Abstract
Li-ion batteries are currently considered promising energy storage devices for the future. However, the use of liquid electrolytes poses certain challenges, including lithium dendrite penetration and flammable liquid leakage. Encouragingly, solid electrolytes endowed with high stability and safety appear to be a potential solution to these problems. Among them, ionic liquids (ILs) packed in metal organic frameworks (MOFs), known as ILs@MOFs, have emerged as a hybrid solid-state material that possesses high conductivity, low flammability, and strong mechanical stability. ILs@MOFs plays a crucial role in forming a continuous interfacial conduction network, as well as providing internal ion conduction pathways through the ionic liquid. Hence, ILs@MOFs can not only act as a suitable ionic conduct main body, but also be used as an active filler in composite polymer electrolytes (CPEs) to meet the demand for higher conductivity and lower cost. This review focuses on the characteristic properties and the ion transport mechanism behind ILs@MOFs, highlighting the main problems of its applications. Moreover, this review presents an introduction of the advantages and applications of Ils@MOFs as fillers and the improvement directions are also discussed. In the conclusion, the challenges and suggestions for the future improvement of ILs@MOFs hybrid electrolytes are also prospected. Overall, this review demonstrates the application potential of ILs@MOFs as a hybrid electrolyte material in energy storage systems.
1. Introduction
The increasing demand of clean energy calls for the progression of advanced energy storage systems, which helps to regulate the unstable energy output using renewable energy [1]. Nowadays, electrochemical energy storage, such as Li-ion batteries, is considered to be one of the most promising future energy storage techniques [2]. The rapid development of Li-ion batteries has drawn much attention from researchers due to their distinct advantages, such as high theoretical energy density, stable energy output and low memory effect. However, the highly flammable electrolytes, complex temperature management and limited practical capacity still restrict the further development of Li-ion batteries. In comparison, lithium batteries which utilize an Li-metal anode show significant superiority in high energy density due to their ultra-high theoretical capacity of 3860 mAh/g and ultra-low electrode potential of −3.04 V (vs. SHE), which reveals promising prospects in alleviating the “range anxiety” of electrical vehicles [3]. Unfortunately, serious challenges remain to be solved before the practical application of lithium batteries, including the infinite volume change of Li metal and the generation of Li dendrites, and even “dead lithium” caused by the pulverization of the Li anode [4].
The emergence of solid-state electrolytes with a comparatively higher safety and longer life span offers a potential solution to the challenges faced in Li metal batteries. Solid-state electrolytes with a high shear modulus can provide sufficient mechanical strength to suppress the uneven Li deposition. In addition, solid-state lithium batteries employing solid electrolytes with high thermal stability prevent the potential thermal runaways, which greatly improves the safety of high energy-density devices. Solid-state electrolyte can be mainly classified into inorganic ceramic electrolyte, solid polymer electrolyte and a combination of the two. As a solid electrolyte with promising prospects for practical application, solid polymer electrolytes which exhibit the advantages of shape versatility, low weight, flexibility and low processing costs have become the research focus [5]. However, on the one hand, the high crystallinity of polymer chains at room temperature results in an undesired ionic conductivity. On the other hand, the low electrochemical stability of polymers can cause interfacial side reactions, resulting in an increased interfacial resistance and structural degradation of electrode materials. Moreover, due to volume effects, poor contact between the electrode and electrolyte can also occur during long-term cycling. These challenges need to be addressed to improve the performance of polymer-based electrolytes in lithium-ion batteries [6,7]. To solve the problem, adding inorganic fillers into solid polymer electrolyte and forming composite polymer electrolyte is regarded as the ultimate approach to construct solid-state electrolyte with advanced comprehensive properties. However, the nano effect of inorganic fillers leads to two challenges: firstly, the unsatisfied dispersity of filler, and secondly, the low upper limit of filler addition. Therefore, the properties of composite polymer electrolyte have not yet met the established standards for practical operation of batteries. For example, the low migration number of lithium ions of composite polymer electrolytes can easily form a lithium-ion concentration gradient on the electrode surface, which accelerates dendrite growth. Moreover, the conduction of Li-ions can easily be impeded by overly added fillers, leading to discontinuous Li+ transmission pathways, which attenuates the high C-rate performance of batteries. Furthermore, during the Li+ deposition process, the uneven electric field on the electrode surface resulting from the inhomogeneous distribution of inorganic fillers can also accelerate electrode decay. Hence, in view of composite polymer systems, the structure and the chemical composition of fillers has a significant impact on the conductive property of the polymer electrolyte chain, and can therefore enhance comprehensive performance of polymer electrolyte [8]. Therefore, developing advanced fillers for high-performance solid electrolyte is considered an urgent requirement for Li-metal solid-state battery manufacturing.
Notably, in view of the advantage of high conductivity, stable structure and high chemical compatibility, other types of newly developed solid electrolytes have become substantial alternatives for ceramic electrolytes and composite polymer electrolytes [9,10,11]. Most recently, ionic liquids (ILs)@metal organic frameworks (MOFs) have emerged as a promising candidate material for potential utility because of their high ion conductivity, abundant metal sites, large specific surface area and modulable ability [12]. Unlike conventional carbonate solvents, ionic liquids are a class of molten salts that exist entirely in ionic form at room temperature. Generally speaking, the cations of ionic liquids are derivatives of 1-methylimidazole and anions are conjugate bases of inorganic acids [13]. Equipped with unique properties such as nonflammability, low vapor pressure and electrochemical stability [14], ionic liquids have been widely used in Li-ion batteries to replace carbonate solvents or participate in the formation of functional SEI [15]. Apart from that, ionic liquids can also be used as stabilizing agents in solid-state batteries to improve interface stability [16]. However, the direct use of ionic liquids as liquid electrolytes still cannot avoid the problem of Li dendrites caused by low Li+ transference number (tLi+) and possible liquid leakage. The low transference number originates from the free anion and cation migration. Although the nonflammable ILs avoid electrolyte combustion, the dendrite remains can penetrate the separator and cause a short circuit under abuse conditions. Meanwhile, the high cost of the ionic liquids system impedes the application progress of lithium-ion batteries. Hence, some researchers use MOFs to confine ILs, which achieves high performance solid-state electrolytes with a low ILs dosage, contributing to the transition metal ion or clusters and organic ligands on MOFs [13]. In the 1990s, Robison and Hoskins reported the first successfully synthesized MOF [N(CH3)4][CuIZnII(CN)4] [17], the stable porous structure, diverse combination of metal and organic units and the tunable electrochemical property of which attracted the interest of researchers [18,19,20,21]. Usually, simple and inexpensive methods such as the microwave-assisted heating method, hydrothermal method and solvent self-assembly methods have been proposed to synthesize MOFs [22,23,24]. Through a self-assembling procedure in a solution experiment, the chemical bonds formed between organic ligands and metal ions can bring the unique properties which cannot be achieved in other skeletal compounds [25]. Compared with ILs hybrid electrolyte and SPEs in Figure 1, using MOFs to confine ILs can not only avoid leakage of ILs and provide mechanical suppor, but also facilitate the ion conductivity of MOFs material. Consequently, with tunable porosity, rich Lewis acidic active sites and a modular nature, ILs@MOFs have been regarded as promising materials for applications as electrolyte of solid-state lithium batteries.
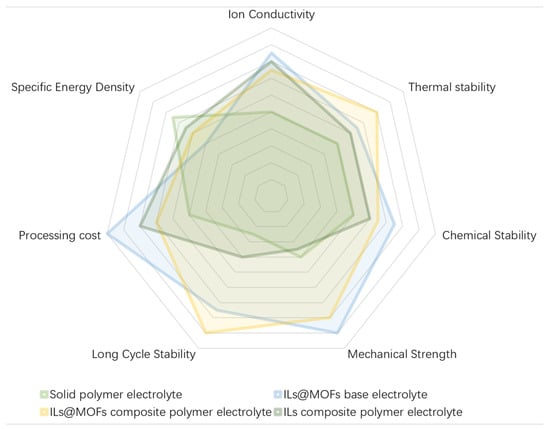
Figure 1.
The performance comparison diagram of different organic electrolyte: SPE, ILs@MOFs-based electrolyte, ILs@MOFs CPE and ILs CPE.
In this review, the application of ILs@MOFs materials as the main body of solid electrolytes in lithium battery was first explored, including MOFs interface layers for solid electrolytes as well as the use of MOFs materials directly as solid electrolytes after modification and compounding with ionic liquids. Meanwhile, the application of MOFs as fillers in composite polymer electrolytes was also reviewed. This review provides guidance in exploring the ionic conduction mechanism inside ILs@MOFs-based solid electrolyte materials, and brings significant suggestions for the future application orientation of MOFs materials in energy storage devices.
2. Ionic Liquids Hybrid MOFs as Electrolyte
2.1. Composition and Structure Introduction of MOFs Hybrid Electrolyte
Traditional ionic liquid electrolytes possess a low Li+ transference number and weak dendrite resistance. To solve these problems, the innovative development of advanced electrolyte by applying MOFs as the main body for ionic conduction, as well as taking advantage of metal nodes in consideration of the distinctive features of MOFs, have been proposed by researchers. Generally, MOFs exhibit three types of features: tunable porous structure, multi metal node properties and modular nature. Firstly, since the pore size is determined by organic linkers, it is feasible to adjust the pore size to suit the desired application by inserting molecules into the MOFs cage or using MOFs as fillers for selective permeating, for example [12,26]. Additionally, high specific surface area allows a high density of charged species and therefore provides abundant Li+ hopping sites in a small volume. The ordered porosity could suppress dendrite formation by promoting uniform Li+ plating [27]. Secondly, the metal nodes of MOFs not only play a role in connecting organic links, but also serve as Lewis acidic active sites which prefer to bind with electronic cloud, and thus anions can be selectively absorbed by MOFs with alternative absorption strength through the proper regulation of metal nodes [28,29]. Finally, in contrast with inorganic compounds, organic segments are also easy to make post-synthesis modifications to, and are endowed with additional features to improve electrolyte behavior. For example, fluoric groups and amination groups can functionalize MOFs through simple aqueous reaction which would simultaneously promote the formation of stable anion-derived SEI [30].
Inspired by porous zeolites, which were investigated as fillers in solid polymer lithium electrolyte systems [31], Long et al. used electrolyte solution-contained MOFs as possible lithium superionic conductors [32,33]. They synthesized MOFs-74 material upon the graft of lithium alkoxide. After soaking the as-prepared MOFs in 1 M LiBF4 in 1:1 ethylene carbonate (EC) and diethyl carbonate (DEC) solution, a maximum conductivity of 4.4 × 10−4 S cm−1 at room temperature and a low Ea of 0.15 eV can be achieved for the obtained electrolyte pellets. It had been speculated that the lithium alkoxide anion binding with exposed metal sites can promote Li+ transportation through one-dimensional hexagonal channels of MOFs and the robust structure prevented dendrite growing in organic solutions. After soaking in carbonate solution, the high density of charge carriers in channels can also facilitate Li+ hopping. Different from the ionic conduction mechanism of inorganic solid-state electrolytes, in which metal ions hop through vacancies to enable the charge transfer process, the main conduction mechanism of MOFs lies in the adsorption of solution anions by metal nodes, which allows the dissociated Li+ to complex and transport along [34]. Nonetheless, the flammable inner solution cannot be avoided, which leaves safety hazards unresolved, and the relatively low conductivity also limits the further application of MOFs.
2.2. Proposal and Development of ILs@MOFs
On the basis of extensive research on MOFs containing carbonate solutions [35,36,37], as well as the application of ILs liquid electrolytes [38], some researchers suggests integrating ILs that possess high stability and nonflammability with MOFs. The combination of ILs with MOF materials is often achieved by encapsulating ILs in porous MOFs using host-guest interactions. In this system, ILs serve as ion conduction electrolytes while MOFs act as a solid supporting framework. As suggested by group interactions in FT-IR and microporous adsorption properties in BET tests, the ILs in the pores of MOFs exists in the form of both physisorption and chemisorption [39]. The first example regarding the incorporation of ILs into the micropores of MOFs was presented by Kitagawa et al. [40]. Figure 2a shows that researchers used a simple mixing and heating method to incorporate ILs of EMI-TFSA (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide) into the pores of MOFs material ZIF-8 ((Zn (MeIM)2; H(MeIM) = 2-methylimidazole)). Kitagawa et al. systematically investigated EMI-TFSA@ZIF-8 and verified that the addition of ionic liquids into the framework of MOFs can lower the melting point of ionic liquids and stabilize the liquid phase of ionic liquids at low temperatures (Figure 2b,c). They also found that ion dynamics can be controlled through this subject-object interaction, which exhibited great potential in realizing the actual ion conduction process [41]. To endow actual ionic conduction capability, researchers mixed EMI-TFSA with LiTFSA to obtain (EMI0.8Li0.2) TFSA, which was then heated and mixed with ZIF-8 to obtain the target product. Although the ionic conductivity of obtained (EMI0.8Li0.2) TFSA@ZIF-8 ions is two orders of magnitude lower than that of the pure (EMI0.8Li0.2) TFSA due to the lower fluidity, the activation energy in (EMI0.8Li0.2) TFSA is nearly as high as that in bulk (EMI0.8Li0.2) TFSA, which suggests that the diffusing mechanisms of Li+ are the same (Figure 2d). Experiments demonstrate that Li+ can dissociate from anions in the framework of MOFs and achieve ion conduction by diffusion through the internal micropores.
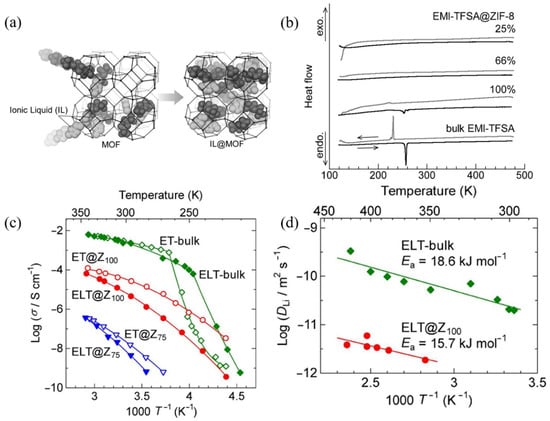
Figure 2.
(a) The incorporation of ionic liquids into micro pores of MOFs. Reproduced with permission [40]. Copyright 2014, Wiley. (b) The DSC curves of bulk EMI-TFSA and EMI-TFSA@ZIF-8 at different volumetric occupancies. The sharp peak in 257 K and 231K represent melting and freezing of liquid state. Reproduced with permission [40]. Copyright 2014, Wiley. (c) Arrhenius plot of the ionic conductivity in heating process, the slope indicates the pseudoactivation energy changes around phase transition point, the green line stands for pure ILs and the red and blue lines stand for 100% and 75% ILs occupied MOFs respectively. Reproduced with permission [41]. Copyright 2015, American Chemical Society. (d) Arrhenius plot of the self-diffusion coefficient of lithium nucleus in ILs and ILs@MOFs. Reproduced with permission [41]. Copyright 2015, American Chemical Society.
2.3. Advantages and Function Mechanism of ILs@MOFs Electrolyte
Inspired by the research of Kitagawa’s group, Pan’s group further proposed the practical application of MOFs-based composite ionic liquids in battery systems, and summarized the three advantages brought by ILs@MOFs comprehensively, including high conductivity, mechanical support and dendrite suppression [42]. (EMI0.8Li0.2) TFSI ionic liquid and MOF-525 were selected for compounding based on the electrochemical stability and the appropriate pore size of the MOFs. Firstly, in terms of the high conductivity, it was found that the conductivity of the electrolyte increased substantially with the increase of ionic liquids loading, which proves that the ionic conductivity of the electrolyte is dependent on the bulk phase transport within the nanocrystal. In addition to conductor phase transport, the lithium-ion transport mechanism of ILs@MOF also includes intercrystal transport mechanism. Studies revealed that the mid-frequency spectrogram of EIS reflects liquid-like electrolyte properties, from which the researchers concluded that ionic liquids infiltrate the interface between nanocrystals at the atomic level. The nano-wetting interface enables the direct interfacial connection of the internal ILs, and X-ray photoelectron spectroscopy (XPS) characterization confirmed that the nature of interface lied in nanocrystals. Apart from that, MOFs play an important role in hindering the movement of large ions while the migration of lithium ions of small size remains unaffected, thus increasing the Li+ transference number. Secondly, in terms of mechanical strength, the MOFs skeleton in ILs@MOF also provides the framework for alternative physical properties and presents a dry powder appearance rather than gel even with the encapsulation of ILs, which improves the overall mechanical strength. Finally, the ability to suppress dendrites’ growth has been strengthened through the regulation of chemical environment and construction of physical barrier. Researchers discovered that ILs@MOFs possessing both high mechanical strength and nano-wetting properties are capable of not only impeding the penetration of dendrites, but also filling the gap between the electrolyte layer and metal electrode by utilizing dendrites for self-healing, ultimately resulting in reduced resistance after cycling.
The ability to suppress lithium dendrite is essential for ILs@MOFs, as dendrite may induce a short circuit and even thermal runaway. The factors influencing dendrite formation in solid-state batteries can be summarized as follows: (i) the high electric driving force for dendrite tips to extend into defects or grain boundaries of SSEs; (ii) the low dendrite consumption rate induced by the sluggish ionic transport kinetics of SSEs; and (iii) the high interface impedance of the solid-solid interphase in solid-state batteries that cause retardance in ionic transportation, which induces surface Li+ deficiency and aggravates tip ion deposition and dendrite growth [43,44]. To overcome dendrite formation, three improvement strategies of ILs@MOFs have been proposed. First, a conformal coating layer of ILs@MOFs can be formed between the electrolyte and Li metal anode. Pan et al. prepared hybrid-sized ILs@MOF nanoparticles as novel electrolyte. The larger particles effectively suppressed Li dendrites’ propagation while smaller particles filled the gap of larger particles to achieve better contact and further barricade dendrites [45]. Second, ILs@MOFs can inhibit dendrite through Li ion flux regulation. Zhang et al. constrained glymes ILs in Uio-66 to accelerate Li ions’ transportation and confine ILs anion which relieved the difference between Li+ ion diffusion and deposition rates and prevented inhomogeneous Li dendrite formation [46]. Finally, ILs can form a nanowetted interface between MOFs and Li metal to improve their compatibility, which ameliorates the detrimental point contact. Pan et al. found that in combination with ILs@MOFs electrolyte, the Li electrode surface was flat and composed by plate-like nanostructures after cycling [47]. The appearance of S and F elements belonging to ILs on Li surface after nanowetting ensured good contact between electrolyte and anode at first, and the formed stable interphase can also protect the battery from dendrite-induced short circuit.
Despite the many benefits of ILs@MOFs, the combination of solid-state frameworks and liquids also increases the research complexity in ion transport mechanisms. In pure ILs systems, the coordination environment and solvation pattern of ILs have been investigated by researchers [48,49]. Through molecular dynamics simulations, researchers calculated the free energy and transition barrier of alkali metal ions, as well as mapping the free energy landscape of alkali metal ions in ILs, which provided further instructions on restricting anion mobility. However, unlike pure ionic liquids, the ionic conduction of ILs@MOF contains both the contribution of highly mobile ILs at the nano-wetting interface and the conduction contribution of ILs in the pores of MOFs. Conventionally, EIS has been widely used to determine the transport mechanism of ILs, in which results showed that the ion transport was mainly determined by the migration and transport of ILs wrapped in the outer layer of MOFs, while the ILs in the pores would not participate [50]. In Figure 3a, the broad NMR lines above −20 °C indicated that there existed two lithium spin reservoirs, and the fast Li+ fraction appears small. The result has shown that with ILs, most Li+ did not change dynamics properties, which means that only Li+ near surface can interact with surface ILs to facilitate transportation. Researchers also found that after flooding the MOFs crystals with excess Li-ILs to form a gel electrolyte, the overall Li-ion transport became faster and the measured conductivity also enhanced as expected (Figure 3b) [51]. However, if the isolated ILs on MOFs surface dominate the transport of lithium ions, the advantages of MOFs in avoiding ionic liquid leakage would be counterbalanced. Hence, the utilization of IL in the pores of MOFs and research on its intrinsic ion transport are of greater significance. Micaela et al. prepared layered ILs@MOF films and washed off the excess ILs, and then systematically investigated the mechanism of Li-based ionic liquid conduction in MOF [52]. They found that the conductivity of HKUST-1 MOF was many orders of magnitude higher when containing pure [BMIM][TFSI] ionic liquid inside, indicating that the measured conductivity was mainly attributed to the internal ions. The ion mobility in subsequent tests decreased by two orders of magnitude with the increase loading of ILs. Therefore, researchers believed that the internal anions and cations with a large radius would hinder ion conduction by ion bunching and pore blocking, as reproduced by the molecular dynamics simulations (Figure 3c). Meanwhile, if a more mobile Li+ is added to the ionic liquid to form [Li0.2BMIM0.8] [TFSI], the full-load ionic conductivity is as high as 70 times that of the original ionic liquid. In addition, researchers demonstrated that the high ionic conductivity originated from the role of ionic liquids by loading samples with only LiTFSI. Li+ in ILs attenuates ionic bunching as well as pore blocking through the effect of Li-TFSI neutrals. Simulations also revealed that Li+ in ILs conducts via the typical Grotthuss mechanism, binding with TFSI− and then releasing to bind with TFSI− at the next site repeatedly.
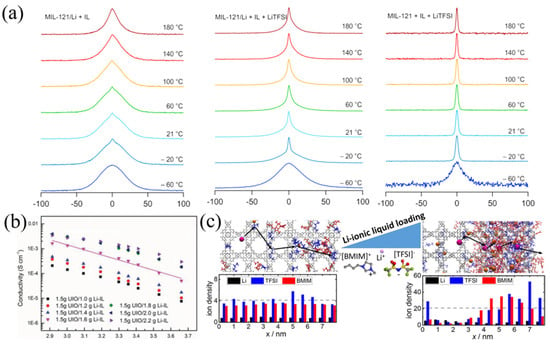
Figure 3.
(a) The 7Li NMR lines of MOFs hybrid Li+ with ILs, MOFs hybrid Li+ with ILs and Li salt and pure MOFs with ILs and Li salt. Reproduced with permission [50]. Copyright 2021, Roman Zettl and Ilie Hanzu. (b) Arrhenius plots of the nanostructured MOFs/Li-ILs with different composition. Reproduced with permission [51]. Copyright 2019, Wiley. (c) The snapshot of the ions of [Li0.2BMIM0.8][TFSI] IL in HKUST-1 MOF at different loading and the ion distribution of Li-free reference system under identical conditions. Reproduced with permission [52]. Copyright 2021, American Chemical Society.
2.4. Improvement Direction of ILs@MOFs Electrolyte
Although research on the ionic conduction mechanism is not extensive, the general direction for improving ILs@MOFs is clear: enhancing the IL accommodation capacity of MOFs with high stability and striving to control the addition of ILs while ensuring overall conductivity. Among those efforts on the performance improvement of ILs@MOFs electrolyte, the synthesis of MOFs with core-shell structure has been extensively studied. Mai et al. prepared the first MOF-in-MOF core-shell structure of UiO-66@UiO-67 and filled the core-shell structure with the ionic liquid Li-[EMMI][TFSI] [53]. This unique structural design combined the advantages of different types of MOFs, using UiO-67 material with large pore size and high specific surface area as the external bulk of the ionic liquid load, and small pore size UiO-66 material as the inner core to restrict the movement of large ions inside ionic liquid (Figure 4a). Upon the same Li-IL addition, the compact granular electrolyte (CSIL) showed a high room-temperature ionic conductivity of 2.1 × 10−3 S cm−1, which exceeded that of UiO-66 by nearly five times. The tLi+ is also twice as high as that of UiO-67, reaching 0.63 (Figure 4b). The researchers found that the fabricated materials did not form ILs@MOFs, which are prone to leakage, but formed nano-wetting interfaces between adjacent nanoparticles in a dry powder state. The prepared liquid-containing nanoparticles displayed excellent thermal, structural, and electrochemical stability and can withstand a compression pressure of 30 MPa, exhibited a thermal degradation temperature of over 360 °C, and showed an oxidative potential of up to 5.2 V. During a cycle stability test under rate of 2C, the Li/CSIL/LiFePO4 (LFP) battery exhibited specific capacity of 158 mAh g−1 and capacity retention of 99% after 100 cycles (Figure 4c). Although in-depth investigations regarding the maximum internal liquid capacity of MOFs before and after the formation of the core-shell structure are still required, their pioneering work demonstrating the potential of core-shell structured MOFs as high-performance solid-state electrolyte bodies remains relevant. Based on a similar thought, Tian et al. also proposed MOFs as cores to form the MOF@PIN (polymerized ionic net-work) structure [54]. PIN as a network-like polyconic liquid was used as a shell to adsorb IL to prevent leakage and provide conductive pathways, and HKUST MOFs as a core to support the shell and improve the structural ability to withstand pressure. Compared to solid PIN solids, HKUST@PIN provides internal frame space which obtained an ILs loading of 250% (mass ratio). In contrast to hollow PIN, HKUST@PIN revealed a stronger interaction tendency towards TFSI anions and thus exhibited a higher tLi+. Other researchers have also used MOFs as shells and ceramic particles as cores inside the MOFs to increase the mechanical strength and provide internal ion pathways [55]. On the one hand, ILs were stored in the external HKUST-1 shell as well as in the voids of the reinforcement layer to facilitate the interfacial ion diffusion of the nanomaterials. On the other hand, Li6.75La3Zr1.75Nb0.25O12 (LLZN) ceramic cores not only provide additional Li+ pathways that facilitate ion transport such as vacancy diffusion, interstitial atom diffusion and substitution diffusion [56,57], but also provide a thermal stability of 300 °C and a high modulus of 71.49 MPa for the electrolyte due to its excellent mechanical strength and heat endurance [58]. To illustrate the enhancing effect of ILs@MOFs, the performance of the above-mentioned ILs@MOFs in batteries has been summarized in Table 1.
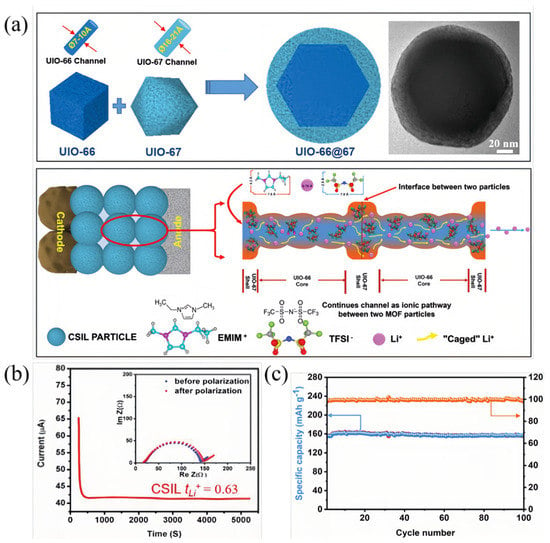
Figure 4.
(a) Schematic diagram of the architecture UIO-66@67 with corresponding TEM image and continuous ionic pathways within nanowetted interface and particles in UIO-66@67 with Ils (CSIL). Reproduced with permission [53]. Copyright 2021, Wiley. (b) DC polarization curve and tLi+ of UIO-66@67 with Ils. Reproduced with permission [53]. Copyright 2021, Wiley. (c) Room temperature cycling stability (blue line) and Coulombic efficiencies (yellow line) of Li/CSIL/LFP under 0.2C. Reproduced with permission [53]. Copyright 2021, Wiley.

Table 1.
The conductivity and battery performance of different Ils@MOFs electrolytes.
However, as far as the current studies are concerned, several unsolved problems still exist, including the enhancement of mechanical strength, which remains speculative with indirect evidence, with the actual performance enhancement not as effective as claimed. In view of this, future efforts on improving comprehensive performances of ILs@MOFs need to focus more on the mechanism analysis and the use of more complete argumentation methods. From another point of view, using MOFs-in-Polymer (such as HKUST@PIN), which can combine the advantages of polymer, ILs and MOFs, and ILs@MOFs, as polymer electrolyte filler can also be promising.
3. ILs@MOFs as Filler of Composite Electrolyte
3.1. Advantages and Bottlenecks of ILs/MOFs in Composite Electrolyte
As an important functional filler of CPEs, MOFs have been widely used [59,60,61,62]. On the one hand, the surface metal nodes as well as functional groups can interact with lithium salt and polymer, therefore facilitating the solvation of lithium salt and the Li ion transport through segment movement [63]. The defect of metal nodes may bring ion conduction to the crystal, making MOFs competitive fillers in enhancing ionic conduction efficiency for composite polymer electrolytes. On the other hand, as with traditional filler, rapid interphase conduction between polymer electrolyte and fillers can improve electrochemical performance [64].
Since MOFs have been extensively studied as CPE fillers, the interaction of polymers with ionic liquids has also attracted the attention of researchers. In general, ionic liquids are able to form ILs-Gel gel electrolytes with suitable polymers, acting as plasticizers [65]. To reduce the crystallinity of polyethylene oxide (PEO), ionic liquids [EMIM][TFSI] were added to PEO solid electrolytes to obtain a room-temperature ionic conductivity of up to 1.85 × 10−4 S cm−1 by Polu et al. [66]. Considering the narrow electrochemical window of PEO, Hofmann added 1-ethyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)azanide (EMPyrr-TFSA) ILs into PEO, which achieved an electrochemical stabilization window of 0~5.2 V while obtaining a room temperature ionic conductivity at the mS cm−1 level [67]. Further substituting PEO matrix with PVDF-HFP, a wider electrochemical stable window of 0~6.2 V can be obtained. Apart from that, researchers also used ionic liquids to replace flammable organic solutions, thus improving the thermal stability and safety of electrolytes [68].
Previous studies revealed that as framework structure compounds rich in OMS, MOFs materials can encapsulate organic solutions to form quasi-solid electrolytes [41,57]. Therefore, the application of MOFs can be extended into polymer electrolytes to act as fillers which would bind ILs (Figure 5). Firstly, the MOFs framework provided suppor to ILs, which prevents the leakage of ILs during tight battery assembling and also enhances the mechanical strength of electrolyte. Secondly, the ILs provide extra ionic transport pathways inside MOFs to enhance conductivity (Figure 6a). Finally, the interaction of MOFs particles and ILs reduces the addition of Ils to achieve approximate performance, which lowers the production cost. Guo et al. synthesized a series of PEO-n-UiO CPE containing Li-[EMIM][TFSI] for the first time in regards to reducing the mass fraction of Ils@MOFs fillers based on the study of Ils@MOFs electrolytes [42,57,69]. Combining SEM, XRD and DSC characterization results, the researchers found that the addition of ILs@MOFs still retained the plasticizer effect of ILs in reducing the crystallinity of PEO. The resulting CPE reached an optimized room temperature ionic conductivity of 1.7 × 10−5 S cm−1, and the Li+ transference number was increased to 0.35 due to the pore absorption effect of MOFs on anions.
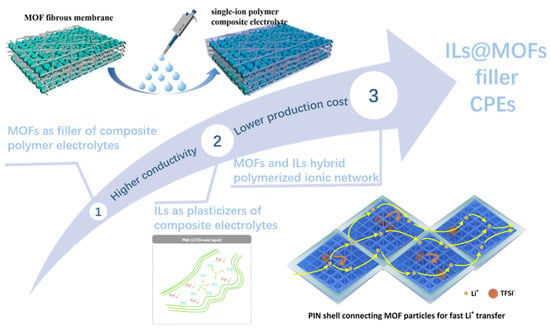
Figure 5.
The development from MOFs filler CPEs, ILs plasticized CPEs and MOFs and ILs PIN to ILs @MOFs filler CPEs. Reproduced with permission [54]. Copyright 2023, Elsevier. Reproduced with permission [70]. Copyright 2016, Wiley. Reproduced with permission [71]. Copyright 2022, American Chemical Society.
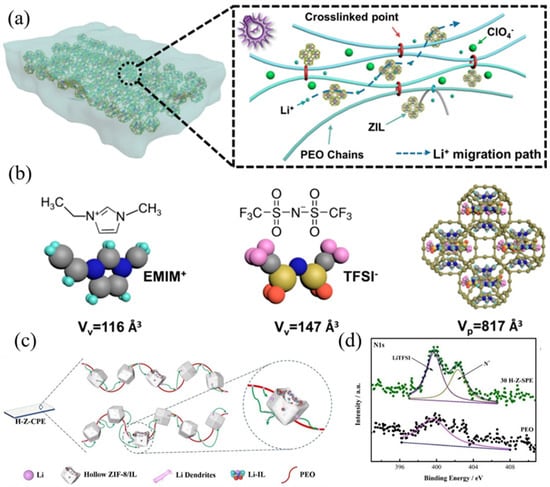
Figure 6.
(a) The crosslinked composite solid electrolyte and Li+ migration pathway. Reproduced with permission [72]. Copyright 2020, American Chemical Society. (b) The van der Waals volumes of EMIM+ and TFSI− and structure of ZIF-based ionic conductor after incorporating (EMIM0.83Li0.17) TFSI. Reproduced with permission [72]. Copyright 2020, American Chemical Society. (c) Schematic illustration of structure of hollow ZIF-8/IL filler and the its function in storage LiTFSI. Reproduced with permission [73]. Copyright 2022, Wiley. (d) XPS N 1s spectra of PEO CPE and ILs@MOFs CPE, reflected the extra N+ from [EMIM][TFSI] ILs. Reproduced with permission [73]. Copyright 2022, Wiley.
Mai et al. selected ZIF-8 materials with micropore sizes that matched the size of EMIM+ ions as MOFs framework to accommodate ionic liquids, which obtained a tLi+ up to 0.67 [72]. Explanation for this performance enhancement lies in the confining effect of ZIF framework on EMIM+ and TFSI− in lithium-containing ionic liquids with a large ionic radius, making them difficult to diffuse under the drive of electric field (Figure 6b). Meanwhile, the zinc ion in ZIF-8 which acts as a Lewis acid center, can adsorb TFSI− based on Lewis acid-base interaction, which immobilizes anion movement and promotes Li+ dissociation at the same time. The Lewis acid site also reduced the crystallinity of polymer by the reduction of ion coupling; therefore, the ion migration ability is enhanced. Consequently, a room-temperature ionic conductivity of 4.26 × 10−4 and a tLi+ of 0.67 were achieved simultaneously. In addition to the advantages of ionic liquids themselves being retained, the characteristics of MOFs can be better utilized when combined with polymer. Luo et al. used tannic acid for etching to obtain ZIF-8 with larger size after the introduction of ionic liquids (Figure 6c) [73]. They suggested that the effect of ZIF-8 on ionic liquids not only lay in the adsorption of anions by Lewis acid, but also depended on the interaction between imidazole N of ZIF-8 with N+ of EMIM+ [74]. This can be confirmed from N 1s XPS spectra of EMIM+ in Figure 6d. Through density functional theory (DFT), researchers found that the interaction made Li+ inclined to combine with TFSI− site and thus promoted lithium-ion movement. The Li deposition and stripping stability was also found to be improved in the cycling of symmetric lithium batteries. Conclusions were drawn by the authors that, firstly, the insulate hollow ZIF-8 shell layer inhibits the generation of lithium dendrites; secondly, the hollow inner cavity can accommodate the ionic liquid as well as provide low-potential electron deposition of dead lithium, which alleviates the degradation of polymer matrix; and finally, the rigid shell structure can mechanically block lithium dendrites and prevent penetration.
However, as an inert filler, MOFs material not only provides insufficient conductivity, but is also likely to agglomerate after exceeding the percolation threshold, which would in reverse destroy the continuous transport pathways inside electrolyte [75]. Therefore, preparation methodologies regarding the mixing of ILs@MOFs and polymers have also been investigated by researchers. The traditional mixed coating technique inevitably suffers from particle aggregation, which is caused by the decrease tendency of surface energy. Moreover, a single ILs@MOFs filler may not achieve the complex performance requirements. Exploring novel composite approaches using ionic liquids, MOFs, and polymers can facilitate further enhancement of the performance of composite electrolytes. Tu et al. proposed an in-situ growth approach to prepare CPE containing MOFs material, followed by ionic liquid impregnation (Figure 7a). This ensured the uniform distribution of MOFs in CPE which can be considered a promising way to enhance the comprehensive performance of solid-state lithium batteries [76]. The Li+ flux can be modified through uniform growth MOFs to obtain homogenized lithium deposition. Yang et al., on the other hand, used an electrostatic spinning technique to form hybrid composite fillers including ILs@MOFs with flame retardant materials [77]. They poured PEO hybrid ILs@MOFs composite polymer in the electrospinning framework structure constructed by polymer fiber and flame-retardant materials, achieving improved flame retardancy and relieved lithium ion concentration (Figure 7b). As shown in Figure 7c, Sun et al. used an electrospray technique combined with electrostatic spinning to prepare highly stable polymer backbones loaded with ILs@MOFs [78]. The electrospray technique can not only retain the integrity of the fiber skeleton, but also ensure the uniform distribution of nanoparticles. Consequently, more preparation methodologies such as in-situ growth, hybrid fillers and electrospray should be developed to solve problems of particle aggregation as well as performance deficiency. Although great promises have been delivered by these applications in the combination of ionic liquids with polymer electrolytes, shortcomings still exist. For example, the compatibility of ionic liquids with polymers generally limits the loading amount of ionic liquids. Excessive addition not only reduces the mechanical strength of the electrolyte, but also results in the leakage of ILs when the cell is assembled compactly with high pressure loading [59,79]. Moreover, even though the ionic conductivity of the electrolyte can rise to excellent levels, previous studies have demonstrated that the overall conductivity in ionic liquids containing lithium salts is mostly contributed by the ionic liquid anion and cation, while the Li+ transference number is usually lower than 0.3 [80]. This would result in the concentration polarization of effectively conducted lithium ions in the electrolyte, limiting the charge/discharge capability of the battery, especially at a high C-rate [81].
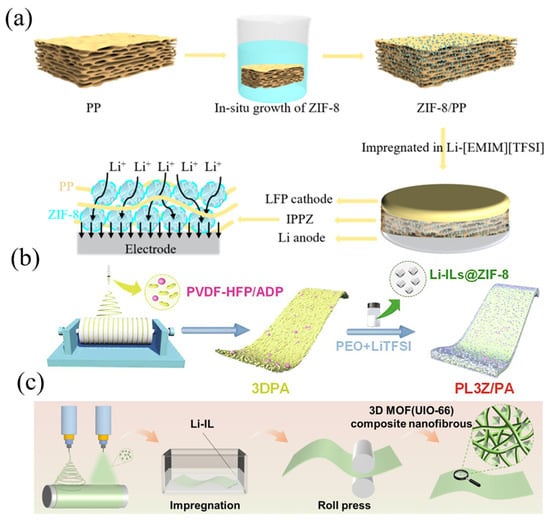
Figure 7.
(a) The preparation method of in-situ growth ILs@MOFs CPEs, Reproduced with permission [76]. Copyright 2022, American Chemical Society. (b) Schematic diagram of electrostatic spinning ILs@MOFs CPEs. Reproduced with permission [82]. Copyright 2022, Elsevier. (c) The preparation process of electrospray and electrostatic spinning ILs@MOFs CPEs. Reproduced with permission [83]. Copyright 2022, Elsevier.
3.2. Ionic Transport Mechanism and Development Strategy of ILs@MOFs CPEs
Although many ILs@MOFs polymer electrolytes have been developed, the ion-dipole interaction and ionic transport mechanism behind it still remain to be discovered. In terms of factors influencing the Li+ transport efficiency, the pore size of MOFs, as well as the loading amount of ionic liquid, are concerned, since only the proper pore size can restrict the movement of large ions and ILs exert great influence on polymer chain mobility and carrier concentration. However, although horrow-ILs-ZIF-8 adds more lithium salts to the ionic liquid than ILs-ZIF-8 in order to increase the lithium-ion migration number, the tLi+ was still lower than ILs-ZIF-8 (0.41 vs. 0.59) even though the etched hollow ZIF-8 exhibited a pore size nine times larger than the normal ZIF-8 [74,84]. Based on this irregular variation trend, future studies on the relationship between pore size of MOFs and Li+ transfer number of electrolytes in the same framework system are particularly important. Moreover, the loading conditions of ionic liquids in the pores of MOFs mainly depend on the variation of particle pore volume and specific surface area, and if only the single specific surface area variation is considered, the possible impact of ionic liquids wrapping around the outside of MOFs will be ignored. Meanwhile, excess ionic liquids may exist outside MOFs and form a triple layer structure of ILs@MOFs@ILs, which can be identified by thermogravimetry analysis (TGA) in Figure 8a [77]. The exposed ionic liquid layer is not only prone to leaking when added to the polymer electrolyte, but certain decomposition may also occur for ionic liquids with narrow electrochemical windows (Figure 8b) [82]. In the study proposed by Yuan et al., it was found that although ILs@MOFs were able to increase the ionic conductivity of the PEO electrolyte, the lithium-ion transfer number decreased from 0.23 to 0.13 compared with pure PEO (Figure 8c). The reason for this phenomenon mainly lies in exceeding ILs addition in consideration of accommodation limit, which resulted in the release of internal uncoordinated anions accompanied by the increase of polarization degree [52,84]. Therefore, favorable ionic transport capabilities can only be achieved with the right amount of ionic liquid. Unlike traditional CPEs, the addition of ionic liquids increases the analysis complexity of the system from the perspective of multi-components. The role of pure MOFs materials without the addition of ILs acts as both solid plasticizer and Lewis acid site to disrupt the segmental regularity of polymer chain and adsorb anions, and no additional Li+ conduction pathways would be formed inside MOFs due to its intrinsic inert nature. The crucial difference between MOFs and other inert fillers is the ion restriction effect of the MOFs framework structure and the enhancement of the Lewis acid-base interaction provided by the abundant metal sites. Upon the addition of ionic liquids, additional ion transport paths can be taken into account in the composite polymer electrolytes, which is similar to the percolation theory in the active filler contained-CPE category [75], and the highest ion transport capacity can be achieved at the optimum content of ILs@MOFs active fillers. The regulation is depicted as follows: at a low addition of active filler, ion transport mainly occurs in the polymer phase, in which an ion migration enhancement of the polymer chain with decreased crystallinity is achieved. When the filler reaches the optimum content, Li+ conduction routes can interconnect along the continuous percolation interface on the MOFs surface and therefore enhance ionic conductivity through the fast pathway (Figure 8d) [82].
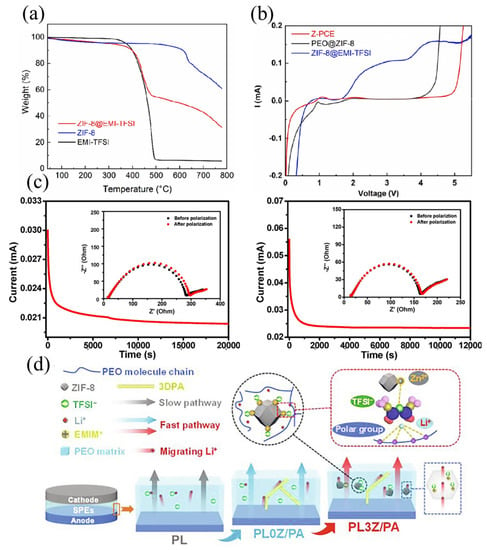
Figure 8.
(a) TGA curves of ILs@MOFs and IL and MOF while the IL is EMI-TFSI and the MOFs is ZIF-8. Reproduced with permission [82]. Copyright 2022, Elsevier. (b) The LSV curve of MOFs ILs CPE, PEO@ZIF-8 and ZIF-8@EMI-TFSI. Reproduced with permission [82]. Copyright 2022, Elsevier. (c) DC curves of PEO-only electrolyte and ILS@MOFs CPEs (insert: AC impedance test of symmetric cells before and after polarization). Reproduced with permission [84]. Copyright 2020, American Chemical Society. (d) The Li+ conductive mechanism of CPEs. Reproduced with permission [82]. Copyright 2022, Elsevier.
However, the highly conductive ionic transport paths would be blocked when the content of particles continuous to increase and unavoidable aggregation occurs, and the ion transport path through polymer matrix is also diluted by the aggregated fillers. Although ions can be transported through the matrix and filler, the polymer phase and the inorganic phase undergo mutual obstruction, which eventually leads to a decrease in ionic conductivity [85]. In the current study on the addition of ILs@MOFs, Sun et al. found a slight increase in polymer ionic conductivity with the addition of small amounts of ILs@MOFs, and a significant increase in conductivity can be obtained after the loading amount achieved a certain extent, which triggered the generation of continuous ion channels [39]. Mai et al., on the other hand, proposed a solid-liquid-like transport interface mechanism [72]. They concluded that the ILs@MOFs material added in the experiment acts as a high-speed migration path for lithium-ion transport between polymer chains through experimental and computational analysis of diffusion energy barriers. Lithium ions bounded by polymer chain segments are solvated on the surface of ILs@MOFs by TFSI− to enter the framework. Simultaneous desolvation of an equal amount of lithium ions is transferred to other PEO chains. The lithium ions in ILs@MOFs exhibit significantly stronger mobility, and the electron density in the micropores changes after the addition of ionic liquids. It is difficult to determine both the lithium ion and external lithium salt concentrations and energy levels inside the filler, which poses a new challenge to the application of space charge layer theory. More scientific evaluation methods for compatibility of ionic liquids, MOFs materials, and polymeric substrates, and Li+ migration paths in the solid-liquid systems of ILs@MOFs are still required for future research applications.
4. Conclusions and Perspectives
In this review, the main advances in hybrid solid-state electrolytes associated with MOFs materials are highlighted. As an important candidate technology for next-generation electrochemical energy storage devices and two application aspect for MOFs-based solid electrolytes are discussed in this paper, including the employment of ILs@MOFs materials as conductive bodies, and ILs@MOFs as composite polymer electrolyte fillers. As an emerging fast ion transport material, ionic liquids in combination with MOFs in framework structures can combine the advantages of ILs and MOFs to enhance ion transport capacity and ion selectivity and improve interfaces while achieving liquid encapsulation to improve the overall structural stability. ILs@MOFs hybrid electrolytes utilize a robust framework to enhance dendrite resistance and restrict internal fluid flow to prevent leakage. The organic framework with metal ions offers an enhanced lithium-ion transference number due to the confining effect on both anions and cations, and the absorption of anions on metal nodes. The internal ionic liquid provides rapid ion transfers within the crystal and intergranular wetting interfaces, which also reduces system flammability. Therefore, the matching of pore size with ions and the adjustment of Lewis acidity can better perform the role of host and guest. Consequently, the possible ion transport mechanism is mainly one-dimensional ion transport within the framework of MOFs, where Li+ ions dissociate and transfer at the solid-phase interface and the internal solvent. Although the complex ion transport mechanism and precise role of ILs in this system still need deeper investigation, the application of ILs@MOFs has shown promising prospects, which provides a structural reference for new solid electrolytes.
The use of ILs@MOFs materials as fillers for polymers originates from the combination of multifunctional MOFs framework structure and ILs plasticizer with high ionic conductivity. While using ILs@MOFs materials as polymer electrolyte fillers, novel low-energy paths for ion transportation can be formed, in which the solid-liquid-like interfacial conduction mode in the polymer greatly improves the Li+ transport speed of the electrolyte. Therefore, the overall ionic conduction network is composed of Li+ transport pathways formed at the interface between MOFs fillers as well as the solvate-desolvate ILs conduction routes inside the polymer matrix, originating from both chain segment movement and interfacial percolation interfacial theory. Moreover, compared to direct IL addition, ILs@MOF also plays a better role in limiting adsorption and stabilization, improving ion selectivity and structural stability effectively. In view of the above-mentioned advantages and prospects, the higher application feasibility of MOFs as solid electrolyte also sheds light on its possible future commercialization.
All in all, ILs@MOFs materials are a promising solid-state electrolyte candidate, either used as bulk material or fillers. Comprehensive performances of ILs@MOFs can be achieved through the complementary individual components including ILs and MOFs, in which ILs provide ion conductivity and surface wettability, and MOFs provide structural support and functional sites. However, the interaction mechanism between ILs and MOFs still requires further research, such as on solvation and desolation behavior at the ILs@MOFs surface. More importantly, the inner ion transportation mechanism of the ILs@MOFs system remains unclear, which needs more illustration in order to guide frontier technologies. Future research can address:
- The development of new MOFs materials. MOFs materials have powerful modular properties due to the rich variety of inorganic metal centers and bridging organic ligands in combination with various grafting methods. For lithium conduction in Li-ion batteries, the composition of the MOFs material will determine the strength of the encapsulant-frame interaction. The reasonable regulation on Lewis acidity and charge density of MOFs material will effectively improve the ionic conductivity and selectivity of the hybrid electrolyte.
- Developing evaluation methods for the performance of ILs@MOFs. While the encapsulation of different kinds of ILs in MOFs has become relatively common, the types of ILs and the generated transference properties are not yet well summarized and are still in the mapping stage. Therefore, theoretical calculations of ILs and systematic encapsulation schemes based on theoretical and practical phenomena are particularly important for the systematic development of ILs@MOFs hybrid electrolytes.
- Deepening the study of key structural factors for MOFs. Due to the complex topology of MOFs materials, the specific surface area, particle size and pore size of the formed structures can have different effects on interaction with ILs and electrochemical properties. The study of these structure-related relationships can better serve the development of new ILs@MOFs materials.
- Carrying out in-depth theoretical studies on ion transport mechanisms. The mechanism of lithium-ion transport can serve as an important guide for the design of both electrolyte systems with ILs@MOFs as the main body and composite polymer systems with ILs@MOFs as fillers. As a new type of solid electrolyte system, the transport mechanism of ILs@MOFs as the main electrolyte system has not been clarified in relation to the type and amount of inner or external ILs. Thus, the basis of the conduction theory still needs to be clarified. Nonetheless, the transport mechanism regarding MOFs in composite polymer systems can be explained using the common theory of composite electrolyte systems to a certain extent, while the influence of their metal sites and encapsulants on conduction still lacks empirical evidence and remains to be explored. Along with more extensive experiments, basic conduction theory research needs to be developed as soon as possible.
- Deepening the analysis of electrolyte/electrode interfacial evolution processes. In electrolyte with ILs@MOFs as the main body, the stability of ILs@MOFs in contact with the lithium anode interface directly affects the SEI generation and the growth of dendrites. Therefore, ensuring good contact between the interface of rigid ILs@MOFs materials and electrodes as well as providing high mechanical strength and thermodynamics stability for electrolyte against electrode is urgent. A thorough theoretical study of the multiple interfaces related to ILs@MOFs can better integrate it with the existing theoretical system and facilitate further theoretical design and practical applications.
- The integrated development of ILs@MOFs in the mass production of electrolytes. In industrialized battery design, factors such as raw material production process, matching degree with present manufacturing procedures, and overall battery performance need to be considered. ILs@MOFs materials can be synthesized at room temperature or using hydrothermal methods, which can meet commercial large-scale production requirements. Due to the precedent of commercial polymers, electrolyte membranes can be easily prepared through solution-casting technique by the simple mixing and flowing of precursor polymer solution containing ILs@MOFs fillers. However, the current problem is the high price of raw materials such as ionic liquids and the difficulty of ensuring uniformity in large-scale production. The development of sustainable, environmentally friendly, low-cost mass production solutions is of great significance for practical production.
Author Contributions
Conceptualization, R.L.; formal analysis, Y.J.; investigation, R.L.; resources, R.L. and X.Z.; data summary, X.Z.; writing original draft preparation, R.L. and Y.L.; writing—review and editing, Y.J. and Y.X.; supervision, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amirante, R.; Cassone, E.; Distaso, E.; Tamburrano, P. Overview on recent developments in energy storage: Mechanical, electrochemical and hydrogen technologies. Energy Convers. Manag. 2017, 132, 372–387. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zong, X.; Zhang, X.; Jia, Z.; Tan, S.; Xiong, Y. Cathode structural design enabling interconnected ionic/electronic transport channels for high-performance solid-state lithium batteries. J. Power Sources 2022, 530, 231297. [Google Scholar] [CrossRef]
- Li, S.Q.; Wang, K.; Zhang, G.F.; Li, S.N.; Xu, Y.A.; Zhang, X.D.; Zhang, X.; Zheng, S.H.; Sun, X.Z.; Ma, Y.W. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Tan, S.J.; Zeng, X.X.; Ma, Q.; Wu, X.W.; Guo, Y.G. Recent Advancements in Polymer-Based Composite Electrolytes for Rechargeable Lithium Batteries. Electrochem. Energy Rev. 2018, 1, 113–138. [Google Scholar] [CrossRef]
- Koerver, R.; Zhang, W.B.; de Biasi, L.; Schweidler, S.; Kondrakov, A.O.; Kolling, S.; Brezesinski, T.; Hartmann, P.; Zeier, W.G.; Janek, J. Chemo-mechanical expansion of lithium electrode materials—on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 2018, 11, 2142–2158. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.Y.; Liang, J.Y.; Liu, C.; Cao, Y.C.; Wei, F.; Mai, L.Q. Interfaces in Solid-State Lithium Batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef]
- Uemura, T.; Yanai, N.; Kitagawa, S. Polymerization reactions in porous coordination polymers. Chem. Soc. Rev. 2009, 38, 1228–1236. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Y.; Li, Q.; Wang, J.; Guo, S.; Li, X.; Ouyang, Y.; Zeng, Q.; He, W.; Huang, S. Design of thiol-lithium ion interaction in metal-organic framework for high-performance quasi-solid lithium metal batteries. Dalton Trans. 2021, 50, 2928–2935. [Google Scholar] [CrossRef]
- Tao, F.; Tian, L.; Liu, Z.; Cui, R.; Liu, M.; Kang, X.; Liu, Z. A novel lithium-impregnated hollow MOF-based electrolyte realizing an optimum balance between ionic conductivity and the transference number in solid-like batteries. J. Mater. Chem. A 2022, 10, 14020–14027. [Google Scholar] [CrossRef]
- Yang, G.; Chanthad, C.; Oh, H.; Ayhan, I.A.; Wang, Q. Organic-inorganic hybrid electrolytes from ionic liquid-functionalized octasilsesquioxane for lithium metal batteries. J. Mater. Chem. A 2017, 5, 18012–18019. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Majid, M.F.; Mohd Zaid, H.F.; Kait, C.F.; Ahmad, A.; Jumbri, K. Ionic Liquid@Metal-Organic Framework as a Solid Electrolyte in a Lithium-Ion Battery: Current Performance and Perspective at Molecular Level. Nanomaterials 2022, 12, 1076. [Google Scholar] [CrossRef]
- Zhou, T.H.; Zhao, Y.; Choi, J.W.; Coskun, A. Ionic Liquid Functionalized Gel Polymer Electrolytes for Stable Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 22791–22796. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.J.; Kim, K.J.; Choi, J.W. The Synergistic Effect of Cation and Anion of an Ionic Liquid Additive for Lithium Metal Anodes. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Lei, W.Y.; Zhang, C.F.; Qiao, R.; Ravivarma, M.; Chen, H.X.; Ajdari, F.B.; Salavati-Niasari, M.; Song, J.X. Stable Li|LAGP Interface Enabled by Confining Solvate Ionic Liquid in a Hyperbranched Polyanionic Copolymer for NASICON-Based Solid-State Batteries. ACS Appl. Energy Mater. 2023, 6, 4363–4371. [Google Scholar] [CrossRef]
- Wu, S.H.; Guo, Z.W.; Sih, C.J. Enhancing the enantioselectivity of Candida lipase-catalyzed ester hydrolysis via noncovalent enzyme modification. J. Am. Chem. Soc. 1990, 112, 1990–1995. [Google Scholar] [CrossRef]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Control of Pore Size and Functionality in Isoreticular Zeolitic Imidazolate Frameworks and their Carbon Dioxide Selective Capture Properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef]
- Cho, S.H.; Ma, B.Q.; Nguyen, S.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A metal-organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem. Commun. 2006, 24, 2563–2565. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Kamachi, Y.; Takai, K.; Imura, M.; Naito, M.; Yamauchi, Y. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chem. Commun. 2013, 49, 2521–2523. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Meng, C.; Zhang, L.; Cai, Y.; Yuan, A. Anion-Immobilized and Fiber-Reinforced Hybrid Polymer Electrolyte for Advanced Lithium-Metal Batteries. Chemelectrochem 2020, 7, 2660–2664. [Google Scholar] [CrossRef]
- Moggach, S.A.; Oswald, I.D.H. Crystallography Under High Pressures. In 21st Century Challenges in Chemical Crystallography I: History and Technical Developments; Mingos, D.M.P., Raithby, P.R., Eds.; Springer: Cham, Switzerland, 2020; Volume 185, pp. 141–198. [Google Scholar]
- AbdelSalam, H.; El-Maghrbi, H.H.; Zahran, F.; Zaki, T. Microwave-assisted production of biodiesel using metal-organic framework Mg3(bdc)3(H2O)2. Korean J. Chem. Eng. 2020, 37, 670–676. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, J.; Zhao, J.; Xu, D.; Wang, F.; Liu, C.; Jiang, Y.; Wu, L.; Cui, P.; Lv, L.; et al. High-Efficiency Electromagnetic Wave Absorption of Cobalt-Decorated NH2-UIO-66-Derived Porous ZrO2/C. ACS Appl. Mater. Interfaces 2019, 11, 35959–35968. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Hosono, N.; Kitagawa, S. Chemistry of Soft Porous Crystals: Structural Dynamics and Gas Adsorption Properties. Angew. Chem.-Int. Ed. 2020, 59, 15325–15341. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Miner, E.M.; Park, S.S.; Dinca, M. High Li+ and Mg2+ Conductivity in a Cu-Azolate Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 4422–4427. [Google Scholar] [CrossRef]
- Li, H.F.; Wu, P.; Xiao, Y.W.; Shao, M.; Shen, Y.; Fan, Y.; Chen, H.H.; Xie, R.J.; Zhang, W.L.; Li, S.; et al. Metal-Organic Frameworks as Metal Ion Precursors for the Synthesis of Nanocomposites for Lithium-Ion Batteries. Angew. Chem.-Int. Ed. 2020, 59, 4763–4769. [Google Scholar] [CrossRef]
- Li, F.L.; Shao, Q.; Huang, X.Q.; Lang, J.P. Nanoscale Trimetallic Metal-Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem.-Int. Ed. 2018, 57, 1888–1892. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Yuan, N.; Sun, C. Molecular design of a metal-organic framework material rich in fluorine as an interface layer for high-performance solid-state Li metal batteries. Chem. Eng. J. 2023, 451, 138819. [Google Scholar] [CrossRef]
- Ben Saad, K.; Hamzaoui, H.; Mohamed, M.M. Ionic conductivity of metallic cations encapsulated in zeolite Y and mordenite. Mater. Sci. Eng. B 2007, 139, 226–231. [Google Scholar] [CrossRef]
- Wiers, B.M.; Foo, M.L.; Balsara, N.P.; Long, J.R. A solid lithium electrolyte via addition of lithium isopropoxide to a metal-organic framework with open metal sites. J. Am. Chem. Soc. 2011, 133, 14522–14525. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Novak, A. Proton transfer and superionic conductivity in solids and gels. J. Mol. Struct. 1988, 177, 277–308. [Google Scholar] [CrossRef]
- Zhao, T.; Kou, W.J.; Zhang, Y.F.; Wu, W.J.; Li, W.P.; Wang, J.T. Laminar composite solid electrolyte with succinonitrile-penetrating metal-organic framework (MOF) for stable anode interface in solid-state lithium metal. J. Power Sources 2023, 554, 232349. [Google Scholar] [CrossRef]
- Colombo, V.; Galli, S.; Choi, H.J.; Han, G.D.; Maspero, A.; Palmisano, G.; Masciocchi, N.; Long, J.R. High thermal and chemical stability in pyrazolate-bridged metal-organic frameworks with exposed metal sites. Chem. Sci. 2011, 2, 1311–1319. [Google Scholar] [CrossRef]
- Sumida, K.; Her, J.-H.; Dinca, M.; Murray, L.J.; Schloss, J.M.; Pierce, C.J.; Thompson, B.A.; FitzGerald, S.A.; Brown, C.M.; Long, J.R. Neutron Scattering and Spectroscopic Studies of Hydrogen Adsorption in Cr3(BTC)2-A Metal-Organic Framework with Exposed Cr2+ Sites. J. Phys. Chem. C 2011, 115, 8414–8421. [Google Scholar] [CrossRef]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef]
- Zhou, Q.; Boyle, P.D.; Malpezzi, L.; Mele, A.; Shin, J.-H.; Passerini, S.; Henderson, W.A. Phase Behavior of Ionic Liquid–LiX Mixtures: Pyrrolidinium Cations and TFSI– Anions—Linking Structure to Transport Properties. Chem. Mater. 2011, 23, 4331–4337. [Google Scholar] [CrossRef]
- Liu, L.; Sun, C. Flexible Quasi-Solid-State Composite Electrolyte Membrane Derived from a Metal-Organic Framework for Lithium-Metal Batteries. Chemelectrochem 2020, 7, 707–715. [Google Scholar] [CrossRef]
- Fujie, K.; Yamada, T.; Ikeda, R.; Kitagawa, H. Introduction of an Ionic Liquid into the Micropores of a Metal-Organic Framework and Its Anomalous Phase Behavior. Angew. Chem. Int. Ed. 2014, 53, 11302–11305. [Google Scholar] [CrossRef]
- Fujie, K.; Ikeda, R.; Otsubo, K.; Yamada, T.; Kitagawa, H. Lithium Ion Diffusion in a Metal–Organic Framework Mediated by an Ionic Liquid. Chem. Mater. 2015, 27, 7355–7361. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, R.; Wang, H.; Yang, L.; Hu, J.; Chen, H.; Pan, F. A Metal-Organic-Framework-Based Electrolyte with Nanowetted Interfaces for High-Energy-Density Solid-State Lithium Battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Huang, J.-Q.; Yuan, H.; Lu, Y.; Yan, C.; Zhu, G.-L.; Xu, R.; Zhao, C.-Z.; Hou, L.-P.; et al. Controlling Dendrite Growth in Solid-State Electrolytes. ACS Energy Lett. 2020, 5, 833–843. [Google Scholar] [CrossRef]
- Liu, Y.D.; Liu, Q.; Xin, L.; Liu, Y.Z.; Yang, F.; Stach, E.A.; Xie, J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2017, 2, 17083. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.Y.; Wang, Z.Q.; Zhao, Y.; Wang, Z.J.; Han, L.; Song, Y.L.; Pan, F. Enhanced lithium dendrite suppressing capability enabled by a solid-like electrolyte with different-sized nanoparticles. Chem. Commun. 2018, 54, 13060–13063. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Jiang, X.; Wang, X.; Li, Z.; Chen, Z.; Zhang, Y.; Zhang, S. Metal-Organic Framework Confined Solvent Ionic Liquid Enables Long Cycling Life Quasi-Solid-State Lithium Battery in Wide Temperature Range. Small 2022, 18, 2203011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Yang, L.; Wang, H.; Song, Y.; Han, L.; Yang, K.; Hu, J.; Chen, H.; Pan, F. Boosting interfacial Li+ transport with a MOF-based ionic conductor for solid-state batteries. Nano Energy 2018, 49, 580–587. [Google Scholar] [CrossRef]
- Kachmar, A.; Carignano, M.; Laino, T.; Iannuzzi, M.; Hutter, J. Mapping the Free Energy of Lithium Solvation in the Protic Ionic Liquid Ethylammonuim Nitrate: A Metadynamics Study. Chemsuschem 2017, 10, 3083–3090. [Google Scholar] [CrossRef]
- Kachmar, A.; Goddard, W.A. Free Energy Landscape of Sodium Solvation into Graphite. J. Phys. Chem. C 2018, 122, 20064–20072. [Google Scholar] [CrossRef]
- Zettl, R.; Hanzu, I. The Origins of Ion Conductivity in MOF-Ionic Liquids Hybrid Solid Electrolytes. Front. Energy Res. 2021, 9, 714698. [Google Scholar] [CrossRef]
- Wu, J.-F.; Guo, X. Nanostructured Metal-Organic Framework (MOF)-Derived Solid Electrolytes Realizing Fast Lithium Ion Transportation Kinetics in Solid-State Batteries. Small 2019, 15, 1804413. [Google Scholar] [CrossRef]
- Vazquez, M.; Liu, M.; Zhang, Z.; Chandresh, A.; Kanj, A.B.; Wenzel, W.; Heinke, L. Structural and Dynamic Insights into the Conduction of Lithium-Ionic-Liquid Mixtures in Nanoporous Metal-Organic Frameworks as Solid-State Electrolytes. Acs Appl. Mater. Interfaces 2021, 13, 21166–21174. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaoula, A.E.; Shu, J.; Cheng, Y.; Xu, L.; Zhang, G.; Xia, Y.; Tahir, M.; Wu, P.; Mai, L. Core-Shell MOF-in-MOF Nanopore Bifunctional Host of Electrolyte for High-Performance Solid-State Lithium Batteries. Small Methods 2021, 5, 2100508. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Yi, Y.K.; Wu, Z.D.; Cheng, G.Y.; Zheng, S.T.; Fang, B.R.; Wang, T.; Shchukin, D.G.; Hai, F.; Guo, J.Y.; et al. Ionic liquid confined in MOF/polymerized ionic network core-shell host as a solid electrolyte for lithium batteries. Chem. Eng. Sci. 2023, 266, 118271. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Sandwich-structural ionogel electrolyte with core-shell ionic-conducting nanocomposites for stable Li metal battery. Chem. Eng. J. 2023, 451, 138993. [Google Scholar] [CrossRef]
- Gao, Z.H.; Sun, H.B.; Fu, L.; Ye, F.L.; Zhang, Y.; Luo, W.; Huang, Y.H. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Jiang, T.L.; He, P.G.; Wang, G.X.; Shen, Y.; Nan, C.W.; Fan, L.Z. Solvent-Free Synthesis of Thin, Flexible, Nonflammable Garnet-Based Composite Solid Electrolyte for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2020, 10, 1903376. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Liu, S.L.; Liu, W.Y.; Ba, D.L.; Zhao, Y.Z.; Ye, Y.H.; Li, Y.Y.; Liu, J.P. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2023, 35, 2110423. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Li, H.; Wu, H.B. Recent Progress of Hybrid Solid-State Electrolytes for Lithium Batteries. Chem.-A Eur. J. 2018, 24, 18293–18306. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, R.; Fang, J.; Liang, Z.; Gao, L.; Bian, J.; Zhu, J.; Zhao, Y. Metal-organic framework (MOF)-incorporated polymeric electrolyte realizing fast lithium-ion transportation with high Li+ transference number for solid-state batteries. Front. Chem. 2022, 10, 1013965. [Google Scholar] [CrossRef]
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T.; Guo, X.; Sun, X. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Liu, W.; Lee, S.W.; Lin, D.C.; Shi, F.F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.-W. Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int. J. Hydrog. Energy 2017, 42, 7212–7219. [Google Scholar] [CrossRef]
- Hofmann, A.; Schulz, M.; Hanemann, T. Gel electrolytes based on ionic liquids for advanced lithium polymer batteries. Electrochim. Acta 2013, 89, 823–831. [Google Scholar] [CrossRef]
- Grewal, M.S.; Tanaka, M.; Kawakami, H. Solvated Ionic-Liquid Incorporated Soft Flexible Cross-Linked Network Polymer Electrolytes for Safer Lithium Ion Secondary Batteries. Macromol. Chem. Phys. 2022, 223, 2100317. [Google Scholar] [CrossRef]
- Chen, L.; Xue, P.; Liang, Q.; Liu, X.; Tang, J.; Li, J.; Liu, J.; Tang, M.; Wang, Z. A Single-Ion Polymer Composite Electrolyte Via In Situ Polymerization of Electrolyte Monomers into a Porous MOF-Based Fibrous Membrane for Lithium Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 3800–3809. [Google Scholar] [CrossRef]
- Wu, J.-F.; Guo, X. MOF-derived nanoporous multifunctional fillers enhancing the performances of polymer electrolytes for solid-state lithium batteries. J. Mater. Chem. A 2019, 7, 2653–2659. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem.-Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, N.; Du, L.; Cheng, Y.; Lei, S.; Li, S.; Liao, X.; Shi, W.; Xu, L.; Mai, L. Rational Design of Ion Transport Paths at the Interface of Metal-Organic Framework Modified Solid Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 22930–22938. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, T.; Xu, L.; Zhang, L.; Luo, L. A Hollow Porous Metal-Organic Framework Enabled Polyethylene Oxide Based Composite Polymer Electrolytes for All-Solid-State Lithium Batteries. Batter. Supercaps 2022, 5, e202100303. [Google Scholar] [CrossRef]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance. ACS Appl. Mater. Interfaces 2016, 8, 30992–31005. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yi, E.; Fici, A.J.; Laine, R.M.; Kieffer, J. Lithium Ion Conducting Poly(ethylene oxide)-Based Solid Electrolytes Containing Active or Passive Ceramic Nanoparticles. J. Phys. Chem. C 2017, 121, 2563–2573. [Google Scholar] [CrossRef]
- Qi, X.; Cai, D.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Ionic Liquid-Impregnated ZIF-8/Polypropylene Solid-like Electrolyte for Dendrite-free Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 6859–6868. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, J.; Chen, J.; Wang, X.; Yang, Z. 3D flame-retardant skeleton reinforced polymer electrolyte for solid-state dendrite-free lithium metal batteries. J. Energy Chem. 2022, 71, 174–181. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Yuan, H.; Zeng, X.; Lan, J.; Yu, Y.; Yang, X. Fast Li+ transport pathways of quasi-solid-state electrolyte constructed by 3D MOF composite nanofibrous network for dendrite- free lithium metal battery. Mater. Today Energy 2022, 29, 101117. [Google Scholar] [CrossRef]
- Long, L.Z.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Frömling, T.; Kunze, M.; Schönhoff, M.; Sundermeyer, J.; Roling, B. Enhanced Lithium Transference Numbers in Ionic Liquid Electrolytes. J. Phys. Chem. B 2008, 112, 12985–12990. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Shen, J.; Lei, Z.; Wang, C. An ion conducting ZIF-8 coating protected PEO based polymer electrolyte for high voltage lithium metal batteries. Chem. Eng. J. 2022, 447, 137503. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Meng, C.; Xiong, W.; Cai, Y.; Hu, P.; Pang, H.; Yuan, A. Enhancing Ion Transport: Function of Ionic Liquid Decorated MOFs in Polymer Electrolytes for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2020, 3, 4265–4274. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Shen, F.-Y.; Long, X.; Zheng, S.; Ruan, Z.; Cai, Y.-P.; Hong, X.-J.; Zheng, Q. Fast Li+ transport and superior interfacial chemistry within composite polymer electrolyte enables ultra-long cycling solid-state Li-metal batteries. Energy Storage Mater. 2022, 52, 201–209. [Google Scholar] [CrossRef]
- Fu, J.L.; Li, Z.; Zhou, X.Y.; Guo, X. Ion transport in composite polymer electrolytes. Mater. Adv. 2022, 3, 3809–3819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).