Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4@C Nanoparticles
2.2. Fabrication of yolk-shelled YS-Fe3O4@C Nanocavities
2.3. Sulfur Cathode Preparation
2.4. Materials Characterization
2.5. Electrochemical Measurement
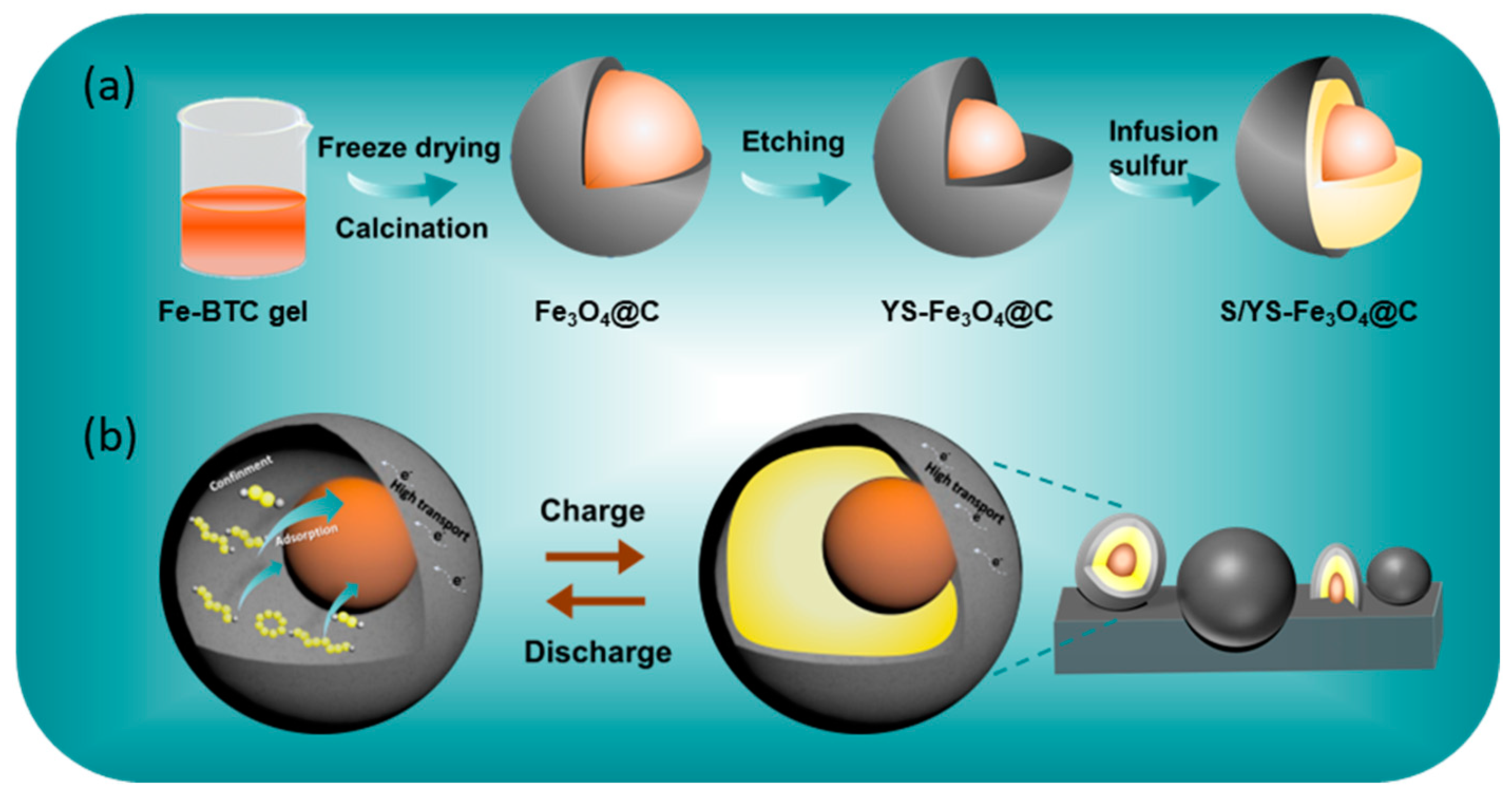
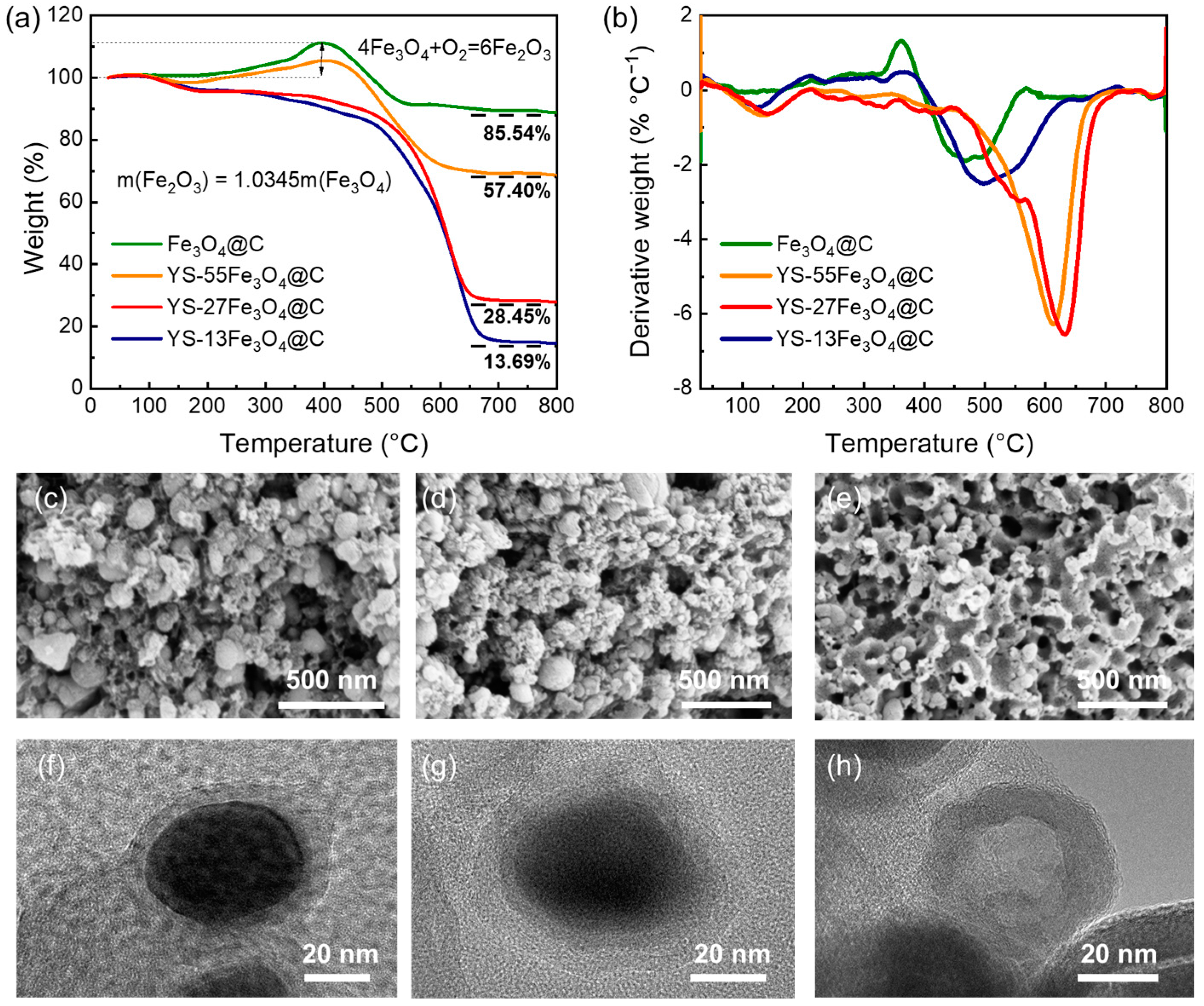
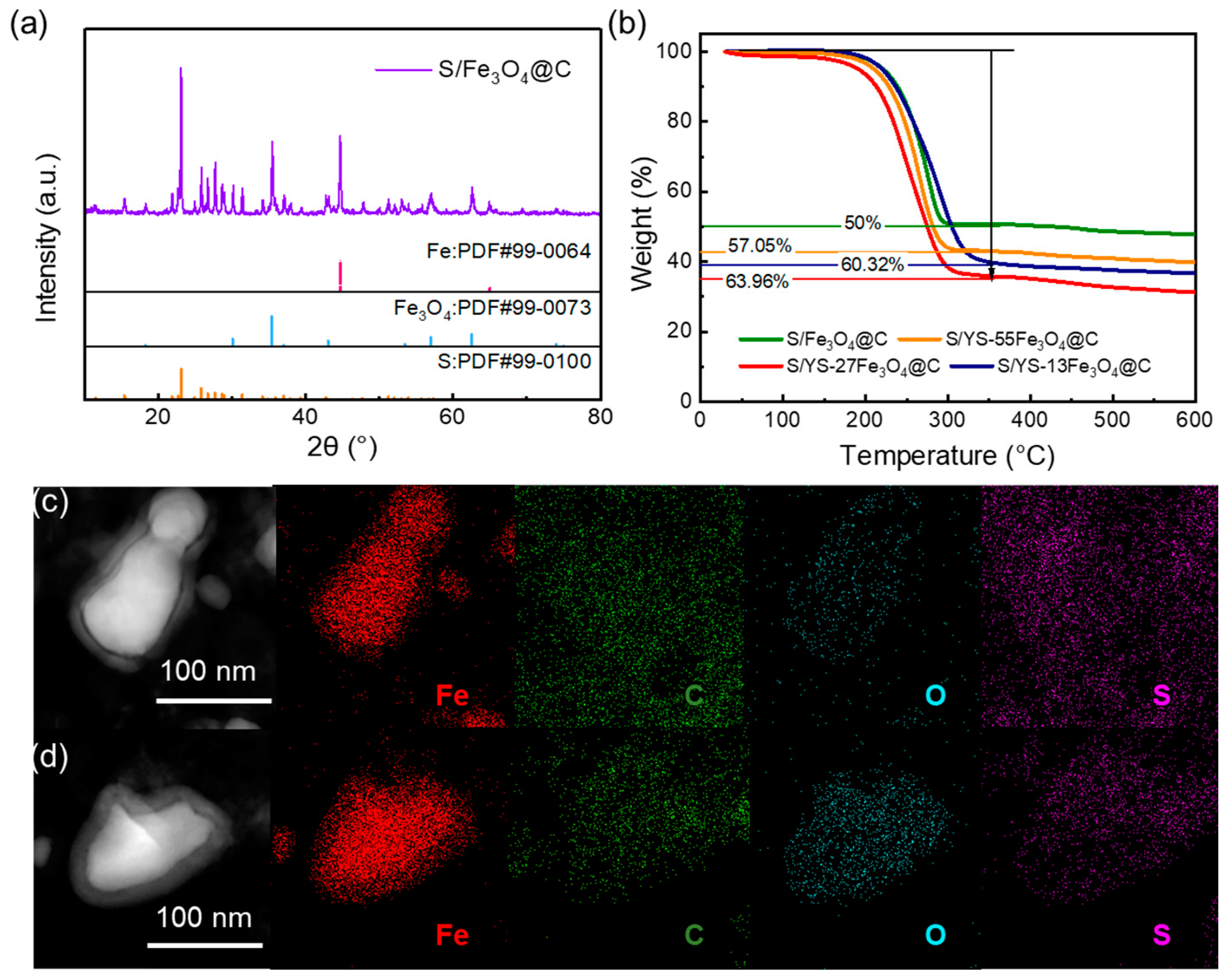
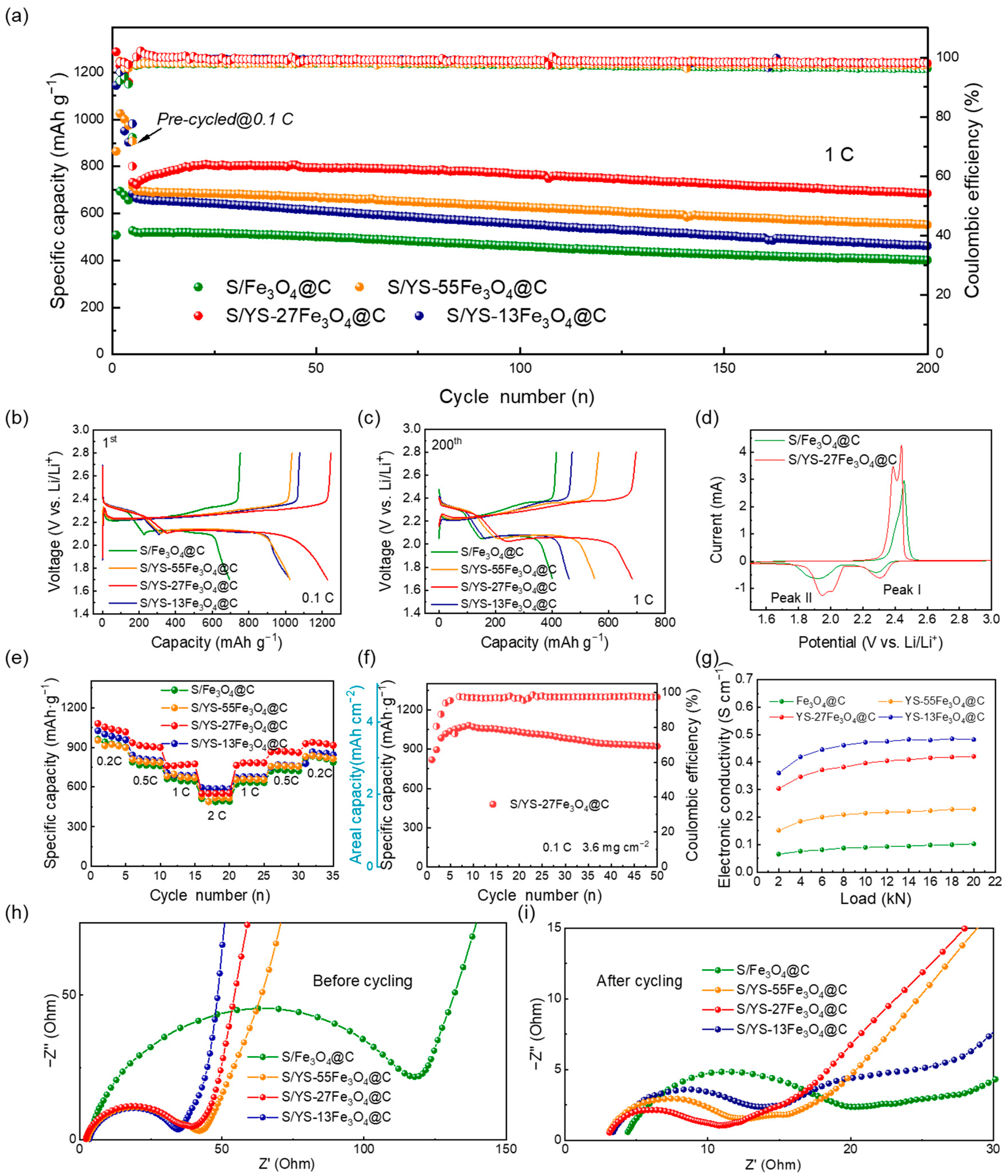
3. Results and Discussion
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Advanced Nanostructured Carbon-Based Materials for Rechargeable Lithium-Sulfur Batteries. Carbon 2019, 141, 400–416. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries—Ten Critical Questions. Chem. Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef]
- Chung, S.-H.; Chang, C.-H.; Manthiram, A. Progress on the Critical Parameters for Lithium-Sulfur Batteries to Be Practically Viable. Adv. Funct. Mater. 2018, 28, 1801188. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.-J.; Li, B.-Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.-Q.; Zhang, Q. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1802768. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Kim, S.J.; Chang, D.; Park, K.-Y.; Dae, K.S.; Dao, K.P.; Yuk, J.M.; Kang, K. Visualization of Regulated Nucleation and Growth of Lithium Sulfides for High Energy Lithium Sulfur Batteries. Energy Environ. Sci. 2019, 12, 3144–3155. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Wen, J.; Hu, X.; Li, Z.; Li, M.; Pan, F.; Xu, C. Separator Engineering toward Practical Li-S Batteries: Targeted Electrocatalytic Sulfur Conversion, Lithium Plating Regulation, and Thermal Tolerance. Nano Energy 2022, 95, 106982. [Google Scholar] [CrossRef]
- Luo, D.; Li, G.; Deng, Y.; Zhang, Z.; Li, J.; Liang, R.; Li, M.; Jiang, Y.; Zhang, W.; Liu, Y.; et al. Synergistic Engineering of Defects and Architecture in Binary Metal Chalcogenide toward Fast and Reliable Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900228. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Wang, J.; Chen, C.; Hu, C. A Multifunctional Separator Based on Dilithium Tetraaminophthalocyanine Self-Assembled on RGO with Improved Cathode and Anode Performance in Li–S Batteries. Carbon 2023, 201, 307–317. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Chen, L.; Lu, Y.; Su, Y.; Jia, Y.; Bao, L.; Wang, J.; Chen, S.; Chen, R. Metal-Organic Frameworks Composites Threaded on the CNT Knitted Separator for Suppressing the Shuttle Effect of Lithium Sulfur Batteries. Energy Storage Mater. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Rafie, A.; Kim, J.W.; Sarode, K.K.; Kalra, V. A Review on the Use of Carbonate-Based Electrolytes in Li-S Batteries: A Comprehensive Approach Enabling Solid-Solid Direct Conversion Reaction. Energy Storage Mater. 2022, 50, 197–224. [Google Scholar] [CrossRef]
- Guo, D.; Li, X.; Ming, F.; Zhou, Z.; Liu, H.; Hedhili, M.N.; Tung, V.; Alshareef, H.N.; Li, Y.; Lai, Z. Electropolymerization Growth of an Ultrathin, Compact, Conductive and Microporous (UCCM) Polycarbazole Membrane for High Energy Li–S Batteries. Nano Energy 2020, 73, 104769. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Shi, X.; Zhang, H.; Song, D.; Ding, F.; Zhang, L. Outstanding Cycle Stability and Rate Capabilities of the All-Solid-State Li–S Battery with a Li7P3S11 Glass-Ceramic Electrolyte and a Core–Shell S@BP2000 Nanocomposite. J. Mater. Chem. A 2019, 7, 3895–3902. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards High-Performance Solid-State Li–S Batteries: From Fundamental Understanding to Engineering Design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Z.; Fang, R.; Shi, Y.; Cheng, H.-M.; Li, F. Metal-Organic Frameworks (MOFs)-Derived Nitrogen-Doped Porous Carbon Anchored on Graphene with Multifunctional Effects for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707592. [Google Scholar] [CrossRef]
- Wang, J.; Jia, L.; Duan, S.; Liu, H.; Xiao, Q.; Li, T.; Fan, H.; Feng, K.; Yang, J.; Wang, Q.; et al. Single Atomic Cobalt Catalyst Significantly Accelerates Lithium Ion Diffusion in High Mass Loading Li 2 S Cathode. Energy Storage Mater. 2020, 28, 375–382. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Chen, L.; Lu, Y.; Su, Y.; Li, J.; Bao, L.; Yao, J.; Zhou, Y.; Chen, R. Electron Bridging Structure Glued Yolk-Shell Hierarchical Porous Carbon/Sulfur Composite for High Performance Li-S Batteries. Electrochim. Acta 2018, 292, 199–207. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, Fabrication, and Mechanism of Nitrogen-Doped Graphene-Based Photocatalyst. Adv. Mater. 2021, 26, 2003521. [Google Scholar] [CrossRef]
- Yao, W.; Tian, C.; Yang, C.; Xu, J.; Meng, Y.; Manke, I.; Chen, N.; Wu, Z.; Zhan, L.; Wang, Y.; et al. P-Doped NiTe2 with Te-Vacancies in Lithium–Sulfur Batteries Prevents Shuttling and Promotes Polysulfide Conversion. Adv. Mater. 2022, 34, 2106370. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. Sulfur Redox Reactions at Working Interfaces in Lithium-Sulfur Batteries: A Perspective. Adv. Mater. Interfaces 2019, 6, 1802046. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Li, Y.; Luo, S.; Liu, S.; Hong, X.; Xie, K.; Liu, Y.; Tan, X.; Zheng, C. Rational Construction of Fe2N@C Yolk–Shell Nanoboxes as Multifunctional Hosts for Ultralong Lithium–Sulfur Batteries. ACS Nano 2019, 13, 12137–12147. [Google Scholar] [CrossRef]
- Ai, W.; Li, J.; Du, Z.; Zou, C.; Du, H.; Xu, X.; Chen, Y.; Zhang, H.; Zhao, J.; Li, C.; et al. Dual Confinement of Polysulfides in Boron-Doped Porous Carbon Sphere/Graphene Hybrid for Advanced Li-S Batteries. Nano Res. 2018, 11, 4562–4573. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, A.; Wang, Z.; Chen, J.; Zeng, Q.; Chen, P.; Liu, W.; Li, Z.; Zhang, L. A New Cathode Material Synthesized by a Thiol-Modified Metal–Organic Framework (MOF) Covalently Connecting Sulfur for Superior Long-Cycling Stability in Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 24515–24523. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Huang, J.; Yan, C.; Sun, H.; Wang, C.; Zhang, W.; Li, Y.; Xiong, J. Multi-Functional Layered WS2 Nanosheets for Enhancing the Performance of Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1601843. [Google Scholar] [CrossRef]
- Deng, D.-R.; Xue, F.; Jia, Y.-J.; Ye, J.-C.; Bai, C.-D.; Zheng, M.-S.; Dong, Q.-F. Co4N Nanosheet Assembled Mesoporous Sphere as a Matrix for Ultrahigh Sulfur Content Lithium–Sulfur Batteries. ACS Nano 2017, 11, 6031–6039. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, J.; Chen, S.; Li, H.; Sang, M.; Yang, M.; Wang, X.; Yang, S. Integrating Polar and Conductive Fe2O3–Fe3C Interface with Rapid Polysulfide Diffusion and Conversion for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 39772–39781. [Google Scholar] [CrossRef]
- Shen, J.; Xu, X.; Liu, J.; Wang, Z.; Zuo, S.; Liu, Z.; Zhang, D.; Liu, J.; Zhu, M. Unraveling the Catalytic Activity of Fe–Based Compounds toward Li2 Sx in Li–S Chemical System from d–p Bands. Adv. Energy Mater. 2021, 11, 2100673. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, M.; Fan, X.; Jiang, H.; Zhao, T. A Li-S Battery with Ultrahigh Cycling Stability and Enhanced Rate Capability Based on Novel ZnO Yolk-Shell Sulfur Host. J. Energy Chem. 2021, 55, 136–144. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Bin Wu, H.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. Enhancing Lithium–Sulphur Battery Performance by Strongly Binding the Discharge Products on Amino-Functionalized Reduced Graphene Oxide. Nat. Commun. 2014, 5, 5002. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Zhang, B.; Chen, C.; Lin, Z. Integrating Conductivity, Immobility, and Catalytic Ability into High-N Carbon/Graphene Sheets as an Effective Sulfur Host. Adv. Mater. 2020, 32, 1906357. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Wei, Q.; James, S.L. A Metal–Organic Gel Used as a Template for a Porous Organic Polymer. Chem. Commun. 2005, 12, 1555–1556. [Google Scholar] [CrossRef]
- Liu, S. 3D Pomegranate-like Structures of Porous Carbon Microspheres Self-Assembled by Hollow Thin-Walled Highly-Graphitized Nanoballs as Sulfur Immobilizers for Li–S Batteries. Nano Energy 2019, 63, 103894. [Google Scholar] [CrossRef]
- Mahmood, A.; Zou, R.; Wang, Q.; Xia, W.; Tabassum, H.; Qiu, B.; Zhao, R. Nanostructured Electrode Materials Derived from Metal–Organic Framework Xerogels for High-Energy-Density Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 2148–2157. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, Y.; Meng, Z.; Xie, Q.; Lin, L.; Zheng, H.; Sa, B.; Lin, J.; Wang, L.; Peng, D. Anchoring Polysulfides and Accelerating Redox Reaction Enabled by Fe-Based Compounds in Lithium–Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100970. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Lu, J.; Skrabutenas, J.C.; Xu, T.; Xiao, Z.; Maguire, J.A.; Hosmane, N.S. Conversion of Carbon Dioxide to Few-Layer Graphene. J. Mater. Chem. 2011, 3, 9491–9493. [Google Scholar] [CrossRef]
- Gao, Z.; Schwab, Y.; Zhang, Y.; Song, N.; Li, X. Ferromagnetic Nanoparticle–Assisted Polysulfide Trapping for Enhanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1800563. [Google Scholar] [CrossRef]
- Tan, G.; Xu, R.; Xing, Z.; Yuan, Y.; Lu, J.; Wen, J.; Liu, C.; Ma, L.; Zhan, C.; Liu, Q.; et al. Burning Lithium in CS2 for High-Performing Compact Li2S–Graphene Nanocapsules for Li–S Batteries. Nat. Energy 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- He, J.; Luo, L.; Chen, Y.; Manthiram, A. yolk-shelled C@Fe3O4 Nanoboxes as Efficient Sulfur Hosts for High-Performance Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1702707. [Google Scholar] [CrossRef]
- Liu, G. MOF Derived In-Situ Carbon-Encapsulated Fe3O4@C to Mediate Polysulfides Redox for Ultrastable Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 381, 122652. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, F.; Cao, H.; Liu, L.; Wang, Y.; Niu, Z. Integration of Binary Active Sites: Co3V2O8 as Polysulfide Traps and Catalysts for Lithium-Sulfur Battery with Superior Cycling Stability. Small 2020, 16, 1907153. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, H.; Yu, M.; Li, X.; Dai, Y.; Zheng, W.; Jiang, X.; He, G. Sandwich-Structured Flexible Interlayer with Co3O4 Nanoboxes Strung along Carbon Nanofibers on Both Sides for Fast Li+ Transport and High Redox Activity in High-Rate Li-S Batteries. Chem. Eng. J. 2022, 449, 137777. [Google Scholar] [CrossRef]
- Deng, D.R.; Xue, F.; Bai, C.-D.; Lei, J.; Yuan, R.; Zheng, M.S.; Dong, Q.F. Enhanced Adsorptions to Polysulfides on Graphene-Supported BN Nanosheets with Excellent Li–S Battery Performance in a Wide Temperature Range. ACS Nano 2018, 12, 11120–11129. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.-C.; Wu, Y.; Shu, Y.; Gong, X.; Ke, F.-S.; Deng, H. Metal–Organic Frameworks for High Charge–Discharge Rates in Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 15, 3916–3921. [Google Scholar] [CrossRef]
- He, Y.; Li, M.; Zhang, Y.; Shan, Z.; Zhao, Y.; Li, J.; Liu, G.; Liang, C.; Bakenov, Z.; Li, Q. All-Purpose Electrode Design of Flexible Conductive Scaffold toward High-Permanence Li–S Batteries. Adv. Funct. Mater. 2020, 30, 2000613. [Google Scholar] [CrossRef]
- Ding, M. Promoting Polysulfide Conversion by Catalytic Ternary Fe3O4/Carbon/Graphene Composites with Ordered Microchannels for Ultrahigh-Rate Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 7, 25078–25087. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Ahad, S.A.; Sun, D.; Wang, L. Cyclic Voltammetry in Lithium-sulfur Battery—Challenges and Opportunities. Energy Technol. 2019, 7, 1801001. [Google Scholar] [CrossRef]
- Ye, Z. Enhanced Catalytic Conversion of Polysulfide Using 1D CoTe and 2D MXene for Heat-Resistant and Lean-Electrolyte Li–S Batteries. Chem. Eng. J. 2022, 430, 132734. [Google Scholar] [CrossRef]
- Cañas, N.A.; Wolf, S.; Wagner, N.; Friedrich, K.A. In-Situ X-Ray Diffraction Studies of Lithium–Sulfur Batteries. J. Power Sources 2013, 226, 313–319. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Hu, J.; Lim, Y.V.; Kong, D.; Zheng, Y.; Ding, M.; Pam, M.E.; Yang, H.Y. Mechanism Investigation of High-Performance Li–Polysulfide Batteries Enabled by Tungsten Disulfide Nanopetals. ACS Nano 2018, 12, 9504–9512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, Y.; Zhu, Y.; Wang, Q.; Zhang, F.; Dong, X.; Alshareef, H.N. Hierarchically Structured Ti3C2 T MXene Paper for Li-S Batteries with High Volumetric Capacity. Nano Energy 2021, 86, 106120. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhao, C.; Lu, Y.; Wan, L.; Yan, K.; Bai, Y.; Liu, Z.; Yang, X.; Su, Y.; Wu, F. Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries. Batteries 2023, 9, 295. https://doi.org/10.3390/batteries9060295
Chen L, Zhao C, Lu Y, Wan L, Yan K, Bai Y, Liu Z, Yang X, Su Y, Wu F. Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries. Batteries. 2023; 9(6):295. https://doi.org/10.3390/batteries9060295
Chicago/Turabian StyleChen, Lai, Chenying Zhao, Yun Lu, Lingyi Wan, Kang Yan, Youxiang Bai, Zhiyu Liu, Xulai Yang, Yuefeng Su, and Feng Wu. 2023. "Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries" Batteries 9, no. 6: 295. https://doi.org/10.3390/batteries9060295
APA StyleChen, L., Zhao, C., Lu, Y., Wan, L., Yan, K., Bai, Y., Liu, Z., Yang, X., Su, Y., & Wu, F. (2023). Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries. Batteries, 9(6), 295. https://doi.org/10.3390/batteries9060295





