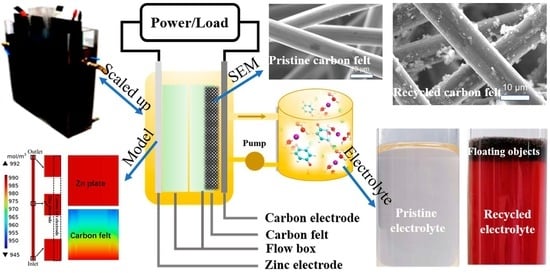
Performance Evaluation of a Scaled-Up Membraneless Organic-Based Hybrid Flow Battery
Abstract
1. Introduction
2. Experimental Details
2.1. Experimental Materials
2.2. Electrode Pretreatment
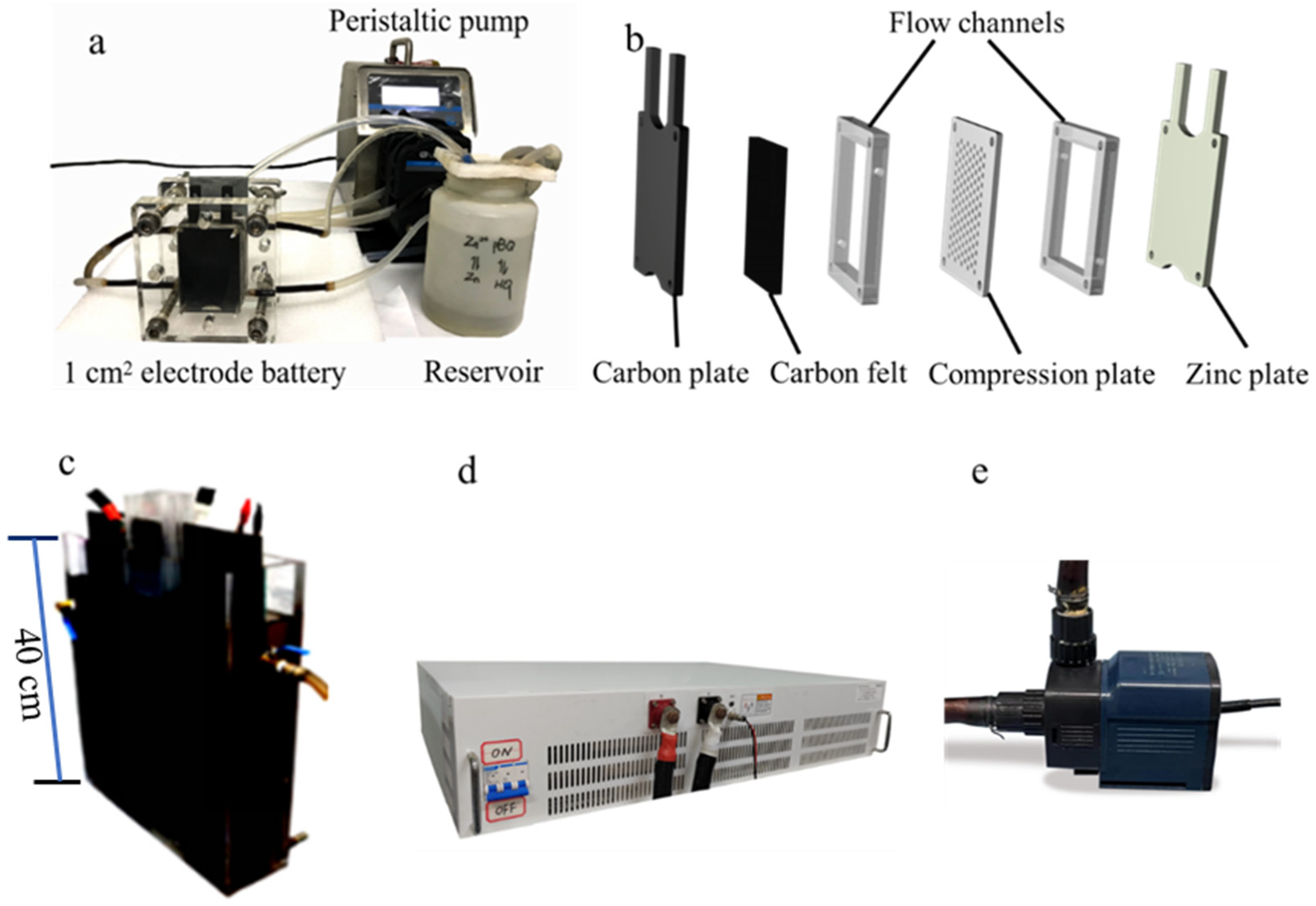
2.3. Flow Battery Experiment
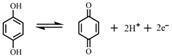 Eo = +0.64 V vs. SHE
Eo = +0.64 V vs. SHE
- (i)
- Laboratory-scale battery (1 cm2 electrode):
- (ii)
- Scaled-up battery (1600 cm2 electrode):
2.4. Electrochemical Impedance Spectroscopy
2.5. Physicochemical Characterizations
- (i)
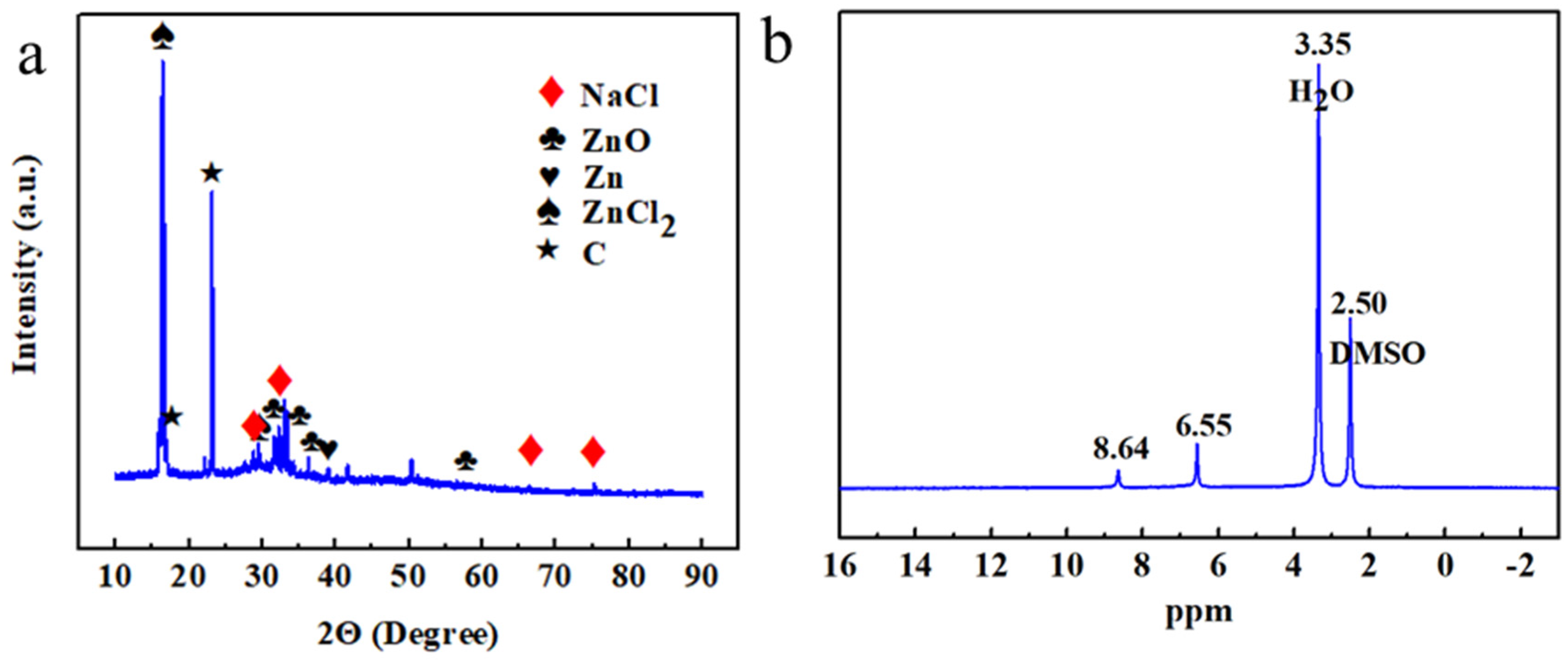
- Electrolyte characterizations: X-ray diffraction (XRD, D8 Advance, Brucke Corp., Germany) analysis was conducted on the dried products, i.e., sediment and floating foam, obtained from the electrolytes after prolonged charge–discharge cycling, and finely powdered using an agate mortar.
- (ii)
- The aim of this analysis was to investigate the crystal structure and phase composition of the dried substances under test conditions of 5–90° 2θ and a scan rate of 10° min−1. X-ray diffraction profiles were matched with standard Powder Diffraction FileTM (PDF) cards using analysis software (Jade6.5). Fourier Transform Infrared Spectroscopy (FTIR, Nicolet IS 10, Thermo Scientific, Waltham, MA, USA) was used to characterize the functional groups present in the organic dried substances, which were subsequently analyzed to determine the molecular structures of organic compounds. The sample to be tested was thoroughly crushed by mixing it with potassium bromide at a mass ratio of 10:1 and then pressed into a specific mold to form nearly transparent circular pellets. Finally, the resulting pellets were placed in the infrared sample tank for testing, and the wave number range was set to 400–4000 cm−1.
- (iii)
- Nuclear Magnetic Resonance Spectroscopy (NMR, JNM-ECZ600R, Japan) was utilized to qualitatively analyze the composition and structure of the dissolved organic compounds, thereby providing insights into the underlying reaction mechanisms. The solvent for nuclear magnetic resonance spectroscopy is methyl sulfoxide (DMSO).
- (iv)
- Positive electrode characterizations: scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) was used to analyze the surface morphology, element composition, and organics adhesion of the carbon felt electrode before and after charge–discharge cycling. Scanning electron microscopy (SEM, TM4000Plus, Hitachi, Japan) was performed to observe the morphology and microstructure, operated at 20 kV and 10 mA, and analysed the adhesion and distribution of the sediments in the carbon felt. Energy dispersive spectroscopy (EDS, Hitachi, Japan) was performed to analyse the sediments, and the samples were irradiated with an electron gun to obtain the compositions and content of precipitates on carbon felt.
- (v)
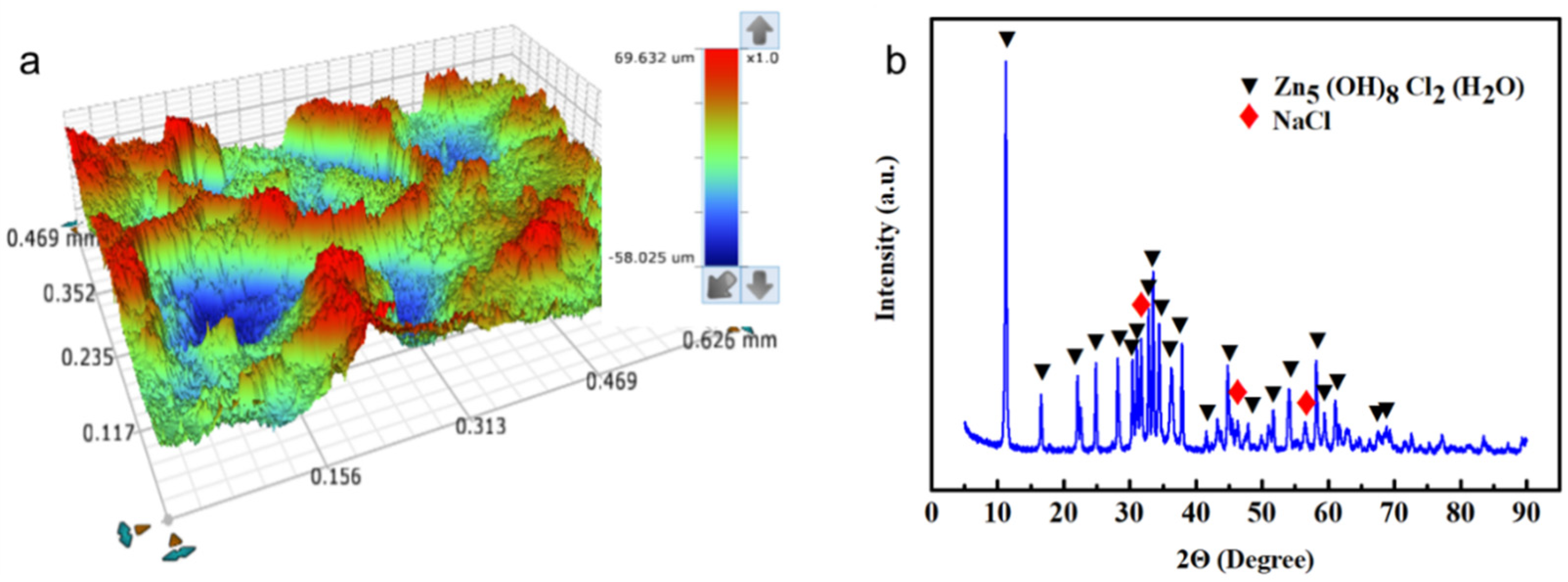
- Negative electrode characterizations: three-dimensional contour scanner (Contoure GT-X, Bruker Corp., Germany) and scanning electron microscopy (SEM) were employed to investigate the crystal structure and phase composition of the zinc anode surface after prolonged charge–discharge cycling. The three-dimensional contour scanner was used to provide a high-resolution topographic map of the zinc anode surface by providing the precise measurements of surface roughness. These two techniques provided direct microscopic information on the morphologies of the zinc anode.
3. Results and Discussions
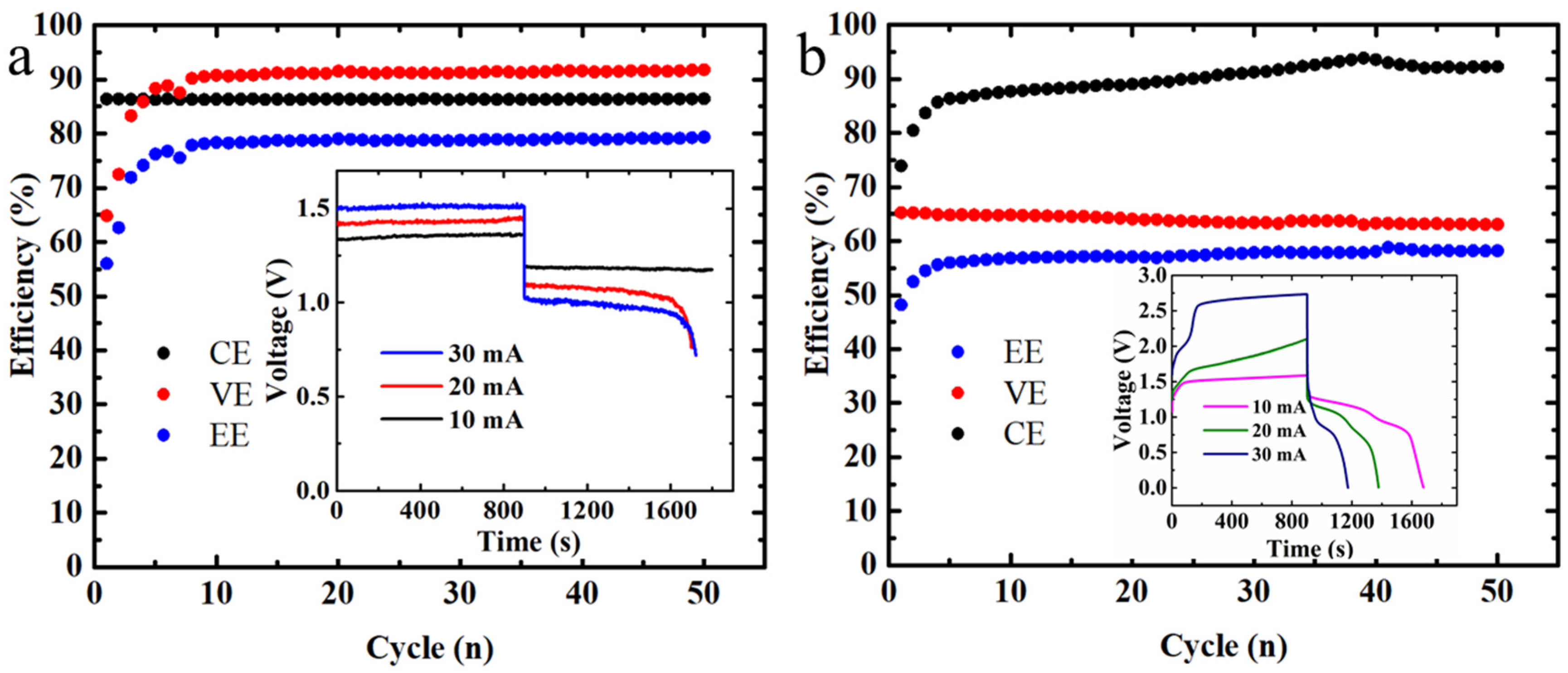
3.1. Performance Comparison with the Scaled-Up Hybrid Flow Batteries
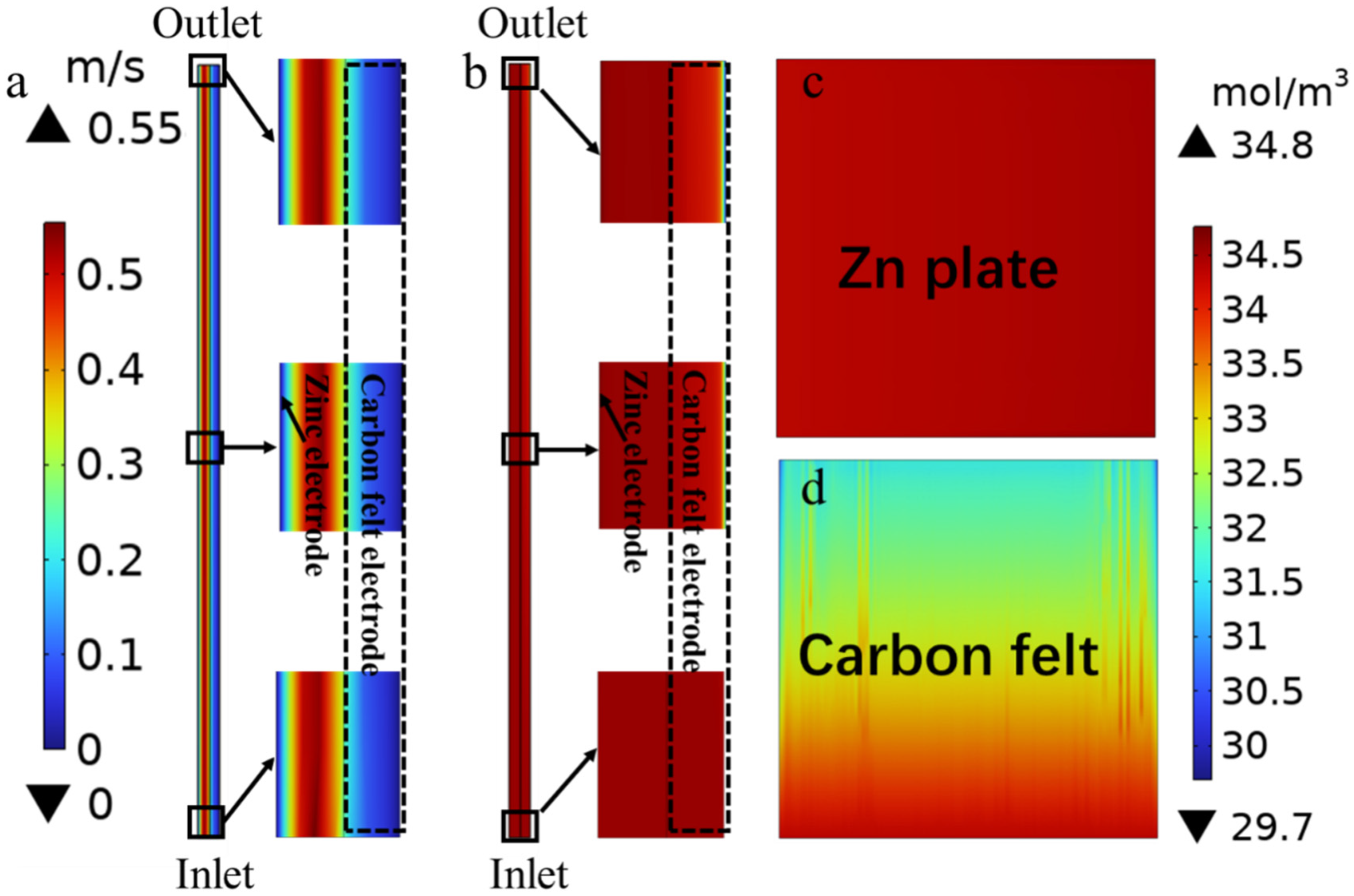
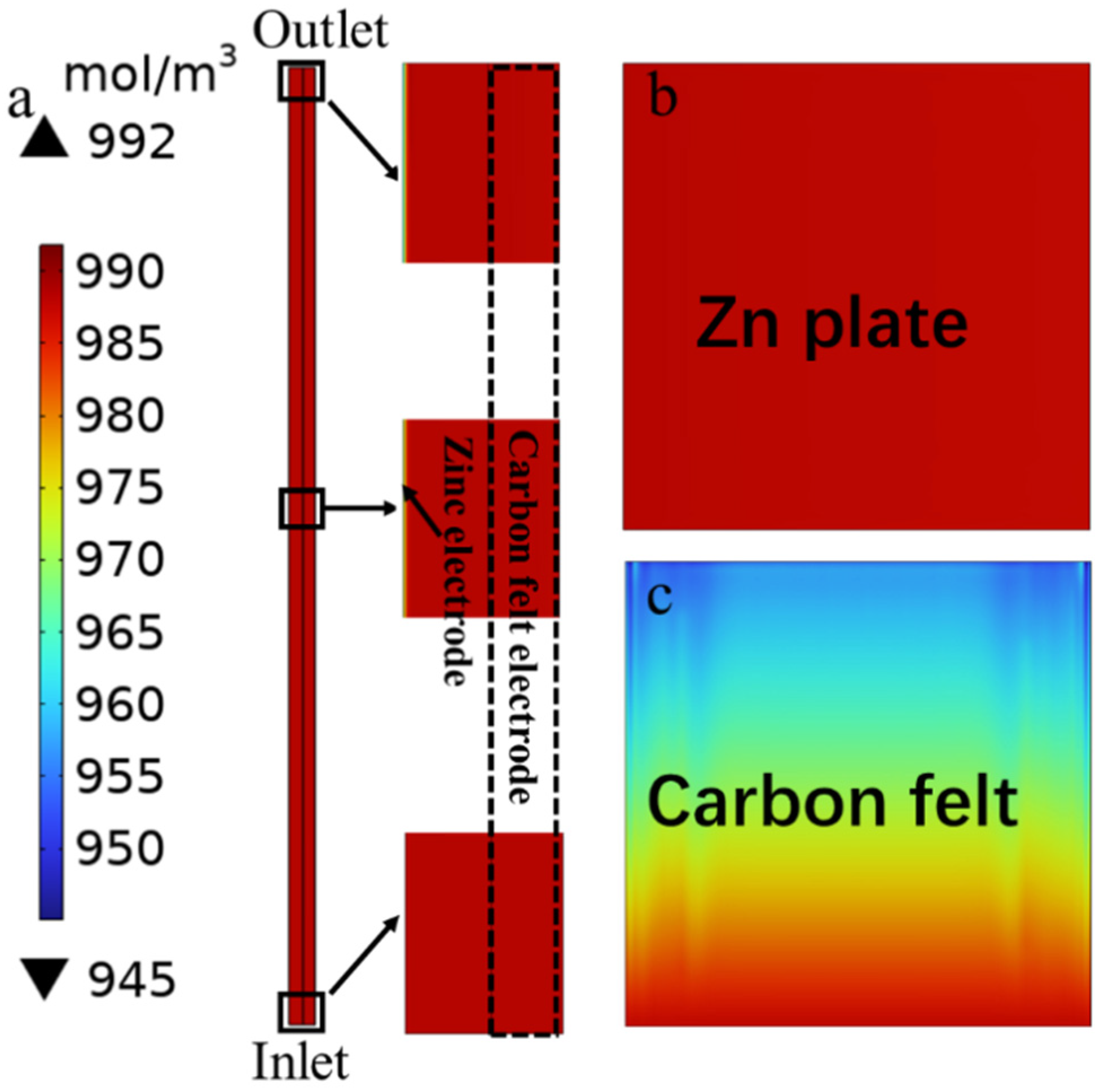
3.2. Mass Transport Analyses Based on Multiphysics Models
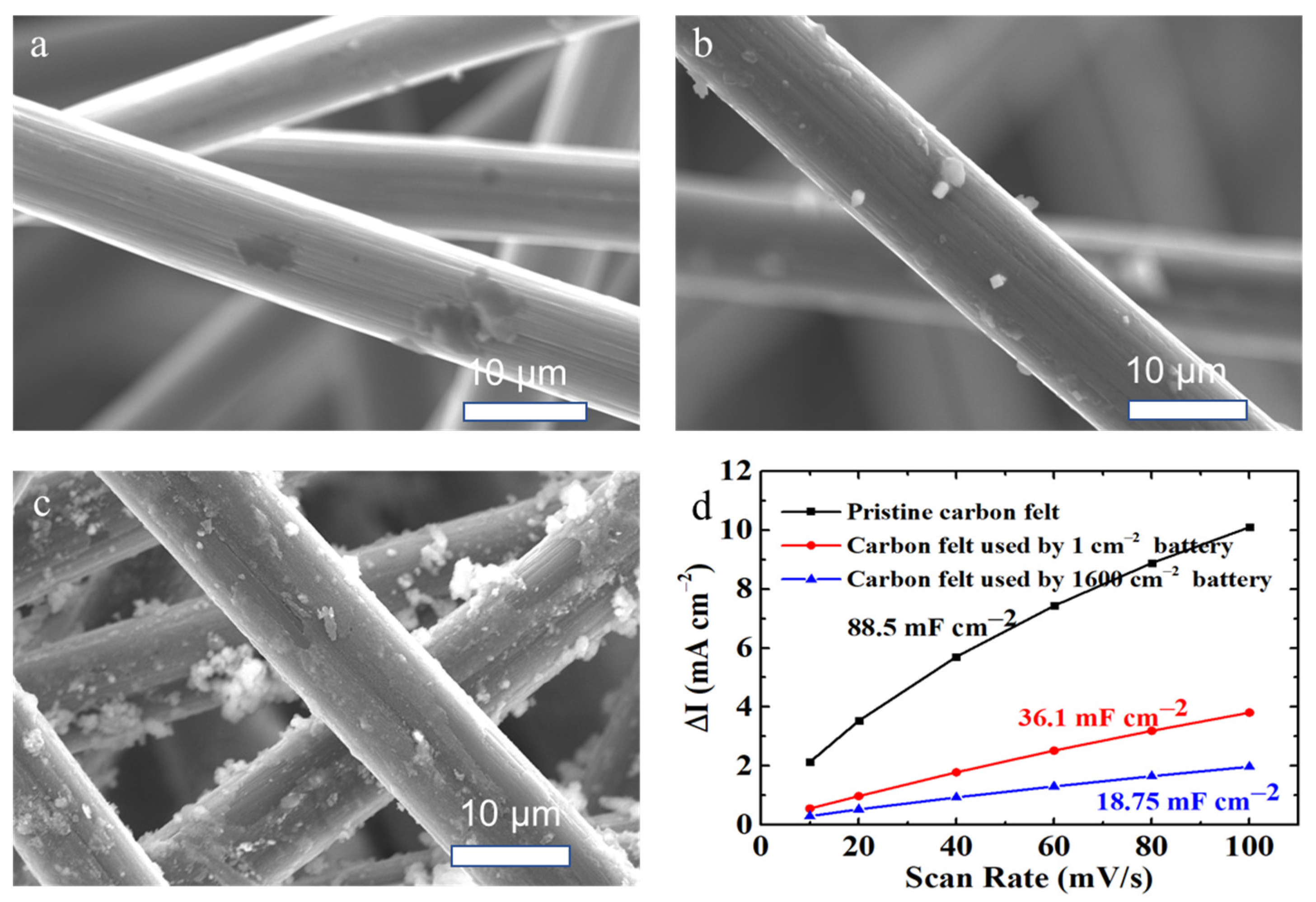
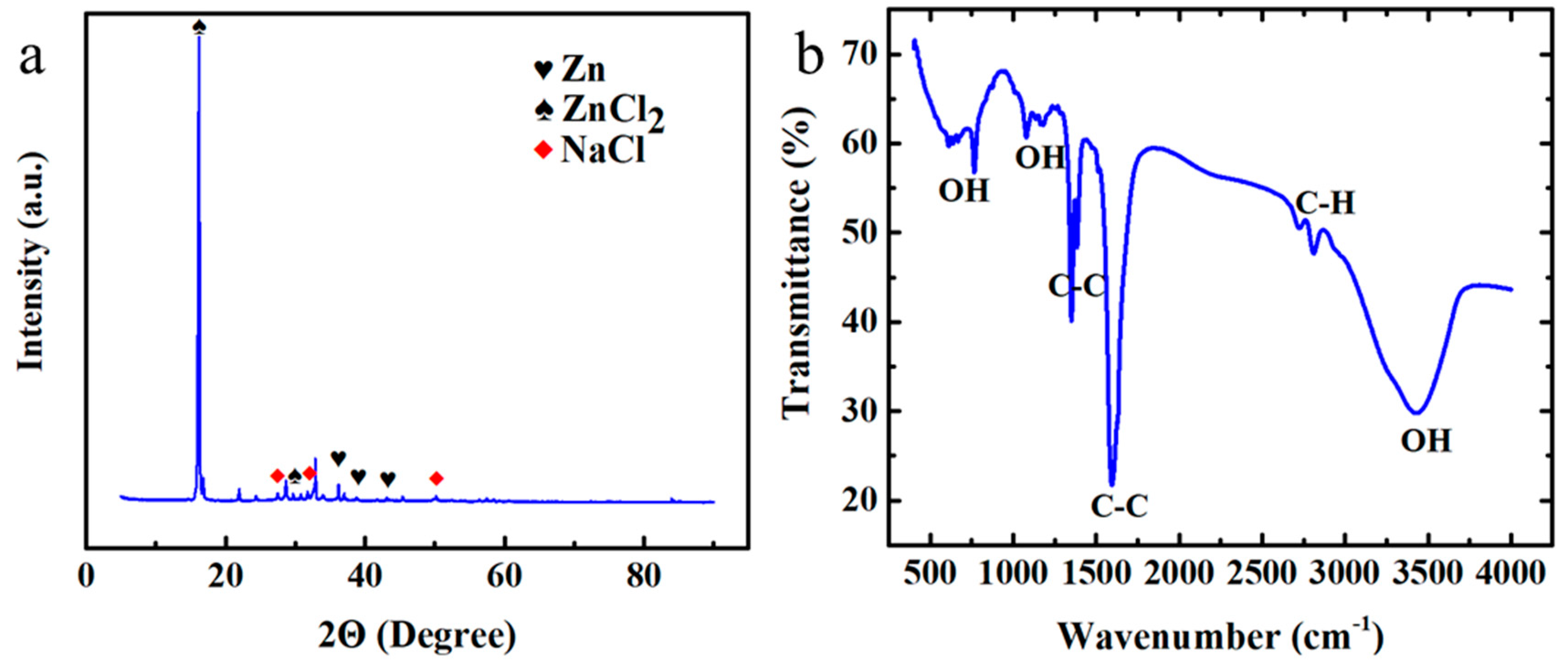
3.3. Characterization Analyses of Cell Components after Charge–Discharge Cycling
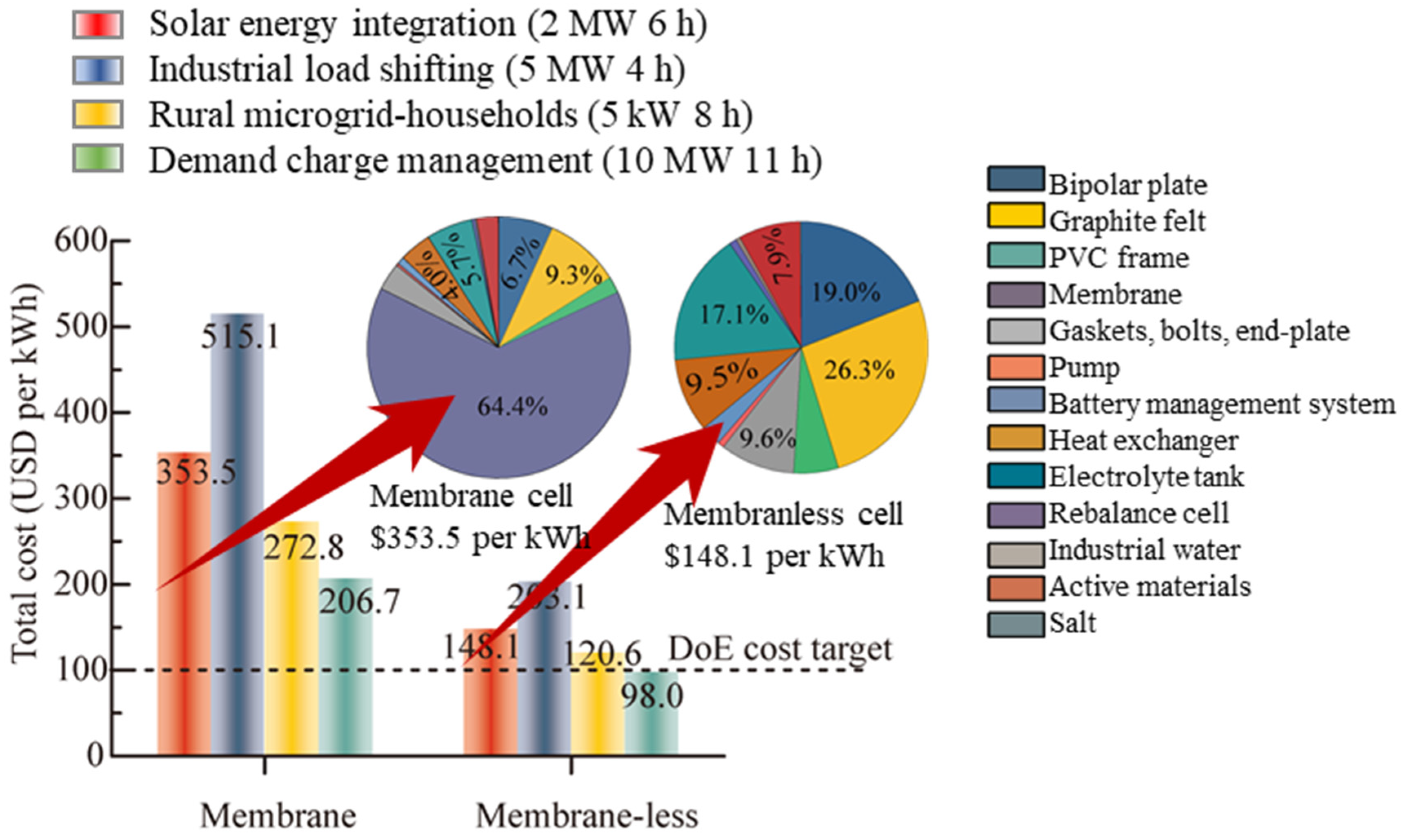
3.4. Capital Cost Analysis of Membraneless Hybrid Flow Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salvia, M.; Reckien, D.; Pietrapertosa, F.; Eckersley, P.; Spyridaki, N.-A.; Krook-Riekkola, A.; Olazabal, M.; De Gregorio Hurtado, S.; Simoes, S.G.; Geneletti, D.; et al. Will climate mitigation ambitions lead to carbon neutrality? An analysis of the local-level plans of 327 cities in the EU. Renew. Sustain. Energy Rev. 2021, 135, 110253. [Google Scholar] [CrossRef]
- Yangka, D.; Rauland, V.; Newman, P. Carbon neutral policy in action: The case of Bhutan. Clim. Policy 2019, 19, 672–687. [Google Scholar] [CrossRef]
- Gil, L.; Bernardo, J. An approach to energy and climate issues aiming at carbon neutrality. Renew. Energy Focus 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Menanteau, P.; Finon, D.; Lamy, M.-L. Prices versus quantities: Choosing policies for promoting the development of renewable energy. Energy Policy 2003, 31, 799–812. [Google Scholar] [CrossRef]
- Aflaki, S.; Netessine, S. Strategic Investment in Renewable Energy Sources: The Effect of Supply Intermittency. Manuf. Serv. Oper. Manag. 2017, 19, 489–507. [Google Scholar] [CrossRef]
- Al-Shahri, O.A.; Ismail, F.B.; Hannan, M.; Lipu, M.H.; Al-Shetwi, A.Q.; Begum, R.; Al-Muhsen, N.F.; Soujeri, E. Solar photovoltaic energy optimization methods, challenges and issues: A comprehensive review. J. Clean. Prod. 2021, 284, 125465. [Google Scholar] [CrossRef]
- Hannan, M.; Wali, S.; Ker, P.; Rahman, M.A.; Mansor, M.; Ramachandaramurthy, V.; Muttaqi, K.; Mahlia, T.; Dong, Z. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Crawford, A.J.; Thomsen, E.C.; Shamim, N.; Li, G.; Huang, Q.; Reed, D.M. An Overview of the Design and Optimized Operation of Vanadium Redox Flow Batteries for Durations in the Range of 4–24 Hours. Batteries 2023, 9, 221. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Pathways to Widespread Applications: Development of Redox Flow Batteries Based on New Chemistries. Chem 2019, 5, 1964–1987. [Google Scholar] [CrossRef]
- Díaz-González, F.; Sumper, A.; Gomis-Bellmunt, O.; Villafáfila-Robles, R. A review of energy storage technologies for wind power applications. Renew. Sustain. Energy Rev. 2012, 16, 2154–2171. [Google Scholar] [CrossRef]
- Schubert, C.; Hassen, W.F.; Poisl, B.; Seitz, S.; Schubert, J.; Usabiaga, E.O.; Gaudo, P.M.; Pettinger, K.-H. Hybrid Energy Storage Systems Based on Redox-Flow Batteries: Recent Developments, Challenges, and Future Perspectives. Batteries 2023, 9, 211. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, W.; Li, X. Progress and Perspectives of Flow Battery Technologies. Electrochem. Energy Rev. 2019, 2, 492–506. [Google Scholar] [CrossRef]
- Brushett, F.R.; Vaughey, J.T.; Jansen, A.N. An All-Organic Non-aqueous Lithium-Ion Redox Flow Battery. Adv. Energy Mater. 2012, 2, 1390–1396. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Martin, U.; Friebe, C.; Morgenstern, S.; Hiller, H.; Hager, M.D.; Schubert, U.S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Choi, J.; Lee, S.; Kim, J.; Han, D.; Jang, H.; Park, M. Recent Progress in High-voltage Aqueous Zinc-based Hybrid Redox Flow Batteries. Chem.—Asian J. 2022, 18, e2022010. [Google Scholar] [CrossRef]
- Pollet, B.G.; Pasupathi, S.; Swart, G.; Mouton, K.; Lototskyy, M.; Williams, M.; Bujlo, P.; Ji, S.; Bladergroen, B.J.; Linkov, V. Hydrogen South Africa (HySA) Systems Competence Centre: Mission, objectives, technological achievements and breakthroughs. Int. J. Hydrogen Energy 2014, 39, 3577–3596. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Mohamed, M.; Xu, Q.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.; Liao, Q. Capital cost evaluation of conventional and emerging redox flow batteries for grid storage applications. Electrochim. Acta 2023, 437, 141460. [Google Scholar] [CrossRef]
- Leung, P.K.; Martin, T.; Shah, A.A.; Anderson, M.A.; Palma, J. Membrane-less organic–inorganic aqueous flow batteries with improved cell potential. Chem. Commun. 2016, 52, 14270–14273. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, M. Toward Dendrite-Free Deposition in Zinc-Based Flow Batteries: Status and Prospects. Batteries 2022, 8, 117. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Xiang, W.; Xu, H.; Ding, M.; Li, L.; Tang, A.; Gao, R.; Zhou, G.; Jia, C. High performance and long cycle life neutral zinc-iron flow batteries enabled by zinc-bromide complexation. Energy Storage Mater. 2022, 44, 433–440. [Google Scholar] [CrossRef]
- Modestov, A.D.; Andreev, V.N.; Antipov, A.E.; Petrov, M.M. Novel Aqueous Zinc–Halogenate Flow Batteries as an Offspring of Zinc–Air Fuel Cells for Use in Oxygen-Deficient Environment. Energy Technol. 2021, 9, 2100233. [Google Scholar] [CrossRef]
- Winsberg, J.; Janoschka, T.; Morgenstern, S.; Hagemann, T.; Muench, S.; Hauffman, G.; Gohy, J.F.; Hager, M.D.; Schubert, U.S. Poly(TEMPO)/Zinc Hybrid-Flow Battery: A Novel, “Green,” High Voltage, and Safe Energy Storage System. Adv. Mater. 2016, 28, 2238–2243. [Google Scholar] [CrossRef]
- Zhou, R.; Yao, S.; Zhao, Y.; Cheng, J. Tab Design Based on the Internal Distributed Properties in a Zinc–Nickel Single-Flow Battery. Ind. Eng. Chem. Res. 2021, 60, 1434–1451. [Google Scholar] [CrossRef]
- Amit, L.; Naar, D.; Gloukhovski, R.; la O’, G.J.; Suss, M.E. A Single-Flow Battery with Multiphase Flow. ChemSusChem 2021, 14, 1068–1073. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef]
- Khetan, A. High-Throughput Virtual Screening of Quinones for Aqueous Redox Flow Batteries: Status and Perspectives. Batteries 2022, 9, 24. [Google Scholar] [CrossRef]
- Kortekaas, L.; Fricke, S.; Korshunov, A.; Cekic-Laskovic, I.; Winter, M.; Grünebaum, M. Building Bridges: Unifying Design and Development Aspects for Advancing Non-Aqueous Redox-Flow Batteries. Batteries 2022, 9, 4. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Y.; Cheng, J.; Cao, G.; Yang, Y. Study on a single flow acid Cd–chloranil battery. Electrochem. Commun. 2009, 11, 1422–1424. [Google Scholar] [CrossRef]
- Li, B.; Liu, J. Progress and directions in low-cost redox-flow batteries for large-scale energy storage. Natl. Sci. Rev. 2017, 4, 91–105. [Google Scholar] [CrossRef]
- Wills, R.G.A.; Collins, J.; Stratton-Campbell, D.; Low, C.T.J.; Pletcher, D.; Walsh, F.C. Developments in the soluble lead-acid flow battery. J. Appl. Electrochem. 2010, 40, 955–965. [Google Scholar] [CrossRef]
- Turney, D.E.; Shmukler, M.; Galloway, K.; Klein, M.; Ito, Y.; Sholklapper, T.; Gallaway, J.W.; Nyce, M.; Banerjee, S. Development and testing of an economic grid-scale flow-assisted zinc/nickel-hydroxide alkaline battery. J. Power Sources 2014, 264, 49–58. [Google Scholar] [CrossRef]
- Leung, P.; Martin, T.; Shah, A.; Mohamed, M.; Anderson, M.; Palma, J. Membrane-less hybrid flow battery based on low-cost elements. J. Power Sources 2017, 341, 36–45. [Google Scholar] [CrossRef]
- Leung, P.; de León, C.P.; Walsh, F. An undivided zinc–cerium redox flow battery operating at room temperature (295 K). Electrochem. Commun. 2011, 13, 770–773. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Mathammal, R. Density functional theory calculations and vibrational spectra of 3,5 dichloro hydroxy benzaldehyde and 2,4 dichloro benzaldehyde. J. Raman Spectrosc. 2008, 39, 1890–1899. [Google Scholar] [CrossRef]
- Guerrero, S.S.M.; Durmus, Y.E.; Dzieciol, K.; Basak, S.; Tempel, H.; van Waasen, S.; Kungl, H.; Eichel, R. Improved Electrochemical Performance of Zinc Anodes by EDTA in Near-Neutral Zinc−Air Batteries. Batter. Supercaps 2021, 4, 1830–1842. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2020, 31, 2006484. [Google Scholar] [CrossRef]
- Sheng, S.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. Simultaneously boosting hydrogen production and ethanol upgrading using a highly-efficient hollow needle-like copper cobalt sulfide as a bifunctional electrocatalyst. J. Colloid Interface Sci. 2021, 602, 325–333. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Zhao, W.; Leung, P.; Mohamed, M.R.; Wei, L.; Shah, A.; Liao, Q. Performance Evaluation of a Scaled-Up Membraneless Organic-Based Hybrid Flow Battery. Batteries 2023, 9, 336. https://doi.org/10.3390/batteries9070336
Yu F, Zhao W, Leung P, Mohamed MR, Wei L, Shah A, Liao Q. Performance Evaluation of a Scaled-Up Membraneless Organic-Based Hybrid Flow Battery. Batteries. 2023; 9(7):336. https://doi.org/10.3390/batteries9070336
Chicago/Turabian StyleYu, Feilin, Wenbo Zhao, Puiki Leung, Mohd Rusllim Mohamed, Lei Wei, Akeel Shah, and Qiang Liao. 2023. "Performance Evaluation of a Scaled-Up Membraneless Organic-Based Hybrid Flow Battery" Batteries 9, no. 7: 336. https://doi.org/10.3390/batteries9070336
APA StyleYu, F., Zhao, W., Leung, P., Mohamed, M. R., Wei, L., Shah, A., & Liao, Q. (2023). Performance Evaluation of a Scaled-Up Membraneless Organic-Based Hybrid Flow Battery. Batteries, 9(7), 336. https://doi.org/10.3390/batteries9070336