In-Situ Polymerized Solid-State Polymer Electrolytes for High-Safety Sodium Metal Batteries: Progress and Perspectives
Abstract
:1. Introduction
2. SPEs Prepared Using Photoinduced In-Situ Polymerization
3. SPEs Prepared by Thermally Induced In-Situ Free Radical Polymerization
3.1. Conventional Polymer Electrolytes
3.2. Poly(ionic liquid)-Based Polymer Electrolytes
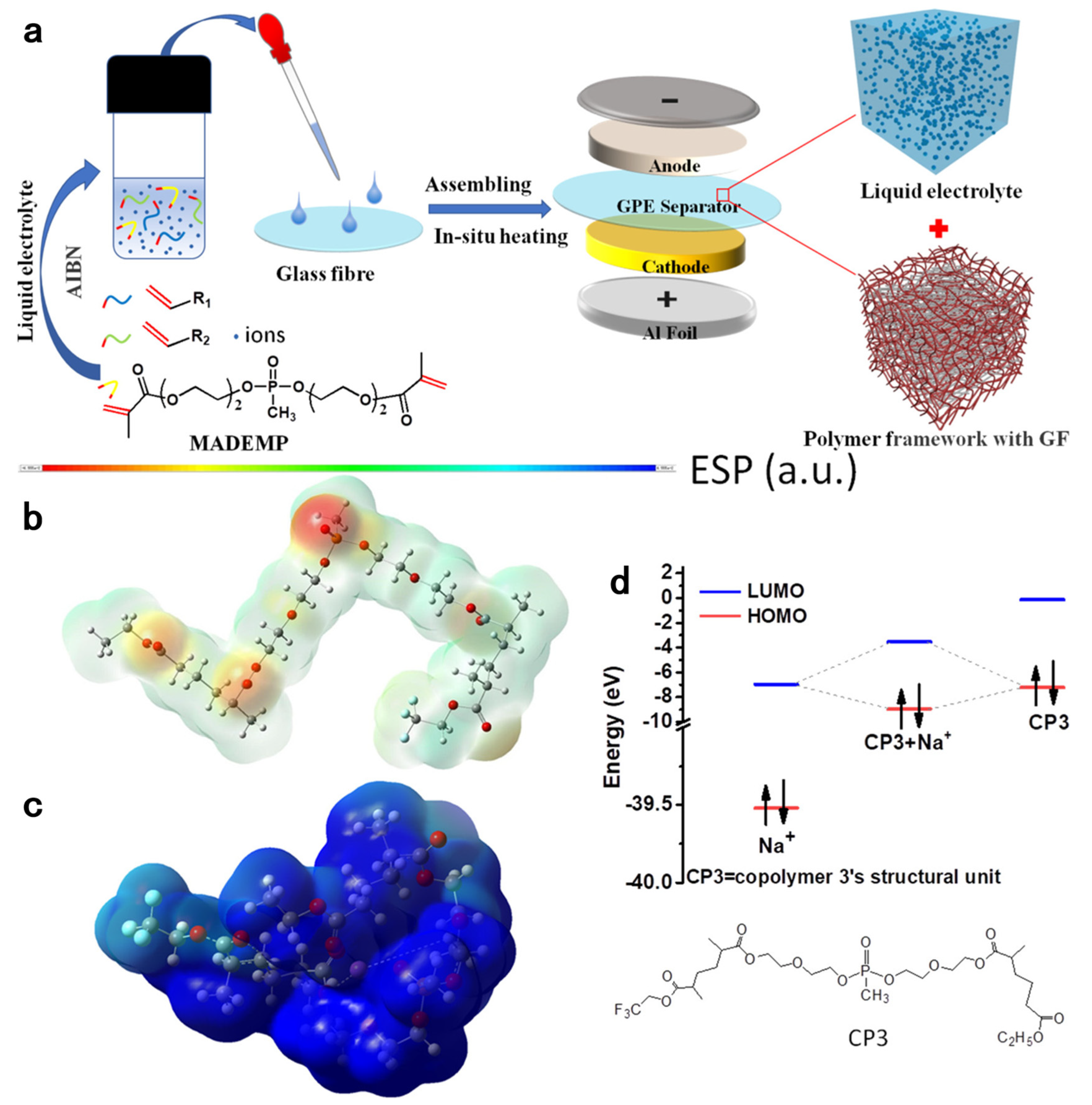
3.3. Flame-Retardant Polymer Electrolytes
4. SPEs Manufactured Using In-Situ Cationic Polymerization
5. SPEs Prepared through a Cross-Linking Reaction
6. Conclusions and Perspectives
- Sodium salt containing large anions is crucial to improve sodium-ion transference and ionic conductivity. However, almost all of the SPEs employ small anion during the in-situ polymerization in the previous works. Small anions will lead to lower sodium-ion transference numbers and increase the polarization of polymer cells. Hence, it is necessary to design and prepare SPEs using sodium salts containing large anions in future research.
- At present, the reported in-situ polymerization employs the glass fiber separator or other separators, which separate the positive and negative electrodes before the polymerization reaction occurs. Nevertheless, the homogeneity and the consistency of the polymerized product in either the coin cells or pouch cells are not well studied. The inhomogeneous polymerization may cause unwanted results that decrease the battery performance, e.g., uneven electrode reactions would cause severe localized degradations of the electrode materials. Hence, the polymerization reactions occurring inside the cells are worth investigating in future work.
- With the rapid deployment of rechargeable batteries in harsh operating conditions, such as deep-sea, deep-space, and deep-ground, it is important to design and develop fluorine-free, environmentally friendly polymer electrolytes to build next-generation fluorine-free SMBs to meet these requirements.
- Although many in-situ polymerization methods have been demonstrated successful for building solid-state sodium metal batteries, from a practical point of view, it is the sodium ion batteries that employ carbon anode will be widely used in grid energy storage or low-speed EVs. Hence, it is suggested that great effects are devoted to developing in-situ polymerization methods for sodium-ion batteries built with carbon-based anodes.
- The interfacial compatibility of the SPEs with both sodium anode and high-voltage cathode is crucially important to achieve high-energy-density and high-safety SIBs. Therefore, more ingenious and advanced characterization methods (e.g., synchrotron X-ray tomography, in-situ X-ray diffraction, operando nuclear magnetic resonance, in-situ Fourier-transform infrared, in-situ X-ray absorption near-edge structure) are necessary to further explore the interfacial compatibility and its dynamic evolution, which can provide mechanistic insights for developing advanced SPEs for SMBs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomich, A.W.; Park, J.; Son, S.-B.; Kamphaus, E.P.; Lyu, X.; Dogan, F.; Carta, V.; Gim, J.; Li, T.; Cheng, L.; et al. A carboranyl electrolyte enabling highly reversible sodium metal anodes via a “fluorine-free” SEI. Angew. Chem. Int. Ed. 2022, 61, e20220. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, Y.B.; Le, P.M.L.; Vo, T.D.; Zhou, Q.; Qi, X.G.; Engelhard, M.H.; Matthews, B.E.; Jia, H.; Nie, Z.M.; et al. Highly reversible sodium ion batteries enabled by stable electrolyte-electrode interphases. ACS Energy Lett. 2020, 5, 3212–3220. [Google Scholar] [CrossRef]
- Ruiz-Martínez, D.; Gómez, R. Sulfur dioxide and sulfolane as additives in organic electrolytes to develop room-temperature sodium batteries. Batteries 2022, 8, 127. [Google Scholar] [CrossRef]
- Singh, P.; Dixit, M. Opportunities and challenges in the development of layered positive electrode materials for high-energy sodium ion batteries: A computational perspective. Langmuir 2023, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Song, K.M.; Liu, C.T.; Mi, L.W.; Chou, S.L.; Chen, W.H.; Shen, C.Y. Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries. Small 2021, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.; Mathew, V.; Sambandam, B.; Hwang, J.Y.; Kim, J. A new tellurium-based Ni3TeO6-carbon nanotubes composite anode for Na-ion battery. Int. J. Energy Res. 2022, 46, 16041–16049. [Google Scholar] [CrossRef]
- Fang, H.Y.; Suning Gao, S.N.; Ren, M.; Huang, Y.H.; Cheng, F.Y.; Chen, J.; Li, F.J. Dual-function presodiation with sodium diphenyl ketone towards ultra-stable hard carbon anodes for sodium-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202214717. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.N.; Wang, J.Z.; Chou, S.L.; Liu, H.K.; Dou, S.X. Prussian blue analogues for sodium-ion batteries: Past, present, and future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Yao, Q.; Shao, R.W.; Wang, C.; Yan, W.S.; Ma, J.Z.; Liu, D.; Yang, J.; Qian, Y.T. Microsized gray tin as a high-rate and long-life anode material for advanced sodium-ion batteries. Nano Lett. 2022, 22, 7976–7983. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Huang, X.X.; Tang, T.Y.; Hou, Y.L. Hierarchically porous Fe2CoSe4 binary-metal selenide for extraordinary rate performance and durable anode of sodium-ion batteries. Adv. Mater. 2018, 30, 1802745. [Google Scholar] [CrossRef]
- Hou, P.Y.; Sun, Y.Y.; Li, F.; Sun, Y.M.; Deng, X.L.; Zhang, H.Z.; Xu, X.J.; Zhang, L.Q. A high energy-density P2-Na2/3[Ni0.3Co0.1Mn0.6]O2 cathode with mitigated P2-O2 transition for sodium-ion batteries. Nanoscale 2019, 11, 2787–2794. [Google Scholar] [CrossRef]
- Zhang, W.C.; Lu, J.; Guo, Z.P. Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today 2021, 50, 400–417. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.H.; Hu, X.D.; Fan, F.R.; Cai, S.; Zheng, C.M.; Stucky, G.D. Review on comprehending and enhancing the initial Coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries. Nano Energy 2020, 77, 105143. [Google Scholar] [CrossRef]
- Che, H.Y.; Chen, S.L.; Xie, Y.Y.; Wang, H.; Amine, K.; Liao, X.-Z.; Ma, Z.-F. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.J.; Hu, X.L. Ionogel-based membranes for safe lithium/sodium Batteries. Adv. Mater. 2022, 34, 2200945. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery–A short review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Monti, D.; Ponrouch, A.; Palacin, M.R.; Johansson, P. Towards safer sodium-ion batteries via organic solvent/ionic liquid based hybrid electrolytes. J. Power Sources 2016, 324, 712–721. [Google Scholar] [CrossRef]
- Ma, Q.; Tietz, F. Solid-state electrolyte materials for sodium batteries: Towards practical applications. ChemElectroChem 2020, 7, 2693–2713. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Han, J.; Guo, C.; Tan, Y.; Jiang, K.; Wang, K. Progress on polymer electrolyte in sodium ion batteries. Energy Storage Sci. Technol. 2020, 9, 1300–1308. [Google Scholar]
- Su, Y.; Rong, X.H.; Gao, A.; Liu, Y.; Li, J.W.; Mao, M.L.; Qi, X.G.; Chai, G.L.; Zhang, Q.H.; Suo, L.M.; et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 2022, 13, 4181. [Google Scholar] [CrossRef]
- Lei, D.N.; He, Y.-B.; Huang, H.J.; Yuan, Y.F.; Zhong, G.M.; Zhao, Q.; Hao, X.G.; Zhang, D.F.; Lai, C.; Zhang, S.W.; et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Xue, L.G.; Goodenough, J.B.; Manthiram, A. Ambient-temperature all-solid-state sodium batteries with a laminated composite electrolyte. Adv. Funct. Mater. 2021, 31, 2002144. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhang, M.; Chen, Z.; Du, X.F.; Huang, S.Q.; Tang, B.; Dong, T.T.; Wu, H.; Yu, Z.; Zhang, J.J.; et al. Flame-retardant quasi-solid polymer electrolyte enabling sodium metal batteries with highly safe characteristic and superior cycling stability. Nano Res. 2019, 12, 2230–2237. [Google Scholar] [CrossRef]
- Yin, H.; Han, C.J.; Liu, Q.R.; Wu, F.Y.; Zhang, F.; Tang, Y.B. Recent advances and perspectives on the polymer electrolytes for sodium/potassium-ion batteries. Small 2021, 17, 2006627. [Google Scholar] [CrossRef]
- Natarajan, S.; Subramanyan, K.; Aravindan, V. Focus on spinel Li4Ti5O12 as insertion type anode for high-performance Na-ion batteries. Small 2019, 15, 1904484. [Google Scholar] [CrossRef]
- Liang, H.P.; Chen, Z.; Dong, X.; Zinkevich, T.; Indris, S.; Passerni, S.; Bresser, D. Photo-cross-linked single-ion conducting polymer electrolyte for lithium-metal batteries. Macromol. Rapid Commun. 2022, 43, 2100820. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Li, H.H.; Passerini, S.; Bresser, D. Single-ion conducting polymer electrolyte for superior sodium metal batteries. Angew. Chem. Int. Ed. 2023, 62, e202308699. [Google Scholar] [CrossRef] [PubMed]
- Zamory, J.; Bedu, M.; Fantini, S.; Passerini, S.; Paillard, E. Polymeric ionic liquid nanoparticles as binder for composite Li-ion electrodes. J. Power Sources 2013, 240, 745–752. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J.L.; Meabe, L.; Riva, R.; Guzmán-González, G.; Porcarelli, L.; Forsyth, M.; Mugica, A.; Calafel, I.; Müller, A.J.; Lecomte, P.; et al. Flame retardant polyphosphoester copolymers as solid polymer electrolyte for lithium batteries. Polym. Chem. 2021, 12, 3441. [Google Scholar] [CrossRef]
- Nair, J.R.; Bella, F.; Angulakshmi, N.; Stephan, A.M.; Gerbaldi, C. Nanocellulose-laden composite polymer electrolytes for high performing lithium–sulphur batteries. Energy Storage Mater. 2016, 3, 69–76. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhu, H.J.; Wang, X.E.; Armand, M.; MacFarlane, D.R.; Forsyth, M. Synthesis of sodium poly [4-styrenesulfonyl(trifluoromethylsulfonyl)imide]-co-ethylacrylate] solid polymer electrolytes. Electrochim. Acta 2015, 175, 232–239. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the solid electrolyte interphase (SEI) in sodium ion batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Grundish, N.S.; Goodenough, J.B.; Khani, H. Designing composite polymer electrolytes for all-solid state lithium batteries. Electrochemistry 2021, 30, 100828. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.T.; Li, S.P.; Fan, L.Z.; Nan, C.W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Fang, R.Y.; Li, Y.T.; Wu, N.; Xu, B.Y.; Liu, Y.J.; Manthiram, A.; Goodenough, J.B. Ultra-thin single-particle-layer sodium beta-alumina based composite polymer electrolyte membrane for sodium-metal batteries. Adv. Funct. Mater. 2023, 33, 2211229. [Google Scholar] [CrossRef]
- Guo, J.Z.; Yang, A.B.; Gu, Z.Y.; Wu, X.L.; Pang, W.L.; Ning, Q.L.; Li, W.H.; Zhang, J.P.; Su, Z.M. Quasi-solid-state sodium-ion full battery with high-power/energy densities. ACS Appl. Mater. Interfaces 2018, 10, 17903–17910. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, C.; Zeng, R.; Liu, Y.J.; Zhang, W.; Wang, Y.J.; Liu, Q.J.; Huang, Y.H. In situ-formed hierarchical metal-organic flexible cathode for high-energy sodium-ion batteries. ChemSusChem 2017, 10, 4704–4708. [Google Scholar] [CrossRef]
- Chae, W.; Kim, B.; Ryoo, W.S.; Earmme, T. A brief review of gel polymer electrolytes using in situ polymerization for lithium-ion polymer batteries. Polymers 2023, 15, 803. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.T.; Stalin, S.; Khan, K.; Archer, L.A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 2019, 4, 365–373. [Google Scholar] [CrossRef]
- Liu, T.T.; Zhang, J.J.; Wu, H.; Zhang, J.N.; Ding, G.; Dong, S.M.; Cui, G.L. In situ polymerization for integration and interfacial protection towards solid state lithium batteries. J. Electrochem. Soc. 2020, 167, 070527. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, Y.Z.; Liu, Y.; Qin, B.S.; Yang, Z.H.; Sun, Y.B.; Zeng, D.L.; Varzib, A.; Passerinib, S.; Liu, Z.H.; et al. Highly porous single-ion conductive composite polymer electrolyte for high performance Li-ion batteries. J. Power Sources 2018, 397, 79–86. [Google Scholar] [CrossRef]
- Nair, J.R.; Colò, F.; Kazzazi, A.; Moreno, M.; Bresserb, D.; Lin, R.L.; Bella, F.; Meligrana, G.; Fantini, S.; Simonetti, E.; et al. Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J. Power Sources 2019, 412, 398–407. [Google Scholar] [CrossRef]
- Nair, J.R.; Cíntora-Juárezb, D.; Pérez-Vicenteb, C.; Tiradob, J.L.; Ahmadc, S.; Gerbaldia, C. Truly quasi-solid-state lithium cells utilizing carbonate free polymer electrolytes on engineered LiFePO4. Electrochim. Acta 2016, 199, 172–179. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Yoon, H.; Forsyth, M.; MacFarlane, D.R. Gelled ionic liquid sodium ion conductors for sodium batteries. Electrochim. Acta 2015, 169, 376–381. [Google Scholar] [CrossRef]
- Sun, B.; Liao, I.-Y.; Tan, S.; Bowden, T.; Brandell, D. Solid polymer electrolyte coating from a bifunctional monomer for three-dimensional microbattery applications. J. Power Sources 2013, 238, 435–441. [Google Scholar] [CrossRef]
- Fang, R.Y.; Xu, B.Y.; Grundish, N.S.; Xia, Y.; Li, Y.T.; Lu, C.W.; Yijie Liu, Y.J.; Wu, N.; Goodenough, J.B. Li2S6-integrated PEO-based polymer electrolytes for all-solid-state lithium-metal batteries. Angew. Chem. Int. Ed. 2021, 60, 17701–17706. [Google Scholar] [CrossRef]
- Khani, H.; Kalami, S.; Goodenough, J.B. Micropores-in-macroporous gel polymer electrolytes for alkali metal batteries. Sustain. Energ. Fuels 2020, 4, 177. [Google Scholar] [CrossRef]
- Meisner, Q.J.; Jiang, S.S.; Cao, P.F.; Glossmann, T.; Hintennach, A.; Zhang, Z.C. An in situ generated polymer electrolyte via anionic ring-opening polymerization for lithium-sulfur batteries. J. Mater. Chem. A 2021, 9, 25927–25933. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Anothumakkool, B.; Kurungot, S.; Winter, M.; Nair, J.R. In situ polymerization process: An essential design tool for lithium polymer batteries. Energy Environ. Sci. 2021, 14, 2708–2788. [Google Scholar] [CrossRef]
- Ma, J.; Feng, X.Y.; Wu, Y.Y.; Wang, Y.D.; Liu, P.C.; Shang, K.; Jiang, H.; Hou, X.L.; Mitlin, D.; Xiang, H.F. Stable sodium anodes for sodium metal batteries (SMBs) enabled by in-situ formed quasi solid-state polymer electrolyte. J. Energy Chem. 2023, 77, 290–299. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhang, H.R.; Zhou, Q.; Qu, H.T.; Dong, T.T.; Zhang, M.; Tang, B.; Zhang, J.J.; Cui, G.L. Safety-enhanced polymer electrolytes for sodium batteries: Recent progress and perspectives. ACS Appl. Mater. Interfaces 2019, 11, 17109–17127. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Li, W.J.; Liu, X.X.; Zhang, J.W.; Feng, X.M.; Chen, W.H. Progress in gel polymer electrolytes for sodium-ion batteries. Energy Environ. Mater. 2022, 6, e12422. [Google Scholar] [CrossRef]
- Gebert, F.; Knott, J.; Gorkin III, R.; Chou, S.-L.; Dou, S.-X. Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Qiao, L.X.; Judez, X.; Rojo, T.; Armand, M.; Zhang, H. Polymer electrolytes for sodium batteries. J. Electrochem. Soc. 2020, 167, 070534. [Google Scholar] [CrossRef]
- Bella, F.; Colý, F.; Nair, J.R.; Gerbaldi, C. Photopolymer electrolytes for sustainable, upscalable, safe, and ambient-temperature sodium-ion secondary batteries. ChemSusChem 2015, 8, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wei, Z.Y.; Wang, H.Y.; Huang, H.J.; Jiang, Y.; Wu, X.J.; Yao, X.Y.; Wu, Z.-S.; Yu, Y. Toward high energy density all solid-state sodium batteries with excellent flexibility. Adv. Energy Mater. 2020, 10, 1903698. [Google Scholar] [CrossRef]
- Wen, P.C.; Lu, P.F.; Shi, X.Y.; Yao, Y.; Shi, H.D.; Liu, H.Q.; Yu, Y.; Wu, Z.-S. Photopolymerized gel electrolyte with unprecedented room-temperature ionic conductivity for high-energy density solid-state sodium metal batteries. Adv. Energy Mater. 2021, 11, 2002930. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Y.; Li, B.H.; Fan, H.B.; Cheng, F.L.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G.X. A stable quasi-solid-state sodium-sulfur battery. Angew. Chem. Int. Ed. 2018, 57, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Panzer, M.J. Zwitterionic copolymer-supported ionogel electrolytes featuring a sodium salt/ionic liquid solution. Chem. Mater. 2020, 32, 7951–7957. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.G.; Ahn, Y.; Kim, D.; Park, J.H. Optimized ion-conductive pathway in UV-cured solid polymer electrolytes for all-solid lithium/sodium ion batteries. J. Membr. Sci. 2021, 619, 118711. [Google Scholar] [CrossRef]
- Chen, S.L.; Che, H.Y.; Feng, F.; Liao, J.P.; Wang, H.; Yin, Y.M. Poly(vinylene carbonate)-based composite polymer electrolyte with enhanced interfacial stability to realize high-performance room temperature solid-state sodium batteries. ACS Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef]
- Gao, H.C.; Zhou, W.D.; Park, K.; Goodenough, J.B. A sodium-ion battery with a low-cost cross-linked gel-polymer electrolyte. Adv. Energy Mater. 2016, 6, 1600467. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Xiao, Z.C.; Han, D.Z.; Yang, L.P.; Zhang, J.Y.; Tang, W.; Shu, C.Y.; Peng, C.X.; Zhou, D.P. Approaching practically accessible and environmentally adaptive sodium metal batteries with high loading cathodes through in situ interlock interface. Adv. Funct. Mater. 2022, 32, 2111314. [Google Scholar] [CrossRef]
- Zhang, W.C.; Zhang, J.; Liu, X.C.; Li, H.; Guo, Y.; Geng, C.N.; Tao, Y.; Yang, Q.-H. In-situ polymerized gel polymer electrolytes with high room-temperature ionic conductivity and regulated Na+ solvation structure for sodium metal batteries. Adv. Funct. Mater. 2022, 32, 2201205. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.L.; Zhang, J.; Qi, X.G.; He, Y.-B.; Li, B.H.; Yang, Q.H.; Hu, Y.-S.; Kang, F.Y. In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 2017, 33, 45–54. [Google Scholar] [CrossRef]
- Chen, G.H.; Zhang, K.; Liu, Y.R.; Ye, L.; Gao, Y.S.; Lin, W.R.; Xu, H.J.; Wang, X.R.; Bai, Y.; Wu, C. Flame-retardant gel polymer electrolyte and interface for quasi-solid-state sodium ion batteries. Chem. Eng. J. 2020, 401, 126065. [Google Scholar] [CrossRef]
- Park, T.-H.; Park, M.-S.; Ban, A.-H.; Lee, Y.-S.; Kim, D.-W. Nonflammable gel polymer electrolyte with ion-conductive polyester networks for sodium metal cells with excellent cycling stability and enhanced safety. ACS Appl. Energy Mater. 2021, 4, 10153–10162. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhao, Y.H.; Feng, X.M.; Chen, W.H.; Zhao, Y.F. Novel safer phosphonate-based gel polymer electrolytes for sodium-ion batteries with excellent cycling performance. J. Mater. Chem. A 2018, 6, 6559–6564. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Liu, X.L.; Duan, Y.L.; Chen, L.J.; Zhang, X.C.; Feng, X.M.; Chen, W.H.; Hao, Y.F. Stable cross-linked gel terpolymer electrolyte containing methyl phosphonate for sodium ion batteries. J. Membrane Sci. 2019, 58, 163–170. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Yang, Y.W.; Li, W.J.; Feng, X.M.; Chen, W.H.; Zhao, Y.F. Novel flame retardant rigid spirocyclic biphosphate based copolymer gel electrolytes for sodium ion batteries with excellent high-temperature performance. J. Mater. Chem. A 2020, 8, 22962–22968. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Sun, Y.K.; Li, W.J.; Feng, X.M.; Chen, W.H.; Zhao, Y.F. Effects of comonomers on the performance of stable phosphonate-based gel terpolymer electrolytes for sodium-ion batteries withultralong cycling stability. ACS Appl. Mater. Interfaces 2021, 13, 25024–25035. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, W.; Li, S.; Li, X.; Sun, C. Durable sodium battery with a flexible Na3Zr2Si2PO12-PVDF-HFP composite electrolyte and sodium/carbon cloth anode. ACS Appl Mater Interfaces 2018, 10, 35039–35046. [Google Scholar] [CrossRef]
- Xu, X.; Lin, K.; Zhou, D.; Liu, Q.; Qin, X.; Wang, S.; He, S.; Kang, F.; Li, B.; Wang, G. Quasi-solid-state dual-ion sodium metal batteries for low-cost energy storage. Chem 2020, 6, 902–918. [Google Scholar] [CrossRef]
- Li, W.; Yao, Z.; Zhang, S.; Wang, X.; Xia, X.; Gu, C.; Tu, J. High-performance Na3V2(PO4)2F2.5O0.5 cathode: Hybrid reaction mechanism study via ex-situ XRD and sodium storage properties in solid-state batteries. Chem. Eng. J. 2021, 423, 130310. [Google Scholar] [CrossRef]
- Luo, C.; Li, Q.; Shen, D.; Zheng, R.; Huang, D.; Chen, Y. Enhanced interfacial kinetics and fast Na+ conduction of hybrid solid polymer electrolytes for all-solid-state batteries. Energy Storage Mater. 2021, 43, 463–470. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wen, H.J.; Yue, L.P.; Chai, J.C.; Ma, J.; Hu, P.; Ding, G.L.; Wang, Q.F.; Liu, Z.H.; Cui, G.L.; et al. In situ formation of polysulfonamide supported poly(ethylene glycol) divinyl ether based polymer electrolyte toward monolithic sodium ion batteries. Small 2017, 13, 1601530. [Google Scholar] [CrossRef]
- Niu, Y.B.; Yin, Y.X.; Wang, W.P.; Wang, P.F.; Ling, W.; Xiao, Y.; Guo, Y.G. In situ copolymerizated gel polymer electrolyte with cross-linked network for sodium-ion batteries. CCS Chem. 2019, 1, 589–597. [Google Scholar] [CrossRef]
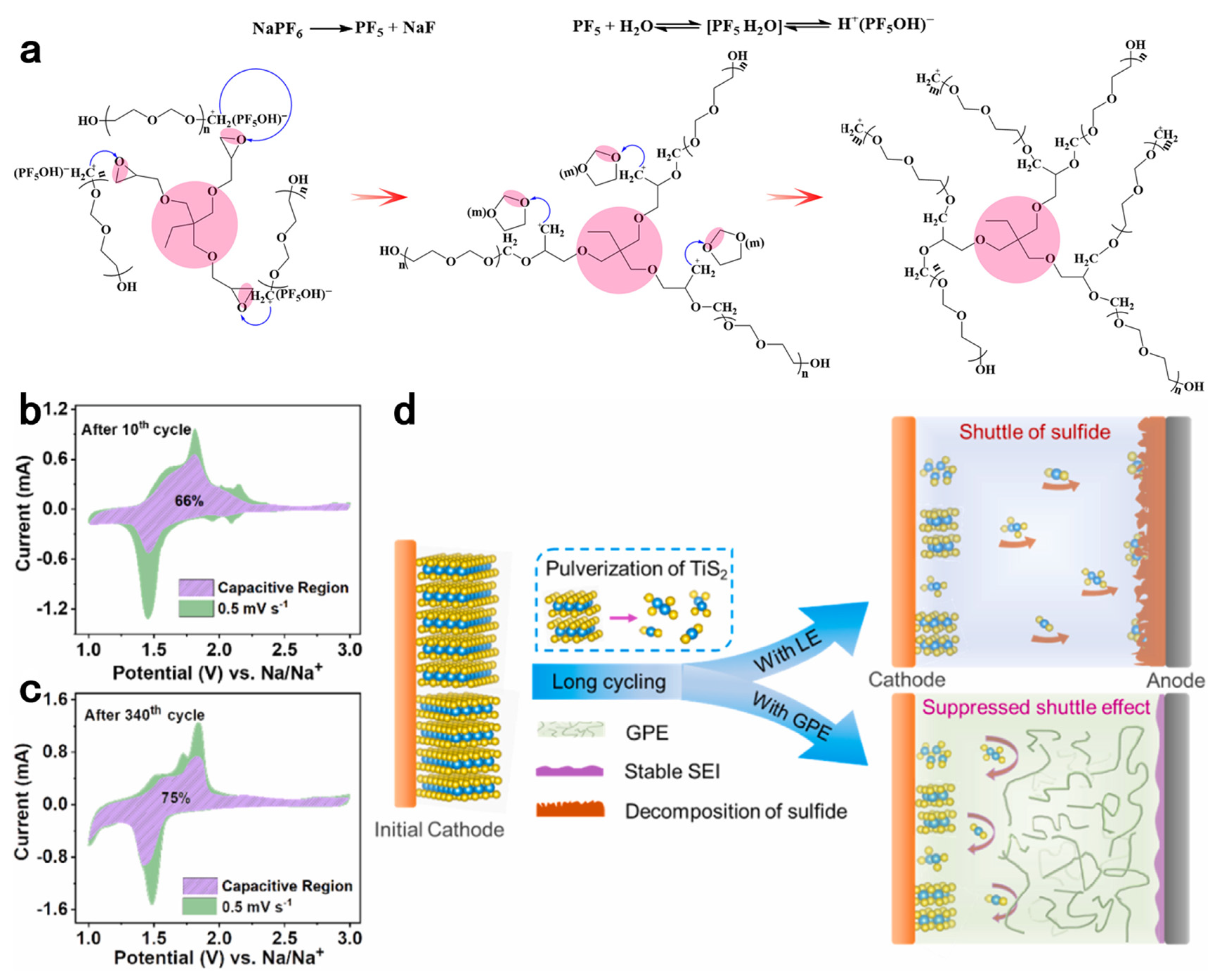
- Zhang, X.Q.; Tang, S.; Li, X.; Guo, W.; Fu, Y.Z. Ultrastable Na-TiS2 battery enabled by in situ construction of gel polymer electrolyte. J. Power Sources 2021, 516, 230653. [Google Scholar] [CrossRef]
- Liu, X.Z.; Lei, X.F.; Wang, Y.-G.; Ding, Y. Prevention of Na corrosion and dendrite growth for long-life flexible Na-Air Batteries. ACS Central Sci. 2021, 7, 335–344. [Google Scholar] [CrossRef]
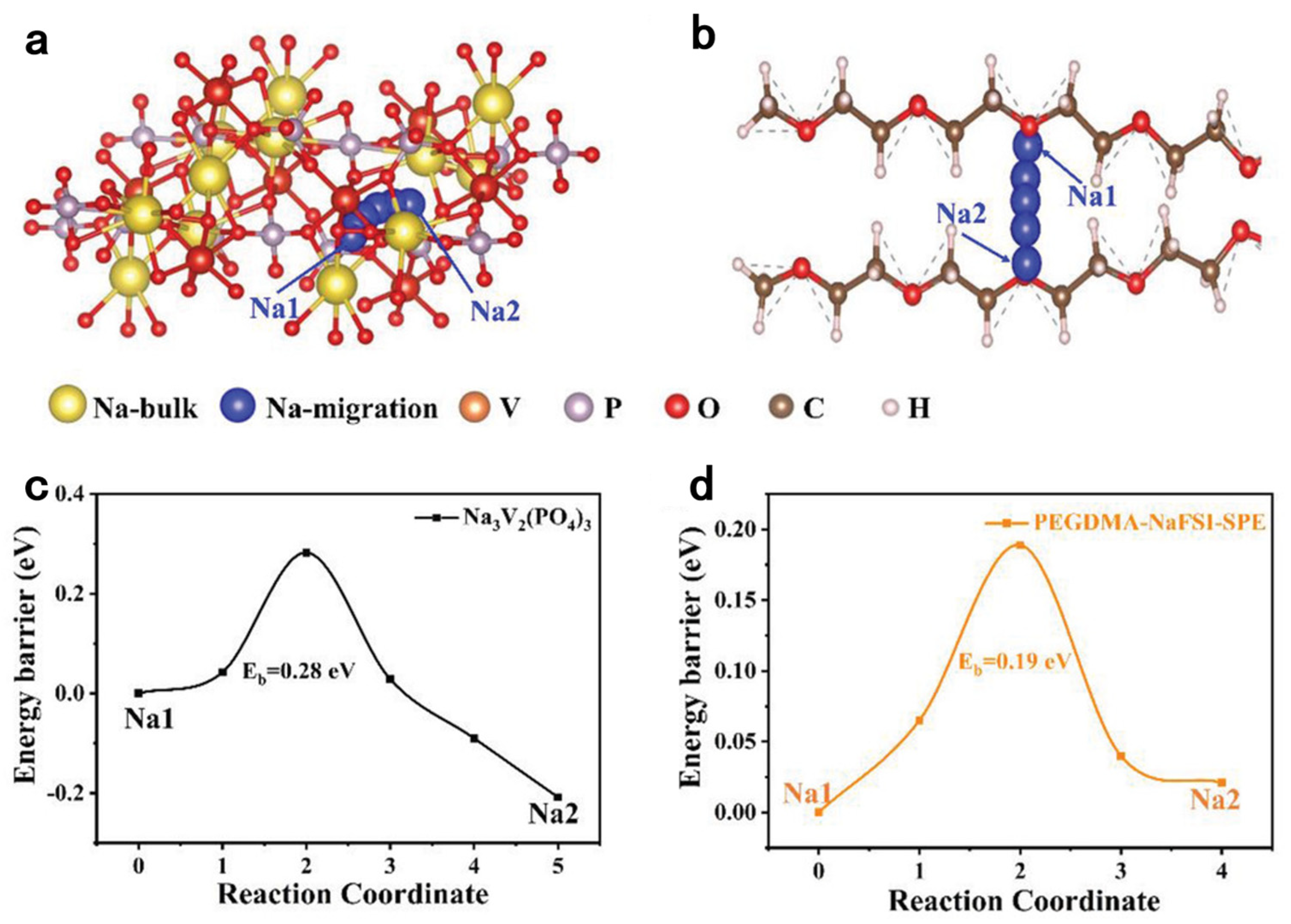
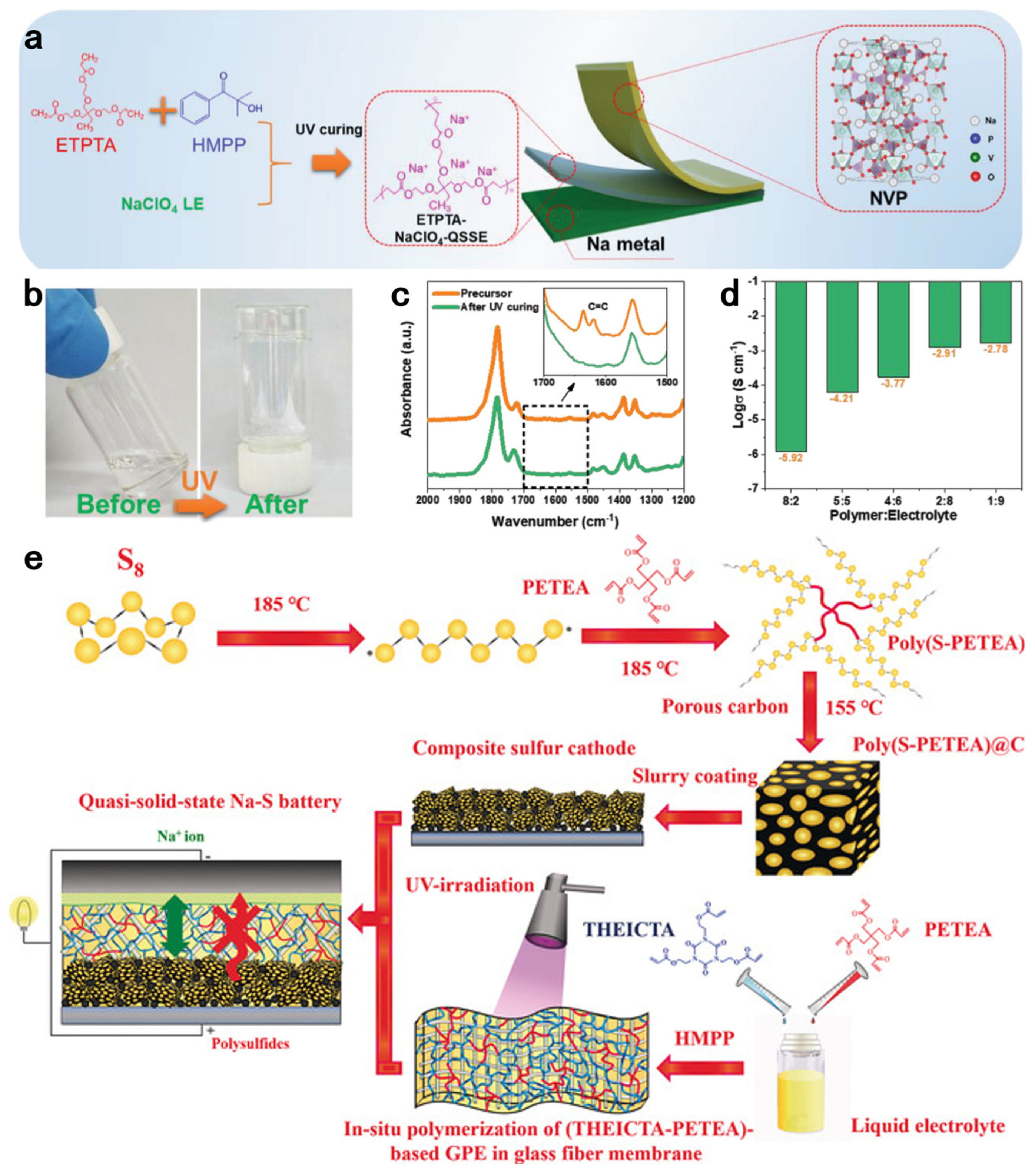

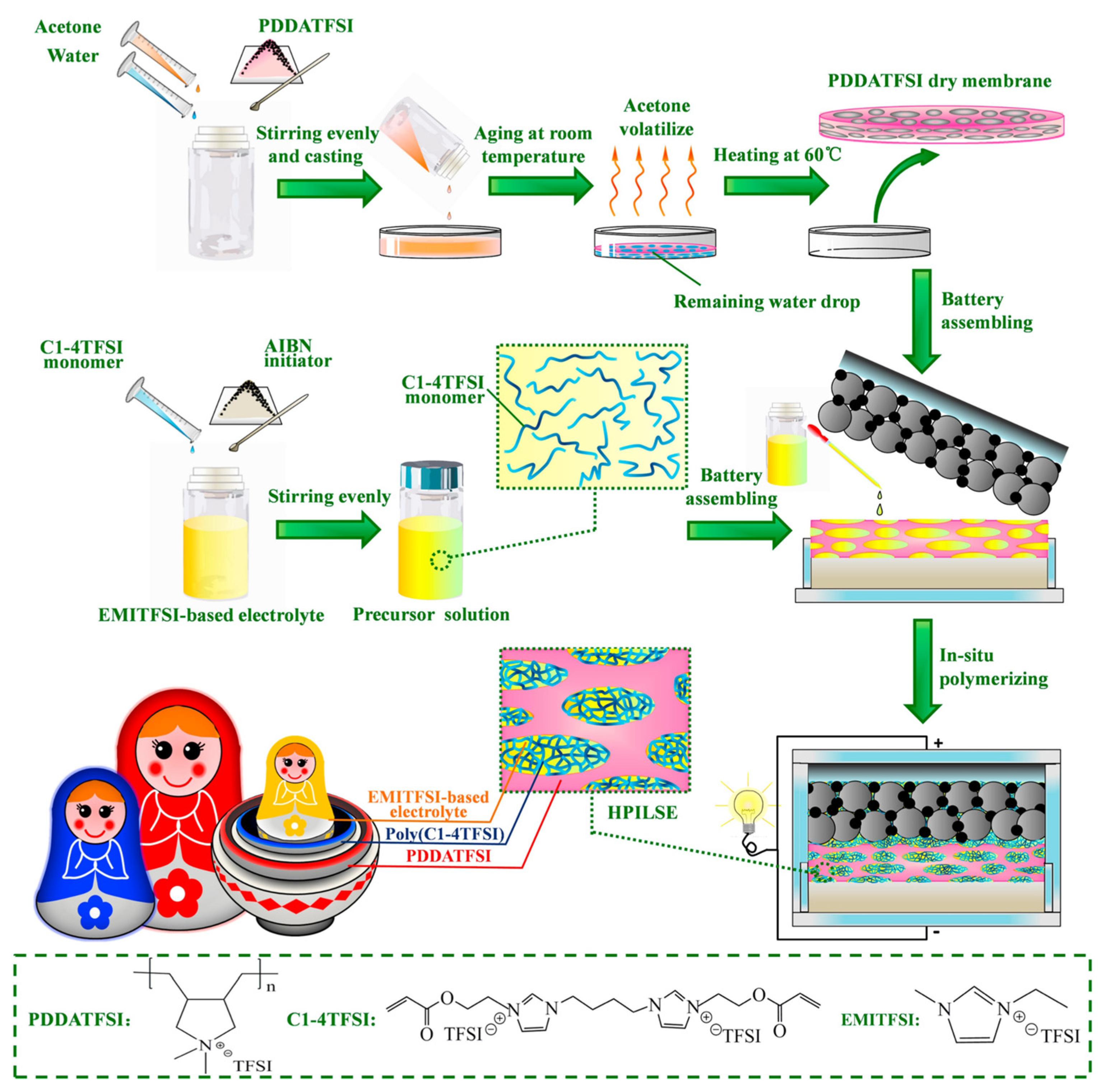
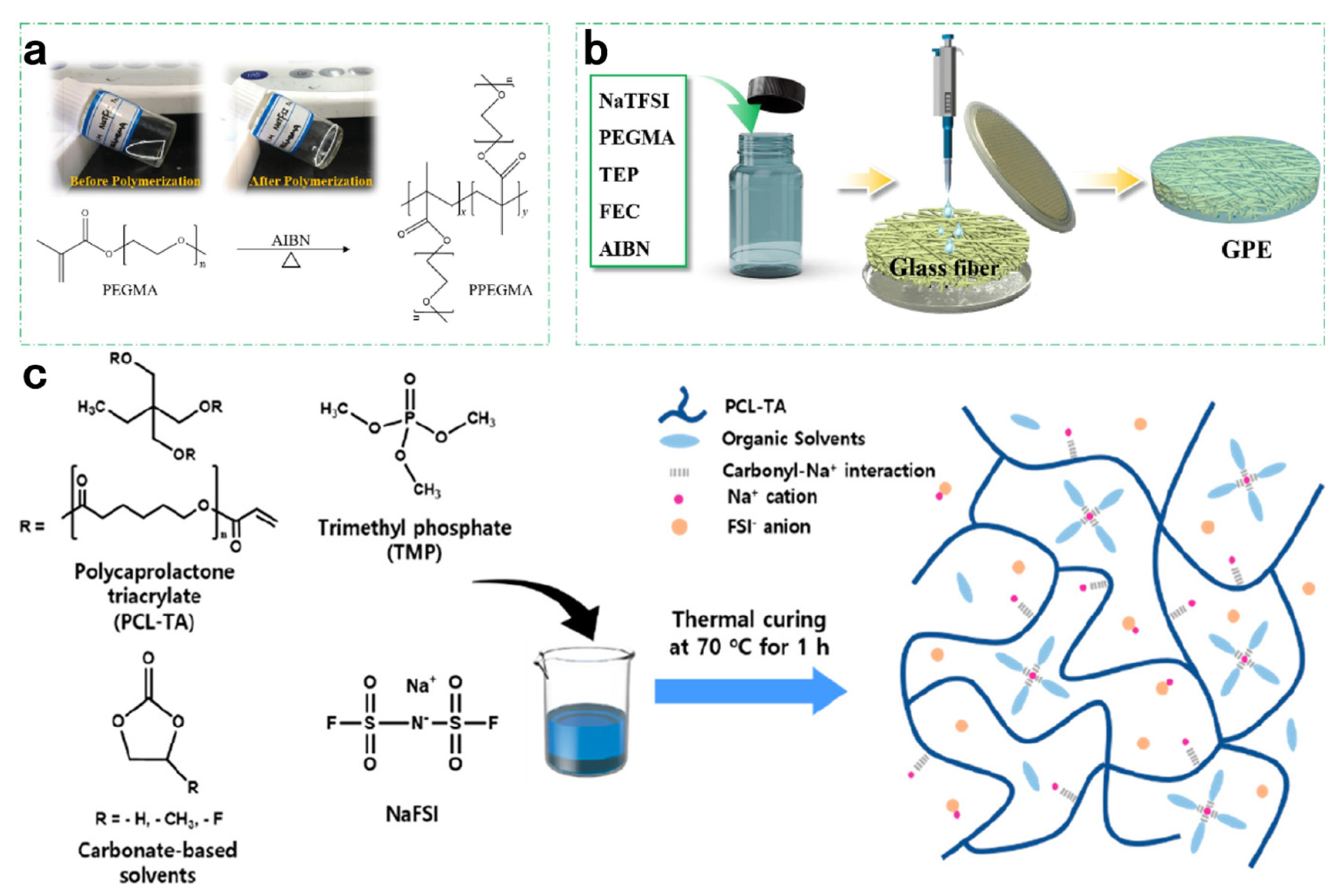

| Initiation Conditions | Solid-State Polymer Electrolyte | Ionic Conductivity σ, S/cm | Transference Number | Test Temperature | Ref |
|---|---|---|---|---|---|
| photoinduced | BEMA/PEGMA | 5.1 × 10−3 | 0.53 | 20 °C | [56] |
| PEGDMA-NaFSI-SPE | 1.1 × 10−4 | 30 °C | [57] | ||
| ETPTA-based SPE | 1.2× 10−3 | 0.62 | room temperature | [58] | |
| (PETEA-THEICTA)-based SPE | 3.85 × 10−3 | 0.34 | 25 °C | [59] | |
| thermally induced | PVC-CPE | 1.2 × 10−4 | 0.6 | 25 °C | [62] |
| PMMA-based GPE | 6.2 × 10−3 | 25 °C | [63] | ||
| TMPTA-based SPE | 7.16 × 10−4 | 0.62 | 30 °C | [64] | |
| poly(BA)-based SPE | 1.6 × 10−3 | 0.39 | room temperature | [65] | |
| HPILSE | 1.15 × 10−3 | 25 °C | [66] | ||
| (PEGMA)-based SPE | 9.1 × 10−4 | 0.24 | 27 °C | [67] | |
| (PCL-TA)-based SPE | 6.3 × 10−3 | room temperature | [68] | ||
| (MADEMP)-based SPE | 3.37 × 10−3 | 0.52 | room temperature | [72] | |
| cationic induced | PPDE-CPE | 1.2 × 10−3 | 0.46 | room temperature | [77] |
| GPE-CPN | 8.2 × 10−4 | 0.46 | room temperature | [78] | |
| DOL-based SPE | 3.66 × 10−4 | 0.66 | room temperature | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Wang, D.; Yuan, Z.; Zhang, H.; Tian, S.; Zhang, Y.; Zhang, B.; Han, Y.; Zhang, J.; Cui, G. In-Situ Polymerized Solid-State Polymer Electrolytes for High-Safety Sodium Metal Batteries: Progress and Perspectives. Batteries 2023, 9, 532. https://doi.org/10.3390/batteries9110532
Hu S, Wang D, Yuan Z, Zhang H, Tian S, Zhang Y, Zhang B, Han Y, Zhang J, Cui G. In-Situ Polymerized Solid-State Polymer Electrolytes for High-Safety Sodium Metal Batteries: Progress and Perspectives. Batteries. 2023; 9(11):532. https://doi.org/10.3390/batteries9110532
Chicago/Turabian StyleHu, Sijia, Duo Wang, Zhixiang Yuan, Hao Zhang, Songwei Tian, Yalan Zhang, Botao Zhang, Yongqin Han, Jianjun Zhang, and Guanglei Cui. 2023. "In-Situ Polymerized Solid-State Polymer Electrolytes for High-Safety Sodium Metal Batteries: Progress and Perspectives" Batteries 9, no. 11: 532. https://doi.org/10.3390/batteries9110532
APA StyleHu, S., Wang, D., Yuan, Z., Zhang, H., Tian, S., Zhang, Y., Zhang, B., Han, Y., Zhang, J., & Cui, G. (2023). In-Situ Polymerized Solid-State Polymer Electrolytes for High-Safety Sodium Metal Batteries: Progress and Perspectives. Batteries, 9(11), 532. https://doi.org/10.3390/batteries9110532








