible zinc metal anodes enabled by a three-dimensional silver host for aqueous batteries. J. Mater. Chem. A 2022, 10, 10043–10050. [Google Scholar] [CrossRef]
- Zhao, R.; Dong, X.; Liang, P.; Li, H.; Zhang, T.; Zhou, W.; Wang, B.; Yang, Z.; Wang, X.; Wang, L.; et al. Prioritizing Hetero-Metallic Interfaces via Thermodynamics Inertia and Kinetics Zincophilia Metrics for Tough Zn-Based Aqueous Batteries. Adv. Mater. 2023, 35, e2209288. [Google Scholar] [CrossRef]
- Zeng, Y.; Sun, P.X.; Pei, Z.; Jin, Q.; Zhang, X.; Yu, L.; Lou, X.W.D. Nitrogen-Doped Carbon Fibers Embedded with Zincophilic Cu Nanoboxes for Stable Zn-Metal Anodes. Adv. Mater. 2022, 34, e2200342. [Google Scholar] [CrossRef]
- Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.G. A Seamless Metal-Organic Framework Interphase with Boosted Zn(2+) Flux and Deposition Kinetics for Long-Living Rechargeable Zn Batteries. Nano Lett. 2023, 23, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yuan, X.; Xia, Y.; Zhang, Y.; Fu, L.; Liu, L.; Yu, N.; Huang, Q.; Wang, B.; Hu, X.; et al. An Artificial Polyacrylonitrile Coating Layer Confining Zinc Dendrite Growth for Highly Reversible Aqueous Zinc-Based Batteries. Adv. Sci. 2021, 8, e2100309. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Liu, P.; Ran, R.; Jiang, S.P.; Wu, H.; Shao, Z. A Function-Separated Design of Electrode for Realizing High-Performance Hybrid Zinc Battery. Adv. Energy Mater. 2020, 10, 2002992. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Zhang, M.; Wang, Y.; Ding, S.; Song, A.; Yi, H.; Yang, L.; Song, Y.; Cui, Y.; et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478. [Google Scholar] [CrossRef]
- Yi, J.; Liang, P.; Liu, X.; Wu, K.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc–air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar] [CrossRef]
- Qi, C.; Weinell, C.E.; Dam-Johansen, K.; Wu, H. Assessment of Anticorrosion Performance of Zinc-Rich Epoxy Coatings Added with Zinc Fibers for Corrosion Protection of Steel. ACS Omega 2023, 8, 1912–1922. [Google Scholar] [CrossRef]
- Qiu, M.; Jia, H.; Lan, C.; Liu, H.; Fu, S. An enhanced kinetics and ultra-stable zinc electrode by functionalized boron nitride intermediate layer engineering. Energy Storage Mater. 2022, 45, 1175–1182. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, J.; Lu, Q.; Dong, H.; Karnaushenko, D.D.; Becker, C.; Karnaushenko, D.; Li, Y.; Tang, H.; Qu, Z.; et al. A Patternable and In Situ Formed Polymeric Zinc Blanket for a Reversible Zinc Anode in a Skin-Mountable Microbattery. Adv. Mater. 2021, 33, e2007497. [Google Scholar] [CrossRef]
- Michlik, T.; Rosin, A.; Gerdes, T.; Moos, R. Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries. Batteries 2019, 5, 32. [Google Scholar] [CrossRef]
- Yu, F.; Shah, A.; Leung, P. A numerical model of a zinc-para-benzoquinone membrane-free organic flow battery. Energy Rep. 2022, 8, 1158–1165. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Fu, J.; Chu, F. Mass Transfer Behaviors and Battery Performance of a Ferrocyanide-Based Organic Redox Flow Battery with Different Electrode Shapes. Energies 2023, 16, 2846. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, H.M. Size dependent interface energy and its applications. Surf. Sci. Rep. 2008, 63, 427–464. [Google Scholar] [CrossRef]
- Roeber, E.F.; Parmelee, H.C. Electrochemical and Metallurgical Industry; Electrochemical Publishing Company: Pennington, NJ, USA, 1907. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Dai, S.; Leung, P.; Mohamed, M.R.; Zeng, Y.; Zhu, X.; Flox, C.; Shah, A.A.; Liao, Q. Exploring the Performance and Mass-Transfer Characteristics of Porous Zinc Anodes for Membraneless Hybrid-Flow Batteries. Batteries 2023, 9, 340. https://doi.org/10.3390/batteries9070340
Tang L, Dai S, Leung P, Mohamed MR, Zeng Y, Zhu X, Flox C, Shah AA, Liao Q. Exploring the Performance and Mass-Transfer Characteristics of Porous Zinc Anodes for Membraneless Hybrid-Flow Batteries. Batteries. 2023; 9(7):340. https://doi.org/10.3390/batteries9070340
Chicago/Turabian StyleTang, Lina, Shuyang Dai, Puiki Leung, Mohd Rusllim Mohamed, Yikai Zeng, Xun Zhu, Cristina Flox, Akeel A. Shah, and Qiang Liao. 2023. "Exploring the Performance and Mass-Transfer Characteristics of Porous Zinc Anodes for Membraneless Hybrid-Flow Batteries" Batteries 9, no. 7: 340. https://doi.org/10.3390/batteries9070340
APA StyleTang, L., Dai, S., Leung, P., Mohamed, M. R., Zeng, Y., Zhu, X., Flox, C., Shah, A. A., & Liao, Q. (2023). Exploring the Performance and Mass-Transfer Characteristics of Porous Zinc Anodes for Membraneless Hybrid-Flow Batteries. Batteries, 9(7), 340. https://doi.org/10.3390/batteries9070340







